Abstract
The lens of the eye is a radiosensitive tissue in the human body. The main detriment following an exposure by ionizing radiation is a cataract of the eye lens but not tumor induction. In 1990, the ICRP has recommended an annual dose limit for the eye lens of 150 mSv for occupational exposed persons and 15 mSv for the public. These limits have again been recommended by ICRP in 2007, but in a recent ICRP statement in 2011, it has been recommended to lower the limit from 150 to 20 mSv per year due to various new epidemiological data dealing with cataracts. The specific structure of the lens, where the stem cells are located in the frontal outer equator region of the lens only, results in a very different radiosensitivity within the eye lens. This becomes important in cases of exposure by low-penetrating radiation, e.g., electrons. The lens of the eye cannot be well modeled by the reference voxel phantoms of ICRP. A detailed geometrical model developed by Behrens et al. is described, and calculations of dose distribution and conversion coefficients for incident electrons and photons are presented. It is discussed which operational dose quantity is appropriate for measurements assessing the equivalent dose to the lens of the eye for incident electrons or photons. Neutrons are of less importance for this discussion.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Conversion Coefficient
- Electron Exposure
- Individual Monitoring
- Secondary Charged Particle
- Photon Exposure
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The lens of the eye is a specific part of the human body which is very sensitive to exposure by ionizing radiation, not with respect to cancer induction but mainly due to the induction of a cataract in the lens of the eye. In the past, the cataract induced by ionizing radiation has been seen as a deterministic effect (non-stochastic) with an absorbed dose threshold of 0.5–2.0 Gy for short-time exposures and 5–6 Gy for long-time exposure with low dose rate. This means that it was assumed that a cataract is not induced if the mean absorbed dose in the lens is less than about 0.5 Gy. As a consequence, the International Commission on Radiological Protection (ICRP) has not included the lens of the eye into the system of organs and tissues specified for the definition of an effective dose, E (see Table A.3.1 in ICRP Publication 103 [1]), a quantity which is mainly designed for an application at low doses where only stochastic effects, e.g. induction of cancer, and no deterministic effects are present.
Therefore, ICRP had defined a specific annual dose limit for the lens of the eye of 150 mSv for occupationally exposed persons and 15 mSv for the public and hence by values far below the dose threshold for cataract induction. Recently, however, some studies have shown [2, 3] that a cataract may be induced at even lower doses of ionizing radiation, and in its Publication 103 [1], the ICRP has mentioned that further research is seen to be necessary before deciding if annual dose limits for the lens of the eye should be lowered. During recent years, more epidemiological data became available from cataract studies on atomic bomb survivors in Japan [4, 5], from studies among Chernobyl cleanup workers [2], from occupational exposure of persons in radiology [3], and from studies on pilots and astronauts in space [6]. Based on these new data, the ICRP in a statement [7] has recently recommended to lower the limit for the lens of the eye from 150 to 20 mSv per year averaged over 5 years but not exceeding 50 mSv in a single year. This new recommendation lowers the limit by more than a factor of 7 and may have strong consequences for applications of interventional treatments in medicine where the physicians and other medical persons may achieve higher eye lens doses. Obviously, the dosimetry of the eye lens will also get more attention than before.
In the following, some information is given about the eye and its lens and the sensitivity of the lens to ionizing radiation. The modeling of the eye and its lens and the determination of doses from external exposure by electrons and photons are described. Furthermore, it is discussed which operational dose quantity is appropriate for monitoring the dose to the lens of the eye sufficiently precise for applications in radiological protection. Exposure to neutrons or heavy ions is not discussed because such exposures are restricted to very few cases, e.g. to astronauts in space or in accidental situations.
2 The Eye and Its Lens
It is not the aim to describe the eye and its function in detail. A schematic model of the eye is given in Fig. 4.1a. Most data of the eye geometry are from Charles and Brown [8]. The lens of the eye is small with a volume of about 0.216 cm3 corresponding to a mass of 229 mg. The lens is positioned near the front of the eye in a depth of about 3.2 mm. The lens (see Fig. 4.1b) contains in the central part the embryonic nucleus which is surrounded by fiber cells without any inner structure (no cell nucleus, no protein structures) in order to improve the optical properties of the lens.
