Abstract
Reproductive fitness may be regarded as the most important criteria for studying or evaluating animal adaptation. Body systems activated by stress are considered to influence reproduction by altering the activities of the hypothalamus, pituitary gland, or gonads. Activation of stress pathways may directly affect the activity of Gonadotropin-releasing hormone (GnRH) neurons within the hypothalamus or higher neural centers which in turn affects the synthesis or secretion of GnRH into the hypophysial portal blood. It is also possible that stress directly influences the responsiveness of gonadotrophin cells in the anterior pituitary gland via the action of GnRH. A further potential action of stress is to alter the feedback actions of sex steroids in the hypothalamus or pituitary and inhibin in the anterior pituitary gland. Reproduction processes in animals may be impacted during heat exposure and glucocorticoids are paramount in mediating the inhibitory effects of stress on reproduction. Glucocorticoids are capable of enhancing the negative feedback effects of estradiol and reducing the stimulation of GnRH receptor expression by estrogen. Glucocorticoids may also exert direct inhibitory effects on gonadal steroid secretion and sensitivity of target tissues to sex steroids. Heat stress (HS) influences estrous incidences and embryo production. The birth weights of lambs of heat stressed ewes are generally lower than the unstressed animals. This could be attributed to the fact that HS may cause a temporal impairment of placental size and function, resulting in a transient reduction in fetal growth rate. Secretion of the hormones regulating reproductive tract function may also be altered by HS. Further, HS can inhibit 3-beta-hydroxysteroid dehydrogenase (3β HSD) thereby minimizing progesterone secretion from luteal cells. Aromatase is an enzyme that converts androgens into estrogens and is present in the granulosa cells. By inhibiting the expression of this enzyme, HS may induce follicular atresia and consequently anestrus. Effects of steroid hormones on reproductive tract tissue could be reduced during exposure to HS due to increased synthesis of heat shock proteins (HSPs)—HSP 70 and HSP 90. Increased synthesis of HSP might alter assembly, transport, or binding activities of steroid receptors. Further, increased magnitude of these stresses will increase secretion of prostaglandin and reduce the secretion of interferon tau which affects the maternal recognition of pregnancy. In male, HS adversely affects spermatogenesis by inhibiting the proliferation of spermatocytes. This chapter will address the effect of environmental stresses on livestock reproduction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Conception
- Embryo
- Fertilization
- Heat stress
- Lambing rate
- Nutritional stress
- Oocyte
- Reproductive endocrinology
1 Introduction
Environmental stress is not limited to climatic factors but extends to nutrition, housing, and any stimuli that demand a response from the animal to adapt to new circumstances. Low energy and low or excessive protein levels in the diet are detrimental to reproduction. Likewise, high ambient temperatures, radiation, and humidity alter the intricate balance of endocrine profiles, leading to lower intensity of estrus behavior, anestrus, poor semen quality, embryonic death, and infertility. Various chemical substances present in the environment may lead to infertility in animals. Polluted soil, water, and air are major sources through which animals are exposed to xenobiotics. Substances such as pesticide, dioxins, and organic solvents which alter hormonal balance (endocrine disruptors) cause major damage to livestock reproduction. However, other inherent (genetic) and environmental (feed, climate, geographical location, diseases, etc.) factors also affect the reproductive efficiency of animals. In ruminants, for example sheep, the reproductive efficiency is determined by factors such as age of puberty, interlambing period, ovulation rate, estrus incidences, fertilization, embryo implantation, pregnancy, parturition, lactation, and mothering ability (Snowder 2008). A greater variability in various genotypes of animal has been reported for each factor (Safari et al. 2005).
Reproductive efficiency of animals generally improves the production and economic efficiency of any farm animal production system (Dickerson 1970). Animal farming has a great significance in human activities. Globally, livestock account for 40% of the world’s agricultural gross domestic product (GDP) and generating employment for more than 1.3 billion people (FAO 2008, 2009). It also contributes significantly to the livelihood of people (about 1.0 billion) living below poverty level worldwide. Heat stress (HS) is known to have adverse impact on reproductive functions in animals. Disruption of follicular development, oocyte maturation, fertilization, embryogenesis and development, placental and fetus growth in female animals are major events caused by HS. These deleterious effects are manifested due to rise in the body temperature that could not be mitigated by inefficient thermolytic mechanism.
In order to maintain body function in steady state, homeotherms are required to maintain body temperature within a narrow range. Deviation from the set level of body temperature under stressful hot environment leads to interference with physiological events and consequently negative impacts on animal productivity. The maintenance of homeotherms is dependent upon the energy flow from animal to environment and vice versa. Effective ambient temperature (EAT) is a major environmental factor controlling the energy flow. Due to the fact that various factors influence the EAT viz. dry bulb temperature, wet bulb temperature, humidity, wind speed, heat radiation, contact surfaces, etc., no satisfactory measures have been developed so far to quantify EAT and hence ambient temperature is the most commonly used indicator. Sheep possess thick insulating boundary on the body and only about 10% of solar radiation received by fleeced sheep reaches to the skin. The exogenous (solar) heat load of the shorn sheep standing in sun may be about 5–6 times greater than its internal heat production (resting metabolic heat). Consequently, adaptation and mitigation of detrimental effects of extreme climates have played a major role in combating the climatic impact in livestock production (Khalifa 2003). Among the environmental variables affecting animals, HS and nutritional stress seem to be the more intriguing factors making animal production of many world areas difficult (Shelton 2000; Koubková et al. 2002). Animals can adapt to the hot climate; nevertheless, the response mechanisms are helpful for survival but are detrimental to productive and reproductive performance. Hence, attempt will be made in this chapter to particularly address the effect of HS and nutrition stress on reproduction in livestock.
Animals live in complex environments in which they are constantly confronted with short- and long-term changes due to a wide range of factors such as environmental temperature, photoperiod, geographical location, nutrition, and socio-sexual signals. Environment plays an important role in influencing the reproductive performance of farm animals. Fertility of farm animals is affected by high ambient temperature, excess humidity, severe cold, and lesser access to drinking water. HS, due to high ambient temperature accompanied with excess humidity during summer months, causes infertility in most of the farm species. Dairy cattle are particularly susceptible to HS because of higher metabolic heat produced during milk production. Furthermore, high yielding cows are most sensitive to HS. Nutrition modulates reproductive functions in many species including sheep (Naqvi and Rai 1991). Failure to rebreed due to decline in average sheep flock fertility results in more serves per successful conception, extended lambing intervals, and increased culling (Naqvi et al. 2002). Genetics, management, and nutrition have all contributed to this decline in fertility. Nutritional deficiencies and imbalances are frequently implicated as an important cause of infertility in sheep (Naqvi et al. 2011).
2 Probable Mechanisms of Stress Affecting Reproduction
Figure 5.1 describes different possible mechanisms by which stress affects reproduction in livestock. These possible mechanisms are outlined below:
-
The principal biological mechanism by which HS impacts on livestock reproduction is partly explained by reduced feed intake, and also includes altered endocrine status, reduction in rumination and nutrient absorption, and increased maintenance requirements resulting in a net decrease in nutrient/energy availability for reproduction
-
Stress (heat, nutritional, pH, immunological, physiological, etc.) prevents the basic cell function by causing improper folding of proteins. To cope with this, expressions of HSPs like HSP70 are stimulated/upregulated. The basic function of HSPs is to help in ‘proper folding’ of proteins so that the three-dimensional structure of the protein is not compromised and thus maintaining its normal function. Further, the cell diverts its entire transcriptional and translational machinery to synthesize proteins that are required for maintaining ‘house keeping functions’ such as cellular respiration, ATP synthesis, excretion of protons (H+ ions) at the expense of other functions of the cell, e.g., testosterone production. To combat stress, the Adrenocorticotropic hormone (ACTH)–Cortisol axis is activated and cortisol and other glucocorticoids are produced to enhance stress tolerance.
-
Glucocorticoids are anti-inflammatory. They prevent the expression of cyclo-oxygenase-2 (cox2) and lipoxygenase which gives rise to prostaglandins and leukotrienes, respectively. Luteinizing hormone (LH) surge induces cox2 expression and PGE2 production. PGE2 is essential for ovulation but may be inhibited by glucocorticoids.
-
The growing fetus is under stress due to space and nutrient constraint. This fetal stress activates ACTH-Cortisol axis. The fetal cortisol enters maternal circulation and activates the expression of 17 alpha-hydroxylase enzyme in the placenta. This enzyme converts progesterone into estrogen. Declining progesterone levels with concomitant increase in estrogen levels induce parturition. Injection of glucocorticoids (dexa, beta, prednisone, triamcenalone, etc.) any time after first month of pregnancy induces abortion in sheep and goat. Glucocorticoids prevent the ‘maturation of placentome’. Therefore, if parturition is induced with steroids, retained fetal membranes are common sequel.
-
Cortisol ‘stabilizes’ the lysosomal membrane and thereby prevents the release of vasoactive substances (such as histamine, serotonin, heparin, substance P, proteolytic enzymes) and subsequently prevents inflammatory response. Most of the common reproductive events such as ovulation, luteolysis, implantation, parturition, and involution of the uterus are ‘physiological inflammation’. You may expect an adverse effect on these events. Reproduction is basically a ‘luxurious phenomenon’ and appropriate when the animal is in just perfect homeostasis. In case of severe stress, reproduction is typically the first physiological event to let go by the body.
-
In female animals, stress can inhibit 3β HSD and minimize progesterone secretion from luteal cells. Aromatase, an enzyme present in the granulosa cells, may be inhibited to induce follicular atresia and anestrus. In male animals, stress adversely affects spermatogenesis perhaps by inhibiting the proliferation of spermatocytes. At cellular level, testosterone is converted into dihydrotestosterone (bioactive form of testosterone) by 5 alpha reductase and stress may adversely affect the expression of this enzyme.
Different mechanisms with which stress affects reproduction in livestock. Stress stimulus on hypothalamus produces CRH which in turn acts on anterior pituitary to release ACTH. ACTH atimulates cortisol release from adrenal cortex. Cortisol is the principal glucocorticoid which inhibits reproductive function by acting on animal cell, ovary, reproductive track in female and testis in male. Corticotrophin releasing hormone (CRH); Adrenocorticotropic hormone (ACTH), Cycloxigenase 2 (COX2); Prostaglandin E2 (PGE2)
3 Environmental Stresses and Female Reproduction
3.1 Effect on Superovulation
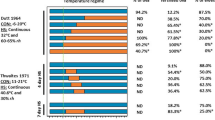
The reproductive efficiency of sheep is known to be adversely affected by hyperthermia (Thwaites 1971; Sawyer 1979). HS is also known to influence the superovulation response in sheep (Gordon 1997) and cattle (Hansen et al. 2001; Alfujairi et al. 1993; Gordon et al. 1987; Monty and Racowsky 1987) in a multiple ovulation and embryo transfer programme. Ewes exposed to HS produced relatively poor quality embryos when compared to ewes that were not exposed to HS. The results indicate that HS could adversely affect the quality of the embryos in Bharat Merino sheep reared in semi-arid tropics (Naqvi et al. 2004). The effect of HS on superovulation has been reported to vary in cattle. Monty and Racowsky (1987) reported no influence on superovulation response in Holstein cows, while adverse effects were observed by other workers (Gordon et al. 1987; Alfujairi et al. 1993). Alfujairi et al. (1993) reported a negative effect of hot summer on ovulation rate, total ova/embryos, and quality of embryos in cows. Similarly, Gordon et al. (1987) registered a highly significant difference in values recorded for Holstein cows treated for superovulation during midsummer and winter/spring.
