Abstract
As the adult population is increasing, prostate cancer (PCa) will become a considerable health problem in the next millennium. This has raised the public interest in potential chemoprevention of this disease. As PCa is extremely common and generally slow to progress, it is regarded as an ideal candidate for chemoprevention. At present, 5 alpha-reductase inhibitors finasteride and dutasteride have been identified as preventive agents that effectively reduced PCa incidence. However, this was limited to Gleason score ≤6 and associated with an apparent increase in the detection of higher-grade cancers, hindering registration in that indication. In parallel, Selenium (Se), α-tocopherol, isoflavones, lycopene, and green tea polyphenols have been studied for decreasing the risk of PCa. Although encouraging observations have been made, most of the large studies show negative results. Differences in study design, sample size, doses administered and/or concentrations achieved in the body may be the reason for these inconsistencies. Although larger randomized controlled studies are needed and epidemiologic evidence should be placed in a clinical context, physicians must be aware of these preventive opportunities in PCa care. Combinations of chemopreventive agents should be carefully investigated because mechanisms of action may be additive or synergistic.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Androgen Receptor
- Benign Prostatic Hyperplasia
- Androgen Deprivation Therapy
- Digital Rectal Examination
- Prostate Cancer Prevention Trial
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
2.1 Introduction
Prostate cancer (PCa) is a very heterogeneous disease with a wide spectrum of clinical presentations and consequences. Indeed, if microscopic foci of adenocarcinoma can be found in the prostate of many men, only a minority will progress to clinically relevant, symptomatic, or potentially lethal disease. This explains the striking difference between the incidence of PCa and its mortality rate. In Europe in 2008, an estimated 382,000 cases were diagnosed while 90,000 deaths have occurred in 2008 (Ferlay et al. 2010).
The natural history of PCa is usually slow, evolving over decades from a preclinical tumour to a detectable tumour. Many low-volume/well-differentiated cancer foci never develop into clinically relevant cancer, never cause symptoms, and would probably remain undetected throughout men’s lifetime if aggressive PSA screening was not advocated. Indeed, most of the deaths come from a pool of poorly undifferentiated aggressive cancer (Albertsen et al. 2005). Whether these more rapidly progressing, poorly differentiated PCa are derived from pre-existing, well-differentiated “latent” PCa or develop de novo with a much shorter preclinical phase is still unknown.
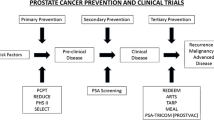
Chemoprevention implies that a disease can be prevented. Primary chemoprevention refers to reducing the risk of cancer development. Secondary chemoprevention involves reducing the risk of progression of a cancer that is already present. In the case of PCa, these two concepts overlap. The “holy grail” of prevention with respect to PCa is to avoid high-volume/high-grade aggressive PCa since low-volume/low-grade cancers are supposedly neither morbid nor lethal diseases. These “indolent” cancers, that for the sake of the patients should remain undiagnosed, have emerged as a major public health concern because they surface with PSA screening and are the matter of aggressive (over)treatments (Daskivich et al. 2011; Schroder et al. 2009). This poses a huge burden on the health-care system because of the costs associated with increase diagnosis and therapy. Prevention of PCa can thus be seen as reducing the rate of transformation of normal cells into premalignant cells but also reducing the rate of transformation from low-grade to high-grade disease. This should be kept in mind when interpreting the data of chemoprevention trials. Some author referred to risk reduction rather than true chemoprevention. Even a moderate reduction or even delay in the development of PCa accomplished through pharmacologic or dietary intervention could result in a considerable reduction in the incidence of PCa, and thus in the health and economic burden of the disease.
The genetic, epigenetic and environmental factors driving transformation from normal cells into malignant cells and then into aggressive prostate cancers remain largely unknown. Amongst the identified pathways that can be targeted by chemoprevention studies, two have been more extensively studied in large randomized trials: inflammation and hormonal stimulation of the prostate (Nelson 2007). In addition, because several epidemiological studies have suggested geographical variations in the risk of PCa potentially linked to dietary and lifestyle factors, several studies have been conducted with dietary elements and food supplements.
Here, we will review the main trials of chemoprevention for PCa trying to provide recommendations to the reader.
2.2 Anti-inflammatory and Antioxidants
Inflammation has been associated with the development of lung cancer in smokers, hepatic cancer in chronic hepatitis and bowel cancer in inflammatory bowel disease.
Prostate inflammation may contribute to prostatic carcinogenesis. Inflammation may promote carcinogenesis by causing cell and genome damage, promoting cellular turnover and creating a tissue microenvironment that can enhance cell replication, angiogenesis and tissue repair (Bardia et al. 2009). Inflammatory situations are characterized by the production of free radicals or reactive oxygen species (ROS) that damage cell membranes. ROS cause oxidative damage to LDL and damage cell membranes by means of lipid peroxidation. Interesting, one of the earlier and most ubiquitous epigenetic phenomenon identified in prostatic carcinogenesis is the somatic silencing of GSTP1, encoding a glutathione S-transferase capable of detoxifying ROS, and this defends against oxidant cell and genome damage (Nelson et al. 2004). Proliferative inflammatory atrophy (PIA), a lesions containing activated inflammatory cells and proliferating epithelial cells, has been identified as a precursor lesion to prostatic intraepithelial neoplasia (PIN) and PCa. Finally, epidemiological data have correlated prostatitis and sexually transmitted infections with increased PCa risk and intake of anti-inflammatory drugs and antioxidants with decreased PCa risk (Nelson et al. 2004).
2.2.1 COX-2 Inhibitors
Studies have shown that essential fatty acids, linoleic acid (LA) and arachidonic acid (AA), and their prostaglandin metabolite PGE2 stimulate tumour growth. The COX-1 and COX-2 enzymes catalyze the conversion of AA to prostaglandins and are therefore amongst the most critical key enzyme of the inflammatory process.
Aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) antagonize COX-2 and reduce the incidence of malignancy. High doses of COX-2 inhibitor, celecoxib, prevent precancerous adenomatous polyps from progressing to overt colon cancer (Arber et al. 2011). In vitro, COX-2 inhibitors celecoxib and rofecoxib suppress carcinogenesis by both COX-2-dependent and COX-2-independent mechanisms (Patel et al. 2005).
The ViP study was a double-blinded, randomized, placebo-controlled (RCT) trial evaluating the effects of rofecoxib 25 mg compared with placebo in decreasing PCa incidence in high-risk men. The initial trial plan was to recruit 15,000 men, but the trial was terminated when only 4,741 men were enrolled because rofecoxib was withdrawn from the market due to an excess of ischemic cardiac toxicity. Antonarakis et al. have investigated the effect of celecoxib administered for 4–6 weeks before radical prostatectomy (RP) in men with localized PCa (Antonarakis et al. 2009). The endpoints were tissue celecoxib concentration and difference in prostatic prostaglandin levels, COX-1 and COX-2 expressions, oxidized DNA bases, and markers of proliferation, apoptosis and angiogenesis. Unfortunately, treatment with 4–6 weeks of celecoxib had no effect on intermediate biomarkers of prostate carcinogenesis, despite the achievement of measurable tissue levels. Because of the cardiovascular toxicity of the class in chronic administration, it is unlikely that the efficacy of this approach will be tested again in the future.
2.2.2 Selenium (Se) and Vitamin E
Selenium (Se) is an essential trace element found in vegetables, grains, red meat, fish, poultry, and eggs. Se helps to make antioxidant enzymes, which play a role in preventing cell damage. Epidemiological evidence provides support for a global cancer prevention effect. Vitamin E is an essential lipid-soluble antioxidant found in plant oils such as soy, corn and olive oil. Other sources include nuts and seeds, and green leafy vegetables. It protects cells from free radicals. Several forms of vitamin E have been identified. The most active form with highest bioavailability in human tissues is α-tocopherol. The body is not capable of producing this substance, and it must be consumed in the diet or supplements for proper health.
The rationale for using Se to prevent PCa originates in the Nutritional Prevention of Cancer (NPC) trial. On secondary analysis, this RCT for skin cancer prevention trial showed that Se significantly reduced the overall incidence of PCa with a relative risk (RR) of 0.51 (95% confidence interval (CI): 0.29–0.87) (Duffield-Lillico et al. 2003). The unadjusted estimate showed a significant 65% reduction in PCa incidence with Se supplementation. The protective effect of Se supplementation (200 μg daily) was restricted to men with lower baseline PSA (≤4 ng/ml) and men with a low baseline plasma Se concentration (<123.2 ng/ml). The rationale for using α-tocopherol was based on the Alpha-Tocopherol, Beta-carotene Cancer Prevention (ATBC) study (1994). On secondary analysis, the ATBC lung cancer prevention trial found a 32% reduction in PCa incidence (95% CI: 12–47; P = 0.002) in men receiving 50 mg/day α-tocopherol. In addition, a 41% reduction in PCa mortality (95% CI: 1–65%) was observed in the α-tocopherol group (Heinonen et al. 1998). An additional follow-up of 12 years confirmed that higher serum α-tocopherol at baseline was associated with improved PCa survival (HR: 0.67; 95% CI: 0.45–1.00) (Watters et al. 2009). The strongest survival relationship was seen for those who received α-tocopherol supplementation and were in the highest serum α-tocopherol quintile at baseline (HR: 0.51; 95% CI: 0.20–0.90) or at 3-year follow-up measurement (hazard ratio (HR): 0.26; 95% CI: 0.09–0.71).
Based on these indirect evidences, Se and vitamin E were tested separately and in combination for the prevention of PCa in a large trial, the Selenium and Vitamin E Cancer Prevention Trial (SELECT). As for today, SELECT remains the largest PCa prevention study ever performed. It randomized 35,533 men to four groups: Se (200 μg/day) + placebo, vitamin E (400 IU/day) + placebo, Se + vitamin E, or placebo + placebo. Eligibility criteria were age 50 years or older for African Americans, 55 years or older for Caucasians, a serum PSA level of 4 ng/ml or less, a digital rectal examination (DRE) not suspicious for cancer and normal blood pressure. The primary endpoint was biopsy-confirmed PCa. The first analysis of SELCT, released in 2009, had failed to show a benefit for selenium and vitamin E, alone or in combination (Lippman et al. 2009). The study was then preliminary terminated at 7 years (planned duration was 12 years). Even worse, latest results, released in 2011, demonstrated that dietary supplementation with vitamin E significantly increased the risk of PCa among healthy men. Indeed, at this second analysis 529 men from the placebo had developed PCa, vs. 620 men in the vitamin E group (HR, 1.17; 99% CI, 1.004–1.36, P = .008), 575 in the selenium group (HR, 1.09; 99% CI, 0.93–1.27; P = .18), and 555 in the selenium plus vitamin E group (HR, 1.05; 99% CI, 0.89–1.22, P = .46) (Klein et al. 2011). Compared with placebo, the absolute increase in risk of prostate cancer per 1000 person-years was 1.6 for vitamin E, 0.8 for selenium, and 0.4 for the combination.