Epithelial cells with a cell nucleus are mainly positioned near to the front surface of the lens especially at the outer region near to the equator. While those cells are most sensitive to radiation exposure and are seen to be the target for mutations in the cell nucleus induced by the radiation, the cataract itself occurs mostly near to the back surface of the lens (cortical posterior cataract), but generally, also cataracts at other places within the lens are possible.
While for most organs and tissues ICRP recommends to use the mean absorbed dose in an organ or tissue, D T, as the basis in radiation protection applications, the ICRP stated already in 1955 in the Supplement 6 of its first general recommendation [9]: “When the spatial distribution of radiation in the organ is very non-uniform, an average of the physical dose is not necessarily indicative of the potential damage to the organ in its relation to the normal physiological functions of the body as a whole. Therefore, in such cases it is necessary to consider a local volume within the organ in which the dose is highest. This may be called the significant volume… For the lens of the eye the significant volume is that in which the cell nuclei are located.” Hence, a realistic model of the eye lens should consider this situation.
The latency time between an eye exposure and the appearance of a cataract varies from some months up to about 20 years depending on the applied dose. Low doses result in longer latency times.
3 Modeling of the Eye and Its Lens
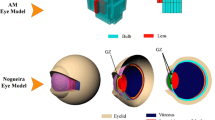
Doses to the lens of the eye cannot be measured directly. They are generally determined by Monte Carlo calculations using appropriate models of the eye and the surrounding head. Especially for low-penetrating radiation with its small range in tissue, the modeling of the eye plays a very important role in those calculations. For example, data of conversion coefficients H T/Φ (H T: equivalent dose to the eye lens, Φ : fluence of incident electrons) were published in ICRP Publication 74 [10] based on data from Schultz and Zoetelief [11] which were calculated using a geometrical phantom ADAM of Kramer et al. [12] with the eye lens positioned at the surface and no material in front of the lens. Also, the reference voxel phantoms recommended by ICRP in 2007 [1] are not well suited for modeling of the eye lens. A voxel phantom simulates the human body by a large number of small volume elements (voxels) where each element is related to a specific type of tissue with a given density and atomic composition. Because of the specific selection of the voxel sizes which are too large compared to the small size of the eye lens and the determination of a mean dose value in each voxel, it is difficult to calculate eye lens doses. In addition, eyelids are not considered in both models. Hence, a more realistic geometrical model of the eye has recently been developed by Behrens et al. [13]. The main information on the geometry of the eye, its atomic composition, and densities was taken from Charles and Brown [8]. This includes also information on the most radiation-sensitive part of the lens positioned near the front surface of the lens at the outer region of the equator. Figure 4.2 shows the geometrical model in detail which also includes a simple model for the eyelids. The lids are important for dose determination in the eye, when low-penetrating radiations, e.g. electrons, are considered. For example, electrons with energies below about 1.5 MeV will not penetrate the lids. All calculations described in this chapter, however, are performed for open eyes. In the lens of the eye, a small volume is separated in order to model the position of the epithelial cells, the most sensitive region within the lens with respect to cataract induction by ionizing radiation.
Geometrical model of the eye (left) and the eye lens (right) from Behrens et al. [13]
While for low-penetrating radiation, e.g. electrons, the modeling of the eye is sufficient for the calculation of doses to the eye lens, for photons and other types of penetrating radiation, the modeling of the head is also necessary in order to consider the scattering and absorption of the radiation in the surrounding tissues.
Hence, a geometrical model of the head and the body based on the geometrical ADAM and EVA phantoms of Kramer et al. [12] has been developed by Behrens et al. [14] where the medium of the head and body was simply chosen to be ICRU 4-element standard tissue [15], however, with a density of 1.11 g/cm³. This simple model which is shown in Fig. 4.3 is sufficient when only scattering and absorption need to be considered and not the doses in these tissues.
