Abstract
External beam irradiation is a common and standard treatment for prostate cancer. Intensity modulated radiotherapyv (IMRT) is a technique of irradiation which allows to generate concave isodoses and then to reduce the dose delivered to organs at risk of toxicities. In prostate cancer, IMRT reduces the rates of acute and late rectal and urinary toxicities. Therefore, IMRT appears as an optimal technique to escalate the dose of irradiation without causing increased toxicities. It could make easier the development of new approaches of irradiation of prostate cancers such as hypofractionated irradiation or stereotactic irradiation. The preservation of healthy tissues in IMRT could be improved by combination with the progress in imaging (fusion CT/MRI) and a systematic association with a daily repositioning using the image-guided radiotherapy (IGRT).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Prostate Cancer
- Biochemical Recurrence
- Rectal Toxicity
- Hypofractionated Radiotherapy
- Biological Equivalent Dose
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
12.1 Concept of Intensity Modulated Radiotherapy (IMRT)
IMRT is a highly conformal radiotherapy technique able to optimize the shape of the dose distribution and to generate a concave isodose profile by intensity modulated beams, which can deliver more than two intensity levels for a single beam direction and a single source position in space. IMRT is designed using inverse planning method (computer optimization) based on dose–volume criteria, in which above all the radiation oncologist prescribe the target volume dose coverage “objectives” and normal tissue protection “objectives,” and then, the computer creates a custom intensity modulation plan to satisfy the prescribed objectives (Intensity Modulated Radiation Therapy Collaborative Working Group 2001).
The major IMRT advantage is the better sparing of close-proximity organs at risk (OAR) for an identical tumor dose and consecutively the reduction of adverse event rates with no difference in disease-related outcomes. Moreover, IMRT can allow theoretically dose escalation to the primary tumor, keeping safe dose–volume constraints to organs at risk. Because of the close relation between the prostate and the rectum and the bladder, IMRT seems particularly adapted for prostate irradiation (Martin et al. 2010) (Fig. 12.1).
In a systematic review, Veldeman et al. (2008) analyze the toxicity events reported in comparative (IMRT against non-IMRT) studies on head and neck, prostate, gynecological, CNS, breast, and lung cancer and in noncomparative studies on mesothelioma and gastrointestinal malignancies. It demonstrated that compared with classical 3D irradiation, IMRT is not inferior in terms of local tumor control and survival and results in a decrease in toxic effects. Regarding the possibility of safe total-dose or fractionated-dose escalation to improve cancer control, future randomized clinical trials (RCT) to directly compare standard dose with total or fraction-dose escalation should be performed.
To summarize, IMRT generates concave isodoses which can bypass some organs at risk of toxicities. Thus, the interest of IMRT in prostate cancer could be important by reducing the doses received by the rectum and the bladder. A reduction of toxicities could be expected, allowing new approaches of doses escalation.
12.2 3D Conformal Radiation Therapy (3DCRT) Versus IMRT
12.2.1 Irradiation of Prostate and Seminal Vesicles Only
To date, no randomized study has compared the 3DCRT with IMRT. In several publications, the authors proposed to compare the two irradiation techniques. Thus, Kupelian has compared 166 patients treated with IMRT with 116 patients treated with 3DCRT (Kupelian et al. 2002). The results are in favor of a significant reduction (p = 0.002) of acute rectal toxicity and nonsignificant of late toxicities of grade ≥2 (5% vs. 12%, p = 0.24) with IMRT. However, a hypofractionated schema was used for IMRT and normofractionated for 3DCRT, making it difficult to distinguish between fractionation and intensity modulated in the differences of the obtained results between the two groups. In the study of Vora et al., 145 patients were treated on prostate and seminal vesicles with IMRT at dose of 76.5 Gy and 271 patients with 3DCRT at dose of 68.4 Gy (Vora et al. 2007). Despite a difference in total dose irradiation of 8 Gy, there was no significant difference found between groups for acute and late urinary and rectal toxicities. A benefit found in terms of biochemical-recurrence-free survival at 5 years (74.1% vs. 60.4%, p < 0.0001) suggested that IMRT would increase the control rates of the disease by increasing the delivered dose without increasing the toxicities. In another publication, Lips et al. also concluded that IMRT allows an irradiation dose escalation without increasing the toxicities (Lips et al. 2007). In this study, the 78 patients treated with conformal radiotherapy had received a dose of 70 Gy, and 92 patients treated with IMRT had received a dose of 76 Gy. In a series of 1,571 patients treated for a T1–T3 prostate cancer by radiotherapy alone Zelefsky et al. (2008a, b), found a significant reduction in gastrointestinal toxicities when IMRT is used (5% vs. 13% p < 0.001). Finally in 2011, Sharma et al. assessed the IMRT contribution when the irradiation is associated with hormone therapy (Sharma et al. 2011). Data from two groups of 123 patients treated with IMRT and 170 patients treated with IMRT were analyzed. Again, the benefit of the IMRT was found in terms of reduced acute and late gastrointestinal toxicities.
