Abstract
Seaweeds are photoautotrophs which dominate benthic marine ecosystems and like other aquatic algae show efficient adaptations to incorporate and process dissolved inorganic carbon. The carbon concentrating mechanisms (CCMs) widely found in seaweeds convert HCO −3 to CO2 enhancing the CO2 environment to RUBISCO, the central photosynthetic enzyme. As a collateral benefit, the high intracellular CO2 levels inhibit photorespiration. Seaweeds can be morphologically complex, and hence, carbon metabolism and its products are functionally integrated in growth patterns. For example, dark-independent carbon fixation pathways (LICF) and translocation along the thallus are relevant processes. As carbon metabolism is regulated by the interplay between environmental and endogenous forces, its involvement in adaptive mechanisms underlying, e.g., biogeographic patterns is being increasingly emphasized. Although there are still important areas that are poorly known, the database on carbon metabolism is a starting point to understand present and future responses of seaweeds to large-scale environmental shifts.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The process by which inorganic carbon is converted into organic forms driven by the solar energy is unique to photoautotrophic organisms (some types of prokaryotes, cyanobacteria, eukaryotic algae, and plants). Although in many cases the underlying mechanisms and enzymatic machinery associated with carbon metabolism are essentially similar to those found in higher plants, seaweeds show some differences, especially related with carbon acquisition and concentration, biochemical strategies to avoid the oxygen/CO2 interference of RUBISCO (photorespiration), and C4 metabolism. Other striking characteristic lies in the remarkable versatility of the different biochemical pathways and products that allow seaweeds to operate under resource limitation (e.g., CO2, light, nutrients) and under changing environments. On the other hand, seaweeds exhibit a great variety of storage carbohydrates (e.g., mannitol, floridoside). These compounds can be remobilized during growth and reproduction, and are also normally involved in a series of reactions associated with osmoregulation and antioxidant activity (see Lobban and Harrison 2000 for an overview).
Seaweeds are multicellular, macroscopic organisms and thus a major aspect of carbon metabolism that makes them different from other groups of algae and cyanobacteria is its dependency on gross morphology. The morpho-functional processes of carbon assimilation occurring in foliose and finely branched seaweeds can be considerably different from those of, e.g., coarsely branched and leathery species (Ramus 1978; Rosenberg et al. 1995). In many large brown algae, seasonal decoupling of carbon assimilation and anabolic processes, long-distance transport of photoassimilates, and biomass formation restricted to meristematic cells are conceived as fundamental adaptations for optimal allocation of energy in changing environments (Gómez et al. 2007).
In terms of its ecological importance, the photosynthetic transformation of inorganic carbon into organic molecules carried out by benthic seaweeds accounts for an important fraction of the coastal primary production and biomass. Furthermore, the fate of seaweed-derived organic material is central for the higher trophic levels and geochemical processes of the coastal ecosystems (Mann 1973).
2 Inorganic Carbon Acquisition
Seaweeds must use CO2 dissolved in seawater as inorganic carbon source. In the surface water the different species of inorganic carbon (CO2, HCO −3 , and CO 2−3 ) are in equilibrium in the so-called carbonate buffer system (see also Chap. 19 by Roleda and Hurd). For example, at partial pressure of 365 μatm, pH 8.1, 25°C, and salinity of 35 psu, the CO2 concentration is close to 10.4 μmol kg−1, while HCO −3 and CO 2−3 have values of 1,818 and 272 μmol kg−1, respectively. Thus, only a small fraction (~0.5%) is in the form of CO2 (Zeebe and Wolf-Gladrow 2001). Diffusion of CO2 in water (0.16 × 10−4 cm−2 s−1) is four orders of magnitude lower than in air (0.16 cm−2 s−1), which has important consequences for photosynthesis (Badger and Spalding 2000). In the case of aquatic organisms, the entry of CO2 into the cell is normally limited by the diffusion boundary layer, whose thickness, and hence its resistance, depends on the form and volume of the alga as well as the speed of the water flow around it. For example, for an aqueous phase system with a diffusion coefficient of 1.5 × 10−5 m−2 s−1 and a boundary layer of 15 μm, a maximum flux of CO2 close to 2.6 m−2 s−1 can be estimated (Falkowski and Raven 1997). Due to these constraints the sole diffusive entry of CO2 does not support photosynthetic demands and thus algae can suffer carbon limitation. This situation has been documented for some subtidal red algae that apparently rely on CO2 diffusion as the only mechanism of inorganic carbon uptake (Raven and Beardall 1981; Maberly 1990). The majority of seaweeds, however, have developed the capacity to concentrate CO2 in order to guarantee an adequate supply to RUBISCO. One of the most efficient carbon concentrating mechanism (CCM) is the active transport not only of CO2 but also HCO −3 , which accounts for up to 95% of the total dissolved inorganic carbon in seawater (reviewed by Raven 2010; see also Chap. 4 by Gordillo). In many cases, the HCO −3 is an intermediate pool, which is converted to CO2 in the vicinity of RUBISCO (Badger and Price 1994). Similar to strict CO2 users, there is depth dependence in the ability of seaweeds to use HCO −3 with a tendency of higher affinity for HCO −3 in seaweeds occupying the upper littoral zones compared to their counterparts from deeper locations (Sand-Jensen and Gordon 1984; Mercado et al. 1998; Murru and Sandgren 2004).
The principal way by which algae utilize HCO −3 is through the enzyme carbonic anhydrase (CA). This enzyme catalyzes the interconversion between HCO −3 and CO2 in distinct sites outside or inside the cell (Badger and Price 1994; Badger 2003) and its activity has experimentally been demonstrated in different seaweeds (Haglund et al. 1992; Mercado and Niell 1999). CA activities can vary considerably depending on various environmental factors (e.g., temperature, UV radiation) and at different temporal scales (Flores-Moya et al. 1998; Gómez et al. 1998a, b; Choo et al. 2005). Various surveys carried out in North Atlantic (Giordano and Maberly 1989), Mediterranean (Mercado et al. 1998, 2009), southern Chile (Huovinen et al. 2007), and the Arctic (Gordillo et al. 2006) have confirmed that the CA-based inorganic carbon acquisition is broadly extended in seaweeds, suggesting that this metabolic ability is advantageous in coping with changes in the availability of ambient CO2. Other mechanisms include the nondiffusive incorporation of HCO −3 via a specific transporter or proton pump, e.g., ATPase, an OH−/HCO −3 antiport system, which have been reported, e.g., in the brown alga Laminaria digitata (Klenell et al. 2002), and the green alga Cladophora (Choo et al. 2005), or an anion exchanger at the plasmalemma as has been postulated for Ulva sp. (Drechsler et al. 1994). Many aspects dealing with the nature of the transporter or the unbalance in the electrochemical potential across membranes remain unknown, but apparently its operation does not preclude the action of any intracellular CA (Raven and Lucas 1985).
Unlike terrestrial C3 plants that base their inorganic acquisition on diffusive CO2 entry, the majority of seaweeds exhibit functional CCMs (Raven 2010). For example, the model of carbon acquisition/assimilation in the Chlorophyta Ulva sp. is based on the capacity of this alga to convert HCO −3 into CO2 via external CA and also to transport actively HCO −3 through the plasmalemma (Beer 1996). If one considers that at normal atmospheric CO2 level (350 ppm, chloroplast flux of CO2 of about 2.8 mM s−1), the uncatalyzed rate of interconversion of CO2 to HCO −3 is 10,000 times slower than the biological flux via CO2 fixation by RUBISCO, then the action of CA is necessary in seaweeds (Badger and Price 1994). In the case of some intertidal algae such as Fucus, CCMs have also been proposed to serve as an inhibitor of the oxygenase activity of RUBISCO (photorespiration) during emersion periods (Kawamitsu and Boyer 1999) (see below). Overall, the ecological significance of these mechanisms in seaweeds, as well as their prevalence in relation to phylogeny and biogeography, has been proposed (Surif and Raven 1990; Raven 1991; Mercado et al. 2009). An increasingly relevant issue is the unpredictable effect of present and future global change-driven increases in CO2 concentration, which probably have impact on carbon acquisition patterns of seaweeds (Raven et al. 2002; Hurd 2000; Mercado et al. 2009, see Chap. 19 by Roleda and Hurd).
