Abstract
Industrialization and globalization are causing numerous fluctuations in our ecosystem including increased level of heavy metals. Bioextraction is an alternative to the existing chemical processes for better efficiency with least amount of by-products at optimum utilization of energy. Here is an overview of bioextraction methodology and its associated biological processes, and discussion of the approaches that have been used successfully for withdrawal of heavy metals using metal selective high biomass transgenic plants and microbes from contaminated sites and sub-graded ores. The future of bioextraction is challenging as it offers advantages of operational simplicity, low capital and operating cost, and shorter construction times that no other alternative process can provide. Above all, due to environmental friendliness use of this technology is set to increase.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
The interfacial face of bioextraction arises as it uses the technologies in which plants clean up the contaminated sites by immobilizing the contaminants in the soil. This technique is mostly applied to heavy metals in soil sediments and sludges. These metals are either trapped within the root system or taken up to the tissues by selected fast growing plant species. These species are grown under normal farming conditions until they reach their maximum size. Throughout the growth period, amendments are added to soil to increase availability of metals to plants. When the plants are mature, metal specific chelating agents are applied to the harvested biomass for the recovery of accumulated metals [1]. So, selection of plant materials is an important factor for this technique. Therefore, two main strategies are proposed to clean up toxic metals from soil. The first approach is the use of metal hyper-accumulator species for cleaning up of soil, as they can take up significant amount of metals from contaminated soils, but their low annual biomass production tends to limit its ability. This problem can be overcome by using high biomass plants that can be easily cultivated. So, the efficiency of this technique is determined by two key factors: metal hyper-accumulating capacity and biomass production [2].
Plant-based environmental remediation technology has been widely pursued in recent years as greener cost effective strategy to trap metals and radionuclide contaminants that are in mobile chemical forms which are most threatening to human and environmental health. Once the removal of contaminants is complete the soil generated from this process is fertile and is able to support the growth of plants [1].
This technology has been applied at a number of sites all over the world. Examples include Magic Marker site in New Jersey and a Daimler Chrysler site in Detroit, Michigan (induced accumulation of lead in soil); Argonne National Laboratory-West (mercury, silver, chromium in soil/sediment removed by whole plant harvesting). This technique can also be merged with other techniques, like it can be used in conjunction with electrochemical technology to remove contaminants (such as cesium-137), which bioextraction technology alone cannot remove and many more permutations and combinations can be tried [1].
14.1 Disadvantages of Metal Extraction Process, its Environmental Concerns and Need of Bioextraction
The tremendous increase in the use of heavy metals over the past few decades has inevitably resulted in an increased flux of metallic substances in the environment. Industrial processes like petroleum refining, metal refining, coal combustion, tanning, metal extraction, electroplating, paints and pigments, the manufacture of batteries etc. discharge effluents in solid, liquid, and gaseous forms. They contain heavy metals such as lead, chromium, cadmium, nickel, arsenic, etc. But the major sources of heavy metals in the environment are traditional chemical processes for extraction of heavy metals. There are various disadvantages of these metal extraction processes like requirement of sufficient concentrations of elements in ores, environmental unfriendliness as huge amount of waste is generated, economically noncompetitive, nonrecovery of metals from low grade deposits i.e. minerals and inefficient use of energy.
Also, lot of metal containing effluent is produced during these processes, which is discharged as such without any treatment. This causes heavily loaded metal contaminated sites due to metal toxicity and non-biodegradability. The heavy metals are easily percolated through the soil and further trapped and biomagnified along the food chain via consumption of affected plants and animals. The increased concern about the environment and stringent national and international regulations on water pollution and the discharge of heavy metals makes it essential to develop efficient and cost effective technologies for their removal. Hence, it is the utmost need to extract metal ions (not only from the low grade ores but also from the contaminated sites) by the methods which are eco-friendly and greener in nature. The answer is provided by the nature itself: bioextraction.
14.2 Brief Description of Bioextraction Process
Bioextraction incorporates a range of technologies that not only use plants to remove, reduce, degrade, or immobilize environmental pollutants from soil and water, for restoration of contaminated sites to a relatively clean, non-toxic environment but also use microbes to extract metals from the low grade ores. This relatively new and growing technology uses natural processes to break down, stabilize, or accumulate pollutants and for extraction of metals [3]. It basically incorporates two phenomena:
-
Phytoextraction
-
Biomining
Phytoextraction is the removal of pollutants by the roots of plants, followed by translocation to aboveground plant tissues, which are subsequently harvested. Biomining is the extraction of metals by the help of naturally growing thermal sensitive microbes.
14.2.1 Phytoextraction
Contamination of soils with toxic metals has often resulted from human activities, especially those related to mining, industrial emissions, disposal or leakage of industrial wastes, application of sewage sludge to agricultural soils, manure, fertilizer, and pesticide use. Excessive metal concentration in soil poses significant hazard to human, animal and plant health, and to the environment. The aim of phytoextraction is to reduce the concentration of metals in contaminated soils to regulatory levels within a reasonable time frame. This extraction process depends on the ability of selected plants to grow and accumulate metals under the specific climatic and soil conditions of the site being remediated.