The negative results of SELECT have caused an immense disappointment, especially amongst vitamins and trace elements aficionados. PCa complementary medicines represent a multibillion over the counter market, and it was expected that “good reasons” to pursue prescription of these drug would emerge rapidly, including criticisms on the dose of vitamin E and type of Se used in SELECT. The high dose of vitamin E (400 IU/D of the alpha-tocopherol form) in SELECT may have been less effective than a lower dose such as the eightfold lower 50 IU/D of the ATBC study (Lippman et al. 2009). In SELECT, 200 μg of l-selenomethionine was chosen whereas in the NPC trial, the 200 μg of high Se yeast contained only 20% of l-selenomethionine (Duffield-Lillico et al. 2003; Lippman et al. 2009). Another drawback of SELECT is the absence of selection of patients since it is likely that personal predispositions may enhance or hinder the benefit of supplementation. For example, several studies have suggested that vitamin E is more protective against PCa in smokers, and in SELECT, less than 60% of men where current or former smokers, whereas in the ATBC study all men were smokers. As for Se, genetic susceptibilities exist may confer different benefit to Se supplementation. Chan et al. have assessed manganese superoxide dismutase (SOD2) gene variants and plasma Se in 489 patients with localized/locally advanced PCa (Chan et al. 2009). SOD2 is an endogenous mitochondrial enzyme that metabolizes reactive oxygen species and superoxide anions to oxygen and hydrogen peroxide. Several polymorphisms of SOD2 have been identified, including a single nucleotide permutation that encodes either an alanine (A) or a valine (V). SOD2 genotype alone was not associated with disease aggressiveness, whereas higher versus lower Se levels were associated with a slightly increased likelihood of presenting with aggressive disease (RR: 1.35; 95% CI: 0.99–1.84). There was evidence of an interaction between SOD2 and Se levels such that among men with the AA genotype, higher Se levels were associated with a reduced risk of presenting with aggressive disease (RR: 0.60; 95% CI: 0.32–1.12), whereas among men with a V allele, higher Se levels were associated with an increased risk of aggressive disease (for VV or VA men, RR: 1.82; 95% CI: 1.27–2.61; P for interaction <0.007) (Chan et al. 2009).
But clearly one of the more consistent hypotheses is that the positive effects of Se in the NPC study and of vitamin E in the ATBC trial could have been due to chance in secondary analyses. Recent results from the Prostate Cancer Prevention Trial found no significant association between vitamin E and Se and the incidence of PCa (Kristal et al. 2010). Long-term supplemental intake of vitamin E (≥400 IU/day) in the VITamins And Lifestyle (VITAL) study was not associated with PCa risk overall; however, the risk of clinically relevant advanced disease was reduced with greater long-term (10-year average intake) vitamin E supplementation (Peters et al. 2008). Currently, several prevention studies are still ongoing or have been completed. A trial by Southwest Oncology Group has evaluated the effectiveness of Se 200 (μg/day) as selenomethionine in preventing PCa in approximately 423 patients aged 40 years or older who have high-grade PIN and PSA level of ≤10 ng/ml. Three-year cancer rates were 36.6% in placebo group versus 35.6% in Se group (P = 0.73, adjusted) (Marshall et al. 2011). The majority of patients who developed cancer on trial (70.8% Se and 75.5% placebo) had a Gleason score ≤6, and there was no difference in Gleason scores distribution between the two arms (Marshall et al. 2011).
To summarize our position regarding Se and Vitamin E supplements, the best is to literately quote P. Gann in its editorial to the publication of SELECT results:
Epidemiology teaches that every statistical association has only 3 possible explanations: bias, chance, and cause. Regarding nutritional prevention of prostate cancer, first-generation phase 3 trials were too reliant on biased interpretation of prior research; second-generation trials may have been too reliant on chance; yet there is every reason to believe that the next generation will have a firmer basis for causal hypotheses. Until then, physicians should not recommend Se or vitamin E—or any other antioxidant supplements—to their patients for preventing prostate cancer.
(Gann 2009)
2.3 Dietary Supplements
The incidence and mortality of PCa shows strong variations worldwide with the highest rates in North America, Australia, Western and Northern Europe and the lowest rates in Japan and other Asian countries. Interestingly, however, the incidence of latent or clinically PCa in autopsy studies among men from Japan and the USA is not substantially different. Migrant studies have shown an increase in PCa incidence in Asian men after emigration to the United States (Shimizu et al. 1991). The underlying theory is that these men adopt a western life style with a high-fat, high-protein, low-fibre diet that lacks certain substances of the Asian diet such as plant-derived antioxidants, isoflavones-containing soy, and tea polyphenols that may protect against the development of cancer. Therefore, it is hypothesized that dietary changes and pharmacological intervention could have an impact on PCa development and progression (Syed et al. 2007).
2.3.1 Isoflavones
Isoflavones, a subclass of the flavonoids, are plant-derived compounds with weak estrogenic activity and therefore classified as phytoestrogens. Phytoestrogens have been suggested to have a preventive effect against various cancers (Adlercreutz 2002). Soyfoods are a rich source of isoflavones. The main isoflavones found in most soy products are genistein, daidzein and glycitein. In vitro, genistein and daidzein inhibit the growth of PCa cells (Swami et al. 2005). The mechanism of action of the isoflavones in soy products is not entirely clear.
Epidemiological surveys have shown that serum isoflavone levels are related to the risk of PCa. Most of them have been conducted in Asian men. A case–control study, including 200 Japanese patients and 200 age-matched Japanese controls, suggested that isoflavones might be protective against PCa. The odds ratio (OR) for the highest quartile (≥89.9 mg/day) compared with the lowest quartile (<30.5 mg/day) of isoflavone intake was 0.42 (P < 0.01) (Nagata et al. 2007). In a nested case–control study on 14,203 Japanese men in which 201 PCa were identified during a 12.8 years of follow-up, plasma genistein and equol, a metabolite of daidzein, levels were inversely associated with the risk of PCa. The ORs of PCa diagnosis in the highest group of plasma genistein and equol compared with the lowest was 0.54 (P: 0.03) and 0.43 (P: 0.02), respectively (Kurahashi et al. 2008).