3 Photosynthetic Carbon Fixation
3.1 Calvin–Benson Cycle and RUBISCO
The process of fixation of CO2 into ribulose 1,5-bisphosphate (RuBP) to form triose phosphate is denominated photosynthetic carbon reduction cycle or Calvin–Benson cycle and occurs in the chloroplasts of seaweeds. The whole cycle consists of three major reactions: carboxylation, reduction, and regeneration. The carboxylation part can be summarized as:
3 Ribulose-1,5-bisphosphate (RuBP) + 3CO2 + H2O + 2 × 3 Phosphoglycerate (PGA)
The chloroplast enzyme ribulose1,5 bis-phosphate carboxylase/oxygenase (RUBISCO) is central in the carboxylation phase. The enzyme has a complex structure and is formed by eight large catalytic subunits of approximately 53 kDa encoded in the chloroplast genome and eight small 15 kDa mass peptides encoded in the nucleus. Different types of RUBISCO (I, II, III, and IV), which vary in different groups of photosynthetic organisms according to their kinetic characteristics, have been recognized (Tabita et al. 2008). Only variants of the type I have been found in seaweeds (Raven 1997). Although the enzyme is dispersed in the chloroplast stroma, a portion of the RUBISCO is located in proteinaceous bodies denominated pyrenoids, which are present in many seaweed groups (McKay et al. 1991). The role of pyrenoids in carbon metabolism is still not well understood; however, the evidence that other enzymes (e.g., RUBISCO activases) are also located in these bodies suggests its functional involvement in carbon fixation in the chloroplast.
The Calvin–Benson cycle is autocatalytic, i.e., its reactions generate the biochemical intermediates, which enhance the rate of carbon fixation in the case of increase in its concentration. It has been demonstrated that thallus regions of seaweeds with higher relative abundances in RUBISCO exhibit the highest rates of carbon fixation (Cabello-Pasini and Alberte 2001b). On the other hand, activity of RUBISCO is highly dependent on the environmental factors (Raven 1997; Bischof et al. 2002). During the photosynthetic carboxylation, CO2 is incorporated into the carboxyl group of the RuBP to form an unstable intermediate (enediol) and finally 3-phosphoglycerate (PGA), which is the first and most important compound of the cycle that is labeled in presence of 14C (Calvin 1956; Beer and Israel 1986). In the reduction phase, the main reaction is the conversion of PGA in glyceraldehyde 3-phosphate (GAP) through the use of ATP and NADPH formed in the photochemical reactions by GAP-dehydrogenase (GAP-DH). Due to this dependence, a feedback between both processes has been examined in some seaweeds, mainly in relation with environmental factors that affect thylakoid membranes (Bischof et al. 2002). The third stage of the carbon fixation process is the regeneration that allows maintaining the operation of the cycle through the constant supply of RuBP from molecules of triose phosphate. In the ATP-consuming process, which includes isomerization, condensation, hydrolization, and phosphorylation reactions, three molecules of RuBP are formed from five molecules of triose phosphate (Nelson and Cox 2004).
3.2 Rates of Carbon Fixation
Rates of RUBISCO-catalyzed carbon fixation measured using radioactive carbon isotopes (H14CO3) vary in seaweeds depending on different environmental and endogenous factors. Values compiled in Table 2.1 indicate that, irrespective of taxa or geographical region, carbon fixation reaches maxima of close to 40–50 μmol 14C g−1 FW h−1. However, age and thallus part can be relevant components of variability. For example, in complex morphs (e.g., some red algae and large brown algae) 14C fixation can considerably increase in mature thallus regions (which attain a well-developed photosynthetic apparatus) compared to meristematic (growing) zones (Küppers and Kremer 1978; Gómez et al. 2007). At a molecular level, the number of active sites (~4–8 mM), the concentrations of CO2, levels of O2 (which competes with CO2), and RuBP are key factors determining the in vivo kinetics of RUBISCO (Woodrow and Berry 1988). In contrast to the terrestrial C4 plants enriching the concentration of CO2 via decarboxylation of C4-acids (van Caemmerer and Furbank 2003), seaweeds increase the availability of inorganic CO2 to RUBISCO (and in parallel inhibits the oxygenase activity of the enzyme) through the action of CCMs (Raven 2010).
3.3 Photorespiration
The oxygenase property of RUBISCO, mainly of organisms with diffusive entry of CO2, is a relevant topic in photosynthetic physiology. In fact, RUBISCO catalyzes the competitive oxidation of RuBP by fixation of O2 to RuBP to form glycolate and PGA, a pathway-denominated C2 oxidative photosynthetic carbon cycle, which coexists with the Calvin–Benson cycle. In strict sense, the term “photorespiration” implies the consumption of O2 and release of CO2 in the light and thus the process depends on the CO2/O2 balance, the so-called CO2 compensation. At low partial pressure of CO2 and high O2, photosynthetic carbon fixation is competitively inhibited by the oxygenase activity of RUBISCO with formation of CO2 from the metabolism of glycolate (Raven et al. 2005). Thus, photorespiration is integrated in the whole photosynthetic carbon metabolism (Tolbert 1997).
Photorespiration in seaweeds has been less studied than in other photosynthetic organisms, probably because seaweeds exhibit CCMs. However, an effect of O2 on carbon fixation has been demonstrated for some representative seaweeds, which exhibit ratios of oxygenase to carboxylase activities between 0.1 and 0.25 (Raven 1997; Giordano et al. 2005). In seaweeds physiologically resembling C3 plants, e.g., understory red algae that acquire carbon via diffusive CO2 entry, the effects of photorespiration on photosynthetic carbon fixation are higher than in other groups (Raven 2010). In addition, the detection of various enzymes involved in the glycolate metabolism (e.g., P-glycolate phosphatase, glycolate oxidase, and glycolate dehydrogenase) (Gross 1990; Suzuki et al. 1991) as well as some of their products in different seaweeds (Reiskind et al. 1988) suggests that photorespiratory carbon oxidation is widespread in these organisms and in many ways similar to terrestrial plants (Raven 1997). The fate of glycolate in the cell, which includes its oxidation to glyoxylate in peroxisomes and further conversion to amino acids and CO2, has been studied only in some seaweeds (Iwamoto and Ikawa 1997). Overall, although the implications of the photorespiratory pathway for seaweed ecology and its reason of maintenance along the evolution of algae are not well understood, its expression has been demonstrated and apparently, under certain environmental conditions, it can have consequences for the whole carbon metabolism of algae (Raven 1997).