12.2.2 Prostate and Pelvic Irradiation
Ashman et al. compared 13 patients treated with IMRT at a dose of 81 Gy with 14 patients treated with 3DCRT at a dose of 75.6 Gy (Ashman et al. 2005). The volumes of irradiation included initially the pelvis. Despite an escalated total dose irradiation, IMRT appeared to give less acute rectal toxicities (7% vs. 36%) and intestinal disorders (0% vs. 43%) of grade 2 than 3DCRT. In another study, Sanguineti et al. (2006) also evaluated the IMRT contribution when a pelvic irradiation (54 Gy) was associated with prostate irradiation (76 Gy). Using the RTOG criteria, the toxicities were evaluated in a group treated with IMRT (45 patients) and a group treated with 3DCRT (68 patients). At 2 years, the cumulative rates for grade 2 rectal toxicities were 4% with IMRT and 21.2% without IMRT. No grade 3 toxicity was observed.
To summarize, compared to 3DCRT, IMRT reduces acute and late rectal and urinary grade ≥2 toxicities. A dose escalation can be achieved without increasing toxicities. This could result in improved biochemical-relapse-free survival. This benefit is found in case of localized prostate and seminal vesicles irradiation, but also when a pelvic irradiation and/or a hormone therapy are associated. However, mainly retrospective studies have been published, and no randomized trial is available.
12.3 Dose Escalation in Prostate Cancer
12.3.1 Interests of Dose Escalation in Prostate Cancer
Several randomized studies (Sathya et al. 2005; Peeters et al. 2006; Dearnaley et al. 2007; Kuban et al. 2008; Zerini et al. 2010; Zietman et al. 2005; Beckendorf et al. 2011) evaluated the impact of a dose escalation on disease control. Doses of 66–70 Gy were compared to doses of 74–80 Gy. In none of these studies, a hormone therapy was associated with radiotherapy. The increase in total dose of about 10 Gy was associated with an improved rates of biochemical-recurrence-free survival at 5 years from 50–60% to 70–85%, all stages of the disease combined. Of these studies, three proposed an irradiation dose of 78–80 Gy in the experimental arm. Thus, in the M.D. Anderson study, a dose of 78 Gy was compared to a dose of 70 Gy (Kuban et al. 2008). In total, 301 patients were included, having an intermediate-to-high-risk cancer. The main objective was to assess the impact of this increase dose on the clinical and/or biological-disease-free survival using the Phoenix definition (nadir + 2 ng/ml). At 5, 8, and 10 years, respectively, it increased from 78% to 85%, 59% to 78%, and 50% to 73% in the 78 Gy arm compared to 70 Gy arm. The increase in irradiation dose was though associated with an increase of late grade ≥2 rectal toxicities (26% vs. 13%) and urinary toxicities (13% vs. 8%). In the Dutch study (Peeters et al. 2006), 669 patients with intermediate-to-high-risk cancers were randomized between two doses of irradiation: 68 and 78 Gy. At 7 years, the biochemical-recurrence-free survival increased with the dose, from 45% to 56%. The cumulative incidence of gastrointestinal toxicity was also increased by 25–35%. The subgroup analysis showed a greater benefit for the intermediate-risk group. Finally, in the GETUG 06 (Beckendorf et al. 2011), a dose of 80 Gy was compared to a dose of 70 Gy in patients having mainly intermediate-risk prostate cancer. At 5 years, the survival rates without biochemical recurrence were respectively 68% and 76.5% in the 70 and 80 Gy arms, using the Phoenix definition. The benefit seemed greater when the PSA rate was higher than 15 ng/ml. In this study, the increase in radiation dose was also associated with an increase of acute and late rectal and urinary toxicities.