It uses plants to remove metals from soils and to transport and concentrate them in above-ground biomass [4]. In this process, plant roots sorb the contaminants along with other nutrients and water. The contaminant mass is not destroyed but ends up in the plant’s shoots and leaves. This method is used primarily for wastes containing metals where water-soluble metals are taken up by plant species selected for their ability to take up large quantities of metals. The metals stored in the plant’s aerial shoots are harvested and smelted for potential metal recycling/recovery which were earlier disposed off as a hazardous waste. As a general rule, readily bio-available metals for plant uptake include cadmium, nickel, zinc, arsenic, selenium, and copper. Moderately bio-available metals are cobalt, manganese, and iron. They can be made much more bio-available by the addition of chelating agents to soils.
Phytoextraction has been growing rapidly in popularity worldwide for the last 20 years or so. In general, this process has been tried more often for extracting heavy metals than for organics. The plants absorb contaminants through the root system and store them in the root biomass and/or transport them up into the stems and/or leaves. A living plant may continue to absorb contaminants until it is harvested. After harvest, a lower level of the contaminant will remain in the soil, so the growth/harvest cycle must usually be repeated through several crops to achieve a significant cleanup. After the process, the cleaned soil can support other vegetation.
There are two main categories of plants to clean up toxic metals from soil:
-
Metal hyper-accumulator plants
-
High biomass plants
14.2.1.1 Metal Hyper-Accumulator Plants
They take up significant amounts of metal from contaminated soil but their low biomass production tends to limit their phytoextraction ability. As these plants have natural ability to extract metal ions, so it is known as natural phytoextraction. Hyper-accumulating plants have natural ability to extract high amounts of metals from soil, have efficient mechanism to translocate metals from roots to shoots, and can accumulate and tolerate high metal concentrations due to inherent mechanisms to detoxify metals in the tissues. Metal hyper-accumulators have the extraordinary capacity to accumulate high concentrations of heavy metals in the above-ground biomass. By virtue of this remarkable characteristic, phytoextraction is economically viable alternative to the extreme expense of conventional remediation methods [5].
Example: Alyssum lesbiacum as Ni hyper-accumulator, Thlaspi caerulescens/Alpine pennycress as Zn/Cd hyper-accumulator (Figs. 14.1, 14.2).
14.2.1.2 High Biomass Plants
They are fast growing plants that can be easily cultivated using established agronomic practices which compensate for their relatively low capacity of metal accumulation. Their metal uptake capacity can further be enhanced by adding conditioning fluid containing a chelator or another agent to soil to upsurge metal solubility or mobilization so that the plants can absorb them more easily. This is known as chemically induced/assisted phytoextraction. Afterwards, the soluble metal (desorbed from soil particles) is easily transported to roots surface via diffusion and translocated from roots to shoots. Complexing with organic ligands, which may occur at any point along the transport pathway, converts the metal into less toxic form thus conferring high metal tolerance in biomass plants [5]. A wide range of synthetic chelates [e.g.Ethylenediaminetetraacetic acid (EDTA), 1,2-cyclohexylenedinitrilotetraacetic acid (CDTA), diethylenetriaminepentaacetic acid (DTPA), EGTA, EDHA, hydroxyethylethylenediaminetriacetic acid (HEDTA), nitriloacetic acid (NTA), and organic acids (e.g. citric acid, oxalic acid, malic acid) are used for enhancing root uptake and translocation of metal contaminants from soil to biomass plants, thereby improving phytoextraction [6] (Fig. 14.3, 14.4, 14.5).
Example: Indian mustard, sunflower, and maize as high biomass crop plants, willows, and poplars as high biomass trees
Advantages. The main advantage of phytoextraction is environmental friendliness. Traditional methods that are used for cleaning up heavy metal contaminated soil disrupt the soil structure and reduce its productivity, whereas phytoextraction can clean up the soil without causing any kind of harm to soil quality. Add on benefit of phytoextraction is that it is less expensive than any other clean-up process (Fig. 14.6) (Table 14.1).
14.2.2 Biomining
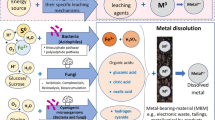
For centuries people have been using microbes to their advantage, turning grapes into wine, milk into cheese, and cabbage into sauerkraut. People benefit from what microbes do naturally: They eat and digest organic compounds, changing the chemical makeup of one product and turning it into a completely different yet tasty food or drink. Now microbes, in form of biomining, are providing efficient helping hand for extraction of heavy metals from sub-graded ores and minerals (Fig. 14.7).
Biomining is the interaction between metals and microbes with the specific aim of converting insoluble metal sulfides to soluble metal sulfates. Bioleaching has been defined as the dissolution of metals from their mineral sources by certain naturally occurring microorganisms or the use of microorganisms to transform elements so that the elements can be extracted from a material when water is filtered through it. So, it is the application of microbial process in the mining industry for economic recovery on a large scale [7] (Fig. 14.8).