Views of the geometry used in the Monte Carlo simulations [14]. Different colors indicate different materials. (left) Complete body, (middle) head, and eyes from the side and (right) head and eyes from the top with a cut at the center of the eyes
4 Monte Carlo Calculations
There exist various Monte Carlo radiation transport codes for the calculation of mean absorbed doses in organs and tissues of the human body which are simulated by geometrically designed anthropomorphic phantoms or anthropomorphic voxel phantoms. For the calculation of doses in the lens of the eye, different codes have been used.
An important point in the calculation of doses in tissue is if the codes use the “kerma approximation” or a full follow-up of the secondary charged particles in the material considered (see Fig. 4.4). Kerma approximation means that the energy transferred to the matter by the production of secondary charged particles is taken to be fully deposited in matter at the point or volume element where the interaction takes place ignoring the finite range of these particles in matter. It may be applied in cases when the incident particles are photons or other uncharged particles, e.g. neutrons. The different calculation procedures mainly result in differences near the entrance surface or near boundaries of different tissues (see Fig. 4.4). As shown below, for high-energy photons, the calculated mean absorbed dose in the eye lens strongly depends on the choice of the calculation procedure.
For dosimetric applications, often conversion coefficients are calculated using Monte Carlo codes, e.g. D T/Φ, H T/Φ, and E/Φ, where D T and H T are the mean absorbed dose and the mean equivalent dose in an organ or tissue T of the body. E is the effective dose and Φ the fluence of the radiation incident on the anthropomorphic phantom. Conversion coefficients are mainly published for simple exposure conditions assuming an external field with a constant fluence over the size of the body, e.g. frontal exposure of the body (AP), exposure from the back (AP), exposure from the left- (LLAT) or right- (RLAT) hand side, isotropic exposure (ISO), or rotational isotropic (ROT) exposure along the vertical axis of the body. For photons, the dose in the body is often related to the air kerma of the incident radiation outside of the body.
For the lens of the eye, most important data are those for AP exposure. For photons and electrons, D T and H T are numerically equal because the radiation weighting factor for photons and electrons of all energies is one. Data of conversion coefficients for the mean equivalent dose of the eye lens for photon and electron exposure published in ICRP Publication 74 [10] were taken from Schultz and Zoetelief [11] which were calculated using the geometrically designed ADAM phantom [12] with no tissue in front of the eye lens and the use of the kerma approximation.
Mean doses of the eye lens for photon exposure have also been calculated by Schlattl et al. [16] using the reference male and female voxel phantoms REX and REGINA defined by the ICRP in its 2007 Recommendations [1] and a full secondary charged particles follow-up. Data for electron exposure have also been obtained using the same voxel phantoms (Zankl M, private communication, 2008).
Recently, Behrens et al. [13, 14] have performed calculations for photons and electrons using the specific model for the eye described above and also a full secondary charged particle follow-up. In addition to the mean eye lens doses, the mean doses of the sensitive part of the eye lens were calculated. Results for photons and electrons are presented below.
5 Personal Dose Equivalent, H p(d)
Mean doses of organs and tissues in the human body cannot be measured. Hence, operational dose quantities have been defined by ICRU [15] and ICRP [1] for area and individual monitoring in situations of external exposure. For individual monitoring, the personal dose equivalent, H p(d), is the quantity used in measurements with individual dosimeters worn on the body. It is defined by:
The personal dose equivalent, H p(d), is the dose equivalent in ICRU (soft) tissue at an appropriate depth, d, below a specified point on the human body.
The specified point is usually given by the position where the individual dosimeter is worn. For the assessment of effective dose, a depth d = 10 mm, and for assessing the equivalent dose to the skin, hands, and feet, a depth d = 0.07 mm is recommended. In cases of monitoring the dose to the eye lens, a depth d = 3 mm is recommended.
ICRU (soft) tissue is an artificial tissue defined with a density of 1 g cm−3 and a mass composition of 76.2 % oxygen, 11.1 % carbon, 10.1 % hydrogen, and 2.6 % nitrogen [15].