3.2 Estrus Intensity and Duration
HS influence on estrus incidences is a well-established fact (Naqvi et al. 2004; Tabbaa et al. 2008). In general, duration and intensity of estrus in animals are reduced due to HS (Younas et al. 1993; Gwazdauskas et al. 1981). Exposure of Rambouillet cross ewes to severe HS from day 12 of the estrous cycle can extend the length of the cycle significantly (Dutt 1963). In addition, HS can influence the onset of estrus (Sejian et al. 2011). Exposure of ewes to high ambient temperatures 1.5–6 days prior to estrus has been reported to reduce estrus occurrence in ewes (Sawyer 1979). An alteration in the pulsatile release of LH and decrease in estrogen secretion is a potential reason for the delay of onset of estrus in ewes after exposure to HS. The normal GnRH release patterns (frequency and amplitude of LH pulses secreted from the pituitary) are reduced by exposure to HS (Dobson and Smith 2000). This results in abnormal ovarian functions and hence causes a delay in the LH surge. Furthermore, HS alters follicular development and dominance which leads to a decrease in estrogen secretion. Badinga et al. (1993) found follicular dominance to be altered in cows that were heat stressed during the first 8 days of the estrous cycle. However, Gangawar et al. (1965) also reported the duration of estrus to average 20 h in cows housed under cool conditions, compared to 11 and 14 h for cows reared in a hot psychrometric chamber and summer season, respectively. The intensity of estrus was also greater under cool than hot environmental conditions. The estrus response, fertilization rate, and neonatal survival may also decrease with HS (Mohamed 1974). The reduced estrus percentage and duration in livestock during summer months could be related to the high plasma progesterone concentration due to HS. Presumably, the longer estrus cycles were due to a slower rate of follicular maturation after corpus luteum (CL) regression. This statement agrees with Stewart and Oldham (1986), who reported that nutritional effect on ovulation rate seems to be more due to mechanisms that are confined to final stages of folliculogenesis rather than change in secretion of GnRH, LH, and follicle-stimulating hormone (FSH).
3.3 Sexual Behavior
HS reduces the normal manifestation of different sexual behaviors, which leads to decrease in productive potential of animals. The variation in sexual behavior pattern occurs in ewes during estrus, i.e., perceptivity (active search of ram) and receptivity (acceptance of mating attempts by ram) (Banks 1964). However, in Merino sheep sexual behavior occurs in the form of circling, tail fanning, head turning, standing, and approaching ram (Lynch et al. 1992).
3.4 Oocyte Maturation
Oocyte maturation process is a complex event and involves nuclear, cytoplasmic, and molecular maturation (Ferreira et al. 2009). Oocyte maturation, in vivo, begins with the resumption of meiotic process which is facilitated by cumulus cells. Calcium ions present in cumulus of oocyte brings about this nonhormone-mediated meiotic induction (Webb et al. 2002).
A sizeable population of livestock species in tropical and subtropical regions of the world are exposed to elevated ambient temperature and thus experience hyperthermia. HS, at the time of oocyte maturation (near estrus/breeding), is most susceptible to hyperthermic condition and lead to reduction in infertility (Cavestany et al. 1985; Putney et al. 1989). The magnitude of the effect of HS on fertility is dependent on the intensity and duration of HS on the animals (Cavestany et al. 1985; Barati et al. 2008). Ozawa et al. (2005) reported that HS in goats reduced circulating concentrations of estradiol and lowered follicular estradiol concentration, aromatase activity and LH receptor level, and delayed ovulation. Elevated temperature condition during in vitro culture of follicular cells of cattle reduced the steroid production (Bridges et al. 2005). Estradiol secretion in response to GnRH diminished in goats exposed to HS (Kanai et al. 1995). Figure 5.2 describes the direct and indirect effects of HS (green arrows) on ovarian follicles, oocyte, and granulosa cells.
Direct (hyperthermia) and indirect (negative energy balance associated with reduced dry matter intake) effects of HS (green arrows) on ovarian follicles, oocyte, and granulosa cells. Insulin-like growth factor-1 (IGF1), non-esterified fatty acids (NEFA). Metabolic parameters are altered in blood (red text) and have been reflected in the follicular fluid (white text) (Source Shehab-El-Deen 2011)
3.5 Nuclear Damage
Many research findings suggested that the exposure of oocytes to elevated temperature induced DNA damage in oocytes prior to fertilization. Exposure of oocytes to elevated temperature (41.8°C) for 12 h reduces their ability to complete nuclear maturation and development after fertilization. Similarly, Roth and Hansen (2004) and Ju and Tseng (2004) reported that elevated temperature during maturation culture of oocytes induced DNA fragmentation and cytoskeleton disruption. When Porcine oocytes are exposed to temperatures 41.0 or 38.5°C (sham control) for 0, 0.5, 1.0, or 1.5 h, followed by culture for 44 h, meiotic competence was compromised. However, nuclear maturation can be improved with the use of antioxidants in heat stressed oocytes (Lawrence et al. 2004; Maya-Soriano et al. 2010).
3.6 Molecular Changes
Heat exposure of animals may alter the biochemical composition of follicles which indirectly affects the developmental competence of oocyte and quality of granulosa cells. Molecular changes occurring during oocyte meiotic maturation affect developmental competence of later stage embryos. Later stage embryos derived from the oocytes exposed to HS do not have optimum development competence as compared to that of non-stressed oocytes (Edwards et al. 2009). Shehab-el-Deen (2011) has demonstrated that exposure of high yielding dairy cows to summer HS early postpartum reduces the diameter of dominant follicle and alters its biochemical concentrations of glucose, IGF-1, NEFA, urea, and total cholesterol in the follicular fluid. Figure 5.3 describes the effects of HS on the organization of the oocyte at the cellular level.
Effects of HS on the organization of the oocyte at the cellular level (Source Shehab-El-Deen 2011). Left an unstressed oocyte, right oocyte under heat stress. Heat stress leads to damage of the cytoskeleton and as such to poor alignment of the chromosomes (brown filaments). Organelles such as the Golgi and the endoplasmic reticulum (blue) become fragmented and disassemble. The number and integrity of mitochondria (dark blue) decreases. The synthesis of HSPs decreases (purple). Also, there are changes in the membrane morphology, aggregation of membrane proteins, and an increase in membrane fluidity. Together, all these effects stop cell growth and lead to cell-cycle arrest
Furthermore, protein synthesis in heat stressed oocytes is compromised (Edwards and Hansen 1996). Rodriguez and Farin (2004) have described that mRNA and proteins get accumulated in the oocyte during maturation and these are used during process of fertilization and early cleavage of embryos. Oocytes are incapable of synthesizing proteins during maturation and ovulation because they are transcriptionally inactive after reaching a diameter of about 110 μm in the tertiary follicle (Hyttel et al. 1997) or soon after GVBD (Rodman and Bachvarova 1976). Edwards and Hansen (1997) have demonstrated that exposure of oocyte to HS does not increase concentration of HSP 70 in cattle. As is evident, HSPs play an important role in protection of cells against HS through refolding the damaged proteins and stabilizing rRNA as a protection mechanism (Duncan and Hershey 1989). But the inability of maturing oocyte to respond to HS and express HSP 70 render them vulnerable except getting some protection by utilization of maternal RNA pools previously accumulated during oocyte growth for protein synthesis (Wassarman and Letourneau 1976). Further, HS conditions could have deleterious effects on oocyte growth, protein synthesis, and formation of transcripts required for subsequent embryonic development. The consequence of HS on the maturing oocyte can ultimately lead to a reduction in the capacity of an oocyte to be fertilized and to develop into a blastocyst.
3.7 Fertilization and Embryo Development
Lenz et al. (1983) and Edwards and Hansen (1996) performed in vitro maturation (IVM) and reported reduced maturation rate when bovine oocytes are exposed to 41°C. Subsequently, they also reported reduction in fertilization rate following in vitro fertilization (IVF) at 41°C. Rivera and Hansen (2001) showed that bovine oocytes fertilized at 41°C for 8 h had lower cleavage rates than did oocytes fertilized at 38.5°C in vitro. However, incubation at 40°C during IVF had no effect on cleavage rate and tended to increase the rate of oocytes forming blastocysts compared with oocytes fertilized at 38.5°C. This study mimicked rectal temperatures recorded during very hot mid-summer days in southeast Queensland, Australia. Sugiyama et al. (2007) recorded poor fertilization rates compared to the control group confirming that not only do high ambient temperature during IVF impairs fertilization, but also that on exposure to a maximum of 41°C for only 4 h is sufficient to adversely affect the outcome of IVF. Al-Katanani et al. (2002) collected oocytes from cows during the summer and reported that they have a reduced ability to develop to the blastocyst stage after IVF.
Most study suggests that early cleavages of embryos are sensitive to the exposure to elevated temperature (Putney et al. 1989). The adverse impact of HS declines as the embryo develops from 2-cell stage to morula and/or blastocyst (Ealy et al. 1993). The crossbred ewes (Bharat Merino) exposed to HS (40°C and 58.4% RH) of 6 h/d (10.00–16.00 h) for 4 weeks, yielded relatively poor quality embryos (Naqvi et al. 2004). In their study, the fertilization rate of the ewes was not affected by HS. Exposing Holstein cows to elevated ambient temperature (42°C for 10 h) during the periovulatory period increased the incidence of retarded and/or abnormal embryos (Putney et al. 1989). Similarly, Dutt (1963) reported that elevated ambient temperature 24 h prior to fertilization has no effect on the fertilization rate but increases the incidences of embryonic abnormalities. Similar results have been found in mice by Baumangartner and Chrisman (1987), who indicated that HS prior to ovulation did not interfere with oocyte fertilization but resulted in extensive developmental embryonic retardation.