All these data support the benefit of an irradiation dose escalation mainly for the intermediate-risk cancer. However, the dose augmentation may also be a benefit for the high-risk cancer patients. In fact, in the study of MD Anderson, if a majority of patients have had low-to-intermediate-risk prostate cancers, 30% (70 Gy) to 35% (78 Gy) of patients had a high-risk prostate cancer. Specific analysis in this group of patients shows a benefit at 5 years in biochemical-recurrence-free survival, local-progression-free, and without metastasis, in favor of dose increasing. In the Dutch study, half of the patients had high-risk prostate cancers. This benefit of increasing the radiation dose in high-risk patients had already been suspected in most retrospective studies. Thus, in the study of Zelefsky et al., on 752 patients irradiated for a high-risk cancer, increasing the radiation dose from 70.2 to 86.4 Gy improved the survival without metastatic evolution from 77% to 82% (Zelefsky et al. 2008a, b). The question remains whether this benefit persists when a 3-year hormone therapy is associated with radiotherapy. The GETUG 18 study aims to answer this question by randomizing patients into two levels of dose (70 vs. 80 Gy) in combination with 3 years of hormone therapy in both arms.
12.3.2 IMRT in Dose Escalation
The first IMRT experiences for prostate cancer treatment were described by Memorial Sloan-Kettering Cancer Center (MSKCC). In the early 2000s, Zelefsky et al. reported the results of a series of 171 patients treated at a dose of 81 Gy with IMRT, between 1992 and 1998 (Zelefsky et al. 2000). A dosimetric study to compare, for the same patient, two treatment plans, with and without IMRT, was also performed on 20 patients. This study showed that the intensity modulation provides a benefit in terms of target volume coverage and rectum and bladder preservation. A comparison of clinical outcomes in the two groups of patients treated with and without IMRT confirmed a reduced actuarial risk of late rectal toxicity of grade 2 at 2 years, from 10% to 2% when using IMRT. The toxicity grades were defined using the radiation therapy oncology group (RTOG) criteria. In 2002, the same author (Zelefsky et al. 2002) published the results of a series of 772 patients treated with IMRT for a prostate cancer at doses between 81 (90% of patients) and 86.4 Gy (10% of patients). The rates of rectal and urinary acute toxicities of grade 2 were respectively 4.5% (0% of grade 3) and 28% (1 toxicity of grade 3). In total, 15% of patients developed a late rectal toxicity of grade 2 and 0.1% a rectal toxicity of grade 3. The probability of developing a rectal toxicity of grade ≥2 was of 4% at 3 years. In terms of urinary toxicity, 9% of patients presented a late toxicity of grade 2 and 0.5% of grade 3. The probability of developing a late urinary toxicity of grade ≥2 was estimated at 15% at 3 years. In 2011, data at 10 years were published for the 170 patients treated with IMRT, at doses of 81 Gy (Alicikus et al. 2011). The actuarial biochemical-recurrence-free survival at 10 years was of 81%, 78%, and 62% respectively for patients with low-, intermediate-, or high-risk prostate cancer. Using the CTC AE V3 criteria, the probabilities at 10 years of developing a rectal toxicity of grades 2 and 3 were respectively 2% and 1%. The 10-year probabilities of grades 2 and 3 urinary toxicities were 11% and 5%. Finally, Cahlon et al. reported the results for 478 patients treated at a dose of 86.4 Gy (Cahlon et al. 2008). With a median follow-up of 53 months, the rates for rectal toxicities of grade 2 were 8%, and no higher grade toxicity was observed. Using the Phoenix definition of biochemical recurrences (nadir + 2 ng/ml), the actuarial rate of biochemical-recurrence-free survival at 5 years was respectively 98%, 85%, and 70% for low, intermediate, and high risks. Other IMRT experiences were published for prostate irradiation with at least 80 Gy. Thus, Ghadjar et al. analyzed the data from 102 patients treated with IMRT at 80 Gy and with IMRT and daily image-guided radiotherapy (IGRT) of the prostate (Ghadjar et al. 2010). A total of 5% late rectal toxicities of grade 2 were observed, and no grade 3 toxicity. The rates of late urinary toxicities of grades 2 and 3 were respectively 21% and 1%. Azria et al. reported a French series of 373 patients treated at a total dose of 80 Gy with IMRT (Azria et al. 2009). The rates of late rectal and urinary toxicities ≥2 are respectively 5.3% and 5.9%.