4 Light-Independent Carbon Fixation
Carboxylation is not an exclusive feature of RUBISCO; seaweeds are equipped with a suite of diverse nonphotosynthetic enzymes that, like C4 and CAM in plants, are able to carboxylate and decarboxylate various C3 and C4 compounds. Light-independent carbon fixation (LICF) is also called “dark carbon fixation” or “β-carboxylation,” since inorganic carbon is fixed into the β-site of acceptors such as phosphoenolpyruvate (PEP) or pyruvate. Two enzymes, phosphoenolpyruvate carboxylase (PEPC) and phosphoenolpyruvate carboxykinase (PEP-CK), are especially important in seaweeds. The role of PEP-CK, which uses CO2 as inorganic carbon source, in LICF has been demonstrated for various species of seaweeds, in particular large brown algae (Küppers and Kremer 1978; Johnston and Raven 1986; Cabello-Pasini et al. 2000). In contrast to PEPC, during the PEP-CK catalysis the energy of the phosphorylated group of PEP is saved by phosphorylation of nucleoside diphosphates. The first studies using radiocarbon (14C) in different groups of seaweeds revealed that amino acids such as aspartate, glutamate, citrate, and alanine were primarily 14C labeled (Akagawa et al. 1972; Kremer 1981; Kerby and Evans 1983). The formation of oxalacetic acid (OAA) as a key intermediate of the Krebs cycle, suggested a link with anabolic processes (Kremer 1981). In fact, an apparent function of LICF is the replenishing of carbon via “anaplerotic” reactions, especially when pyruvate is degraded to acetyl-CoA during glycolysis (Kremer 1981). However, LICF reactions do not increase the net fixed carbon but are essential for cell metabolism, i.e., the pathway provides indispensable C4 acids that are not synthesized in the Calvin–Benson cycle.
Like photosynthetic C-fixation rates, LICF rates show considerable variation among different seaweeds; however, there is a tendency of higher values in brown algae compared to Chlorophytes and Rhodophytes (Table 2.1). In Chlorophytes and Rhodophytes, LICF rarely exceeds 1 μmol 14C g−1 FW h−1, which in terms of their contribution to the photosynthetic carbon is normally <1% (Cabello-Pasini and Alberte 1997). In the case of brown algae, values can be considerably higher (up to 9.6 μmol 14 C g−1 FW h−1), accounting for up to 48% of the photosynthetic fixation (Kremer 1981). Especially high LICF rates have been reported in growing thallus areas of Laminariales (e.g., Laminaria and Lessonia ) and during the spring/summer season for temperate and cold-temperate species. In the case of temperate red and brown algae, values of LICF can also be important (Cabello-Pasini and Alberte 1997). Carboxylation measured as the activity of PEP-CK is also linked to growth requirements, especially in species with marked seasonality in growth and photosynthetic carbon fixation, e.g., polar seaweeds (Weykam 1996; Weykam et al. 1997; Wiencke et al. 2009). Interestingly, algae from cold regions have exploited very efficiently the potential for LICF as a strategy to minimize the carbon losses due to high respiration and to optimize the supply of carbon skeletons during rapid growth during the short open water season (Drew and Hastings 1992; Gómez and Wiencke 1998) (see below). For example, in the kelp-like Antarctic brown alga Ascoseira mirabilis, LICF represents approximately 9.5% of light C-fixation (Gómez et al. 1995a, b), which is comparable to ratios found in species of Laminaria (Küppers and Kremer 1978) (Table 2.1). Despite the potential for LICF that seaweeds exhibit, it is not clear whether this pathway may compensate for C losses due to respiration as pointed by Kremer (1981). Thomas and Wiencke (1991) did not conclusively demonstrate its relationship with dark respiration in several Antarctic marine algae. In general, LICF was between 4.9 and 31% of dark respiration in five brown algae and one red alga. In species such as Himantothallus grandifolius and Desmarestia anceps, low LICF values were coupled with high respiration rates (Thomas and Wiencke 1991). This situation confirms the findings reported in Ascophyllum nodosum where a net C loss due to respiration was estimated in the dark (Johnston and Raven 1986). Recent studies revisiting the role of LICF in carbon metabolism of seaweeds have demonstrated that these reactions can be functional to morpho-physiological strategies to cope with, e.g., enhanced solar UV radiation. In blades of Lessonia nigrescens, LICF decreased 70% whereas light carbon fixation decreased by 90% under elevated doses of UV-B radiation. This suggests that LICF could be regarded a compensating mechanism necessary to keep physiological performance of algae during severe photodamage (Gómez et al. 2007). The findings that LICF is also well expressed in temperate and polar Rhodophytes such as Cryptopleura lobulifera, Palmaria decipiens, and Iridaea cordata (Thomas and Wiencke 1991; Weykam 1996; Weykam et al. 1997; Cabello-Pasini and Alberte 1997) open questions related with its involvement in morpho-functional processes that allow these organisms to cope with stressful conditions. Involvement of LICF as a mechanism to reduce photorespiration has only been reported in the Chlorophyte Udotea (Reiskind et al. 1988). For most of seaweed groups, especially green and red algae, data on LICF are lacking and thus further studies are required in order to outline accurate conclusions on the significance of this pathway for the ecology of seaweeds.
5 Morpho-functional Aspects of Carbon Metabolism
Carbon metabolism in seaweeds is integrated in multicellular organization that in many groups exhibits several plant-like traits. Although seaweeds do not display the structural complexity of vascular plants, the integration of form and function is an important factor even in the simplest groups, e.g., uncorticated filaments and sheet-like species. Thus, gross morphology of seaweeds has been related with ecophysiological adaptations (especially photosynthetic performance and carbon production) in response to abiotic and biotic determinants (Littler and Littler 1980; Steneck and Watling 1982). It is now well established that thallus morphology defines much of the carbon physiology of large brown and red algae as well as some siphonal Chlorophytes.
5.1 The Role of Storage Carbohydrates
Storage carbohydrates of seaweeds are normally formed in the chloroplasts. Starch, a common compound of Chlorophyta and plants, is an insoluble storage polysaccharide constituted by units of ADP-glucose, which is synthesized from the triose phosphate generated in the Calvin–Benson cycle. Alternatively, triose phosphate can be exported via an antiport system to the cytosol to form sucrose. A significant part of the pool of sucrose is recycled to RuBP and thus the formation of starch or sucrose in the cell is a highly regulated process, closely synchronized with the carbon requirements of the Calvin–Benson cycle (Nelson and Cox 2004). The d-glucose monomers are linked to form branched polymers of starch composed of two types of chains: α 1,4-d-glucans (amylose) and/or additional α-1,6-d-glucans (amylopectin). In Rhodophyta, carbohydrates synthesized from carbon fixation are stored as floridean starch, which is characterized by α 1,4-d and α 1,6-glucans. The brown algae have storage laminaran (β-d-glucopyranose), a combination of soluble and insoluble chains of the type β-1,3 and β-1,6-d-glucans. Seaweeds contain also important amounts of low-molecular-weight compounds such as sucrose (green algae), mannitol (which can form part of laminaran chains of brown algae), and floridoside (red algae). These compounds are not only reserve products but also have a series of intracellular functions (e.g., osmolytes; see also Chap. 5 by Karsten) or are also precursors of cell wall polysaccharides (reviewed by Craigie 1974; Wëiwer et al. 2008).
Seasonal variation in major organic compounds, especially carbohydrates, is well known since 60 years and primarily based on studies of large brown algae, especially Laminariales and Fucales (Black 1950; Haug and Jensen 1954; Jensen and Haug 1956) and some Rhodophyta (Dawes et al. 1974). Although these changes were related to gradients of environmental variations (salinity, temperature, light, etc.), the importance of these compounds in life strategy, morpho-functional processes, and stress tolerance mechanisms was addressed later. Only after the classic works by Chapman and Craigie (1977, 1978), the relationship between nutrient availability, growth, photosynthesis, and organic composition in Laminariales could be comprehensively understood. In these seaweeds, degradation of storage carbohydrates, which are built up in summer (when net photosynthetic C assimilation occurs), supplies the energy requirements for growth during high nutrient availability in winter-early spring (Hatcher et al. 1977). In species such as Saccharina latissima, mannitol and laminaran vary from total absence in winter (4.5 and 0% DW, respectively) to high values close to 26% DW in summer (Black 1950). This strategy is extreme in the Arctic species Laminaria solidungula, which grow only in darkness in winter powered by the carbohydrates (laminaran/mannitol) synthesized during the previous season. About 25% of the original carbon content of the thallus is depleted during the dark winter period while it completes nearly 90% of its annual linear growth (Dunton and Schell 1986). In Antarctic brown algae, due to the seasonally constant levels of nutrients, the dynamics of synthesis and utilization of storage carbohydrates mostly depend on the availability of light. In Antarctic Desmarestiales and Ascoseirales, depletion of laminaran during spring and summer results in increases of mannitol, suggesting that these compounds support requirements during lamina elongation (Drew and Hastings 1992; Gómez and Wiencke 1998; Wiencke et al. 2009).