The mechanism through which HS exerts influence on early embryos is not fully understood. Some evidence suggests increased production of reactive oxygen species (ROS) during preimplantation embryos following exposure to HS. Sakatani et al. (2004, 2008) reported increased production of ROS in cattle preimplantation embryos in response to elevated culture temperature. With the use of antioxidant, the negative effect of HS on the developing bovine embryos could be alleviated (Sakatani et al. 2008), but such effect was not reported by de Castroe Paula and Hansen (2008). Impaired function of oocytes and embryos were seen when they were exposed to heat during different stages of oocyte maturation and early embryo development in both in vivo and in vitro systems (Edwards et al. 2001; Naqvi et al. 2004). Naqvi et al. (2004) reported no affect on fertilization rate but found abnormal embryos from the Bharat Merino ewes exposed to HS during follicular phase. Further, Ealy et al. (1993) found that HS on day 1 after breeding decreased subsequent embryonic development. They further reported that HS on days 3, 5, or 7 after breeding, did not affect embryonic development. Therefore, the period of embryonic sensitivity to HS begins early during the development of the follicle and continues until about 1 day after breeding. Other studies based on in vitro culture system in cattle reported that the HS effect on embryo is stage dependent and zygote being most sensitive than the morula or blastocyst to high temperatures (Ealy et al. 1993; Edwards et al. 2001).
Exposure of bovine embryos to in vitro HS induces a range of effects including increased apoptosis (Paula-Lopes and Hansen 2002), increased expression of HSPs by porcine embryos (Bernardini et al. 2004), disruption of microtubule, and microfilaments (Rivera et al. 2004).
HS at and immediately after the time of breeding results in lower conception rates. Heat stressed dairy cows tend to have a decrease in dry matter intake thus reducing the amount of energy in their diet. In order to maintain pregnancy, it is critical to sustain a healthy diet (Al-Katanani et al. 2002). Further, Du Preez et al. (1991) developed three regression models relating conception rate (CR) and mean monthly temperature-humidity index (THI) and reported that CR decreased with increasing THI during summer season. Chronic HS leads to delayed ovulation, low CR, as well as to a higher rate of abortions (Ben and Bouraoui 2009). Figure 5.4 represents the schematic description of the possible mechanisms generated by HS which may affect reproduction in the lactating dairy cow.
A schematic description of the possible mechanisms with which HS may affect reproduction in the lactating dairy cow. HS can act in more than one way to reduce fertility in lactating dairy cows. HS can reduce dry matter intake and indirectly inhibit GnRH and LH secretion from the hypothalamo-pituitary system (dashed red lines). It is not clear if HS can directly affect the hypothalamo-pituitary system (dashed blue line) to reduce GnRH and LH secretion. HS can directly compromise the uterine environment (solid lines) to cause embryo loss and infertility (Source Shehab-El-Deen 2011)
HS reduces the length and intensity of estrus behavior, modifies endocrine function, alters the oviductal and uterine environments, interrupts early embryonic development, and ultimately, lowers the conception rates of dairy cattle (Hansen 2009; Wolfenson 2009). Collier et al. (2006) reported a 10% reduction in CR when cows were mated during summer months. Similarly, HS can cause reduction in CR in sheep and goats, and sheep embryo is most susceptible (Thwaites 1971).
4 Mechanisms of Nutritional Stress in Livestock Reproduction
Figure 5.5 describes the mechanism with which nutritional stress affects reproduction. Nutrient deficiency decreases the blood leptin concentration. This acts on the appetite control center in the brain and increases NPY which acts on the hypothalamus to decrease GnRH secretion. This decreased GnRH simultaneously leads to lower LH and FSH secretion from anterior pituitary. This in turn affects all the reproductive organs that control specific events of reproduction.
Mechanism of nutritional stress affecting livestock reproduction. Deprived nutrition reduces blood leptin concentration. This stimulates the hypothalamus to reduce secretion of GnRH. This leads to reduced secretion of FSH and LH in the anterior pituitary. This leads to reduced sex steroids production in the reproductive tract and affects all the reproductive processes
5 Effect of Nutritional Stress on Livestock Reproduction
Nutritional stress is an important environmental factor that influences ruminant fertility directly because it supplies specific nutrients required for oocyte development, ovulation, fertilization, embryo survival, and the establishment of pregnancy. Impact of nutritional stress on the circulating concentrations of the reproductive hormones and other nutrient-sensitive metabolites required for the physiological function has been highlighted (Robinson et al. 2006). Undernutrition delays the onset of sexual maturation and negatively affects sexual behavior. Food availability is the most important factor that influences mammalian reproduction because undernutrition slows down development of ovarian follicles and reduces lifetime reproductive performance in livestock (Rae et al. 2001). Changes in dietary intake promote variations in concentrations of metabolic [insulin, leptin, and IGF1] and reproductive hormones and consequently affect the developing ovarian follicle and/or the composition of reproductive tract secretions which provides histiotrophic nutrition to early embryos.
HS and feed scarcity often occur simultaneously and are the major predisposing factors that cause low livestock productivity in tropical environment. Undernutrition of donor ewes results in lower body weight (BW) and body condition score (BCS) with a negative effect on oocyte quality such as low rates of cleavage. Further, low BCS affects hormone production, fertilization, and early embryonic development (Boland et al. 2001; Armstrong et al. 2003; Boland and Lonergan 2005; Sejian et al. 2010).
While reviewing the relationship between nutrition and reproduction, Scaramuzzi et al. (2006) reported that energy balance (positive or negative) is regulated by a series of complex interaction of metabolites and hormones. Feed restriction and negative energy balance have been shown to alter follicular growth characteristics in cattle (Murphy et al. 1991) and sheep (Yaakub et al. 1997) and ultimately, estrus response. Restricted feeding (30% of ad lib intake) can significantly reduce the duration of estrus (15 vs 26 h) and increase the estrus interval (31.5 vs 18.6 days) in Malpura sheep (Maurya et al. 2004). The underlying mechanism for suppressed intensity of estrus and estrus duration has not been elucidated (Rhind 1992). However, it has been postulated that ovarian responses are influenced by the availability of nutrients, e.g., glucose and amino acids (Downing et al. 1995).
Optimal reproductive rates are essential for profitable sheep production (Vinoles et al. 2005). It has been postulated that nutrition is one of the main factors affecting ovulation rate and sexual activity in sheep (Forcada and Abecia 2006; Naqvi et al. 2011). Undernutrition during late pregnancy or in early postnatal life can irreversibly reduce the lambing rates of ewes (Gunn et al. 1995). Nutritional status has also been correlated with embryo survival in ewes and marked as key factor influencing efficiency in animal reproduction technology (Armstrong et al. 2003; Webb et al. 2004).
There are reports indicating the level of feeding affecting oocyte quality in ewes (Robinson et al. 2002). Ewes fed 50% of their maintenance requirements for two weeks had reduced expression of glucose transporter 3 (SLC2A3), sodium/glucose co-transporter 1 (SLC5A1), and Naþ/Kþ ATPase mRNA in oocytes, while expression of PTGS2, HAS2, and the leptin receptor in granulosa cells was increased (Pisani et al. 2008). Reduced expression of SLC2A3 is potentially relevant in the light of its significant role in post-implantation embryonic development (Schmidt et al. 2009).
Inadequate nutrition prior to calving results in emaciation of cows which delays the onset of estrual activity post calving. This delay influences the percent of cows available for breeding during the breeding season, thus reducing overall conception rates (Robinson et al. 2006; O’Callaghan et al. 2000). Lawson and Cahill (1983) postulated that variations in the physiological range of peripheral progesterone concentration due to management factors such as nutrition may induce asynchrony between the embryo and uterus resulting in failure of establishing pregnancy. Further, undernutrition in ewes before and after mating can increase embryonic mortality which consequently reduces the lambing rate (Rhind et al. 1989; Abecia et al. 2006).
6 Effect of Heat and Nutritional Stress on the Circulating Reproductive Hormones
Reproductive endocrinology plays a key role in livestock productivity during extreme climatic conditions. Endocrine responses to stress work toward suppressing productive functions such as growth and reproduction while favoring maintenance and survival (Rivest and Rivier 1995; Lindsay 1996). Heat and nutritional stress may cause infertility in farm animals. Notably, stress response involves the release of ACTH by the posterior pituitary gland and release of cortisol by the adrenal cortex (Minton 1994; Aoyama et al. 2003). High cortisol concentration in the system inhibits the pituitary response to GnRH (Dobson et al. 2001). However, cortisol decreases gonadal activity by reducing pulse frequency and amplitude of the LH released by the gonadotrophs (Dobson et al. 2001; Breen and Karsch 2004). The reduction of LH pulses in the luteal phase may induce follicular atresia. Suppression of LH release patterns during the follicular phase delays or inhibits the preovulatory LH surge and consequently disrupts the oocyte maturation and embryo quality (Mihm et al. 1994).
The association between HS and increased secretion of cortisol, the principal glucocorticoid hormone in small ruminants, is well documented (Ali and Hayder 2008; Sejian et al. 2008). Further, it is recognized that reproduction processes are influenced during heat exposure (Tilbrook et al. 2002; Naqvi et al. 2004; Kornmatitsuk et al. 2008). From these studies, it is pertinent to conclude that glucocorticoids are paramount in mediating the inhibitory effects of stress on reproduction. In support to this notion, various studies have shown that administration of natural or synthetic glucocorticoids can inhibit the secretion of the gonadotrophins in sheep (Juniewicz et al. 1987; Tilbrook et al. 2000). Further, glucocorticoids are capable of enhancing the negative feedback effects of estradiol and reducing the stimulation of GnRH receptor expression by estrogen (Adams et al. 1999; Daley et al. 1999). Glucocorticoids may also exert direct inhibitory effects on gonadal steroid secretion and sensitivity of target tissues to sex steroids (Magiakou et al. 1997). Inadequate nutrition delays or prevents the onset of puberty, interferes with normal cyclicity in the female, and results in hypogonadism (Sejian et al. 2010). This in turn affects all other reproductive processes.
Most reproductive responses to environmental factors are coordinated at brain level, where all external and internal inputs ultimately converge into a final common pathway that controls the secretion of GnRH. In turn, this hormone controls the secretion of gonadotrophins, the pituitary hormone, that determines the activity of reproductive axis. The effects of HS on LH concentration in peripheral blood plasma are inconsistent. Some studies report unchanged concentrations (Gwazdauskas et al. 1975; Howell et al. 1994). There are several researchers who reported increased plasma LH concentrations (Roman-Ponce et al. 1981) while others reported decreased concentrations (Gilad et al. 1993; Lee 1993) following HS. Plasma inhibin concentrations in summer are lower in heat stressed cows (Wolfenson et al. 1993) and in cyclic buffaloes, in India (Palta et al. 1997), perhaps reflecting reduced folliculogenesis since a significant proportion of plasma inhibin comes from small- and medium-sized follicles. Inhibin is an important factor in the regulation of FSH secretion (Findlay 1993; Kaneko et al. 1993). A clear inverse relationship between plasma FSH and immunoreactive inhibin concentrations was found throughout the oestrous cycle (Kaneko et al. 1997). The small amount of published information suggests that FSH is increased by HS and this may be due to decreased plasma inhibin production by compromised follicles.