An operational quantity for individual monitoring should provide a conservative estimate under most conditions of external irradiation. This requires that a personal dosimeter for the assessment of the dose to the eye lens must be worn at a position on the body near to the eyes, e.g. on the forehead. Calibration of individual dosimeters in terms of H p(d) is generally performed in front of standardized phantoms (see, e.g. ISO 4037-3 [17]) which simulates the backscattering of the human body. In most countries, however, the quantity H p(3) has not been introduced, and specific dosimeters for the measurements of doses to the eye lens are not yet available. In addition, there exists no international standard for the calibration of eye-lens dosimeters, and no corresponding reference phantom has yet been defined. For the calculation of conversion coefficient for H p(d) given below, therefore, the standard ISO slab and finger phantom [17] are applied. Recently, a cylinder phantom has been proposed for use in eye lens dosimetry as a cylinder much better approximates the shape of a head [18].
6 Photon Exposure of the Eye Lens
As mentioned above, conversion coefficients H eye lens/K a for exposure by monoenergetic photons have been calculated by various authors (see Fig. 4.5). It is seen that at photon energies below about 1 MeV, the various calculations agree sufficiently well, but at higher energies, there are strong differences. The highest values are those calculated using the kerma approximation (ICRP 74 data), while the other data are absorbed dose calculations with full follow-up of secondary particles. This is a typical situation for dose calculations near the surface, especially when the calculations were performed with a phantom positioned in vacuum. Rex and Regina data differ at high energies due to the differences in the material in front of the lens for the male and female voxel phantom applied. In the following, the data calculated by Behrens et al. [14] are used for further discussion.
The question will now be discussed which personal dose quantity is best suited for measurements and for the assessment of an eye lens dose. Figure 4.6 shows the various ratios of H p(10)/H eye lens and H p(0.07)/H eye lens where for H p(0.07) always two different values are shown. For photon radiation, the value of H p(0.07) depends on the size of the body or phantom in front of which a dosimeter is deposited, because of the variation in the backscattering. While usually a rod phantom simulating a finger is used for calibration of dosimeters in terms of H p(0.07), for eye-lens dosimeters, a calibration in front of a larger slab phantom (30 cm × 30 cm × 15 cm) is more appropriate. The ratio of H p(3)/H eye lens is not shown but would be about 1, if H p(3) would be determined on a head phantom. For a slab phantom, the ratio is higher than 1 because of higher backscattering. Obviously, the quantity H p(3) would be the first choice for use in eye-lens dosimetry. Actually, however, no type-tested dosimeters for H p(3) exist and also no international agreement on calibration procedures and phantoms. For photons with energies >30 keV, however, H p(0.07) on a slab phantom approximates H eye lens sufficiently precise for applications in radiation protection.
In practice, exposures of physicians and medical staff by backscattered X-rays during interventional procedures in radiology are most important when doses to the eye lens are considered. Behrens et al. [14] have, therefore, performed some calculations by simulating a situation in interventional radiology in order to check which operational quantity can be used for monitoring the dose to the eye lens. Typically, a patient may be irradiated by x-rays from above, and medical staff may stay at the side looking to the patient.
The situation used for the calculations is schematically shown in Fig. 4.7. The body is simulated by a slab of ICRU (soft) tissue (40 cm in diameter, 15 cm thick) which is exposed by a beam of x-rays of, e.g. 100 kV (radiation qualities of RQR8 (100 kV accelerating voltage)), according to the standard IEC 61267 [19]). The beam size at the phantom surface was 20 cm in diameter. Staff is standing at the side nearby and viewing to the patient. For the calculations, the backscattered radiation under 135° to the normal axis is considered, and the spectral-photon fluence at the position of the eye is determined for a phantom-eye distance of 50 cm (more details see Behrens et al. [14]). Mean conversion coefficients for different quantities are then calculated by applying the corresponding conversion coefficients for monoenergetic photons and averaging over the photon spectrum. Figure 4.8 shows results for the quantities H eye lens, H p(10), H p(3), and H p(0.07). The conversion coefficients for H p(d) are not those for a dosimeter on a head phantom but always those for the phantoms used for calibration of the individual dosimeters, hence the ISO slab phantom for H p(10) and H p(3) and for H p(0.07) both the ISO rod phantom (a) and the ISO slab phantom (b).