In cold-temperate and Arctic Laminariales, the significant direct relation between seasonal changes of amino acids to mannitol and the inverse relationship between amino acids and laminaran content confirms that N availability regulates remobilization of stored carbon (Lüning et al. 1973; Küppers and Kremer 1978; Cagné et al. 1982). In the northern hemisphere, high ambient N supply in winter exceeds substantially the N requirements for protein and amino acid synthesis and free N is accumulated in surplus (Chapman and Craigie 1977). In the case of Antarctic algae, seasonal changes in mannitol and laminaran seem to be mainly triggered by daylength, as seasonal carbon budget of Himantothallus grandifolius, Ascoseira mirabilis,, and Desmarestia menziesii is not affected by nutrients in summer like in Laminaria (Drew and Hastings 1992; Gómez and Wiencke 1998; Gómez et al. 1995b; Gómez and Wiencke 1998). As for kelps, Antarctic algae suffer a photosynthetic carbon deficit during the growth period, i.e., carbon losses due to anabolism and dark respiration exceed photosynthetic carbon fixation, which may be compensated by reutilization of storage carbohydrates (Gómez and Wiencke 1998). In any case, daylength-dependent variations of storage carbohydrates have been documented in cultured Laminaria hyperborea, a species exposed generally to a severe N limitation in summer (Schaffelke 1995). Furthermore, exposure to constant short day alters the seasonal growth cycle of Laminaria digitata by preventing the decrease of growth rates in summer (Gómez and Lüning 2001), suggesting that not only nutrients but also photoperiodic responses are crucial in carbon metabolism in this group of algae.
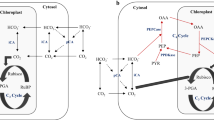
Figure 2.1 describes a model of synthesis, remobilization, and utilization of storage carbohydrates during the growth phase for Laminariales. Laminaran, which is built up mainly in distal regions of various species of kelps (Küppers and Kremer 1978; Lüning 1979; Cabello-Pasini and Alberte 2001a), is degraded with release of mannitol, which is transported (along with various amino acids) via translocation to the meristematic region (see below). Mannitol enters in the LICF pathway as a precursor of 3-phosphoglycerate (PGA), which is transformed to phosphoenol pyruvate (PEP) in the mitochondria. One molecule of mannitol generates two molecules of PEP: one can be converted to oxaloacetate (OAA) by the enzyme PEP-CK following the biosynthetic pathway in the Krebs cycle while the other is transformed to acetyl-CoA by the successive action of pyruvate kinase (PK) and pyruvate dehydrogenase (PD) with loss of one molecule of CO2, which is saved by PEP-CK (Kremer 1981).
Schema indicating the morpho-functional processes during active growth in Laminariales. In distal thallus regions, photosynthetic carbon fixation via RUBISCO and biosynthetic processes in the Calvin–Benson cycle are connected through translocation of low-weight molecular sugars (e.g., mannitol) with anaplerotic processes mediated by LICF in the intercalary meristem. PGA, 3-phosphoglycerate; GAP, glyceraldehyde-3-phosphate; PK, Pyruvate kinase; PD, Pyruvate dehydrogenase; PEP-CK, Phosphoenolpyruvate carboxykinase; PEP, Phospoenolpyruvate; RuBP, ribulose 1,5-bisphosphate
The glycolysis of mannitol, as well as the 14C labeling of carboxylic diacids (e.g., malate, aspartate) in the meristematic zones of the blade, has unequivocally confirmed that growth in large brown algae can be supported by nonphotosynthetic, anaplerotic reactions (Kremer 1981; Cabello-Pasini and Alberte 2001b). In Rhodophyta, anaplerotic consumption of storage carbohydrates has been less studied. In Gelidium coulteri grown under N starvation, rates of LICF were relatively low (1–7%), floridoside was rapidly consumed in glycolysis, and an important fraction of the 14C labeling was allocated to amino acids of the tricarboxylic acids cycle (Macler 1986). This pattern has been found in other Rhodophyta and apparently indicates that, while present, the LICF pathway has much less importance in the whole carbon assimilation in temperate algae (Cabello-Pasini and Alberte 2001b). However, results from the two contrasting polar red algae, the endemic Palmaria decipiens and the widely distributed Iridaea cordata, indicated that accumulation of floridean starch allows the first species to overwinter in darkness. In the case of Iridaea, use of floridean starch to power growth was less marked. In both cases, LICF accounting for up to 9% of the total carbon assimilation was active during a part of the dark period (Weykam et al. 1997), suggesting that nonphotosynthetic carbon metabolism in Rhodophyta may have similar ecophysiological importance in extreme environments as has been reported for large brown algae (Wiencke et al. 2009).
5.2 Thallus Anatomy and Long-Distance Transport of Photoassimilates
Unlike vascular plants, where long-distance transport represents the exchange of resources between genuine, highly differentiated tissues, the transport of substances in seaweeds, called translocation, is normally a strategy to redistribute via mass flux diverse organic compounds towards zones of high metabolic activity (Lüning et al. 1973; Schmitz 1981). In Laminariales and Fucales, carbon metabolism is spatially separated in carbon “source” and “sink” regions (Küppers and Kremer 1978; Arnold and Manley 1985; Cabello-Pasini and Alberte 2001a). As is shown in Fig. 2.1 for Laminariales, photoassimilates are stored in the mature, commonly distal regions of the algae, and then transported as mannitol and amino acids to the meristematic region. This morpho-functional arrangement is a consequence of the allometric growth and the action of an intercalary meristem that normally results in tissues with different metabolic activity.
Different types of sieve elements, such as the “trumpet” cells, have been identified in members of Laminariales and Phyllariaceae (Buggeln 1983; Schmitz 1981; Gómez et al. 2007). These structures are formed by specialized, normally vacuolated cells that are longitudinally arranged in the medulla or below the cortex in parenchymatous and peudo-parenquimatous thalli. Cell lengths measured in different Laminariales vary considerably and can reach several millimeters. Most of the sieve tubes end in the so-called sieve plates, which present pores of up to 100 nm diameter permitting the connection between adjacent cells (Schmitz 1990).
Apart from Laminariales, other groups of seaweeds have been shown to have sieve elements or at least a translocation function has been hypothesized. This is the case of the Antarctic genera Ascoseira and Himantothallus, whose advanced structural organization resembles that of Laminaria species from the northern Hemisphere. For example, members of the Ascoseirales are characterized by a strap-like lamina with an intercalary basally located meristem forming new tissue during each growth phase. Thus, the blade in this species is formed by tissues differing in age and developmental stage (Gómez et al. 1995a; Gómez et al. 1996). Histological studies have revealed the presence of medullar structures denominated “conducting channels”. Apparently, putative translocation could occur only in young plants, as early “conducting channels” are metabolically active, possess plasmodesmata, and contain relatively few physodes (Clayton and Ashburner 1990). Long-distance transport of substances has also been documented in members of the brown algal orders Scytosiphonales (Guimaraes et al. 1986), Desmarestiales (Moe and Silva 1981; Wiencke and Clayton 1990), and Fucales (Moss 1983). In Rhodophyta, evidence for translocation of photoassimilates using 14C labeling has been obtained in Polysiphonia sp. (Wetherbee 1979), Delesseria sanguinea (Hartman and Eschrich 1969), and Gracilaria cornea (Gonen et al. 1996). Although the structures and probably the mechanisms of translocation in red algae are different compared to brown algae, a relationship between carbon fixation and translocation has been clearly demonstrated in Gracilaria (Gonen et al. 1996).