Plasma estradiol concentrations are reduced by HS in dairy cows (Wilson et al. 1998), an effect that is consistent with decreased concentrations of LH. The mechanisms by which HS alters the concentrations of circulating reproductive hormones are not known. Some effects of HS may involve ACTH. HS can cause increased cortisol secretion (Elvinger et al. 1992; Sejian et al. 2011), and ACTH has been reported to block estradiol-induced sexual behavior (Hein and Allrich 1992). Decrease of estradiol concentration in the follicular fluid is more likely to occur after exposure to long-term, chronic (summer) HS than to acute HS. The effect of HS on plasma progesterone concentration is controversial. Wilson et al. (1998) found that HS had no effect on the plasma progesterone concentrations but that luteolysis was delayed. Several other studies have reported increased (Trout et al. 1998; Sejian et al. 2011), decreased (Younas et al. 1993; Ronchi et al. 2001) or unchanged (Guzeloglu et al. 2001) blood concentrations of this hormone during summer HS in dairy cows. These differences probably arise because of uncontrolled changes in other factors that affect blood progesterone concentrations.
It is generally accepted that nutrition modulates reproductive endocrine function in many species including sheep (Lindsay et al. 1993; Polkowska 1996). Kiyma et al. (2004) reported that serum concentrations of estradiol were lower in undernourished ewes. Similar results were reported in other species (Morin 1986; Otukongyong et al. 2000). Decreased concentration of estrogen may result from diminished ovarian follicular development caused by suppressed peripheral concentration of gonadotrophins (Gougeon 1996). Adams et al. (1994) contradicted this finding that undernourished ewes had lower plasma estradiol 17-β concentration. They established that food restriction is clearly associated with higher plasma concentration of estradiol 17-β concentration in ewes. Bell et al. (1989) reported that chronic HS exposure reduced plasma progesterone concentration in ewes. But Sejian et al. (2011) contradicted the above findings in which heat exposure significantly increased the plasma progesterone concentration. The level of nutrition and peripheral progesterone concentrations are inversely related (Parr 1992; Lozano et al. 1998) in ewes. This inverse relationship between the level of feed intake and plasma progesterone concentration was attributed to difference in metabolic clearance rate of progesterone (Parr et al. 1993). This view was further strengthened by the finding of Forcada and Albecia (2006) which states that difference in the rate of clearance rather than differences in secretion levels can explain the apparent inverse relationship between nutrition and peripheral progesterone concentrations in ewes. Plasma progesterone concentrations are determined by the differences between the rate of luteal production and the rate of hepatic metabolism. Both are affected by changes in dry matter intake. The increased plasma progesterone concentration in undernourished animals might be due to the limited extravascular pool in such animals with low body fat content (Lamond et al. 1972; Sejian et al. 2011). The reduced plasma progesterone concentration in the ad libitum fed ewes could be attributed to a consequence of higher metabolism of steroid by the liver (Parr et al. 1982).
7 Environmental Stresses and Male Reproduction
7.1 Scrotal and Testicular Morphology
Scrotal circumference and testicular consistency, tone, size, and weight are excellent indicators of sperm production capacity and spermatogenic functions. HS reduces these testicular measurements due to the degeneration of the germinal epithelium and partial atrophy in semniferous tubules. Mikelsen et al. (1981) recorded lowest scrotal circumference values during summer and the highest in autumn in rams. However, Hafez et al. (1955) reported that testes size of farm animals is not affected by seasonal changes. However, exposure to cold induces morphological, histopathological, and biochemical effects in rat testes (Blanco et al. 2007; El-Shahat et al. 2009).
7.2 Spermatogenesis
The testes of most mammalian species are located extra abdominally in the scrotum and function at a temperature that is a few degrees lower than normal body temperature. In addition, there is an intricate thermoregulatory system in the testis involving countercurrent exchange of heat from warm blood entering the testis and cool blood draining from the testis through an arterio-venous plexus called the pampiniform plexus. The degree of cooling is further controlled by two muscles: the tunica dartos in the scrotum that regulates scrotal surface area and the cremaster muscle that controls the position of the scrotum relative to the body. The lower intratesticular temperature is necessary for spermatogenesis and any disruption to thermoregulatory system of testis may cause problems in spermatogenesis. It can be observed when a local heat source is applied to the testis, the scrotum is insulated, the testes are internalized (i.e., cryptorchidism induced) or body temperature is raised because of fever or hot environment (Setchell 1998).
Exposure of testis to high temperature impairs spermatogenesis by elimination of spermatogonial germ cells in the seminiferous tubules and degeneration of sertoli and leydig cells. The heat damage in the testes is thought to be due to hypoxia causing oxidative stress and consequently germ cell apoptosis and DNA strand breaks (Perez-Crespo et al. 2008; Paul et al. 2008, 2009) mainly in pachytene spermatocytes and round spermatids (Lue et al. 2002). Spermatogonia are relatively resistant to heat compared to spermatocytes and spermatids, because of the fact that the number of spermatogonia remains unchanged and the morphological characteristics are less sensitive to heat exposure (Yin et al. 1997; Lue et al. 2000). Additionally, the testis is able to be repopulated with germ cells following a relative brief or mild temperature exposure (Lue et al. 1999; Yin et al. 1997).
Spermatogenic defects of HS are associated with decreased cytoplasmatic HSP60 immunoreactivity in spermatogonia (Werner et al. 1997). This decreased cytoplasmic HSP60 may negatively affect the mitotic proliferation of spermatogonia because of the fact that HSP60 is necessary for normal functioning of mitochondria. Normal spermatogonia proliferation continues to be drastically reduced for weeks even after the end of the heat treatment. The effects of heat on the spermatogonia seem to be dependent on the method, temperature, duration of heat application, and the livestock species. Exposure of rat testes to 43°C for 15 min causes only a slight increase of ‘‘undetermined’’ tubules 2, 8, and 26 days after heat exposure, whereas rats acclimatized to an environment of 35°C for 3 months showed 20% of severely affected tubular cross- sections. In llama, 38.2% of the tubular cross-sections showed ‘‘no stage’’ directly after the heat period, indicating that the llama is less able to stabilize an optimal temperature within the testes in elevated ambient heat conditions compared to the rat (Schwalm et al. 2007).
7.3 Seminal Attributes
Semen characteristics are not immediately affected by changes in testicular temperature because damaged spermatogenic cells do not enter ejaculates for some time after HS. In the bull, for example, where spermatogenesis takes about 61 days, alterations in semen occur about two weeks after HS and do not return to normal until up to eight weeks following the end of HS.
HS has a negative effect on semen attributes, such as sperm concentration, sperm motility, sperm viability, sperm morphology, and acrosome integrity. The seasonal infertility may be due, at least in part, to a combination of these parameters. Lower semen quality has been reported in bulls exposed to ambient temperatures over 27°C for as little as 6 h per day for several weeks. At 30°C semen quality was affected in 5 weeks, whereas at 38°C lower quality was observed in 2 weeks. In a study by Voglera et al. (1993) in bulls, the sperm motility starts declining on day 12 and reach lowest on day 15 after scrotal insulation for 48 h. Morphological changes first appear on day 12 and progress to peak on day 18 in a chronological sequence, i.e., tailless, (Days12–15); diadem, (Day 18); pyriform and nuclear vacuoles, (Day 21); knobbed acrosome, (Day 27); and Dag defect, (Day 30). Spermatozoa that appeared before 12 days of insulation were presumed to be in the epididymis or rete testes during scrotal insulation and the spermatozoa appeared after 12 days of insulation were presumed to be in spermatogenesis during scrotal insulation.
In rabbits, Marai et al. (2002) have reported that the HS did not have significant effect on reaction time, semen pH, sperm motility, percentages of dead sperm, sperm abnormalities, and acrosomal damage; however, ejaculate volume, sperm concentration, and total sperm output were significantly lower under HS. In seminal plasma, effects of HS were not significant on total protein, globulin, total lipid, cholesterol, creatinine, and alkaline phosphatase, while seminal plasma albumin, acid phosphatase were significantly lower, and GPT and GOT were significantly higher in HS conditions.
7.4 Sperm Capacitation and Fertilization
A satisfactory level of rams’ fertility may be retained throughout the whole year, but in many instances, fertility is depressed when mating occurs during the hot months of the year (Hafez 1987). A high percentage of rams could be sterile during the summer time, especially under conditions of high humidity. Conception failure in ewes mated to heat stressed ram was related to a failure to fertilize than to embryonic mortality (Curtis 1983). Fertility of the rams is related to several phenomena: the ability to mate, sexual desire, sperm production and viability, and fertilizing capacity of ejaculated sperm, which are influenced by elevated ambient temperatures, as well as nutritional level.
The seasonal infertility may be due to early occurrence of the acrosome reaction in response to stimulus, possibly resulting from a decrease in acrosomal stabilizing proteins in the seminal plasma during summer (Murase et al. 2007). These changes may be modulated by heat/humidity stress and/or photoperiod-regulated testosterone. The decrease in seminal plasma and intracellular ion (Ca, Na+ and Cl−) concentrations due to high temperatures can also contribute to decreasing male fertility (Karaca et al. 2002).
There are a number of reports in the literature that suggest males exposed to HS can produce sperm which do not produce normal offspring in unexposed females. Sperm produced by mice which had been exposed to a hot environment, bind to ova normally but are less able to fertilize in vivo and in vitro, even when motile sperms are selected by a swim-up procedure, and many of the resultant embryos do not develop normally.
In two different studies involving male mice exposed in a microclimate chamber to 36°C for 24 h Zhu and Setchell (2004) and Zhu et al. (2004) reported that there were no changes in the proportion of eggs showing two polar bodies if the mice were mated after subjecting them to HS on 7 or 35 days. However, the proportion of zygotes progressing to 2-cell stage by 34–39 h after mating was reduced when mating occurred 21 or 35 days after subjecting them to HS. Likewise, the proportion progressing to 4-cell or morulae by 61–65 h was reduced when mating occurred 7 or 21 days after HS. The proportion reaching blastocyst stage by 85–90 h after mating was reduced when mating occurred 7 or 21 days after HS, and the proportion of expanded blastocysts was reduced by mating on day 35. The proportion of abnormal embryos at 61–65 h and 85–90 h was increased by mating at 21 days post heat. When embryos were collected 25–28 h after human chorionic gonadotrophin (hCG) from mated superovulated females and cultured in vitro, development to 2-cell stage after 24 h of culture was normal with mating on day 3 or day 42, but reduced for days 7, 14, 21, 28, and 35; the 4-cell or morulae after 48 h culture, the 8-cell or blastocysts after 72 h, and blastocysts after 96 or 120 h culture were reduced with mating on the same days post heat. The percentage of abnormal embryos was increased after 48, 72, or 96 h of culture with mating on days 14–35, and the proportion of degenerating embryos after 72, 96, or 120 h culture was increased with mating on days 7–28, 7–35, and 3–35 post heat, respectively.