Mean conversion coefficients H eye lens/K a, H p(10)/K a, H p(3)/K a, and H p(0.07)/K a for backscattered x-rays as a function x-ray quality (data from Behrens et al. [14]). H p(10) and H p(3) data are those obtained in front of a slab phantom, H p(0.07) data are taken for a rod phantom (a) and for a slab phantom (b)
Obviously, both quantities H p(3) and H p(0.07) when calibrated on a slab phantom are appropriate for an assessment of the dose of the eye lens in x-ray radiation stray fields. At higher energies, however, H p(3) might be a better choice.
7 Electron Exposure of the Eye Lens
Electrons are charged particles with a relative short range in tissue. Electrons below about 0.7 MeV have a mean range of less than 3 mm in tissue (see Figs. 4.9 and 4.10) and will, therefore, not reach the lens of the eye, if electron range straggling is not considered. Dose calculations for low-energy electrons depend on the model used for the eye. Due to the different models used as has been shown in Chap. 3, dose calculations show large variations at low electron energies (see Fig. 4.10) especially at energies below 1 MeV.
Mean range of electrons in tissue versus electron energy [20]
For further discussion, the data from Behrens et al. [13] with the detailed model of the eye are used and especially those obtained for the sensitive region of the eye lens. Figure 4.11 shows data for frontal (AP) incidence of monoenergetic electrons on the eye.
Conversion coefficients H eye lens/Φ and H p(3)/Φ for electrons versus electron energy. The data are for the sensitive (s) and the insensitive (i) region of the lens and for the whole lens [13]. The data for H p(3)/Φ are for the ISO slab phantom where they are equal to H′(3,0°)/Φ. They are taken from ICRP 74 [10]
Obviously, below about 2 MeV, the data for the sensitive region of the lens agree well with the data for H p(3) especially near to the threshold between 0.7 and 1.0 MeV. This is clearly shown in Fig. 4.11, where the data for the three different operational quantities for individual monitoring are shown together with those for the eye lens. It is, therefore, recommended to use this quantity for individual monitoring in beta-radiation fields (Fig. 4.12).
Conversion coefficients H eye lens/Φ, H p(10)/Φ, H p(3)/Φ, and H p(0.07)/Φ for frontal incidence (AP) of electrons versus electron energy. The data for H p(d)/Φ are for the ISO slab and were taken from ICRP 74 [10]
All these calculations of conversion coefficients were performed with phantoms positioned in vacuum. Obviously, realistic situations are always phantoms or bodies positioned in air. This may have a strong impact on the situation with electron exposure depending on the distance between the source and the exposed phantom or body, when the energy loss of the electrons in the air cannot be ignored. Behrens et al. [21] have investigated this situation in more detail especially for realistic beta-ray sources with broad beta-energy distributions. One of the results is that for most realistic beta-ray sources, then H p(0.07) provides also a conservative assessment of the eye lens dose. For low-energy sources, however, H p(0.07) can be conservative by more than a factor of 100.
A final remark with respect to radiation protection of the eye lens is that it should always be considered that in beta-radiation fields shielding of the eye by specific spectacles can generally avoid or strongly reduce the exposure of the eye lens. In photon fields, however, spectacles are not as effective as in beta-radiation fields. Sufficient distance from a radiation source is always the best way of reducing doses to the eye lens.