Electron micrographs of typical bacteria used in biomining a Leptospirillum ferrooxidans. b Thiobacillus ferrooxidans [7]
In short, biomining is a term that describes the processing of metal containing ores and concentrates of metal containing ores using microbiological technology. It is often called bioleaching.
By convention bioleaching has been divided into two approaches:
-
Direct bioleaching
-
Indirect bioleaching
Direct bioleaching entails an enzymatic attack by the bacteria on components of the mineral that are susceptible to oxidation. In the process of obtaining energy from the inorganic material the bacteria cause electrons to be transferred from iron or sulfur to oxygen. In many cases the more oxidized product is more soluble. It should be noted that the inorganic ions never enter the bacterial cell; the electrons released by the oxidation reaction are transported through a protein system in the cell membrane and then (in aerobic organisms) to oxygen atoms, forming water. The transferred electrons give up energy, which is coupled to the formation of adenosine triphosphate (ATP), the energy currency of the cell.
Indirect bioleaching, in contrast, does not proceed through a frontal attack by the bacteria on the atomic structure of the mineral. Instead the bacteria generate ferric iron by oxidizing soluble, ferrous iron; ferric iron in turn is a powerful oxidizing agent that reacts with other metals, transforming them into the soluble oxidized form in a sulfuric acid solution. In this reaction ferrous iron is again produced and is rapidly reoxidized by the bacteria. Indirect bioleaching is usually referred to as bacterially assisted leaching. In an acidic solution without the bacteria, ferrous iron is stable and leaching mediated by ferric iron would be slow. T ferrooxidans can accelerate such an oxidation reaction by a factor of more than a million.
Biomining is applied using four different engineered methods:
-
Dump bioleaching
-
Heap bioleaching
-
Heap minerals biooxidation
-
Stirred-tank bioleaching
-
Minerals biooxidation
Dump bioleaching extracts copper from sulfide ores that are too low grade to process by any other method. This process has been used since the mid-1950s.
Heap bioleaching, which has been used since the 1980s, extracts copper from crushed sulfide minerals placed on engineered pads.
Heap minerals biooxidation pretreats gold ores in which the gold particles are locked in sulfide minerals, significantly enhancing gold recovery.
Stirred-tank bioleaching extracts base metals from concentrates of metal containing sulfide ores.
Stirred-tank minerals biooxidation enhances gold recovery from mineral concentrates in which the gold is locked in sulfide minerals [8].
Advantages
The advantages of biomining process over chemical leaching are:
-
(i)
Biomining is a way to exploit low grade ores and mineral resources located in remote areas that would otherwise be too expensive to mine.
-
(ii)
It is more environmentally friendly than the conventional (smelting) method, since it uses less energy and does not produce SO2 emissions. This also translates into profit, as the companies have to spend huge sums finding ways of limiting their SO2 emissions.
-
(iii)
Less landscape damage occurs, since the bacteria grow naturally. Native bacteria can operate over a wide temperature range between 20 and 55°C. Other materials for the process are also natural such as air and water.
-
(iv)
The bacteria breed on their own, i.e. they are self-sustaining. Since there is no need to pay for heating and chemicals required in a conventional operation, companies may be able to reduce the price of metal production by nearly a half.
-
(v)
It is a less energy intensive process.
-
(vi)
It is simpler and therefore cheaper to operate and maintain, as no technical specialist is needed to operate complex chemical plants [9].
-
(vii)
Even the dumps left behind after traditional mining processes can be reprocessed to extract residual metal [10].
So, biomining is the process of extracting valuable metals from ores and mine tailings with the assistance of microorganisms. It is a green technology that can help mine valuable metals with minimal impact on the environment. It requires low energy, causes low gaseous emission and is not labor intensive.
14.3 Contribution of Microbes/Microorganisms in Bioextraction
The microbes are single-celled organisms that multiply by simple cell division and derive energy for growth and cell functioning by oxidizing iron and sulfur. Oxidation involves the removal of electrons from a substance. In biomining process, the microbes remove electrons from dissolved iron (ferrous iron) converting it to another form of iron (ferric iron); electrons are removed from sulfur converting it to sulfuric acid. They obtain carbon for their cellular bodies from carbon dioxide (CO2) in the atmosphere and also require a sulfuric acid environment to grow. This acidic environment is helpful in growth of these microorganisms but acidity must be less than pH 2.5, which is more acidic than vinegar.