To summarize, several randomized trials demonstrated the benefit of a dose escalation in prostate cancer with an increased rate of biochemical relapse free survival. Using IMRT, a dose escalation above 80 Gy can be performed with a low rate of grade ≥2 late rectal toxicities (<10%). The impact of IMRT on the urinary tract seems smaller with rates of late grade ≥2 toxicities, often above 10–20%.
12.4 Optimal IMRT Approach for Prostate Cancer
If currently, IMRT appears as the optimal technique of irradiation of prostate cancer, the preservation of healthy tissues in IMRT could be optimized by the contribution of imaging (fusion CT/MRI) and a systematic association with a daily repositioning using the image-guided radiotherapy (IGRT).
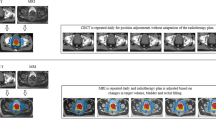
12.4.1 CT/MRI Fusion (Fig. 12.2)
Prostate definition on the CT scan is associated with high interphysician and interscan variations (Mitchell et al. 2009; Valicenti et al. 1999; Gao et al. 2007) and an overestimation of the clinical target volume (CTV), with CT images was confirmed (Sannazzari et al. 2002). However, these differences are significantly reduced when an MRI is used (Smith et al. 2007; Rasch et al. 1999; Jackson et al. 2004; Freedman et al. 2001; Roach et al. 1996; Usmani et al. 2011). MRI allows a better definition and delineation of the apex and base of the prostate (Milosevic et al. 1998; Jackson et al. 2004).
Excepting the prostate contouring, the importance of using a CT/MRI fusion method or an MRI exam for prostate radiotherapy was also demonstrated for:
-
Reducing the dose to the rectum, penile bulb, and the erectile arteries in order to improve the patient’s posttherapy sexual functioning and quality of life (Perna et al. 2009; Steenbakkers et al. 2003; Meirovitz et al. 2003)
-
A better visualization of the prostate for patients with bilateral hip prostheses (Rosewall et al. 2009)
-
For the tumor localization into the prostate using different MRI sequences (Groenendaal et al. 2010a, b; Franiel et al. 2009; Kajihara et al. 2009)
Most of the studies, that evaluated the interest of a CT/MRI combination, used registrations based on the bony landmarks (Milosevic et al. 1998; Roach et al. 1996; Acher et al. 2010; Chen et al. 2004; Petersch et al. 2004). Intraprostatic gold markers are recommended to be implanted for a better daily repositioning before and/or during the irradiation, improving the prostate localization, but the interest in using intraprostatic markers, rather than bony structures for the CT/MRI registration, was also presented (Parker et al. 2003).
Contouring protocols were published in order to improve the radiation therapists’ technique (McLaughlin et al. 2010; Villeirs et al. 2005), but still, it is important that the physician has a good experience in prostate MRI description when using a CT/MRI fusion (Tanaka et al. 2006).
12.4.2 Image Guidance Radiation Therapy IGRT
IGRT means that imaging is used at each fraction of irradiation for high precision of repositioning of the target volume. In prostate cancers, the interfraction variation of the prostate position within the pelvis makes IGRT particularly interesting. In fact, as there is a dose-effect relation on the local control, a precise prostate positioning at each session of irradiation might have an important clinical impact by insuring that the dose of irradiation is well delivered into the prostate. Several studies demonstrated that the variation of prostate position according to the rectal volume and the rectal distension on the planning CT scan significantly increases the risk of local recurrence in multivariate analysis (de Crevoisier et al. 2005; Heemsbergen et al. 2007; Pinkawa et al. 2006). When daily IGRT is used for registration, the overall outcomes appear to be very favorable (Kupelian et al. 2008). A recent study of Haverkort et al. evaluated, using electronic portal images (EPIs), the effect of gold markers-based position correction on the cumulative dose in the rectal wall, when changes in the rectum anatomy and position appear (Haverkort et al. 2011). Compared to bony anatomy-based correction, the rectal wall D50% and D70% and the mean anal wall dose were significantly lower when using gold markers.