5.3 Patterns of Carbon Allocation
Large and complex seaweeds show a differential allocation of carbon fixation products along the thallus. Various brown algal genera such as Sargassum (Gorham and Lewey 1984), Macrocystis (Wheeler and North 1981; Gerard 1982), Lessonia (Percival et al. 1983; Westermeier and Gómez 1996), Durvillaea (Cheshire and Hallam 1985; Lawrence 1986; Gómez and Westermeier 1995), and Desmarestia (Carlberg et al. 1978) show longitudinal variation in organic composition. Primarily, changes in carbon allocation can be directly caused by differential capacity for carbon uptake among parts of thallus. Using 13C/12C ratios (δ 13C), it was possible to identify active HCO −3 uptake sites along the thallus of Antarctic seaweeds correlated to growth activity (Wiencke and Fischer 1990, 1992). For example, δ13C values between −12 and −16.8% (indicating 13C enrichment) were measured in new blade regions of Ascoseira mirabilis during high irradiances and summer daylength (Gómez 1997). Apparently, enhanced carboxylation rates during high light compensate for the energy costs of active HCO −3 incorporation by decreasing the C supply via diffusive CO2 entry, and thus the heavier C isotope is preferentially assimilated (Kübler and Raven 1994; Raven et al. 1995). On the other hand, changes in light use and carbon fixation efficiency along with increasing thallus size and age affect the carbon uptake and allocation. In cultures of Desmarestia menziesii, δ 13C values >−29% were found in small algae, but with increasing size, δ 13C signatures increased accordingly (−32%) (Gómez 1997).
Hydrodynamic processes regulate also the allocation of photoassimilated carbon in the thallus. In many large brown algae, carbon (normally in the form of structural carbohydrates) is preferentially allocated in the basal structures, which are biomechanically designed to attach algae to the substrate and to withstand drag forces from water movement (Hurd 2000). In the fucoid Durvillaea antarctica, characterized by large and floating laminar blades, 85% of the total energy contents from organic compounds is allocated to fronds, while the rest is allocated to the holdfast (Lawrence 1986). In the case of the kelp Postelsia palmaeformis, 63% of the total organic carbon is allocated to holdfast and stipe, which is in line with a gross morphology designed to resist the direct impact of waves (Lawrence and McClintock 1988). In the southern kelp Lessonia nigrescens, carbon allocation changed with increasing size and age: adult plants deposited higher proportion of energy in the holdfast and stipes than young plants with important consequences for population density and local demography (Westermeier and Gómez 1996).
Differential allocation of organic carbon along the thallus can also be functional to withstand other environmental stressors (Wakefield and Murray 2009). The optimal defense theory (ODT) has been proposed to understand the mechanisms that control the interaction between algal allocation of organic compounds and the action of, e.g., herbivores (Cronin and Hay 1996). For example, secondary metabolites are normally allocated in the structures with high fitness value, probably where the investment in energy is higher (Pansch et al. 2008). In brown algae, phlorotannins may function as deterrents for many grazers, but additionally, due to their primary role as cell wall precursors, these compounds act also as cell-wall hardening, conferring mechanical resistance and toughness (Lucas et al. 2000). In the intertidal kelp Lessonia nigrescens, holdfast and stipes contain higher concentrations of phlorotannins, and hence are better defended than transient fronds (Gómez et al. 2005; Gómez and Huovinen 2010, see also Chap. 8 by Iken).
Carbon fixation, biomass, and overall the sum of the morpho-functional processes of seaweeds define much of the primary productivity and energy fluxes in the coastal ecosystems (Mann 1973). Due to their size and patterns of substrate occupation, seaweeds represent habitat for other organisms and also modify the physical and chemical environment (Jackson 1998; Delille et al. 2000). In coastal areas of cold-temperate and polar regions, seaweeds can account for >50% of the total fixed carbon (Gattuso et al. 2006). The outcome of seaweed carbon metabolism is transferred to the food web not only via direct consumption by herbivores but also as secondary product via detritus in near shore (Duggins et al. 1989) and abyssal areas (Wiencke and Fisher 1992).
5.4 Concluding Remarks
Overall, carbon metabolism of seaweeds is highly versatile and has allowed these organisms to thrive in all types of habitats and environmental conditions. However, despite the considerable advances in our knowledge on mechanisms and pathways, the ecological consequences of many photosynthetic adaptations are not well understood. For example, carbon acquisition patterns in relation with morpho-physiological processes, biomass allocation, reproduction, and development of adult and early stages of seaweeds have been hitherto overlooked (Raven 2003). The significance of endogenous processes regulating carbon metabolism, which has been studied in few brown algae, is also a relevant topic that should be expanded to other seaweed groups in order to gain insights into possible evolutionary issues (Schmid et al. 1996). Probably, one of the most important issues in seaweed physiology will be the understanding of the complex interaction between the expression and modulation of carbon metabolism, and processes underlying large biogeographical patterns of seaweeds. For many aspects related to regulation of carbon metabolism in seaweeds, proteomic and genomic studies are urgently needed and fundamental in order to understand the role of seaweeds in present and future scenarios of global change.
The consequences of increasing CO2 levels and related phenomena such as the ocean warming and ozone depletion on seaweed ecophysiology have begun to be explored and different surveys describe various biological and geochemical scenarios modified by anthropogenic activities (Israel and Einav 2010). Due to the dependence of RUBISCO on CO2, it has been postulated that photosynthetic organisms will respond positively to present and future increases in atmospheric CO2 (Amthor 1995). However, some experimental evidence suggests that large increases in photosynthetic carbon fixation are not expected mainly because most of the studied seaweeds exhibit CCMs (Gao et al. 1993; Beer and Koch 1996; Israel and Hophy 2002). Apparently, the focus could be on the increased competiveness of algae without or with poorly developed CCM, which will depend on a series of other environmental and geographic factors (Raven et al. 2002). Overall, although the studies compiled here give important insights into the potential responses of organisms, the central question whether the mechanisms exploited by seaweeds today allow them to adapt to future scenarios remains open.