A few studies have been performed on Caucasian men. Travis et al. have examined plasma concentrations of phytoestrogens in relation to risk for subsequent PCa in a case–control study nested in the European Prospective Investigation into Cancer and Nutrition (EPIC) (Travis et al. 2009). Higher plasma concentrations of genistein were associated with lower risk of PCa, OR for men in the highest versus the lowest quintiles being 0.71 (P: 0.03). A meta-analysis of 14 epidemiological studies, including eight on isoflavones, suggests that soy and isoflavone consumption is associated with a decreased risk of PCa of approximately 26% in men when highest reported intake is compared with lowest reported intake (Yan and Spitznagel 2009). The protective effect is related to the type and quantity of soy food consumed. The analysis on soy intake yielded a combined OR of 0.74 (95% CI: 0.63–0.89; P = 0.01). The analysis of studies on non-fermented soy foods yielded an OR of 0.70 (95% CI: 0.56–0.88; P = 0.01) and those on fermented soy foods yielded a combined OR of 1.02 (95% CI: 0.73–1.42; P = 0.92). The analysis of studies on isoflavones yielded a combined OR of 0.88 (95% CI: 0.76–1.02; P = 0.09). Further separate analyses showed a combined OR of 0.52 (95% CI: 0.34–0.81; P = 0.01) from studies with Asian populations and 0.99 (95% CI: 0.85–1.16; P = 0.91) from studies with Western populations.
However, beyond these convincing epidemiological, case–control, and in vitro/vivo studies, there are no published robust prospective RCTs with sufficient statistical power to confirm that isoflavone supplementation can reduce PCa development or delay PCa progression.
2.3.2 Lycopene
Lycopene is a carotenoid that gives the red colour to tomatoes and tomato-derived products. It is also available in other red fruits and vegetables such as red carrots, watermelons, pink grape fruit and papayas. It possesses potent antioxidant activity and appears to have anti-cancer properties (Levy et al. 1995).
One of the first observation pinpointing at a potential benefit of lycopene for PCa prevention comes from the Health Professionals Follow-Up Study, a trial initiated in 1986 with the purpose of evaluating a series of hypotheses about men’s health relating nutritional factors to the incidence of serious illnesses, such as cancer, heart disease and other vascular diseases. An interim analysis of semi-quantitative food-frequency questionnaires published in 2002 suggested that high lycopene intake was associated with a reduced risk of PCa (RR for high versus low quintiles: 0.84; P = 0.003). Intake of tomato sauce, the primary source of bioavailable lycopene, was associated with an even greater PCa risk reduction: RR for more than two servings/week versus less than one serving/month: 0.77 (P < 0.001) (Giovannucci et al. 2002). This was confirmed by a study on plasma lycopene concentrations suggesting a statistically significant inverse association between higher lycopene plasma concentration and lower risk of PCa in younger patients (>65 years old; OR: 0.47); and in patients without a family history of PCa (OR: 0.43) (Wu et al. 2004). A meta-analysis of 11 case–control studies and 10 cohort studies or nested case–control studies showed that tomato products and lycopene may play a role in the prevention of PCa although the effect is modest and limited to high amounts of tomato products (Etminan et al. 2004). The main findings were that, compared with non-frequent users of tomato product (1st quartile of intake) the OR of PCa among consumers of high amounts of raw tomato (5th quintile of intake) was 0.89 (95% CI: 0.80–1.00). For a high intake of cooked tomato products, the corresponding OR was 0.81 (95% CI: 0.71–0.92). The OR of PCa related to an intake of one serving/day of raw tomato (200 g) was 0.97 (95% CI: 0.85–1.10) for the case–control studies and 0.78 (95% CI: 0.66–0.92) for cohort studies. For serum- or plasma-based studies, the corresponding ORs were 0.74 (95% CI: 0.59–0.92) for all studies, 0.55 (95% CI: 0.32–0.94) for case–control studies and 0.78 (95% CI: 0.61–1.00) for cohort studies. The World Cancer Research Fund (WCFR) estimates that there is a sufficient body of evidence for protective effect of lycopene-containing foods, especially tomatoes and its derivatives on PCa. This tentative health claim is based on a different meta-analysis including 5 cohort and 9 case–control studies with tomatoes, 3 cohort and 14 case–control studies with dietary lycopene and 6 cohort and 2 case studies based on serum or plasma lycopene. Most of the studies decreased risk with increased intake (www.dietandcancerreport.org) (2007). In contrast, a large nested case–control study in the prostate, lung, colorectal and ovarian cancer screening study including 692 PCa cases (Peters et al. 2007) and the recently published Prostate Cancer Prevention Trial (Kristal et al. 2010) including 9,559 participants found no correlation between lycopene and the incidence of PCa.
2.3.3 Polyphenols
Polyphenols are the largest group of constituents found in tea. Green tea contains catechins, a category of water-soluble polyphenolic substances. The four principal catechins are epicatechin (EC), epicatechin-3-gallate (ECG), epigallocatechin (EGC) and epigallocatechin-3-gallate (EGCG) (Balentine et al. 1997). EGCG, found in the highest concentration in green tea, is the most studied and most active of all green tea catechins (GTCs) for the inhibition of oncogenesis and reduction of oxidative stress. The mode of action of polyphenols is not yet fully determined. Several epidemiologic studies have focused on the lower incidence of PCa in Asian populations where green tea is consumed regularly as compared with Western populations, suggesting that green tea is protective against PCa.