The biomining microorganisms do not cause diseases in humans, animals, or plants. Because their food source is inorganic (sulfur and iron) and because they must live in a sulfuric acid environment, they cannot survive in or on plants and animals. These microbes are conveniently grouped within temperature ranges at which they grow and where they are found in the natural environment:
-
Ambient temperature bacteria (mesophiles)
-
Moderately-thermophilic (heat-loving) bacteria
-
Extremely-thermophilic (heat-loving) bacteria
Ambient temperature bacteria (mesophiles). These cylindrical-shaped biomining bacteria are about 1 μm long and ½ μm in diameter (1 μm is 4/100,000 of an inch). About 1,500 of these bacteria could lay end-to-end across a pin head. They only grow and function from 10 to 40°C (50 to 104°F). If the temperature is too low, these bacteria become dormant. If the temperature exceeds 45°C (113°F), the organisms die as their proteins coagulate similar to cooking an egg. Acidithiobacillus ferrooxidans belong to this group of bacteria. Others include Leptospirillum ferrooxidans and species of Ferroplasma.
Moderately-thermophilic (heat-loving) bacteria. These bacteria are similar to the “mesophilic” biomining bacteria, except they are somewhat larger in length —about 2–5 μm long and they only grow and perform when the temperature exceeds 40°C (104°F). The moderate thermophiles die when the temperature exceeds 60°C (140°F). Examples of moderate thermophiles are species of Sulfobacillus and Acidithiobacilluscaldus.
Extremely-thermophilic Archaea. While similar in size (one micrometer in diameter) to ambient temperature bacteria, Archaea have a different molecular organization. In the tree of life, Archaea occupy the lowest branch and are extant members of an offshoot of primitive microbes. They have a spherical shape and characteristically lack a rigid cell wall, rather the contents of the single cell are enclosed by a membrane. These microbes, nevertheless, are extremely robust growing and performing only at temperatures between 60 (140°F) and 85°C (185°F). Examples of extremely-thermophilic Archaea used in biomining are Acidianus brierleyi, Sulfolobus metallicus and Metallosphaera sedula [8].
14.3.1 Role of Microbes in Biomining
Some microbes float freely in the solution around the minerals and some attach themselves to the mineral particles forming a biofilm. The microbes, whether they are freely floating or whether they are in the biofilm, continuously devour their food sources—iron (chemically represented as Fe2+) and sulfur. The product of the microbial conversion of iron is “ferric iron”, chemically represented as “Fe3+”. Ferric iron is a powerful oxidizing agent, corroding metal sulfide minerals (for example, pyrite, arsenopyrite, chalcocite, and sphalerite) and degrading them into a dissolved metal, such as copper, zinc, and more iron—the latter is the food source for the microbes.
The reaction of the biological oxidation involved in leaching of a mineral sulfide is
where, M is a bivalent metal
There are two major mechanisms involved in microbial metal solubilization of sulfide minerals. One is a direct mechanism that involves physical contact of the organism with the insoluble sulfide.
Microorganisms oxidize the metal sulfides obtaining electrons directly from the reduced minerals. Another, indirect mechanism, involves the ferric-ferrous cycle. The oxidation of reduced metals is mediated by the ferric (III) ion and this is formed by microbial oxidation of ferrous (II) ion present in the minerals. Ferric (III) ion acts as an oxidant and oxidizes metal sulfides and is reduced to ferrous (II) ion that, in turn, can be microbially oxidized. Both direct and indirect mechanisms of bacterial leaching are shown schematically in Fig. 14.9.
Schematic diagram of pyrite leaching showing both mechanisms [7]
14.3.2 Role of Fungi in Biomining
Several species of fungi like Aspergillus niger, Penicillium simplicissimum are used for bioleaching. This form of leaching does not rely on microbial oxidation of metal, but rather uses microbial metabolism as source of acids which directly dissolve the metal.
Microfungi are heterotrophic organisms. They exist in all ecological niches, e.g. supporting the weathering of rocks as well as the mineralization of materials containing metals. Their development is encouraged by the acidic reaction, the presence of sugars, and the appropriate humidity. These microorganisms can produce large amounts of organic acids, such as citric, glycolic, oxalic, and other acids which work as chemical solvents, can be used on an industrial scale in bioleaching processes and impact the change of the environment’s reaction. The microfungi, due to their biochemistry and relatively high immunity to hostile factors (pH, temperature, etc.), provide an excellent alternative in the bioleaching of metals, since the classical chemical methods of acidic bioleaching cannot be used for environmental reasons. The extraction through microfungi consists mainly of producing metabolites like organic acids, amino acids, and peptides that serve as leaching agents for the dissolution of metals [11].
The metabolic process of fungi is similar to a great extent to those of higher plants with the exception of carbohydrate synthesis. The glycolytic pathway converts the glucose into variety of products including organic acids. So, these biomining processes are mediated due to the chemical attack by the extracted organic acids on the ores. The acids usually have dual effect of increasing metal dissolution by lowering the pH and increasing the load of soluble metals by complexion/chelating into soluble organic-metallic complexes [12].
14.4 Various Chemical Processes for Extraction of Heavy Metals
Various physical and chemical processes are involved in the extraction of metals from their ores. Ores generally occur in the form of compounds of metal oxides, sulfides, carbonates, or halides.