References
Akagawa H, lkawa T, Nisizawa K (1972) Initial pathway of dark14 CO2-fixation in brown algae. Bot Mar 15:119–125
Amthor JS (1995) Terrestrial higher‐plant response to increasing atmospheric [CO2] in relation to the global carbon cycle. Global Change Biol 1:243–274
Arnold KE, Manley SL (1985) Carbon allocation in Macrocystis pyrifera (Phaeophyta): intrinsic variability in photosynthesis and respiration. J Phycol 21:154–167
Badger M (2003) The roles anhydrases in photosynthetic CO2 concentrating mechanisms. Photosynth Res 77:83–94
Badger MR, Price GD (1994) The role of carbonic anhydrase in photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 45:369–392
Badger MR, Spalding MH (2000) CO2 acquisition, concentration and fixation in cyanobacteria and algae. In: Leegood RC, Sharkey TD, von Caemmerer S (eds) Photosynthesis: physiology and metabolism. Kluwer, Dordrecht, pp 369–397
Beer S (1996) Photosynthetic utilization of inorganic carbon in Ulva. Sci Mar 60:125–128
Beer S, Israel A (1986) Photosynthesis of Ulva sp. III. O2 effects, carboxylase activities, and the CO2 incorporation pattern. Plant Physiol 81:937–938
Beer S, Koch E (1996) Photosynthesis of marine macroalgae and seagrasses in globally changing CO2 environments. Mar Ecol Prog Ser 141:199–204
Bischof K, Kräbs G, Wiencke C, Hanelt D (2002) Solar ultraviolet radiation affects the activity of ribulose- 1,5-bisphosphate carboxylase-oxygenase and the composition of photosynthetic and xanthophyll cycle pigments in the intertidal green alga Ulva lactuca L. Planta 215:502–509
Black WAP (1950) The seasonal variation in weight and chemical composition of the common British Laminariaceae. J Mar Biol Assoc UK 29:45–72
Buggeln RG (1983) Photoassimilate translocation in brown algae. Prog Phycol Res 2:283–332
Cabello-Pasini A, Alberte RS (1997) Seasonal patterns of photosynthesis and light-independent carbon fixation in marine macrophytes. J Phycol 33:321–329
Cabello-Pasini A, Alberte RS (2001a) Expression of carboxylating enzymes in Laminaria setchellii (Phaeophyceae). Phycologia 40:351–358
Cabello-Pasini A, Alberte RS (2001b) Enzymatic regulation of photosynthetic and light-independent carbon fixation in Laminaria setchelli (Phaeophyta), Ulva lactuca (Chlorophyta) and Iridaea cordata (Rhodophyta). Rev Chil Hist Nat 74:229–236
Cabello-Pasini A, Smith GJ, Alberte RS (2000) Phosphoenol pyruvate carboxykinase from the marine diatom Skeletonema costatum and the phaeophyte Laminaria setchelli I. Isolation and biochemical characterization. Bot Mar 43:559–568
Cagné JA, Mann KH, Chapman ARO (1982) Seasonal patterns of growth and storage in Laminaria longicruris in relation to differing patterns of availability of nitrogen in the water. Mar Biol 69:91–101
Calvin M (1956) The photosynthetic carbon cycle. J Chem Soc 1956:1895–1915
Carlberg GE, Percival E, Rhaman A (1978) Carbohydrates of the seaweeds Desmarestia ligulata and D. firma. Phytochemisty 17:1289–1292
Chapman AR0, Craigie JS (1977) Seasonal growth in Laminaria longicruris: relations with dissolved inorganic nutrients and internal reserves of nitrogen. Mar Biol 40:161–164
Chapman AR0, Craigie JS (1978) Seasonal growth in Laminaria longicruris: relations with reserve carbohydrate storage and production. Mar Biol 46:209–213
Cheshire AC, Hallam ND (1985) The environmental role of alginates in Durvillaea potatorum (Fucales, Phaeophyta). Phycologia 24:147–153
Choo KS, Nilsson J, Pedersén M, Snoeijs P (2005) Photosynthesis, carbon uptake and antioxidant defense in two coexisting filamentous green algae under different stress conditions. Mar Ecol Prog Ser 292:127–138
Clayton MN, Ashburner CM (1990) The anatomy and ultrastructure of “conducting channels” in Ascoseira mirabilis (Ascoseirales, Phaeophyceae). Bot Mar 33:63–70
Craigie JS (1974) Storage products. In: Stewart WDP (ed) Algal physiology and biochemistry. Blackwell Scientific, Oxford, pp 206–235
Cronin G, Hay ME (1996) Within-plant variation in seaweed palatability and chemical defenses: optimal defense theory versus the growth-differentiation balance hypothesis. Oecologia 105:361–368
Dawes CJ, Lawrence JM, Cheney DP, Mathieson AC (1974) Ecological studies of floridian Eucheuma (Rhodophyta, Gigartinales). III. Seasonal variation of carrageenan, total carbohydrate, protein, and lipids. Bull Mar Sci 24:286–299
Delille B, Delille D, Fiala PC, Frankignoulle M (2000) Seasonal changes of pCO2 over a subantarctic Macrocystis kelp belt. Polar Biol 23:706–716
Drechsler Z, Sharkia R, Cabantchik ZL, Beer S (1994) The relationship of arginine groups to photosynthetic HCO -3 uptake in Ulva sp. mediated by a putative anion exchanger. Planta 194:250–255
Drew EA, Hastings RM (1992) A year-round ecophysiological study of Himantothallus grandifolius (Desmarestiales, Phaeophyta) at Signy Island, Antarctica. Phycologia 31:262–277
Duggins DO, Simenstad CA, Estes JA (1989) Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 245:170–173
Dunton KH, Schell DM (1986) Seasonal carbon budget and growth of Laminaria solidungula in the Alaskan High Arctic. Mar Ecol Prog Ser 31:57–66
Falkowski PG, Raven J (1997) Aquatic photosynthesis. Blackwell, Oxford
Flores-Moya A, Gómez I, Viñegla B, Altamirano M, Pérez-Rodríguez E, Maestre C, Caballero RM, Figueroa FL (1998) Effects of solar radiation on the endemic Mediterranean red alga Rissoella verruculosa: Photosynthetic performance, pigment content and the activities of enzymes related to nutrient uptake. New Phytol 139:673–683
Gao KY, Aruga Y, Asada K, Kiyohara M (1993) Calcification in the articulated coralline algae Corallina pilulifera, with special reference to the effect of elevated CO2 concentration. Mar Biol 117:129–132
Gattuso J-P, Gentili B, Duarte CM, Kleypas JA, Middelburg JJ, Antoine D (2006) Light availability in the coastal ocean: impact on the distribution of benthic photosynthetic organisms and contribution to primary production. Biogeosciences Discuss 3:895–959
Gerard VA (1982) Growth and utilization of internal nitrogen reserves by the giant kelp Macrocystis pyrifera in a low-nitrogen environment. Mar Biol 66:27–35
Giordano M, Maberly SC (1989) Distribution of carbonic anhydrase in British marine macroalgae. Oecologia 81:534–539
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Gómez I (1997) Life strategy and ecophysiology of Antarctic macroalgae. Rep Polar Res 238:1–99
Gómez I, Huovinen P (2010) Induction of phlorotannins during UV exposure mitigates inhibition of photosynthesis and DNA damage in the kelp Lessonia nigrescens. Photochem Photobiol 86:1056–1063
Gómez I, Lüning K (2001) Constant short-day treatment of outdoor-cultivated Laminaria digitata prevents summer drop in growth rates. Eur J Phycol 36:391–395
Gómez I, Westermeier R (1995) Energy contents and organic constituents in Antarctic and south Chilean marine brown algae. Polar Biol 15:597–602
Gómez I, Wiencke C (1998) Seasonal changes in C, N, and major organic compounds and their significance to morpho-functional processes in the endemic Antarctic brown alga Ascoseira mirabilis. Polar Biol 19:115–124