In 2006, a 1-year proof-of-principle trial has been conducted to assess the safety and efficacy of GTCs for the chemoprevention of PCa in HGPIN (Bettuzzi et al. 2006). Sixty patients were randomized to 600 mg GTCs per day or placebo. After 1 year, only 1 of 30 (3%) GTCs-treated men were found to have PCa compared to 9 of 30 (30%) placebo-treated men. This is the first study showing that GTCs have potent in vivo chemoprevention activity for human PCa. GTCs treatment did not have a significant effect on PSA values throughout the study. In any case, the mean value of total PSA was always lower in patients randomized to GTCs than in patients on placebo. Secondary observations were reduction in lower urinary tract symptoms as assessed by International Prostate Symptom Score and Quality of Life scores in GTCs-treated men. No significant side or adverse effects have been reported. A 2-year follow-up was performed in a subset of patients and showed that GTCs had a long-lasting effect on PCa prevention (Brausi et al. 2008). A larger, randomized, double-blind, placebo-controlled study in 272 HGPIN patients in the United States will assess the rate of progression to PCa after treatment with either 200 mg EGCG as polyphenon E twice daily (i.e., 400 mg EGCG/day) or placebo over a 1-year period (ClinicalTrials.gov Identifier NCT00596011). Results with green tea polyphenols for PCa chemoprevention are encouraging, and patients should be encouraged to incorporate them in their daily diet. Larger clinical trials of men at risk of PCa or with early stage PCa are needed to better assess the role of green tea polyphenols in the prevention of PCa.
2.4 Hormonal Prevention of PCa
2.4.1 Rationale for Hormonal Prevention of PCa
Testosterone is critical initiator of prostate development and growth. Testosterone suppression, the standard systemic treatment of advanced PCa, induces massive apoptosis of normal and malignant prostate cells (Tombal 2007). The role of testosterone in the early development of PCa is unclear (Tombal 2011). Epidemiological surveys and prospective testosterone supplementations trials have failed to show a consistent association between low- or high-serum testosterone levels and the risk of developing cancer (Morgentaler and Traish 2009). Normal epithelial prostate cells do not express the androgen receptor (AR), and the effect of androgens is mediated by epithelial stromal interactions (Tombal 2011). In contrast to normal epithelial cells, AR expression is found in epithelial PCa cells and more importantly in its traditional precursor, high-grade PIN and PIA (Tombal 2011). This suggests that early during prostatic carcinogenesis, there is a gain-of-function that converts the AR from a growth suppressor gene to an oncogene, allowing the AR to engage the molecular signalling pathways stimulating the proliferation and survival of these initiated prostatic cells directly. In the stromal cells, normal and malignant prostate cells, the primary androgen is dihydrotestosterone (DHT), which results from the transformation of T by the 5α-reductases enzymes. 5α-reductase inhibitors (5ARIs), finasteride and dustasteride, inhibit the transformation of T into DHT. They have been used intensely in the treatment of benign prostatic hyperplasia (BPH) because they significantly reduce the prostatic volume and therefore improve urinary symptoms. In addition, 5ARIs decrease the value of PSA. Since androgen deprivation therapy (ADT) or AR direct blockade are unrealistic methods of chemoprevention because of the side effects of hypogonadism, 5ARIs became ideal chemopreventive agents to interfere with androgen regulations in the early development of PCa.
Similar to testosterone, oestrogens have been implicated in PCa carcinogenesis. Oestrogens have significant direct and indirect effects on prostate gland development and homeostasis and have been long suspected in playing a role in the aetiology of prostatic diseases (Prins and Korach 2008). Direct effects are mediated through prostatic oestrogen receptors (ER) α and β. Therefore, selective oestrogen receptor modulators (SERMs) that interfere with ER have been seen as potential chemoprevention agents.
2.4.2 Randomized Controlled Trials with Chemo “Hormono” Prevention
2.4.2.1 SERMS
The SERM toremifene has been tested in a multicentre, double-blind study on 514 men with HGPIN and no cancer that were re-biopsied at 6 and 12 months(Price et al. 2006). After 12 months, there was a 21.8% reduction in the cumulative risk of PCa in favour of toremifene, PCa being diagnosed in 24.4% of patients receiving 20 mg of toremifene and 31.2% of those taking placebo (P < 0.05). Based on this observation, a larger trial was initiated in 1,590 men with high-grade PIN and no cancer on biopsy to compare 20 mg toremifene to placebo daily for 3 years, with yearly repeat biopsies (NCT00106691). The sponsor GTx issue a press release on May 24, 2010, announcing that toremifene reduced the incidence of prostate cancer by a non-significant 10.2% (P = 0.385) and that the trial was stopped.
2.4.2.2 5ARIs Finasteride and Dutasteride
The Prostate Cancer Prevention Trial (PCPT) has tested the benefit of 5 mg finasteride per day versus placebo for a period of 7 years. In total, 18,882 men ≥55 years old with a PSA ≤ 3.0 ng/ml, a normal digital rectal examination (DRE) and no suspicion of PCa were included (Thompson et al. 2003). There were no baseline biopsies. Patients were followed by PSA and DRE. In the finasteride group, PSA was corrected to adjust for finasteride effect (×2 for year 1–2 and ×2, 3 thereafter) and “for-cause” biopsy with ≥6 cores was recommended in case of PSA >4.0 ng/ml or a suspicious DRE. An end-of-study prostate biopsy was recommended at year 7 for patients remaining undiagnosed with PCa. The final analysis, published in July 2003, included 9,060 men (48%) who had for-cause and/or an end-of-study biopsy. Finasteride reduced by 24.8% the prevalence of PCa during the 7-year period (18.4% in finasteride group vs. 24.4% in placebo group; P < 0.001). For-cause biopsies were done in 39% of the participants, and 52% of the cancers were diagnosed on for-cause biopsies. There were 15% fewer for-cause biopsies and 10% fewer PCa in the finasteride group. Noteworthy, the reduction in overall PCa detection was entirely due to a reduction in Gleason ≤6 cancers, and there was an increase in Gleason ≥7 cancers: 280 (6.4%) in the finasteride group versus 237 (5.1%) in the placebo group (P = 0.005).