These processes are:
14.4.1 Concentration of the Ore (Removal of Unwanted Metals and Gangue to Purify the Ore)
-
Hydraulic washing: This process separates the heavier ore particles from the lighter gangue particles. This is done by washing them in a stream (jet) of water over a vibrating, sloped table with grooves. Denser ore particles settle in grooves. Lighter gangue particles are washed away (Fig. 14.10).
-
Froth floatation: In this process, separation of the ore and gangue particles is done by preferential wetting. This process is generally used for sulfide ores of copper, lead, and zinc. The finely powdered ore is mixed with water and suitable oil in a large tank. A current of compressed air agitates the mixture. The ore particles are wetted by oil and form froth at the top, which is removed. The gangue particles wetted by water settle down. Ore preferentially wetted by oil is removed as froth. Gangue wetted by water is removed after it settles down (Fig. 14.11).
-
Magnetic separation: This process is used in the extraction of metals which exhibit magnetic properties. For example, in the extraction of iron, crushed magnetite ore (iron) particles are separated using their magnetic property. The pulverized ore is moved on a conveyor belt. Electromagnetic wheel of the conveyor attracts only the magnetic particles into a separate heap. Only the magnetic particles are attracted by the magnetic wheel. These particles fall separately into a different heap (Fig. 14.12).
-
Chemical separation: This process utilizes the difference in some chemical properties of the metal and gangue particles for their separation. For example, in the Bayer’s process of aluminium extraction, the bauxite ore is treated with hot sodium hydroxide solution. Water-soluble sodium aluminate formed is filtered to separate the undissolved gangue particles. Sodium aluminate (NaAlO2) is further processed to get aluminium oxide (Al2O3).
14.4.2 Conversion into Metal Oxide
-
Calcination for carbonate ore: In this process carbonate ores when heated in absence of air get converted into into oxides.
-
Roasting for sulfide ore: In this process sulfide ores converted into oxides on heating in the presence of air.
14.4.3 Reduction of Metal Oxide to Metal
-
Reduction: The process can be done by either heating the metal oxide or chemically reducing the metal oxide using chemical reducing agents such as carbon, aluminium, sodium, or calcium.
-
Electrolytic reduction: Electrolytic reduction is the process used to extract oxides (or chlorides) of highly reactive metals like sodium, magnesium, aluminium, and calcium. Molten oxides (or chlorides) are electrolyzed. The cathode acts as a powerful reducing agent by supplying electrons to reduce the metal ions into metal. For example: Fused alumina (molten aluminium oxide) is electolysed in a carbon lined iron box. The box itself is the cathode. The aluminium ions are reduced by the cathode.
14.4.4 Refining of Impure Metal into Pure Metals
-
Electrolytic refining: The process of electrolysis is used to obtain very highly purified metals. It is very widely used to obtain refined copper, zinc, tin, lead, chromium, nickel, silver, and gold metals. In this process, the anode is made as impure slab of metal and cathode as pure thin sheet of same metal and a salt solution of the metal is used as the electrolyte. On passing current, pure metal from the electrolyte is deposited on the cathode. The impure metal dissolves from the anode and goes into the electrolyte. The impurities collect as the anode mud below the anode (Fig. 14.13).
-
Liquation process: In this process, the block of impure metal is kept on the sloping floor of a hearth and heated slowly. The pure metal liquefies (melts) and flows down the furnace. The non-volatile impurities are infusible and remain behind (Fig. 14.14).
-
Distillation process: In this process, metals with low boiling point, such as zinc, calcium, and mercury are vaporized in a vessel. The pure vapor are condensed into pure metal in a different vessel. The non-volatile impurities are not vaporized and so are left behind.
-
Oxidation process: In this process, the impurities are oxidized instead of the metal itself. Air is passed through the molten metal. The impurities like phosphorus, sulfur, silicon, and manganese get oxidized and rise to the surface of the molten metal, which are then removed.
All these methods are effective but result in the generation of toxic chemical sludges and waste products.
Another approach which involves aqueous chemistry for the recovery of pure metals from ores is termed as hydrometallurgy. It is typically divided into three general areas:
-
Leaching
-
Solution concentration and purification
-
Metal recovery
Leaching
Leaching involves the use of aqueous solutions containing a lixiviant is brought into contact with a material containing a valuable metal. The lixiviant in solution may be acidic or basic in nature. In the leaching process, oxidation potential, temperature, and pH of the solution are important parameters, and are often manipulated to optimize dissolution of the desired metal component into the aqueous phase. The three basic leaching techniques are in situ leaching, heap leaching, and vat leaching.
After leaching, the leached solids and pregnant solution are usually separated prior to further processing.