In another study, involving male mice exposed to 35°C for two periods of 12 h on consecutive days (Yaeram et al. 2006), the proportion of ova reaching 2-cell stage by 24 h after mating with superovulated females had fallen slightly when mating occurred 7 days post heat and appreciably with mating after 10 or 14 days. Using IVF, it was found that the same number of motile sperm from the epididymis of males heated 7, 10, or 14 days earlier were less effective in terms of the proportion of ova fertilized, with no effect 3 days after heating. Even when motile sperm were separated from immotile sperm in the sample by a swim-up procedure, a similar result was obtained. This was not due to a reduction in the number of sperm binding to the zona pellucida, but there were reductions in the proportion of ova with sperm in the perivitelline space and in the cytoplasm of the eggs.
In a different study, bulls were subjected to scrotal insulation for 48 h and semen samples were collected and cryopreserved 2 or 3 weeks later. Following IVF with swim-up sperm from these samples, there were decreased rates of sperm penetration, pronuclear formation (Walters et al. 2006), embryo cleavage, development, and blastocyst formation (Walters et al. 2005). The same bulls produced embryos with increased caspase activity after 8 days in culture, but there was no effect on apoptosis, as judged by percentage of cells positive in the TUNEL procedure. Paul et al. (2008) reported that IVF with sperm recovered from male mice in which the scrotum was heated to 42°C resulted in embryos with reduced ability to complete development. In addition, females mated to males exposed to scrotal heating had conceptuses with smaller fetal and placental weights compared with controls.
Data are equivocal as to whether ejaculated spermatozoa can be damaged by heat shock when deposited in the reproductive tract of a hyperthermic female. Culture of bull spermatozoa at 40°C did not alter fertilizing capability or the competence of the resultant embryos to develop to the blastocyst stage (Hendricks and Hansen 2009). In addition, they reported that ejaculated bull and stallion spermatozoa do not undergo apoptosis when cultured at temperatures characteristic of physiological hyperthermia. Nonetheless, there may be epigenetic changes in embryonic development associated with damage to the sperm in the reproductive tract. Insemination of rabbit done with sperm exposed to elevated temperature in vitro (Burfening and Ulberg 1968) or in the female reproductive tract (Howarth et al. 1965) resulted in reduced preimplantation as well as post-implantation survival. There is also evidence that X and Y spermatozoa are affected differentially by elevated temperature. The sex ratio of embryos was skewed toward female when female mice were bred to males experiencing scrotal heat treatment on the day of mating (Perez-Crespo et al. 2008). In contrast, incubation of sperm at 40°C for 4 h when compared with 38.5°C tended to reduce the proportion of embryos that were female following IVF (Hendricks and Hansen 2009).
7.5 Testosterone Concentration
Testes testosterone content fell from 1.1 to 0.4 μg/gm and spermatic vein plasma content from 8.2 to 1.9 μg/dl, when rams were exposed for 14 days to an average environmental temperature of 30°C (Curtis 1983). The lowest serum testosterone level was recorded during hot environmental conditions in Ossimi rams (El-Darawany 1999). Exposure of the intact rat to increased environmental temperatures is accompanied by decreased capacity of the testes to synthesize testosterone and consequently reduced serum testosterone concentration (Chap and Bedrack 1983). However, in developing ram lambs, direct exposure to high ambient temperature has no significant effect on testosterone production during non-breeding season (Rasooli et al. 2010). Short daylight stimulates the secretion of testosterone, FSH and LH in rams, while long daylight inhibits their secretion. Rams sexual activities peak occurs during the autumn breeding season and coincides with a sharp rise in plasma testosterone level. Then it declines in late winter, spring, and summer (Jainuden and Hafez 1987).
7.6 Expression Profile of Male Reproductive Genes During Heat Stress
It is widely accepted that changes in gene expression and in the activity of expressed proteins are an integral part of the cellular response to HS. Although the HSPs are perhaps the best-studied examples of genes whose expression is affected by heat shock, it has become apparent in recent years that HS also leads to induction of a substantial number of genes not traditionally considered to be HSPs. Some of these genes are affected by a wide variety of stressors and probably represent a non-specific cellular response to stress, whereas others may eventually be found to be specific to certain types of stress.
The cellular response to HS characteristically includes an increase in thermotolerance (i.e., the ability to survive subsequent to more severe heat stresses) that is temporarily associated with increased expression of HSPs. Heat-induced changes in gene expression occur both during hyperthermia as well as after return to normothermia.
7.7 Heat Shock Protein Genes
HSPs were originally identified as proteins whose expression was markedly increased by heat shock (Lindquist 1986). Several HSPs are expressed even in unstressed cells and play important functions in normal cell physiology. The intensity and duration of the heat stimulus needed for HSP expression vary considerably from tissue to tissue. A typical in vitro exposure involves heating mammalian cells to 42–45°C for 20–60 min and then reverting to normothermic temperatures (37°C). Induction of HSP expression typically starts within minutes after the initiation of HS, with peak expression occurring up to several hours later. Importantly, several experiments have found that, during the period of hyperthermia and shortly thereafter, HSPs become the predominant proteins synthesized by cells (Lindquist 1986). Interestingly, most HSP genes lack introns (Lindquist 1986), which may facilitate their rapid expression and which may also help explain how they can be expressed in the presence of stressors (such as heat) that can interfere with RNA splicing.
Heat shock factors (HSFs) are transcription factors that regulate HSP expression through interaction with a specific DNA sequence in the promoter [the heat shock element (HSE)]. The HSE is a stretch of DNA, located in the promoter region of susceptible genes containing multiple sequential copies (adjacent and inverse) of the consensus pentanucleotide sequence 5′-nGAAn-3′ (Morimoto 1998) and has been found in both HSPs and in a number of other genes. Three HSFs have been identified in mammalian systems: HSF-1, HSF-2, and HSF-4 (Morimoto 1998). HSF-1 (Morimoto 1998) and HSF-2 (Mathew et al. 2001) are involved in the acute response to heat shock. HSF-2 also has a major function in controlling expression of genes important for embryonic development and maintenance of sperm production (Wang et al. 2003).
Before heat-induced activation, HSF-1 exists as a monomer localized to the cytoplasm. The initial stimulus for activation of HSF-1 appears to take place after exposure of hydrophobic domains of denatured proteins to HS. HSPs preferentially bind to denatured proteins, and hence activation of HSF-1 may occur as a result of competitive release of this transcription factor from HSPs when the concentration of denatured cytoplasmic proteins increases as a result of heat shock (Morimoto 1998). After activation by HS, HSF-1 is found primarily in the nucleus in trimeric form, concentrated (in human cell lines) in granules (Sarge et al. 1993). It is this activated, trimeric form of HSF-1 that binds to the HSE and is involved in increased HSP gene transcription during HS (Sarge et al. 1993).
Evidence has also indicated that HS induces tagging of HSF-1 with SUMO-1, a ubiquitin-like protein that is used by the cell to mark proteins for transport into different cellular compartments and to alter their activities (Hong et al. 2001). Importantly, in these experiments, HSF-1, in vitro, was incapable of binding DNA unless it had first acquired a SUMO-1 tag at lysine 298.
In addition to positive regulation of the HSE through HSF-1, evidence also exists for negative regulation of this promoter element in mice by means of a constitutively expressed protein known as the HSE binding factor (HSE-BF) (Liu et al. 1993; Kim et al. 1995). Thus, in addition to a phosphorylation state, the ability of HSF-1 to activate transcription may also be modulated by regulatory processes that affect the binding of HSE-BF to the HSE.
8 Conclusion
This chapter identifies the various reproductive responses that are sensitive to HS and nutrition stress. Evidently, HS can have profound effects on most of the aspects of reproductive function—male and female gamete formation and function, embryonic development, and fetal growth and development. HS challenges the reproductive performance of livestock through a variety of altered physiologic means including: altered follicular development, lowered estrus activity, and impaired embryonic development. The effect of heat environment on reproduction may take place through a direct action of hyperthermia upon the reproductive tissues or through an indirect manner due to lower nutrients intake, impairment of hypothalamic, pituitary, gonadal, and endometrial secretions. HS reduces reproductive efficiency of livestock through a variety of different mechanisms. The principal biological mechanism by which HS impacts on livestock reproduction is partly explained by reduced feed intake, and also includes altered endocrine status, reduction in rumination and nutrient absorption, and increased maintenance requirements resulting in a net decrease in nutrient/energy availability for reproduction. Food availability is the most important factor that influences mammalian reproduction. Adequate feeding has great influence on animal comfort and reproductive performance. Low energy and low or excessive protein levels in the diet are detrimental to reproduction. Undernutrition affects the development of reproductive axis, delaying the development of ovarian follicles and reducing lifetime reproductive performance in livestock. Undernutrition of livestock results in lower BW and BCS which has a negative effect on oocyte quality, which results in lower rates of cleavage, and numerous reproductive functions including hormone production, fertilization, and early embryonic development. Elevation of ambient temperature affects male reproductive functions deleteriously. Such phenomenon leads to testicular degeneration and reduces percentages of normal and fertile spermatozoa in the ejaculate of males. The ability of the male to mate and fertilize is also affected.