There have many attempts to provide explanation for that increase in high-grade cancer and to answer whether finasteride improves the detection of high-grade PCa or negatively impacts the natural history and behaviour of PCA. Interestingly indeed, the increase in Gleason ≥7 cancers concerns for-cause biopsies. In “end-of-study” biopsies, there were 92 and 89 Gleason 7–10 cancers in the finasteride and placebo groups, respectively. The fact that there was fewer for-cause and end-of-study biopsies in the finasteride arm suggests that finasteride most likely influenced the decision to biopsy. Additional analyses have suggested that finasteride improves the sensitivity of both PSA and DRE to detect PCa, including high-grade cancers (Thompson et al. 2006, 2007). This might be partially explained by the decrease in prostate volume resulting from 5AR inhibition, on average 24% lower in the finasteride arm at the time of biopsy (Serfling et al. 2007). Finally, Lucia et al. have reported extended analysis on biopsies and radical prostatectomies (RP) specimens from 222 patients receiving finasteride and 306 receiving placebo (Lucia et al. 2007). Mean percentage of positive cores was lower in men receiving finasteride (34% vs. 38%, P = 0.016), as well as mean tumour linear extent (greatest [4.4 vs. 4.8 mm, P = 0.19] and aggregate [7.6 vs. 9.2 mm, P = 0.13]), bilaterality (22.8% vs. 30.6%, P = 0.046) and perineural invasion (14.2% vs. 20.3%, P = 0.07). More interestingly, the finasteride-associated increase in Gleason ≥7 PCa at biopsy (42.7% finasteride vs. 25.4% placebo, P < 0.001) was reduced and not significant anymore on the RP specimens (46.4% finasteride vs. 38.6% placebo, P = 0.10). Biopsy identified a greater proportion of patients with high-grade disease present at prostatectomy in the finasteride group than in the placebo group (69.7% vs. 50.5%, P = .01). The rate of upgrading (from low-grade cancer at biopsy to high-grade cancer at prostatectomy) and pathologic stage at prostatectomy were similar in both groups.
Several post hoc analyses have been conducted to attempt to account for these factors in determining the true effect of finasteride on overall and Gleason ≥7 cancers (Cohen et al. 2007; Kaplan et al. 2009; Pinsky et al. 2008; Redman et al. 2008). All these analyses seem to confirm the hypothesis that finasteride increases the detection of high-grade cancer and rule out a negative impact on its natural history.
The Reduction by DUtasteride of prostate Cancer Events (REDUCE) trial has tested the benefit of 0.5 mg dustateride versus placebo daily in 8,122 men to reduce the risk of biopsy-detectable PCa over a period of 4 years (Andriole). Men were aged 50–75 years old, had a PSA between 2.5 and 10.0 g/ml, a prostate volume <80 ml and, in contrast to PCPT, a single, negative previous biopsy of 6–12 cores within 6 months prior to study enrolment. Repeat, study-mandated prostate biopsies were taken after 2 and 4 years; for-cause biopsies could be done at any time. Overall, PCa was diagnosed in 858 men in the placebo group (25.1%) and 659 men in the dutasteride group (19.9%) with a relative risk reduction of 23% (P < 0.0001) (Andriole). Gleason 7–10 cancers were diagnosed in 220 men in the dutasteride group (6.7%) and 233 men in the placebo group (6.8%) (P = 0.81). In the subset of Gleason ≥8 cancers, there were 29 cancers in the dutasteride group and 19 cancers in the placebo group (P = 0.15). During the first 24 months, there were 17 and 18 Gleason ≥8 cancers in the dutasteride and placebo groups, respectively. Subsequently, during years 3 and 4, there were 12 Gleason ≥8 cancers in the dutasteride group and only one in the placebo group, out of 2,343 biopsies.
Similar to PCPT, several hypotheses were generated to explain that apparent small but disturbing increase in high-grade cancers. The low number of Gleason ≥8 cancers in the placebo arm at year 3–4 could be explained by 141 more Gleason ≤7 cancers being diagnosed in the placebo arm during years 1 and 2 and subsequently removed from treatment. There was therefore no opportunity for those cancers to be reclassified or upgraded during years 3 and 4. Another argument against dutasteride increasing the rate of high-grade cancers is the result of CombAT, a 4-year BPH trial comparing dutasteride and tamsulosin monotherapies with the combination of the two in 4,800 patients with lower urinary tract symptoms (Roehrborn et al. 2008). In that trial, prostate biopsies were done by investigators in case of PSA elevation or DRE abnormality, and there was no evidence of an increase in high-grade cancers in the two dutasteride arms compared to the tamsulosin monotherapy arm.
Side effects of dutasteride and finasteride are similar, the most common being related to sexual function. In the PCPT, erectile dysfunction (ED) occurred in 67% of the finasteride group and 61% of the placebo group. Decreased libido occurred in 65% of the finasteride group and 60% of the placebo group (Thompson et al. 2003). In REDUCE, new instances of decreased libido occurred in 5.1% of the dutasteride group and 2.9% of the placebo group (Andriole). New instances of ED occurred in 9.0% of the dutasteride group and 5.7% of the placebo group; 4.3% of the dutasteride group and 2.0% of the placebo group dropped out due to drug-related side effects. Gynecomastia occurred in 4.5% of the finasteride arm of the PCPT and 1.9% of the dutasteride arm of REDUCE, double the incidence of gynecomastia in the placebo group (Andriole et al. 2010; Thompson et al. 2003). There have been no life-threatening or serious side effects proven to be related to either finasteride or dutasteride. Both can occasionally be associated with allergic-type skin reactions.
2.4.3 Balancing the Benefits and Risks of 5ARIs for Prostate Cancer Risk Reduction
In December, 2010, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted against recommending dutasteride and finasteride for the indication to reduce PCa risk because in the view of the ODAC members, the risk for more aggressive tumours outweighed the potential for chemoprevention. ODAC recommended against PCa chemoprevention labelling for both 5α-reductase inhibitors—dutasteride (vote 14 (no) to 2 (yes), with 2 abstentions) and finasteride (vote 17 (no) to 0 (yes), with 1 abstention). Currently so far, neither of these drugs is approved for the indication of chemoprevention, and no trials are planned. As for know, we have to live with the fact that registration authorities refuse to rule out that either dutasteride or finasteride induces the growth of high-grade cancer.