Gómez I, Thomas DN, Wiencke C (1995a) Longitudinal profiles of growth, photosynthesis and light independent carbon fixation in the Antarctic brown alga Ascoseira mirabilis. Bot Mar 38:157–164
Gómez I, Wiencke C, Weykam G (1995b) Seasonal photosynthetic characteristics of Ascoseira mirabilis (Ascoseirales, Phaeophyceae) from King George Island, Antarctica. Mar Biol 123:167–172
Gómez I, Wiencke C, Thomas DN (1996) Variations in photosynthetic characteristic of the Antarctic marine brown alga Ascoseira mirabilis Skottsberg in relation to age and size. Eur J Phycol 31:167–172
Gómez I, Weykam G, Wiencke C (1998a) Photosynthetic metabolism and major organic compounds in the marine brown alga Desmarestia menziesii from King George Island (Antarctica). Aquatic Bot 60:105–118
Gómez I, Pérez-Rodríguez E, Viñegla B, Figueroa FL, Karsten U (1998b) Effects of solar radiation on photosynthesis, UV absorbing compounds and enzyme activities of the green alga Dasycladus vermicularis from southern Spain. J Photochem Photobiol B Biol 47:46–57
Gómez I, Ulloa N, Orostegui M (2005) Morpho-functional patterns of photosynthesis and UV sensitivity in the kelp Lessonia nigrescens (Laminariales, Phaeophyta). Mar Biol 148:231–240
Gómez I, Orostegui M, Huovinen P (2007) Morpho-functional patterns of photosynthesis in the South Pacific kelp Lessonia nigrescens: effects of UV radiation on 14 C fixation and primary photochemical reactions. J Phycol 43:55–64
Gonen Y, Kimmel E, Tel-Or E, Friedlander M (1996) Intercellular assimilate translocation in Gracilaria cornea (Gracilariaceae, Rhodophyta). Hydrobiologia 326(327):421–428
Gordillo FJL, Aguilera J, Jiménez C (2006) The response of nutrient assimilation and biochemical composition of Arctic seaweeds to a nutrient input in summer. J Exp Bot 57:2661–2671
Gorham J, Lewey SA (1984) Seasonal changes in the chemical composition of Sargassum muticum. Mar Biol 108:103–107
Gross W (1990) Occurrence of glycolate oxidase and hydroypyruvate reductase in Egreggia menziesii (Phaeophyta). J Phycol 26:381–383
Guimaraes SMPB, Braga MRA, Cordeiro-Marinho M, Pedrini AG (1986) Morphology and taxonomy of Jolyna laminarioides, a new member of the Scytosiphonales (Phaeophyceae) from Brazil. Phycologia 25:99–108
Haglund K, Björk M, Ramazanov Z, García-Reina G, Pedersén M (1992) Role of carbonic anhydrase in photosynthesis and inorganic carbon assimilation in the red alga Gracilaria tenuistipitata. Planta 187:275–281
Hartman T, Eschrich W (1969) Stofftransport in Rotalgen. Planta 85:303–312
Hatcher BG, Chapman AR0, Mann KH (1977) An annual carbon budget for the kelp Laminaria longicruris. Mar Biol 44:85–96
Haug A, Jensen A (1954) Seasonal variations in chemical composition of Alaria esculenta, Laminaria saccharina. Laminaria hyperborea and Laminaria digitata from northern Norway, Norw Inst Seaweed Res Rep No, 4
Huovinen P, Gómez I, Orostegui M (2007) Patterns and UV sensitivity of carbonic anhydrase and nitrate reductase activities in south Pacific macroalgae. Mar Biol 151:1813–1821
Hurd CL (2000) Water motion, marine macroalgae physiology and production. J Phycol 36:453–472
Israel A, Einav R (eds) (2010) Seaweeds and their role in globally changing environments. Cellular origin, life in extreme habitats and astrobiology, vol 15, 1st edn. Springer, New York, p 480
Israel A, Hophy M (2002) Growth, photosynthetic properties and Rubisco activities and amounts of marine macroalgae grown under current and elevated seawater CO2 concentrations. Global Change Biol 8:831–840
Iwamoto K, Ikawa T (1997) Glycolate metabolism and subcellular distribution of glycolate oxidase in Patoglossum pacificum (Phaeophyceae, Chromophyta). Phycol Res 45:77–83
Jackson GA (1998) Currents in the high drag environment in a coastal kelp stand off California. Cont Shelf Res 17:1913–1918
Jensen A, Haug A (1956) Geographical and seasonal variation in the chemical composition of Laminaria hyperborea and Laminaria digitata from the Norwegian coast. Norw Inst Seaweed Res Rep No 14
Johnston AM, Raven JA (1986) Dark carbon fixation studies on the intertidal macroalga Ascophyllum nodosum (Phaeophyta). J Phycol 22:78–83
Kawamitsu Y, Boyer JS (1999) Photosynthesis and carbon storage between tides in a brown alga, Fucus vesiculosus. Mar Biol 133:361–369
Kerby WN, Evans LV (1983) Phosphoenolpyruvate carboxykinase activity in Ascophyllum nodosum (Phaeophyceae). J Phycol 19:1–3
Klenell M, Snoeijs P, Pedersén M (2002) The involvement of a plasma membrane H+-ATPase in the blue-light enhancement of photosynthesis in Laminaria digitata (Phaeophyta). J Phycol 38:1143–1149
Kremer BP (1981) Metabolic implications of non-photosynthetic carbon fixation in brown algae. Phycologia 20:242–250
Kübler E, Raven JA (1994) Consequences of light limitation for carbon acquisition in three rhodophytes. Mar Ecol Prog Ser 110:203–209
Küppers U, Kremer BP (1978) Longitudinal profiles of carbon dioxide fixation capacities in marine macroalgae. Plant Physiol 62:49–53
Lawrence JM (1986) Proximate composition and standing crop of Durvillaea antarctica (Phaeophyta) in the Bay of Morbihan, Kerguelen (South Indian Ocean). Mar Ecol Prog Ser 33:1–5
Lawrence JM, McClintock JB (1988) Allocation of organic material and energy to the holdfast, stipe, and fronds in Postelsia palmaeformis (Phaeophyta: Laminariales) on the California coast. Mar Biol 99:151–155
Littler MM, Littler DS (1980) The evolution of thallus form and survival strategies in benthic marine macroalgae: field and laboratory tests of a functional form model. Am Nat 116:25–44
Lobban CS, Harrison PJ (2000) Seaweed ecology and physiology. Cambridge University Press, Cambridge
Lucas PW, Turner IM, Dominy NJ, Yamashita N (2000) Mechanical defenses to herbivory. Ann Bot 86:913–920
Lüning K (1979) Growth strategies of three Laminaria species (Phaeophyceae) inhabiting different depth zones in the sublittoral region of Helgoland (North Sea). Mar Ecol Prog Ser 1:195–207
Lüning K, Schmitz K, Willenbrink K (1973) CO2 fixation and translocation in benthic marine algae III. Rates and ecological significance of translocation in Laminaria hyperborea and L. saccharina. Mar Biol 12:275–281
Maberly SC (1990) Exogenous source of inorganic carbon for photosynthesis by marine macroalgae. J Phycol 26:439–449
Macler BA (1986) Regulation of carbon flow by nitrogen and light in the red alga, Gelidium coulteri. Plant Physiol 82:136–141
Mann KH (1973) Seaweeds: their productivity and strategy for growth. Science 182:975–981
McKay RML, Sarah I, Gibbs P, Vaughn KC (1991) RuBisCo activase is present in the pyrenoid of green algae. Protoplasma 162:38–45
Mercado JM, Niell FX (1999) Carbonic anhydrase activity and use of HCO -3 in Bostrychia scorpioides (Ceramiales, Rhodophyceae). Eur J Phycol 34:13–19
Mercado JM, Gordillo FJL, Figueroa FL, Niell FX (1998) External carbonic anhydrase and affinity for inorganic carbon in intertidal macroalgae. J Exp Mar Biol Ecol 221:209–220
Mercado JM, de los Santos CB, Pérez-Lloréns JL, Vergara JJ (2009) Carbon isotopic fractionation in macroalgae from Cádiz Bay (Southern Spain): Comparison with other bio-geographic regions. Estuar Coast Shelf Sci 85:449–458