References
Abecia AJ, Sosa C, Forcada F, Meikle A (2006) The effect of undernutrition on the establishment of pregnancy in the ewe. Reprod Nut Dev 46:367–378
Adams NR, Abrondi JA, Briegel JR, Sanders MR (1994) Effect of diet on the clearance of estradiol-17β in the ewe. Biol Reprod 51:668–674
Adams TE, Sakurai H, Adams BM (1999) Effect of stress like concentrations of cortisol on estradiol dependent expression of gonadotrophin-releasing hormone receptor in orchidectomized sheep. Biol Reprod 60:164–168
Alfujairi MM, Albrahim RM, Elnouty ED (1993) Seasonal variations in superovulatory responses of Holstein cows treated with pregnant mare serum gonadotropin in Saudi Arabia. J Reprod Fertil 11:75
Ali A, Hayder M (2008) Seasonal variation of reproductive performance, foetal development and progesterone concentration of sheep in the subtropics. Reprod Domes Anim 43:730–734
Al-Katanani YM, Paula-Lopes FF, Hansen PJ (2002) Effect of season and exposure to heat stress on oocyte competence in Holstein cows. J Dairy Sci 85:390–396
Aoyama M, Negishi A, Abe A, Maejima Y, Sugita S (2003) Sex differences in stress responses to transportation in goats: effects of gonadal hormones. Anim Sci J 74:511–519
Armstrong DG, Gong JG, Webb R (2003) Interactions between nutrition and ovarian activity in cattle: physiological, cellular and molecular mechanisms. Reprod Suppl 61:403–414
Badinga L, Thatcher WW, Wilcox CJ (1993) Effect of environmental stress on follicular development and steroidogenesis in lactating Holstein cows. Theriogenology 39:797–810
Banks EM (1964) Some aspects of sexual behavior in domestic sheep. Ovis aries Behav 23:249
Barati F, Agung B, Wongsrikeao P, Taniguchi M, Nagai T, Otoi T (2008) Meiotic competence and DNA damage of porcine oocytes exposed to an elevated temperature. Theriogenology 69:767–772
Baumangartner AP, Chrisman CL (1987) Embryonic mortality caused by maternal heat stress during mouse oocyte maturation. Anim Reprod Sci 14:309–316
Bell AW, McBride BW, Slepetis R, Early RJ, Currie WB (1989) Chronic heat stress and prenatal development in sheep: I. Conceptus growth and maternal plasma hormones and metabolites. J Anim Sci 67:3289–3299
Ben Salem M, Bouraoui R (2009) Heat Stress in Tunisia: effects on dairy cows and potential means of alleviating it. South African J Anim Sci 39:256–259
Bernardini C, Fantinati P, Zannoni A, Forni M, Tamanini C, Bacci ML (2004) Expression of HSP70/HSC70 in swine blastocysts: effects of oxidative and heat stress. Mol Reprod Dev 69:303–307. doi:10.1002/mrd.20143
Blanco A, Moyano R, Vivo J, Flores-Acuña R, Molina A, Blanco C, Agüera E, Monterde JG (2007) Quantitative changes in the testicular structure in mice exposed to low doses of cadmium. Environ Toxicol Pharmacol 23:96–101
Boland MP, Lonergan P (2005) Effects of nutrition on fertility in dairy cows. Advances Dairy Tech 15:19–33
Boland MP, Lonergan P, O’Callaghan D (2001) Effect of nutrition on endocrine parameters, ovarian physiology, and oocyte and embryo development. Theriogenology 55:1323–1340
Breen KM, Karsch FJ (2004) Does cortisol inhibits pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology 145:692–698
Bridges PJ, Brusie MA, Fortune JE (2005) Elevated temperature (heat stress) in vitro reduces androstenedione and estradiol and increases progesterone secretion by follicular cells from bovine dominant follicles. Domest Anim Endocrinol 29:508–522
Burfening PJ, Ulberg LC (1968) Embryonic survival subsequent to culture of rabbit spermatozoa at 38 and 40 C. J Reprod Fertil 15:87–92
Cavestany D, El-Whishy AB, Foot RH (1985) Effect of season and high environmental temperature on fertility of Holstein cattle. J Dairy Sci 68:1471–1478
Chap Z, Bedrak E (1983) Interrelationship between pituitary-testicular axis activity and raised environmental temperature in the rat. J Endocrinol 97(2):193–200
Collier RJ, Dahl GE, Vanbaale MJ (2006) Major advances associated with environmental effects in dairy cattle. J Dairy Sci 89:1244–1253
Curtis SE (1983) Environmental management in animal agriculture. Iowa State University Press, Ames
Daley CD, Sakurai H, Adams BM, Adams TE (1999) Effect of stress like concentrations of cortisol on gonadotroph function in orchidectomized sheep. Biol Reprod 60:158–163
de Castroe Paula LA, Hansen PJ (2008) Modification of actions of heat shock on development and apoptosis of cultured preimplantation bovine embryos by oxygen concentration and dithiothreitol. Mol Reprod Dev 75:1338–1350
Dickerson GE (1970) Efficiency of animal production-molding the biological components. J Anim Sci 30:849–859
Dobson H, Smith RF (2000) What is stress and how does it affect fertility? Anim Reprod Sci 60:743–752
Dobson H, Tebble JE, Smith RF, Ward WR (2001) Is stress really all that important? Theriogenology 55:65–73
Downing JA, Joss J, Scarmuzzi RJ (1995) Ovulation rate and the concentration of gonadotrophins and metabolic hormones in ewes infused with glucose during the late luteal phase of the oestrous cycle. J Endocrinol 146:403–410
Du Preez JH, Terblanche SJ, Giesecke WH, Maree C, Welding MC (1991) Effect of heat stress on conception in a dairy herd model under South African conditions. Theriogenology 35:1039–1049
Duncan RF, Hershey JWB (1989) Protein-synthesis and protein-phosphorylation during heat-stress, recovery, and adaptation. J Cell Biol 109(4):1467–1481
Dutt RH (1963) Critical period for early embryo mortality in ewes exposed to high ambient temperature. J Anim Sci 22:713–719
Ealy AD, Drost M, Hansen PJ (1993) Developmental changes in embryonic resistance to adverse effects of maternal heat stress in cows. J Dairy Sci 76:2899–2905
Edwards J, Hansen P (1996) Elevated temperature increases heat shock protein 70 synthesis in bovine two-cell embryos and compromises function of maturing oocyte. Biol Reprod 55:340–346
Edwards JL, King WA, Kawarsky SJ, Ealy AD (2001) Response of early embryos to environmental insults: potential protective roles of HSP70 and glutathione. Theriogenology 55:209–223
Edwards JL, Hansen PJ (1997) Differential responses of bovine oocytes and preimplantation embryos to heat shock. Mol Reprod Dev 46(2):138–145
Edwards JL, Bogart AN, Rispoli LA, Saxton AM, Schrick FN (2009) Developmental competence of bovine embryos from heatstressed ova. J Dairy Sci 92:563–570
El-Darawany AA (1999) Improving semen quality of heat stressed rams in Egypt. Ind J Anim Sci 69:1020–1023
El-Shahat AE, Gabr A, Meki AR, Mehana ES (2009) Altered testicular morphology and oxidative stress induced by cadmium in experimental rats and protective effect of simultaneous green tea extract. Int J Morphol 27(3):757–764
Elvinger F, Natzke RP, Hansen PJ (1992) Interactions of heat stress and bovine somatotropin affecting physiology and immunology of lactating cows. J Dairy Sci 75:449–462
FAO (Food and Agricultural Organization) (2008). The State of Food and Agriculture2008, Rome
FAO (Food and Agricultural Organization) (2009). Rural Income Generating Activities database (available at www.fao.org/es/ESA/riga/english/index_en.htm)
Ferreira EM, Vireque AA, Adona PR, Meirelles FV, Ferriani RA, Navarro PAAS (2009) Cytoplasmic maturation of bovine oocytes: structural and biochemical modifications and acquisition of developmental competence. Theriogenology 71:836–848
Findlay JK (1993) An update on the roles of inhibin, activin, and follistatin as local regulators of folliculogenesis. Biol Reprod 48:15–23
Forcada F, Abecia AJ (2006) The effect of nutrition on the seasonality of reproduction in ewes. Reprod Nutr Dev 46:355–365
Gangawar PC, Branton C, Evans DL (1965) Reproductive and physiological responses Holstein heifers to control and natural climate. J Dairy Sci 48:222
Gilad E, Meidan R, Berman A, Graber Y, Wolfenson D (1993) Effect of heat stress on tonic and GnRH-induced gonadotrophin secretion in relation to concentration of oestradiol in plasma of cyclic cows. J Reprod Fertil 99:315–321
Gordon I (1997) Embryo transfer and associated techniques in sheep. In: Controlled Reproduction in Sheep and Goat. CAB International, Oxon
Gordon I, Boland MP, McGovern H, Lynn G (1987) Effect of season on superovulatory responses and embryo quality in Holstein cattle in Saudi Arabia. Theriogenology 27:231
Gougeon A (1996) Regulation of ovarian follicular development in primates: facts and hypotheses. Endocrine Rev 17:121–155
Gunn RG, Sim DA, Hunter EA (1995) Effects of nutrition in utero and in early-life on the subsequent lifetime reproductive-performance of Scottish Blackface ewes in two management-systems. J Anim Sci 60:223–230
Guzeloglu A, Ambrose JD, Kassa T, Diaz T, Thatcher MJ, Thatcher WW (2001) Long term follicular dynamicsand biochemical characteristics of dominant follicles in dairy cows subjected to acute heat stress. Anim Reprod Sci 66:15–34
Gwazdauskas FC, Wilcox CJ, Thatcher WW (1975) Environmental and managemental factors affecting conception rate in a subtropical climate. J Dairy Sci 58:88
Gwazdauskas FC, De Silva AWMV, Anderson GW, McGilliard ML, Lineweave JA (1981) Interrelationships with estrous behavior and conception in dairy cattle. J Dairy Sci 64:2409–2418
Hafez ESE, Baderldin AL, Darwish YH (1955) Seasonal variations in semen characteristics of sheep in subtropics. J Agricul Sci 45:283–292
Hafez ESE (1987) Reproduction in farm animals, 5th edn. LEA and Febiger, Philadelphia
Hansen PJ, Drost M, Rivera RM, Paula Lopes FF, Al-Katanani YM, Krininger CE III, Chase CC Jr (2001) Adverse impact of heat stress on embryo production: causes and strategies for mitigation. Theriogenology 55:91–103
Hansen PJ (2009) Effects of heat stress on mammalian reproduction. Philos T R Soc B 364(1534):3341–3350
Hein KG, Allrich RD (1992) Influence of exogenous adrenocorticotropic hormone on estrous behavior in cattle. J Anim Sci 70:243–247
Hendricks KE, Hansen PJ (2009) Can programmed cell death be induced in post-ejaculatory bull and stallion spermatozoa? Theriogenology 71:1138–1146
Hong Y, Rogers R, Matunis MJ, Mayhew CN, Goodson ML, Park-Sarge OK, Sarge KD (2001) Regulation of heat shock transcription factor 1 by stress-induced SUMO-1 modification. J Biol Chem 276:40263–40267
Howarth B Jr, Alliston CW, Ulberg LC (1965) Importance of uterine environment on rabbit sperm prior to fertilization. J Anim Sci 24:1027–1032
Howell JL, Fuquay JW, Smith AE (1994) Corpus luteum growth and function in lactating Holstein cows during spring and summer. J Dairy Sci 77:735–739