Solution concentration and purification
After leaching, the leach liquor must normally undergo concentration of the metal ions that are to be recovered. Additionally, some undesirable metals may have also been taken into solution during the leach process. The solution is often purified to eliminate the undesirable components. The processes employed for solution concentration and purification include:
-
Precipitation
-
Cementation
-
Solvent Extraction
-
Ion Exchange
Metal Recovery
Metal recovery is the final step in a hydrometallurgical process. Metals suitable for sale as raw materials are often directly produced in the metal recovery step. Sometimes, however, further refining is required if ultra-high purity metals are to be produced. The primary types of metal recovery processes are electrolysis, gaseous reduction, and precipitation.
14.5 Development of Metal Specific Chelating Resins to Extract Metal Ions
There are number of ligands capable of binding metal ions through multiple sites, usually because they have lone pairs on more than one atom. Ligands that bind via more than one atom are often termed chelating ligands. The organic moiety that can trap or encapsulate the metal ion, forming coordinate bond through two or more atoms, to form a chelate is known as chelating agent/ligand. So, “chelate” denotes a complex between a metal and a chelating agent. A chelating agent can be chemically anchored on various inorganic polymeric solid supports to form “chelating resin”. The ligand/agent attached to chelating resin makes it specific and selective for extraction of a particular metal ion (Fig. 14.15).
Various solid supports that are used for scavenging of metal ion are: Chelamine, Silica gel, Amberlite, XAD, Polyurethane foam, Polyacrylonitrile, and Activated Carbon.
The tremendous amount of biomass which is produced after phytoextraction is rich source of heavy metals drawn from the soil which are otherwise the major environmental concern. This biomass is digested and a particular metal specific chelating resin, which possesses high selectivity to the targeted metal ion in a particular pH- range, is used for separation of metal ion. An assortment of novel metal specific chelating resin has been designed which can be easily recovered and reused several times making the process environmentally benign and green (Table 14.2).
Extraction of metal ions from biomass using specifically designed chelating resin has numerous advantages [26]:
-
Selective extraction of metal ions is possible by using a chelating resin having multidentate ligand as it possesses high selectivity to the targeted metal ion.
-
The chelating sorbent method is an economical method since it uses only a small amount of resin and is free from difficult phase separation and extraction solvent.
-
As the target ion specific chelating agent is enriched on solid phase, even ppb level concentrations can also be extracted.
-
The chelating resin can be recycled and reused several times as they can be easily recovered merely by filtration and have high physical and chemical stability.
14.6 Applications of Bioextraction
Biomining of copper. Copper was the first metal extracted by biomining. During the period 1950–1980, as compared to conventional metallurgical techniques, biomining appeared as economically viable and potential technology to recover Cu from low grade ore, like copper sulfide. It has been reported that the Lo Aguirre mine in Chile processed about 16,000 t ore per day between 1980 and 1996 using biomining [27].
Fungal leaching of manganese ore. Recovery of Mn from low grade ore of Mn by using pyrometallurgical and hydrometallurgical methods is expensive because of high energy and capital inputs. Besides, it also contributes a lot to environmental pollution. On the other hand biomining of Mn from manganiferous ores using microbial leaching is cost effective as well as environment friendly. It has been reported that a fungus Penicillium citrium can solubilize or extract 64.6% of Mn from the low grade ore [28].
Biomining of gold. Using cyanide method, it is very much difficult to extract gold, when gold is covered with insoluble metal sulfides. Biomining of these sulfide films is the best option to achieve satisfactory gold recovery. Gold extraction plants of Sao Benzo in Brazil, Ashanti in Ghana, Tamboraque in Peru are known to have such biomining facilities. A series of demonstration plants was also commissioned during 2002 in the Hutti Gold Mines in Karnataka [27].
Recovery of chromium from tannery sludge. About 40% of total Cr used in tanning industry end up in the sludge. Cr is non-biodegradable and can easily accumulate in food chain causing serious health effects to human beings. Use of microfungi due to their biochemistry and relatively high immunity to hostile conditions such as pH, temperature etc. provide a better alternative to commercial leaching processes. It has been demonstrated that chromium from tannery sludge can be bioleached up to 99.7% using indigenous acidophilic fungi, A. thiooxidans [29]. Another Cr recovery option from tannery waste is to grow potential Cr accumulating fungi in tannery waste and subsequent extraction of Cr from the harvested biomass. In an extensive study on Cr accumulation by fungal biomass, the author identified a fungal strain, Paeciomyces lilacinus which can accumulate Cr up to 18.9% of their dry biomass [30].
Bioleaching of economical metals from electronic and galvanic waste. These contain various valuable metals. Microbial process involving both bacteria and fungi, which produce inorganic and inorganic acids, can mobilize these metals from the waste. Metals such as Al, Ni, Pb, and Zn have been reported to be extracted by this process. Microbial leaching has also been found effective to recover Ni and Cd from spent batteries [31].
Phytoextraction of metal. Phytoextraction of metals from low or moderately contaminated soil or waste material is recommended but not an option for highly contaminated soil. In later case, it may take decades or even centuries to reduce the contaminant concentration to an acceptable limit. Instead of using low biomass hyperaccumulator plants, high yielding plants along with addition of chelating agent proved to be better method to phytoextract metal from soil. Uses of different plants in chelant-induced phytoextractiopn are summarized in Table 14.3.