In the last years, the literature demonstrates the effort in finding the easiest and best method of prostate tracking and repositioning: ultrasound-based (BAT) tracking (Boda-Heggemann et al. 2008; Scarbrough et al. 2006), real-time tumor tracking (Kitamura et al. 2002; Langen et al. 2008; Kupelian et al. 2005), portal images on implanted markers (Balter et al. 1995a, b), cone-beam CT (CBCT) (Pouliot et al. 2006; Sorcini and Tilikidis 2006), etc. Several studies have attempted to compare the different techniques together. Definitive conclusions are difficult to make but however, it seems that a prostate repositioning, using a CBCT or the detection of intraprostatic gold markers (KV/KV) offers the greatest precision (Barney et al. 2011; Clancy et al. 2009; Neicu et al. 2009; Owen et al. 2010).
To summarize, CT/MRI fusion allows a higher precision in the prostate contours delineation especially when fiducial markers are used. By correcting the interfraction motion of the prostate, IGRT should allow a reduction of the margin of the planning tumor volume. A combination of these two approaches with IMRT could improve the prostate coverage and reduce the volume of rectum and bladder irradiated.
12.5 Perspective of Evolution of Prostate Irradiation
12.5.1 Hypofractionated Radiotherapy (HR)
In radiotherapy, the daily dose of reference or standard fractionation (normofractionation) is of 1.8–2 Gy per fraction. The HR is an increase of the radiation dose delivered per fraction and a decrease in total number of irradiation fraction compared to a conventional fractionation.
Three reasons justify the development of HR for prostate cancers:
-
1.
Prostate cancer would have a particular sensitivity to the dose delivered in each session. This sensitivity is defined in radiobiology by the α/β ratio. The closer this ratio is to 0, the cells are more susceptible to the dose per fraction; the more this ratio is greater, the impact of fractionation is low. Many studies consistently show that this ratio would be between 1.5 and 3 Gy for prostate cancer (Leborgne et al. 2012; Miralbell et al. 2012; Carlson et al. 2004; Wang et al. 2003; Brenner and Hall 1999; Brenner et al. 2002).
-
2.
The treatment with external beam radiotherapy of prostate cancer requires between 35 and 40 fractions of irradiation or 8 weeks of treatment with a standard dose per fraction of 1.8–2 Gy per fraction. A reduction of this irradiation time to 4–5 weeks or less would definitely represent an amelioration of the patients’ quality of life.
-
3.
A reduction of several weeks of the radiotherapy treatment duration due to the development of HR would significantly reduce the costs and improve the treatment processing of patients in radiotherapy.
12.5.2 Experiences of Hypofractionated Radiotherapy
Many studies have described the feasibility of the HR using different doses per fraction. Only recent studies using 3D conformal radiotherapy techniques with or without intensity modulation are described below.
12.5.2.1 HR with a Dose per Fraction Inferior to 3 Gy
The study of Kupelian et al. (2007) is probably the reference in the development of HR. The dose per fraction was 2.5 Gy for a total dose of 70 Gy, or 28 fractions. A technique of irradiation with a daily repositioning of the prostate and intensity modulated radiotherapy has been used to treat a total of 770 patients. The biological equivalent dose for a 2 Gy fractionation was estimated at 80 Gy. According to the Phoenix definition (nadir + 2 ng/ml), the biochemical recurrence-free survival at 5 years was 94%, 83%, and 72%, respectively, for patients with low-, intermediate-, and high-risk cancers. These rates are particularly high especially for intermediate-to-high-risk patients. A fractionation close to 72 Gy in 30 fractions of 2.4 Gy was used in an Italian study of 25 patients (Zerini et al. 2010). A 3D conformal radiotherapy with a daily ultrasound repositioning was performed. With a mean follow-up of 45 months, only late rectal (16%) and urinary (32%) grade 1 toxicities were described. Only one biochemical recurrence was reported.