Hyttel P, Fair T, Callesen H, Greve T (1997) Oocyte growth, capacitation and final maturation in cattle. Theriogenology 47(1):23–32
Jainuden MR, Hafez ESE (1987) Sheep and goats. In: Hafez ESE (ed) Reproduction in farm animals, 5th edn. LEA and Febiger, Philadelbhia
Ju JC, Tseng JK (2004) Nuclear and cytoskeletal alterations of in vitro matured porcine oocytes under hyperthermia. Mol Reprod Dev 68:125–133
Juniewicz PE, Johnson BH, Bolt DJ (1987) Effect of adrenal steroids on testosterone and luteinizing hormone secretion in the ram. J Androl 81:90–196
Kanai Y, Yagyu N, Shimizu T (1995) Hypogonadism in heat stressed goats: poor responsiveness of the ovary to the pulsatile LH stimulation induced by hourly injections of a small dose of GnRH. J Reprod Dev 41:133–139
Kaneko H, Nakanishi Y, Taya K, Kishi H, Watanabe G, Sasamoto S, Hasegawa Y (1993) Evidence that inhibin is an important factor in the regulation of FSH secretion during mid-luteal phase in cows. Endocrinology 136:35–42
Kaneko H, Taya K, Watanabe G, Noguchi J, Kikuchi K, Shimada A, Hasegawa Y (1997) Inhibin is involved in the suppression of FSH secretion in the growth phase of the dominant follicle during the early luteal phase in cows. Domes Anim Endocrinol 14:263–271
Karaca AG, Parker HM, Yeatman JB, McDaniel CD (2002) Role of seminal plasma in heat stress infertility of broiler breeder males. Poult Sci 81(12):1904–1909
Khalifa HH (2003) Bioclimatology and adaptation of farm animals in a changing climate. In: Interactions between climate and animal production, EAAP Tech Ser 7:15–29
Kim D, Ouyang H, Li GC (1995) Heat shock protein hsp70 accelerates the recovery of heat-shocked mammalian cells through its modulation of heat shock transcription factor HSF1. Proc Natl Acad Sci U S A 92:2126–2130
Kiyma Z, Alexander BM, Van Kirk EA, Murdoch WJ, Hallford DM, Moss GE (2004) Effect of feed restriction on reproductive and metabolic hormones in ewes. J Anim Sci 82:2548–2557
Kornmatitsuk B, Chantaraprateep P, Kornmatitsuk S, Kindahl H (2008) Different types of postpartum luteal activity affected by the exposure of heat stress and subsequent reproductive performance in Holstein lactating cows. Reprod Domes Anim 43:515–519
Koubkova M, Knizkova I, Kune P, Hartlova H, Flusser J, Dolezal O (2002) Influence of high environmental temperatures and evaporative cooling on some physiological, hematological and biochemical parameters in high-yielding dairy cows. Czech J Anim Sci 47:309–318
Lamond RD, Gaddy GR, Kennedy WS (1972) Influence of season and nutrition on luteal plasma progesterone in Rambuillet Ewes. J Anim Sci 34:626–629
Lawrence JL, Payton RR, Godkin JD, Saxton AM, Schrick FN, Edwards JL (2004) Retinol improves development of bovine oocytes compromised by heat stress during maturation. J Dairy Sci 87:2449–2454
Lawson RAS, Cahill LP (1983) Modification of the embryo-maternal relationship in ewes by progesterone treatment early in the oestrous cycle. J Reprod Fertil 67:473–475
Lee CN (1993) Environmental stress effect on bovine reproduction. Vet Clin North Am 9:263–273
Lenz RW, Ball GD, Leibfried ML, Ax RL, First NL (1983) In vitro maturation and fertilization of bovine oocytes are temperature dependent processes. Biol Reprod 29:173–179
Lindquist S (1986) The heat-shock response. Annu Rev Biochem 55:1151–1191
Lindsay DR (1996) Environment and reproductive behavior. Anim Reprod Sci 42:1–12
Lindsay DR, Martin GB, Williams IH (1993) Nutrition and reproduction. In: King GJ (ed) Reproduction in domesticated animals. World animal science series. Elsevier Science Publishers, Amsterdam, pp 459–491
Liu RY, Kim D, Yang SH, Li GC (1993) Dual control of heat shock response: involvement of a constitutive heat shock element-binding factor. Proc Natl Acad Sci U S A 90:3078–3082
Lozano JM, Albecia JA, Forcada F, Zarazaga L, Alfaro B (1998) Effect of under nutrition on the distribution of progesterone in the uterus of ewes during the luteal phase of the estrous cycle. Theriogenology 49(3):536–546
Lue YH, Lasley BL, Laughlin LS, Swerdloff RS, Sinha Hikim AP, Leung A et al (2002) Mild testicular hyperthermia induces profound transitorial spermatogenic suppression through increased germ cell apoptosis in adult Cynomolgus Monkeys (Macaca fascicularis). J Androl 23:799–805
Lue YH, Sinha Hikim AP, Swerdloff RS, Im P, Taing KS (1999) Single exposure to heat induces stage specific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinology 140:1709–1717
Lue YH, Sinha Hikim AP, Wang C, Im M, Leung A, Swerdloff RS (2000) Testicular heat exposure enhances the suppression of spermatogenesis by testosterone in rats: the ‘‘two-hit’’ approach to male contraceptive development. Endocrinology 141:1414–1424
Lynch JJ, Hinch GN, Adams DB (1992) The behaviour of sheep: biological principles and implications for production. C.A.B. International, Wallingford
Magiakou MA, Mastorakos G, Webster E, Chrousos GP (1997) The hypothalamic-pituitary-adrenal axis and the female reproductive system. Annals Newyork Acad Sci 816:42–56
Marai FM, Habeeb AAM, Gad AE (2002) Reproductive traits of male rabbits as affected by climatic conditions, in the subtropical environment of Egypt. Anim Sci 75:451–458
Mathew A, Mathur SK, Jolly C, Fox SG, Kim S, Morimoto RI (2001) Stress-specific activation and repression of heat shock factors 1 and 2. Mol Cell Biol 21:7163–7171
Maurya VP, Naqvi SMK, Mittal JP (2004) Effect of dietary energy level on physiological responses and reproductive performance of malpura sheep in the hot semi-arid regions of India. Small Rum Res 55:117–122
Maya-Soriano MJ, Andreu-Vazquez C, Lopez-Gatius F, Lopez-Bejar M (2010) Retinol improves in vitro Oocyte nuclear maturation under heat stress in dairy cows. Reprod Dom Anim 45:68
Mihm M, Baguisi A, Boland MP, Roche JF (1994) Association between the duration of dominance of the ovulatory follicle and pregnancy rate in beef heifers. J Reprod Fertil 102:123–130
Mikelsen WD, Paisley LG, Dahmen JJ (1981) The effect of semen on the scrotal circumference and sperm motility and morphology in rams. Theriogenology 16:45–51
Minton JE (1994) Function of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system in models of acute stress in domestic farm animals. J Anim Sci 72:1891–1898
Mohamed AA (1974) Physiological changes in the reproductive organ of buffalo from particular to conception. PhD Thesis, Faculty of Agriculture, Al Ajhar University, Egypt
Monty DE Jr, Racowsky C (1987) In vitro evaluation of early embryo viability and development in summer heat stressed, superovulated dairy cows. Theriogenology 28:451–465
Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12:3788–3796
Morin LP (1986) Environment and hamster reproduction: responses to phase-specific starvation during estrous cycle. Am J Physiol 251:R663–R669
Murase T, Imaeda N, Yamada H, Miyazawa K (2007) Seasonal changes in semen characteristics, composition of seminal plasma and frequency of acrosome reaction induced by calcium and calcium ionophore A23187 in Large White boars. J Reprod Dev 53(4):853–865
Murphy MG, Enright WJ, Crowe MA, McConnell K, Spicer LJ, Boland MP, Roche JF (1991) Effect of dietary intake on pattern of growth of dominant follicles during the oestrus cycle in beef heifers. J Reprod Fertil 92:333–338
Naqvi SMK, Maurya VP, Gulyani R, Joshi A, Mittal JP (2004) The effect of heat stress on superovulatory response and embryo production in Bharat Merino ewes. Small Rum Res 55:57–63
Naqvi SMK, Rai AK (1991) Effect of feed restriction followed by real mentation on growth, physiological responses and blood metabolites of native and crossbred sheep. Ind J Anim Sci 61:629–631
Naqvi SMK, Soren NM, Karim SA (2011) Effect of concentrate supplementation on performance, ovarian response, and some biochemical profile of Malpura ewes. Trop Anim Health Prod 43:905–913
Naqvi SMK, Maurya VP, Joshi A, Sharma RC, Mittal JP (2002) Production of crossbred lambs through artificial insemination of non-prolific medium size Malpura and Avikalin ewes using fresh diluted semen of prolific micro size Garole rams. Asian-Aust J Anim Sci 15:633–636
O’Callaghan D, Yaakub H, Hyttel P, Spicer LJ, Boland MP (2000) Effect of nutrition and superovulation on oocyte morphology, follicular fluid composition and systemic hormone concentrations in ewes. J Reprod Fertil 18:303–313
Otukonyong EE, Okutani F, Takahashi S, Murata T, Morioka N, Kaba H, Higuchi T (2000) Effect of food deprivation and leptin repletion on the plasma levels of estrogen (E2) and NADPH-d reactivity in the ventromedial and arcuate nuclei of hypothalamus in the female rats. Brain Res 887:70–79
Ozawa M, Tabayashi D, Latief TA, Shimizu T, Oshima I, Kanai Y (2005) Alterations in follicular dynamics and steroidogenic abilities induced by heat stress during follicular recruitment in goats. Reproduction 129:621–630
Palta P, Mondal S, Prakash BS, Madan ML (1997) Peripheral inhibin levels in relation to climatic variations and stage of estrous cycle in buffalo (Bubalus bubalis). Theriogenology 47:989–995
Parr RA (1992) Nutrition-progesterone interactions during early pregnancy in sheep. Reprod Fertil Dev 4(3):297–300
Parr RA, Davis IF, Miles MA, Squires TJ (1993) Feed intake affects metabolic clearance rate of progesterone in sheep. Res Vet Sci 55:306–310
Parr RA, Cumming IA, Clark IJ (1982) Effect of maternal nutrition and plasma progesterone concentration on survival and growth of the sheep embryo in early gestation. J Agricul Sci (Cambridge) 98:39–46
Paul C, Murray AA, Spears N, Saunders PT (2008) A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction 136:73–84
Paul C, Teng S, Saunders PT (2009) A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol Reprod 80:913–919
Paula-Lopes FF, Hansen PJ (2002) Heat shock-induced apoptosis in preimplantation bovine embryos is a developmentally regulated phenomenon. Biol Reprod 66:1169–1177
Perez-Crespo M, Pintado B, Gutierrez-Adan A (2008) Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol Reprod Dev 75:40–47
Pisani LF, Antonini S, Pocar P, Ferrari S, Brevini TA, Rhind SM, Gandolfi F (2008) Effects of pre-mating nutrition on mRNA levels of developmentally relevant genes in sheep oocytes and granulosa cells. Reproduction 136:303–312
Polkowska J (1996) Stress and nutritional influences on GnRH and somatostatin neuronal systems in the ewe. Acta Neurobiol Exps 56:797–806