However, often application of chelants can result in residual toxicity in soil on which it is applied. Thus, natural accumulation of metals would be the best option provided application of mycorrhizal fungi, plant growth promoting rhizobacteria and other beneficial microbes in soil that can enhance the efficiency of extraction processes [32]. It has also been reported that plants colonized by the AM fungi not only enhance growth, but also significantly increase Pb uptake in root and higher translocation to the shoot at all given treatments [33]. It has also been seen that three mycorriza inoculated plant glomus species namely G. lamellosum, G. intraradices, G. proliferum and their consortia greatly enhance accumulation of Cr from tannery waste to plants.
14.7 Economization of Bioextraction
For cost effective phyto-extraction, it is essential to create stabilizing plants which produce high levels of root and shoot biomass, high tolerance and resistance for heavy metals. This can be done by mycorrhizal association.
Mycorrhizal association: It is a symbiotic association between a fungus and the roots of a plant. The fungus colonizes the host plants’ roots, either intracellularly or extracellularly. This mutualistic association provides the fungus with relatively constant and direct access to carbohydrates, such as glucose and sucrose supplied by the plant. The carbohydrates are translocated from their source (usually leaves) to root tissue and on to fungal partners. In return, the plant gains the benefits of the mycelium’s higher absorptive capacity for water and mineral nutrients (due to comparatively large surface area of mycelium: root ratio), thus improving the plant’s mineral absorption capabilities. These fungi have a protective role for plants rooted in soils with high metal concentrations. The trees inoculated with fungi displayed high tolerance to the prevailing contaminant, survivorship and growth in several contaminated sites. This was probably due to binding of the metal to the extramatricial mycelium of the fungus, without affecting the exchange of beneficial substances.
So, Mycorrhizal Association enhances plant growth on severely disturbed sites, including those contaminated with heavy metals and plays an important role in metal tolerance and accumulation [34, 35].
14.8 Flow Diagram to Summarize the Chapter and the Process of Bioextraction

14.9 Conclusion
Bioextraction has been identified as a potential technology for effective extraction and removal of metals in metal overburdened sites, hence relieving the environmentally stressed ecosystem. Integration of bioextraction and solid phase extraction methodology helps to recover the heavy metal back by encapsulating precious metals from biomass using metal selective chelating resin, making this approach greener and constructive for mankind. The chapter presents the simplistic understanding of this environmentally benign alternative approach.
References
Technology fact sheet; peconic river remedial alternatives; Phytoextraction. Brookheaven National Laboratory. http://www.bnl.gov/erd/Peconic/Factsheet/Phytoextract.pdf
Zhuang P, Yang QW, Wang HB, Shu WS (2007) Phytoextraction of heavy metals by eight plant species in the field. Water Air Soil Pollut 184:235–242
Smits EAHP, Freeman JL (2006) Environmental cleanup using plants: biotechnological advances and ecological considerations. Front Ecol Environ 4(4):203–210
Nascimento CWAD, Xing B (2006) Phytoextraction: a review on enhanced metal availability and plant accumulation. Sci Agric (Piracicaba, Braz.) 63(3):299–311
Mahmood T (2010) Phytoextraction of heavy metals—the process and scope for remediation of contaminated soils. Soil Environ 29(2):91–109
Song J, Luo YM, Wu LH (2005) Biogeochemistry of chelating agents. In: Nowack B, VanBriesen JM(ed) Chelate-enhanced phytoremediation of heavy metal contaminated soil, American Chemical Society, Washington DC
Hoque ME, Philip OJ (2011) Biotechnological recovery of heavy metals from secondary sources—an overview. Mater Sci Eng C 31(2):57–66
Brierley CL (2008) Biomining: extracting metals with microorganisms. Brierley Consultancy LLC
Swamy KM, Narayana KL, Misra VN (2005) Bioleaching with ultrasound. Ultrason Sonochem 12:301–306
Joubert TM (2008) Towards a genetic system for the genus: Sulfobacillus. Dissertation, University of Stellenbosch
Kisielowska E, Kasińska-Pilut E (2005) Copper bioleaching from after-flotation waste using microfungi. Acta Montan Slovaca Ročník 10, číslo 1:156–160
Ghorbani Y, Oliazadeh M, Shahvedi A, Roohi R, Pirayehgar A (2007) Use of some isolated fungi in biological leaching of aluminum from low grade bauxite. Afr J Biotechnol 6(11):1284–1288