This creates an interesting, although schizophrenic, registration paradigm. Indeed, both finasteride and dutasteride are effective treatments for men with symptomatic BPH. They not only improve urinary symptoms related to an enlarged prostate but also reduce the risk of acute urinary retention and the need for BPH-related surgery. What should we say to these men regarding their subsequent risk of developing PCa? Most of these patients could in theory receive 5ARI for BPH or PCa prevention because they have a moderately enlarged prostate with a moderately elevated PSA and BPH symptoms. Is it for them like choosing between the plague and cholera, balancing a demonstrated risk of reducing urinary retention and surgery and an increased risk of being diagnosed with high-grade Gleason. Very important questions on which, interestingly, the industry has been extremely quiet regarding that issue and most guidelines have avoided tackling the issue.
Finally, one should notice that the long-term effect of 5ARI on the responsiveness to further hormonal manipulation in men needing ADT for advanced cancer is not known. Neither the PCPT nor REDUCE were designed to measure the impact of 5ARIs on PCa survival. One may question if a cancer that progresses under 5ARIs will respond effectively to more aggressive androgen ablation. 5ARIs may or may not induce adaptation mechanisms similar to those observed during castration resistance and therefore decrease the sensitivity to ADT. This should be taken into account when evaluating the benefit of chemoprevention not in terms of reduction of incidence but of PCa mortality. For example, Koivisto et al. have studied six PCa diagnosed during finasteride treatment. Comparative genomic hybridization detected genetic alterations in four tumours, including Xq gains and 6q losses. Some of these abnormalities, including AR amplification and mutation, were consistent with what has previously been shown for PCa progressing under ADT (Koivisto et al. 1999).
2.5 Conclusions and Future Perspectives
So far, neither attempts to claim PCa chemoprevention has been very successful. Randomized phase III with nutrients have been overall negative or difficult to interpret. Differences in study design, sample size, doses administered and/or concentrations achieved in the body may be the reason for the many observed inconsistencies. Therefore, no recommendation can be made beyond a healthy diet, Mediterranean style and a correct load of physical activity. Chemo “hormone” prevention with 5ARIs can be quoted “effective” in reducing PCa incidence, but that effectiveness result largely from reducing the rate of Gleason ≤6 cancer. Today, it is widely accepted by most guidelines that these cancers pose little threat to men with life expectancy of less than 20–10 years. We agree that these cancers are nowadays overtreated and that effective strategies are required to reduce the rate of overtreatment. Overtreatment should be avoided with counselling and education and presently not with 5ARI as long as the controversy on the increase risk of high-grade cancer is not resolved.
Is it then the dusk of chemoprevention? We believe not, but smart adaptation and expectation, especially regarding the definition of risk categories will be needed. It seems reasonable to believe that chemoprevention strategies are more effective in high-risk groups, which, at this moment, are still very difficult to identify. Patients with isolated HGPIN on prostate biopsies constitute a unique and well-demarcated risk group for PCa. Prospective, randomized data on chemopreventive strategies in HGPIN are scarce but seem promising. Other high-risk groups include those above 40 years of age, with elevated PSA levels, rapid PSA velocity, sub-Saharan African ethnicity, with a family history of PCa or with specific genes, obese men with insulin resistance and those who would benefit from early diagnosis and treatment with at least 10–15 years of life expectancy.
References
Adlercreutz H (2002) Phyto-oestrogens and cancer. Lancet Oncol 3:364–373
Albertsen PC, Hanley JA, Fine J (2005) 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA 293:2095–2101
Andriole GL, Bostwick DG, Brawley OW et al (2010) Effect of dutasteride on the risk of prostate cancer. N Engl J Med 362:1192–1202
Antonarakis ES, Heath EI, Walczak JR et al (2009) Phase II, randomized, placebo-controlled trial of neoadjuvant celecoxib in men with clinically localized prostate cancer: evaluation of drug-specific biomarkers. J Clin Oncol 27:4986–4993
Arber N, Spicak J, Racz I et al (2011) Five-year analysis of the prevention of colorectal sporadic adenomatous polyps trial. Am J Gastroenterol 106:1135–1146
Balentine DA, Wiseman SA, Bouwens LC (1997) The chemistry of tea flavonoids. Crit Rev Food Sci Nutr 37:693–704
Bardia A, Platz EA, Yegnasubramanian S et al (2009) Anti-inflammatory drugs, antioxidants, and prostate cancer prevention. Curr Opin Pharmacol 9:419–426
Bettuzzi S, Brausi M, Rizzi F et al (2006) Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res 66:1234–1240
Brausi M, Rizzi F, Bettuzzi S (2008) Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update. Eur Urol 54:472–473
Chan JM, Oh WK, Xie W et al (2009) Plasma selenium, manganese superoxide dismutase, and intermediate- or high-risk prostate cancer. J Clin Oncol 27:3577–3583
Cohen YC, Liu KS, Heyden NL et al (2007) Detection bias due to the effect of finasteride on prostate volume: a modeling approach for analysis of the prostate cancer prevention trial. J Natl Cancer Inst 99:1366–1374
Daskivich TJ, Chamie K, Kwan L et al (2011) Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer 117:2058–2066, Epub 2010 Nov 29
Duffield-Lillico AJ, Dalkin BL, Reid ME et al (2003) Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the nutritional prevention of cancer trial. BJU Int 91:608–612
Etminan M, Takkouche B, Caamano-Isorna F (2004) The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev 13:340–345
Ferlay J, Shin HR, Bray F et al (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Gann PH (2009) Randomized trials of antioxidant supplementation for cancer prevention: first bias, now chance – next, cause. JAMA 301:102–103