References
ICRP (2007) The 2007 recommendations of the international commission on radiological protection. ICRP Publ. 103. Ann ICRP 37(2–4):1–332
Worgul BV, Kundiyev YI, Sergiyenko NM et al (2007) Cataracts among chernobyl cleanup workers: implications regarding permissible eye exposures. Radiat Res 167:233–243
Chodick G, Bekiroglu N, Hauptmann M et al (2008) Risk of cataract after exposure to low doses of ionizing radiation: a 20-year prospective cohort study among US radiologic technologists. Am J Epidemiol 168:620–631
Nakashima E, Neriishi K, Minamoto A (2006) A reanalysis of atomic bomb cataract data, 2000–2002: a threshold analysis. Health Phys 90:154–160
Neriishi K, Nakashima E, Minamoto A, Fujiwara S, Akahoshi M, Mishima HK, Kitaoka T, Shore RE (2007) Postoperative cataract cases among atomic bomb survivors: radiation dose response and threshold. Radiat Res 168:404–408
Cucinotta FA, Manuel FK, Jones J, Iszard G, Murrey J, Djojonegro B, Wear M (2001) Space radiation and cataracts in astronauts. Radiat Res 156:460–466
ICRP (2011) Statement on tissue reactions. ICRP ref 4825-3093-1464 (Approved April 2011)
Charles MW, Brown N (1975) Dimensions of the human eye relevant to radiation protection. Phys Med Biol 20:202–218
ICRP (1955) 1954 Recommendations of the International Commission on Radiological Protection. Brit J Radiol 1955 (Suppl. 6):1–92
ICRP (1996) Conversion coefficients for use in radiological protection against external radiation. ICRP Publ. 74. Ann ICRP 25 (3–4)
Schultz FW, Zoetelief J (1996) Organ and effective doses in the male phantom ADAM exposed in AP direction to broad unidirectional beams of monoenergetic electrons. Health Phys 70:498–504
Kramer R, Zankl M, Williams G, Drexler G (1982) The calculation of dose from external photon exposures using reference human phantoms and Monte-Carlo methods: Part I. The male (ADAM) and female (EVA) adult mathematical phantoms. GSF Bericht S-885 (ISSN 0721-1694)
Behrens R, Dietze G, Zankl M (2009) Dose conversion coefficients for electron exposure of the human eye lens. Phys Med Biol 54:4069–4087 (corrigendum: Phys Med Biol 55: 3937–3945, 2010)
Behrens R, Dietze G (2011) Dose conversion coefficients for photon exposure of the human eye lens. Phys Med Biol 56:415–437
ICRU (1985) Determination of dose equivalents resulting from external radiation sources. ICRU report 39. ICRU Publications, Bethesda, MD
Schlattl H, Zankl M, Petoussi-Henss N (2007) Organ dose conversion coefficients for voxel models of the reference male and female from idealized photon exposures. Phys Med Biol 52:2123–2145
International Organization for Standardization (1999) Calibration of area and personal dosimeters and the measurement of their response as a function of energy and angle of incidence. ISO 4037-3-Part 3, Geneva, Switzerland
Gualdrini G, Mariotti F, Wach S, Bilski P, Denoziere M, Daures J, Bordy J-M, Ferrari P, Monteventi F, Fantuzzi E, Vanhavere F (2011) A new cylindrical phantom for eye lens dosimetry development. Radiat. Meas. 46:1231–1234
International Electrotechnical Commission (2005) Medical diagnostic X-ray equipment – radiation conditions for use in the determination of characteristics IEC 61267. Organization for Standardization, Geneva
Berger MJ et al (1998) Stopping-power and range tables for electrons, protons, and helium ions. NIST standard reference database 124, NISTIR 4999
Behrens R, Dietze G (2010) Monitoring the eye lens: which dose quantity is adequate? Phys Med Biol 55:4047–4062 (corrigendum: Phys. Med. Biol. 56: 511, 2011)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Dietze, G. (2013). Radiobiology and Radiation Dosimetry for the Lens of the Eye. In: Mattsson, S., Hoeschen, C. (eds) Radiation Protection in Nuclear Medicine. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-31167-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-31167-3_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-31166-6
Online ISBN: 978-3-642-31167-3
eBook Packages: MedicineMedicine (R0)