A dose per fraction of 2.64 Gy was evaluated in the study of Junius et al. (2007). The delivered dose to the seminal vesicles was 50 Gy in 25 fractions, while the delivered dose to the prostate during the 25 fractions was 66 Gy. With a median follow-up of 20 months, three biochemical recurrences are described in a population of 38 patients consisting primarily of intermediate stages to high according to D’Amico classification. The limit of these two studies is the use of moderately increased doses per fraction that probably not allows an optimal hypofractionation effect on prostate cancers.
12.5.2.2 HR with a Dose per Fraction Superior or Equal to 3 Gy
Several studies have evaluated a dose per fraction of 3–3.15 Gy. In the study of Leborgne and Fowler (2009), 89 patients with prostate cancer were treated with 20 fractions of 3 (n = 52) or 3.15 Gy (n = 37). The biochemical recurrence-free survival at 5 years was 96%, 84%, and 85% respectively for low-, intermediate-, and high-risk stages. Thirty percent of patients had late rectal toxicity, with 6% of grades 2–3. In the study by Yassa et al. (2008), 19 fractions of 3 Gy were delivered to 42 patients. With a mean follow-up of 46 months, 79% of patients had no biochemical recurrence. Acute rectal toxicity of grade ≥2 was observed in 36% of patients while 12% of them had late rectal toxicity, bleeding symptoms of grades 1–2. In the Canadian study of Faria et al. (2008), 72 patients were treated with a dose of 66 Gy in 22 fractions of 3 Gy. The technique of radiotherapy was 3D conformation without intensity modulation. The margins defining the target volume were limited to 7 mm. In total, 39% of patients experienced late rectal toxicity. In 18% of patients, it was grades 2–3 toxicity. In a more recent publication (Rene et al. 2010) of 129 patients, using the same technique of irradiation and the same fractionation, the rates of late urinary and rectal toxicities of grade ≥2 are respectively 32% and 25% but do not persist in time (only 2% and 1.5%). Akimoto et al. (2004) reported the results of a phase II study in which 52 patients were treated with a hypofractionation schema of 69 Gy in 23 fractions of 3 Gy. The rate of late rectal toxicity of grade ≥2 was 25% and a late rectal toxicity of grade 3 was observed.
12.5.3 Experiences of HR with IMRT
In 2007, Martin et al. (2007) reported the results of radiotherapy of 60 Gy in 20 fractions of 3 Gy delivered with IMRT and daily repositioning of the prostate on gold markers. In total, 36% of 92 enrolled patients showed a gastrointestinal acute toxicity of grades 2–3 in 12% of cases. The late rectal toxicities were less frequent with only 6% of grades 1–2. At 3 years, the rate of biochemical control was 76% as defined by ASTRO (3 successive of PSA increase). More recently, Coote el al. (2009) reported the results in terms of tolerance to an irradiation of 3 Gy per fraction for a total dose of 57–60 Gy. The irradiation technique was based on conformal radiotherapy with modulated intensity. In total, 57% of patients treated with 57 Gy and 70% of patients treated with 60 Gy showed an acute rectal toxicity. This toxicity was grade 2 in 20% and 10% of patients respectively treated with 57 and 60 Gy. No acute rectal toxicity of grade 3 was observed. In the group treated with 57 Gy, 27% of patients presented a late rectal toxicity of grade 1, whereas at 60 Gy, 19% of patients presented a late rectal toxicity of grades 1 or 2 (50% of grade 2). No grade 3 late toxicity was reported. Vesprini et al. reported a series of 121 patients treated with IMRT to a dose of 60–66 Gy using the same fractionation of 3 Gy per fraction (Vesprini et al. 2011). With a follow-up of 47 months, the rates of urinary and rectal late toxicities of grade 2 and more were respectively of 15% and 16%. Finally, the results of a phase II study (Lock et al. 2011) on 66 patients have been published in which the treatment associated an intensity modulated irradiation at a dose of 63.2 Gy in 20 fractions with a daily repositioning on gold markers or using ultrasound. With a median follow-up of 30 months, the rates of late rectal toxicities of grades 2 and 3 were respectively of 25% and 3%. The rates of late urinary toxicities of grades 2 and 3 were respectively 14% and 5%.