Giovannucci E, Rimm EB, Liu Y et al (2002) A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst 94:391–398
Heinonen OP, Albanes D, Virtamo J et al (1998) Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst 90:440–446
Kaplan SA, Roehrborn CG, Meehan AG et al (2009) PCPT: evidence that finasteride reduces risk of most frequently detected intermediate- and high-grade (Gleason score 6 and 7) cancer. Urology 73:935–939
Klein EA, Thompson IM Jr, Tangen CM et al (2011) Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 306:1549–56
Koivisto PA, Schleutker J, Helin H et al (1999) Androgen receptor gene alterations and chromosomal gains and losses in prostate carcinomas appearing during finasteride treatment for benign prostatic hyperplasia. Clin Cancer Res 5:3578–3582
Kristal AR, Arnold KB, Neuhouser ML et al (2010) Diet, supplement use, and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol 172:566–577
Kurahashi N, Iwasaki M, Inoue M et al (2008) Plasma isoflavones and subsequent risk of prostate cancer in a nested case-control study: the Japan Public Health Center. J Clin Oncol 26:5923–5929
Levy J, Bosin E, Feldman B et al (1995) Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene. Nutr Cancer 24:257–266
Lippman SM, Klein EA, Goodman PJ et al (2009) Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 301:39–51
Lucia MS, Epstein JI, Goodman PJ et al (2007) Finasteride and high-grade prostate cancer in the prostate cancer prevention trial. J Natl Cancer Inst 99:1375–1383
Marshall JR, Tangen CM, Sakr WA et al (2011) Phase III trial of selenium to prevent prostate cancer in men with high-grade prostatic intraepithelial neoplasia: SWOG S9917. Cancer Prev Res 4:1761–1769
Morgentaler A, Traish AM (2009) Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 55:310–320
Nagata Y, Sonoda T, Mori M et al (2007) Dietary isoflavones may protect against prostate cancer in Japanese men. J Nutr 137:1974–1979
Nelson WG (2007) Prostate cancer prevention. Curr Opin Urol 17:157–167
Nelson WG, De Marzo AM, DeWeese TL et al (2004) The role of inflammation in the pathogenesis of prostate cancer. J Urol 172:S6–S11; discussion S11–2
Patel MI, Subbaramaiah K, Du B et al (2005) Celecoxib inhibits prostate cancer growth: evidence of a cyclooxygenase-2-independent mechanism. Clin Cancer Res 11:1999–2007
Peters U, Leitzmann MF, Chatterjee N et al (2007) Serum lycopene, other carotenoids, and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 16:962–968
Peters U, Littman AJ, Kristal AR et al (2008) Vitamin E and selenium supplementation and risk of prostate cancer in the vitamins and lifestyle (VITAL) study cohort. Cancer Causes Control 19:75–87
Pinsky P, Parnes H, Ford L (2008) Estimating rates of true high-grade disease in the prostate cancer prevention trial. Cancer Prev Res 1:182–186
Price D, Stein B, Sieber P et al (2006) Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: results of a double-blind, placebo controlled, phase IIB clinical trial. J Urol 176:965–970; discussion 970–1
Prins GS, Korach KS (2008) The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids 73:233–244
Redman MW, Tangen CM, Goodman PJ et al (2008) Finasteride does not increase the risk of high-grade prostate cancer: a bias-adjusted modeling approach. Cancer Prev Res 1:174–181
Roehrborn CG, Siami P, Barkin J et al (2008) The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J Urol 179:616–621; discussion 621
Schroder FH, Hugosson J, Roobol MJ et al (2009) Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 360:1320–1328
Serfling R, Shulman M, Thompson GL et al (2007) Quantifying the impact of prostate volumes, number of biopsy cores and 5alpha-reductase inhibitor therapy on the probability of prostate cancer detection using mathematical modeling. J Urol 177:2352–2356
Shimizu H, Ross RK, Bernstein L et al (1991) Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer 63:963–966
Swami S, Krishnan AV, Peehl DM et al (2005) Genistein potentiates the growth inhibitory effects of 1,25-dihydroxyvitamin D3 in DU145 human prostate cancer cells: role of the direct inhibition of CYP24 enzyme activity. Mol Cell Endocrinol 241:49–61
Syed DN, Khan N, Afaq F et al (2007) Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol Biomarkers Prev 16:2193–2203
The Alpha-Tocopherol BCCPSG (1994) The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 330:1029–1035
Thompson IM, Goodman PJ, Tangen CM et al (2003) The influence of finasteride on the development of prostate cancer. N Engl J Med 349:215–224
Thompson IM, Chi C, Ankerst DP et al (2006) Effect of finasteride on the sensitivity of PSA for detecting prostate cancer. J Natl Cancer Inst 98:1128–1133
Thompson IM, Tangen CM, Goodman PJ et al (2007) Finasteride improves the sensitivity of digital rectal examination for prostate cancer detection. J Urol 177:1749–1752
Tombal B (2007) The importance of testosterone control in prostate cancer. Eur Urol Suppl 6:834–839
Tombal B (2011) What is the pathophysiology of a hormone-resistant prostate tumour? Eur J Cancer 47:S179–S188
Travis RC, Spencer EA, Allen NE et al (2009) Plasma phyto-oestrogens and prostate cancer in the European prospective investigation into cancer and nutrition. Br J Cancer 100:1817–1823
Watters JL, Gail MH, Weinstein SJ et al (2009) Associations between alpha-tocopherol, beta-carotene, and retinol and prostate cancer survival. Cancer Res 69:3833–3841
Wu K, Erdman JW Jr, Schwartz SJ et al (2004) Plasma and dietary carotenoids, and the risk of prostate cancer: a nested case-control study. Cancer Epidemiol Biomarkers Prev 13:260–269
Yan L, Spitznagel EL (2009) Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr 89:1155–1163
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Tombal, B. (2012). Chemoprevention of Prostate Cancer. In: Bolla, M., van Poppel, H. (eds) Management of Prostate Cancer. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-27597-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-642-27597-5_2
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-27596-8
Online ISBN: 978-3-642-27597-5
eBook Packages: MedicineMedicine (R0)