Moe RL, Silva PC (1981) Morphology and taxonomy of Himantothallus (including Phaeoglossum and Phyllogigas) an Antarctic member of the Desmarestiales (Phaeophyceae). J Phycol 17:15–29
Moss BL (1983) Sieve elements in the Fucales. New Phytol 93:431–433
Murru M, Sandgren CD (2004) Habitat matters for inorganic carbon acquisition in 38 species of red macroalgae (Rhodophyta) from Puget Sound, Washington, USA. J Phycol 40:837–845
Nelson DL, Cox MN (2004) Lehninger, principles of biochemistry, 4th edn. W.H. Freeman, New York
Pansch C, Gómez I, Rothäusler E, Veliz K, Thiel M (2008) Defense of vegetative vs. reproductive blades of the Pacific kelps Lessonia nigrescens and Macrocystis integrifolia: A test on species-specific defense strategies. Mar Biol 155:51–62
Percival EE, Venegas MJ, Weigel H (1983) Carbohydrates of the brown seaweed Lessonia nigrescens. Phytochemistry 22:1429–1432
Ramus J (1978) Seaweed anatomy and photosynthetic performance: the ecological significance of light guides, heterogeneous absorption and multiple scatter. J Phycol 14:352–362
Raven JA (1991) Implications of inorganic C utilization: ecology, evolution and geochemistry. Can J Plant Physiol 69:908–924
Raven JA (1997) Putting the C in phycology. Eur J Phycol 32:319–333
Raven JA (2003) Inorganic carbon concentrating mechanisms in relation to the biology of algae. Photosynth Res 77:155–171
Raven JA (2010) Inorganic carbon acquisition by eukaryotic algae: four current questions. Photosynth Res 106:123–134
Raven JA, Beardall J (1981) Carbon dioxide as the exogenous inorganic carbon source for Batrachospermum and Lemanea. Br Phycol J 16:165–172
Raven JA, Lucas J (1985) The energetics of carbon acquisition. In: Lucas WJ, Berry JA (eds) Inorganic carbon uptake by aquatic photosynthetic organisms. Am Soc Plant Physiol, pp 305–324
Raven JA, Walker DI, Johnston AM, Handley LL, Kübler JE (1995) Implications of 13 C natural abundance measurements for photosynthetic performance by marine macrophytes in their natural environment. Mar Ecol Prog Ser 123:193–205
Raven JA, Johnston AM, Kübler JE, Korb RE, McInroy SG, Handley LL, Scrimgeour CM, Walker DI, Beardall J, Clayton MN, Vanderklift M, Chudek JA, Fredriksen S, Dunton KH (2002) Seaweeds in cold seas: evolution and carbon acquisition. Ann Bot 90:525–536
Raven JA, Ball LA, Beardall J, Giordano M, Maberly SC (2005) Algae lacking carbon concentrating mechanisms. Can J Bot 83:879–890
Reiskind JB, Seamon PT, Bowes G (1988) Alternative methods of photosynthetic carbon assimilation in marine macroalgae. Plant Physiol 87:686–692
Rosenberg G, Littler DS, Littler MN, Oliveira EC (1995) Primary production and photosynthetic quotients of seaweeds from Sao Paulo State, Brazil. Bot Mar 38:369–378
Sand-Jensen K, Gordon DM (1984) Differential ability of marine and freshwater macrophytes to utilize HCO -3 and CO2. Mar Biol 80:247–253
Schaffelke B (1995) Storage carbohydrates and abscisic acid contents in Laminaria hyperborea are constrained by experimental daylengths. Eur J Phycol 30:313–317
Schmid R, Mills JA, Dring MJ (1996) Influence of carbon supply on stimulation of light saturated photosynthesis by blue light in Laminaria saccharina: implications for the mechanism of carbon acquisition in higher brown algae. Plant Cell Environ 19:383–391
Schmitz K (1981) Translocation. In: Lobban CS, Wynne MJ (eds) The biology of seaweeds. University of California Press, Berkeley, pp 534–558
Schmitz K (1990) Algae. In: Behnke H-D, Sjolund RD (eds) Sieve elements. Comparative structure, induction and development. Springer, New York, pp 1–18
Steneck RS, Watling L (1982) Feeding capabilities and limitation of herbivorous mollusks—a functional—group approach. Mar Biol 68:299–319
Surif MB, Raven JA (1990) Photosynthetic gas exchange under emersed conditions in eulittoral and normally submersed members of the Fucales and the Laminariales: interpretation in relation to C isotope ratio and N and water use efficiency. Oecologia 82:68–80
Suzuki K, Iwamoto K, Yokoyama S, Ikawa T (1991) Glycolate-oxidizing enzymes in algae. J Pycol 27:492–498
Tabita FR, Satagopan S, Hanson TE, Kreel NE, Scott SS (2008) Distinct form I, II, III, and IV Rubisco proteins from the three kingdoms of life provide clues about Rubisco evolution and structure/function relationships. J Exp Bot 59:1515–1524
Thomas DN, Wiencke C (1991) Photosynthesis, dark respiration and light independent carbon fixation of endemic Antarctic macroalgae. Polar Biol 11:329–337
Tolbert NE (1997) The C2 oxidative photosynthetic carbon cycle. Annu Rev Plant Physiol Plant Mol Biol 48:1–25
van Caemmerer S, Furbank RT (2003) The C4 pathway: an efficient CO2 pump. Photosynth Res 77:191–207
Wakefield RL, Murray SN (2009) Factors influencing food choice by the seaweed-eating marine snail Norrisia norrisi (Trochidae). Mar Biol 130:631–642
Wëiwer M, Sherwood T, Linhardt RJ (2008) Synthesis of floridoside. J Carbohydr Chem 27:420–427
Westermeier R, Gómez I (1996) Biomass, energy contents and major organic compounds in the brown alga Lessonia nigrescens (Laminariaes, Phaeophyceae) from Mehuín, South Chile. Bot Mar 39:553–559
Wetherbee R (1979) “Transfer connections”: specialized pathways for nutrient translocation in red alga? Science 204:858–859
Weykam G (1996) Photosynthetic characteristics and life strategies of Antarctic macroalgae. Rep Polar Res 192:1–132
Weykam G, Thomas DN, Wiencke C (1997) Growth and photosynthesis of the Antarctic red algae Palmaria decipiens (Palmariales) and Iridaea cordata (Gigartinales) during and following extended periods of darkness. Phycologia 36:395–405
Wheeler PA, North WJ (1981) Nitrogen supply, tissue composition and frond growth rates for Macrocystis pyrifera off the coast of Southern California. Mar Biol 64:59–69
Wiencke C, Clayton MN (1990) The anatomy, life history and development of the Antarctic brown alga Phaeurus antarcticus (Desmarestiales, Phaeophyceae). Phycologia 29:303–315
Wiencke C, Fischer G (1990) Growth and stable carbon isotope composition of cold-water macroalgae in relation to light and temperature. Mar Ecol Prog Ser 65:283–292
Wiencke C, Fischer G (1992) Stable carbon isotope composition, depth distribution and fate of macroalgae from the Antarctic Peninsula region. Polar Biol 12:341–348
Wiencke C, Gómez I, Dunton K (2009) Phenology and seasonal physiological performance of polar seaweeds. Bot Mar 52:585–592
Woodrow IE, Berry JA (1988) Enzymatic regulation of photosynthetic CO2 fixation in C3 plants. Annu Rev Plant Physiol Plant Mol Biol 39:533–594
Zeebe RE, Wolf-Gladrow DA (2001) CO2 in seawater: equilibrium, kinetics, isotopes. Els Oceanogr Ser 65:346
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Gómez, I., Huovinen, P. (2012). Morpho-functionality of Carbon Metabolism in Seaweeds. In: Wiencke, C., Bischof, K. (eds) Seaweed Biology. Ecological Studies, vol 219. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-28451-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-642-28451-9_2
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-28450-2
Online ISBN: 978-3-642-28451-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)