Sharma RK, Pant P (2009) Solid phase extraction and determination of metal ions in aqueous samples using quercetin modified Amberlite XAD-16 chelating polymer as metal extractant. Int J Environ Anal Chem 89(7):503–514
Sharma RK, Pant P (2009) Preconcentration and determination of trace metal ions from aqueous samples by newly developed gallic acid modified Amberlite XAD-16 chelating resin. J Hazard Mater 163(1):295–301
Tucker AR (2002) Speciation of Cr(III) and Cr(VI) in water after preconcentration of its 1,5-diphenylcarbazone complex on amberlite XAD-16 resin and determination by FAAS. Talanta 57:1199–1204
Tewari PK, Singh AK (2002) Preconcentration of lead with Amberlite XAD-2 and Amberlite XAD-7 based chelating resins for its determination by flame atomic absorption spectrometry. Talanta 56:735–744
Hutchinson S, Kearney GA, Horne E, Lynch B, Glennon JD, McKervey MA, Harris SJ (1994) Solid phase extraction of metal ions using immobilised chelating calixarene tetrahydroxamates. Anal Chim Acta 291:269–275
Bkjrrijn MCY, Dopazo MCG, Barrera AB, Barrer MPB (1992) Preconcentration of trace amouts of manganese from natural waters by means of a macroreticular poly (dithiocarbamate) resin. Talanta 39(6):671–674
Sengupta B, Das J (1989) Preconcentration of trace amounts of mercury(II) in water on picolinic acid amide-containing resin. Anal Chim Acta 219:339–343
Wen B, Shan XQ, Lian J (2002) Separation of Cr(III) and Cr(VI) in river and reservoir water with 8-hydroxyquinoline immobilized polyacrylonitrile fiber for determination by inductively coupled plasma mass spectrometry. Talanta 56:681–687
Minagawa K, Takizawa Y, Kifune I (1980) Determination of very low levels of inorganic and organic mercury in natural waters by cold vapor atomic absorption spectrometry after preconcentration on a chelating resin. Anal Chim Acta 115:103–110
Sharma RK (1995) Column chromatographic preconcentration and separation of cobalt in a vitamin, an alloy and an ore using chelating resin containing acenaphthenequinone monoxime supported over naphthalene. Anal 120(8):2203–2205
Garg BS, Bist JS, Sharma RK, Bhojak N (1996) Solid-phase extraction of metal ions and their estimation in vitamins, steel and milk using 3-hydroxy-2-methyl-1,4-naphthoquinone-immobilized silica gel. Talanta 43:2093–2099
Garg BS, Sharma RK, Bist JS, Bhojak N, Mittal S (1999) Separation and preconcentration of metal ions and their estimation in vitamin, steel and milk samples using o-vanillin-immobilized silica gel. Talanta 48(1):49–55
Sharma RK, Dhingra S (2011) Fuctionalized silica gel as metal scavenger, sensor and catalyst. LAP Lambert Academic Publishing GmbH & Co, Germany
Garg BS, Sharma RK, Bhojak N, Mittal S (1999) Chelating resin and their applications in the analysis of trace metal ions. Microchem J 61:94–114
Acevedo F (2002) Present and future of bioleaching in developing countries. Electron J Biotechnol 5(2):196–199
Acharya C, Kar RN, Sukla LB, Misra VN (2004) Fungal leaching of manganese ore. Trans Indian Inst Met 57(5):501–508
Wang YS, Pan ZY, Lang J M, Xu JM, Zheng YG (2007) Bioleaching of chromium from tannery sludge by indigenous Acidithiobacillus thiooxidants. J Hazard Mater 147, 1-2(17):319-324
Sharma S, Adholeya A (2011) Detoxification and accumulation of chromium from tannery effluent and spent chrome effluent by Paecilomyces lilacinus fungi. Int Biodeterior Biodegrad 65:309–317
Mishra D, Kim DJ, Ahn JG, Lee JC (2004) Bacterial leaching of metals from sulfide minerals and industrial wastes. KIGAM Bulletin 9(1):48–57
Vivas A, Vörös A, Biró B, Barea JM, Ruiz-Lozano JM, Azcón R (2003) Beneficial effects of indigenous Cd-tolerant and Cd-sensitive Glomus mosseae associated with a Cd-adapted strain of Brevibacillus sp. in improving plant tolerance to Cd contamination. Appl Soil Ecol 24(2):177–186
Punamiya P, Datta R, Sarkar D, Barber S, Patel M, Das P (2010) Symbiotic role of Glomus mosseae in phytoextraction of lead in vetiver grass [Chrysopogon zizanioides (L.)]. J Hazard Mater 177:465–474
Gaur A, Adholeya A (2004) Prospects of arbuscular mycorrhizal fungi in phytoremediation of heavy metal contaminated soils. Curr Sci 86(4):528–534
Adholeya A, Sharma RK (2010) Patent: CBR 4171 1146/del/2010
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Sharma, R.K., Adholeya, A., Puri, A., Das, M. (2012). Bioextraction: The Interface of Biotechnology and Green Chemistry. In: Baskar, C., Baskar, S., Dhillon, R. (eds) Biomass Conversion. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-28418-2_14
Download citation
DOI: https://doi.org/10.1007/978-3-642-28418-2_14
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-28417-5
Online ISBN: 978-3-642-28418-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)