Putney DJ, Mullins S, Thatcher WW, Drost M, Gross TS (1989) Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between the onset of estrus and insemination. Anim Reprod Sci 19:37–51
Rae MT, Palassio S, Kyle CE, Brooks AN, Lea RG, Miller DW, Rhind SM (2001) Effect of maternal undernutrition during pregnancy on early ovarian development and subsequent follicular development in sheep fetuses. Reproduction 122:915–922
Rasooli A, Jalali MT, Nouri M, Mohammadian B, Barati F (2010) Effects of chronic heat stress on testicular structures, serum testosterone and cortisol concentrations in developing lambs. Anim Reprod Sci 117(1–2):55–59
Rhind SM (1992) Nutrition: its effects on reproductive performance and its hormonal control in female sheep and goats. In: Speedy AW (ed) Progress in sheep and goat research. CAB International, UK, pp 25–51
Rhind SM, McKelvey WAC, McMillen SR, Gunn RG, Elston DA (1989) Effect of restricted food intake, before and/or after mating, on the reproductive performance of Greyface ewes. Anim Prod 48:149–155
Rivera RM, Hansen PJ (2001) Development of cultured bovine embryos after exposure to heat temperatures in the physiological range. Reproduction 121:107–115
Rivera RM, Kelley KL, Erdos GW, Hansen PJ (2004) Reorganization of microfilaments and microtubules by heat stress in two-cell bovine embryos. Biol Reprod 70:1852–1862
Rivest S, Rivier C (1995) The role of corticotrophin releasing factor and interleukin- 1 in the regulation of neurons controlling reproductive functions. Endocrine Rev 16:177–199
Robinson JJ, Ashworth CJ, Rooke JA, Mitchell LM, McEvoy TG (2006) Nutrition and fertility in ruminant livestock. Anim Feed Sci Technol 126:259–276
Robinson JJ, Rooke JA, McEvoy TG (2002) Nutrition for conception and pregnancy. In: Freer M, Dove H (eds) Sheep nutrition. CAB International, Wallingford, pp 189–211
Rodman TC, Bachvarova R (1976) RNA synthesis in preovulatory mouse oocytes. Cell Biol 70:251–257
Rodriguez KF, Farin CE (2004) Gene transcription and regulation of oocyte maturation. Reprod Fertil Dev 16(1–2):55–67
Roman-Ponce H, Thatcher WW, Wilcox CJ (1981) Hormonal interrelationships and physiological responses of lactating dairy cows to shade management system in a tropical environment. Theriogenology 16:139–154
Ronchi B, Stradaioli G, Verini Supplizi A, Bernabuci U, Lacetera N, Accorsi PA (2001) Influence of heat stress or feed restriction on plasma progesterone, oestradiol-17beta, LH, FSH, prolactin and cortisol in Holstein heifers. Livestock Prod Sci 68:231–241
Roth Z, Hansen PJ (2004) Involvement of apoptosis in disruption of developmental competence of bovine oocytes by heat shock during maturation. Biol Reprod 71:1898–1906
Safari E, Fogarty NM, Gilmour AR (2005) A review of genetic parameter estimates for wool, growth, meat and reproduction in sheep. Livest Prod Sci 92:271–289
Sakatani M, Kobayashi S, Takahashi M (2004) Effects of heat shock on in vitro development and intracellular oxidative state of bovine preimplantation embryos. Mol Reprod Dev 67:77–82
Sakatani M, Yamanaka K, Kobayashi S, Takahashi M (2008) Heat shock-derived reactive oxygen species induce embryonic mortality in vitro early stage bovine embryos. J Reprod Dev 54:496–501
Sarge KD, Murphy SP, Morimoto RI (1993) Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol 13:1392–1407
Sawyer GJ (1979) The influence to radiant heat load in Merino ewes. II. The relative effects of heating before and after insemination. Aust J Agric Res 30:1143–1149
Scaramuzzi RJ, Cambel BK, Downing JA, Kendall NR, Khalid M, Gutierrez M, Somchit A (2006) A review of the effect of supplementary nutrition in the ewe on the concentration of reproductive and metabolic hormones and the mechanism that regulate folliculogenesis and ovulation rate. Reprod Nut Dev 46:339–354
Schmidt S, Hommel A, Gawlik V, Augustin R, Junicke N, Florian S, Richter M, Walther DJ, Montag D, Joost HG, Schürmann A (2009) Essential role of glucose transporter GLUT3 for post-implantation embryonic development. J Endocrinol 200:23–33
Schwalm A, Gauly M, Erhardt G, Bergmann M (2007) Changes in testicular histology and sperm quality in llamas (Lama glama), following exposure to high ambient temperature. Theriogenology 67(8):1316–1323
Sejian V, Maurya VP, Naqvi SMK, Kumar D, Joshi A (2010) Effect of induced body condition score differences on physiological response, productive and reproductive performance of Malpura ewes kept in a hot, semi-arid environment. J Anim Physiol Anim Nut 94(2):154–161
Sejian V, Srivastava RS, Varshney VP (2008) Pineal-adrenal relationship: modulating effects of glucocorticoids on pineal function to ameliorate heat-stress in Goats. Asian-Austral J Anim Sci 21:988–994
Sejian V, Maurya VP, Naqvi SMK (2011) Effect of heat, nutritional and combined (heat and nutritional) stresses on growth and reproductive performance of Malpura ewes under semi-arid tropical environment. J Anim Physiol Anim Nut 95:252–258
Setchell BP (1998) The parkes lecture. Heat and the testis. J Reprod Fertil 114:179–194
Shehab-El-Deen MAMM (2011) Effects of metabolic stressors and high temperature on oocyte and embryo quality in high yielding dairy cows. PhD thesis submitted to Department of Reproduction Obstetrics and Herd Health, Salisburylaan 133, B-9820, Merelbeke
Shelton M (2000) Reproductive performance of sheep exposed to hot environments. In: Malik RC, Razzaque MA, Al-Nasser AY (eds) Sheep Production in Hot and Arid Zones. Published by the Kuwait Institute for Scientific Research, pp 155–162
Snowder GD (2008) Genetic improvement of overall reproductive success in sheep: a review. Arch Latinoam Prod Anim 16(1):32–40
Stewart R, Oldham CM (1986) Feeding lupins for 4 days during the luteal phase can increase the ovulation rate. In: Proceeding of the Australian society of animal production. Rev Reprod 16:367–370
Sugiyama S, Michael M, Nancy P, Majataba K, Mary Y (2007) Effects of increased ambient temperature during IVM and/or IVF on the in vitro development of Bovine Zygotes. Reprod Dom Anim 42(3):271–274
Tabbaa MJ, Alnimer MA, Shboul M, Titi HH (2008) Reproductive characterstics of Awassi rams. Anim Reprod 5(1):23–29
Thwaites CJ (1971) Short term heat stress and embryo mortality in sheep. Austral J Exp Agricul Anim Husbandry 11(50):265–267
Tilbrook AJ, Turne AI, Clark IJ (2000) Effect of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Reprod 5:105–113
Tilbrook AJ, Turner AI, Clark IJ (2002) Stress and reproduction: central mechanisms and sex differences in non-rodent species. Stress 5(2):83–100
Trout JP, McDowell LR, Hansen PJ (1998) Characteristics of the oestrous cycle and antioxidant status of lactating Holstein cows exposed to stress. J Dairy Sci 81:1244–1250
Vinoles C, Forsberg M, Martin GB, Cajarville C, Repetto J, Meikle A (2005) Short term nutritional supplementation of ewes in low condition affects follicle development due to an increase in glucose and metabolic hormones. Reproduction 129:299–309
Voglera CJ, Bamea JH, DeJarnettea JM, McGilliarda ML, Saacke RG (1993) Effects of elevated testicular temperature on morphology characteristics of ejaculated spermatozoa in the bovine. Theriogenology 40(6):1207–1219
Walters AH, Saacke RG, Pearson RE, Gwazdauskas FC (2005) The incidence of apoptosis after IVF with morphologically abnormal bovine spermatozoa. Theriogeneology 64:1404–1421
Walters AH, Saacke RG, Pearson RE, Gwazdauskas FC (2006) Assessment of pronuclear formation following in vitro fertilization obtained after heat insulation of the testis. Theriogeneology 65:1016–1028
Wang G, Zhang J, Moskophidis D, Mivechi NF (2003) Targeted disruption of the heat shock transcription factor (hsf)-2 gene results in increased embryonic lethality, neuronal defects, and reduced spermatogenesis. Genesis 36:48–61
Wassarman PM, Letourneau GE (1976) RNA synthesis in fully grown mouse oocytes. Nature 361:73–74
Webb R, Garnsworthy PC, Gong JG, Armstrong DG (2004) Control of follicular growth: local interactions and nutritional influences. J Anim Sci 82:E63–E74
Webb RJ, Bains H, Cruttwell C, Carroll J (2002) Gap-junctional communication in mouse cumulus-oocyte complexes: implications for the mechanism of meiotic maturation. Reproduction 123:41–52
Werner A, Meinhardt A, Seitz J, Bergmann M (1997) Distribution of heat-shock protein immunoreactivity in testes of infertile men. Cell Tissue Res 288:539–544
Wilson SJ, Marion RS, Spain JN, Spiers DE, Keisler DH, Lucy MC (1998) Effect of controlled heat stress on ovarian function in dairy cattle: I. Lactating cows. J Dairy Sci 1:2124–2131
Wolfenson D, Bartol FF, Badinga L, Barros CM, Marple DN, Cummings K, Wolfe D, Lucy MC, Spencer TE, Thatcher WW (1993) Secretion of PGF2a and oxytocin during hyperthermia in cyclic and pregnant heifers. Theriogenology 39:1129–1141
Wolfenson D (2009) Impact of heat stress on production and fertility of dairy cattle. In: Proceedings 18th annual tri-state dairy nut conference, pp 55–59
Yaakub H, ÓCallaghan D, ÓDoherty JV, Hyttel P (1997) Effect of dietary intake on follicle numbers and oocyte morphology in unsuperovulated and superovulated ewes. Theriogenology 47:182
Yaeram J, Setchell BP, Maddocks S (2006) Effect of heat stress on the fertility of male mice in vivo and in vitro. Reprod Fertil Dev 18:647–653
Yin Y, Hawkins KL, Dewolf WC, Morgentaler A (1997) Heat stress causes testicular germ cell apoptosis in adult mice. J Androl 8:159–165
Younas M, Fuquay JW, Smith AE, Moore AB (1993) Estrus and endocrine responses of lactating Holsteins to forced ventilation during summer. J Dairy Sci 76:430–434
Zhu BK, Setchell BP (2004) Effects of paternal heat stress on the in vivo development of preimplantation embryos in the mouse. Reprod Nutr Dev 44:617–629
Zhu B, Walker SK, Oakey H, Setchell BP, Maddocks S (2004) Effect of paternal heat stress on the development in vitro of preimplantation embryos in the mouse. Andrologia 36:384–394
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Naqvi, S.M.K., Kumar, D., Paul, R.K., Sejian, V. (2012). Environmental Stresses and Livestock Reproduction. In: Sejian, V., Naqvi, S., Ezeji, T., Lakritz, J., Lal, R. (eds) Environmental Stress and Amelioration in Livestock Production. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-29205-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-642-29205-7_5
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-29204-0
Online ISBN: 978-3-642-29205-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)