12.5.4 Comparison HR and Conventional Fractionation Radiotherapy
In a recent randomized study, Arcangeli et al. compared the efficacy and tolerance of a normofractionation radiotherapy (80 Gy in 40 fractions) and of a hypofractionated radiotherapy (62 Gy in 20 fractions of 3.1 Gy) on 160 patients (Arcangeli et al. 2010). The main quality of this study was to propose in both arms biological equivalent doses by taking an α/β = 1.5 Gy. The first results, with a follow-up of 3 years, were in a favor of an increase in biochemical-recurrence-free survival in the hypofractionated arm (87% vs. 79%). This difference was observed even for the high-risk stages (88% vs. 76%). The rates of late rectal toxicities of grade 2 and more at 3 years were similar in both arms (17% and 16%).
Previously, an Australian randomized study (Yeoh et al. 2010) compared an irradiation of 64 Gy in 32 fractions (n = 109) with and irradiation of 55 Gy in 20 fractions (n = 108). The irradiation was in 2D for the majority of patients. At 90 months, the HR gave a better biochemical-recurrence-free survival (53% vs. 34%) without improvement of toxicity. The results of this study are difficult to interpret because of the low doses delivered in the normofractionated arm. Four randomized studies are underway to compare hypofractionated radiotherapy to conventional radiotherapy (RTOG 0415, MRC trial, NCIC trial, and Fox Chase trial).
To summarize, hypofractionated radiotherapy gives encouraging results in terms of biological control. When the dose per fraction is superior or equal to 3 Gy, the rate of late rectal toxicity grade ≥2 appears to be between 15% and 25%. When IMRT is used, this rate seems closer to 10–15%. Several randomized studies are underway to compare this radiation technique to conventional radiotherapy.
12.5.5 Stereotactic Body Radiotherapy (SBRT)
12.5.5.1 Concept of SBRT
The principle of stereotactic radiotherapy is to deliver a high-radiation dose highly conformed on a small tumor. The result is a “removal” of the tumor while ensuring the preservation of the surrounding tissue. This technique requires great precision in the localization of the tumor and, therefore, was first developed in the treatment of brain metastases. In fact, by immobilizing the skull (and thus the brain), it was possible to pinpoint an intracerebral lesion. More recently, the use of new technologies in the spatial location of tumors (integrated scanner to an accelerator, detection of intratumoral implants, etc.) permits the visualization with a high precision (order of mm), the tumor position, even in the soft tissues (lung, liver, etc.). Stereotactic radiotherapy has been thus developed in the irradiation of tumor sites outside the brain as some small-cell lung cancer (T1 and T2N0). For these tumors, although considered radioresistant, the local control rate passed from 30% to 40%, after conventional radiotherapy, to 80–90% after stereotactic radiotherapy. The prostate, due to its limited volume and easy location (intraprostatic implants), represents a well-suited organ for developing such technique.
12.5.5.2 Stereotactic Radiotherapy and Prostate Cancer
The experiences of various radiotherapy centers, having developed stereotactic radiotherapy in prostate cancer, have been reported. The first experience is the one of Seattle (Madsen et al. 2007). Forty patients were enrolled in a phase I/II study and treated at a dose of 33.5 Gy in 5 fractions of 6.7 Gy with an equivalent dose of 78 Gy using a conventional fractionation of 2 Gy per fraction. With a median follow-up of 41 months, one urinary toxicity of grade 3 was reported. The rate of actuarial survival without biochemical recurrence was 90% at 48 months using the Phoenix definition for local recurrences. A repositioning based on intraprostatic implants was used at each fraction. The irradiation dose was delivered with a linear accelerator. At Stanford University (King et al. 2009), 41 patients were included in a phase I/II study and received a dose of 36.25 Gy in 5 fractions of 7.25 Gy. With a median follow-up of 33 months, no toxicity of grade 4 or more was observed. Two urinary toxicities of grade 3 were reported, but no grade 3 rectal toxicity. At the time of publication, no patient had presented biochemical recurrence. In a more recent publication on 67 patients (King et al. 2012), but with a median follow-up of 27 months, rates of urinary toxicities of grades 1, 2, and 3 were respectively 23%, 5%, and 3% and the rectal toxicities of grades 1, 2, and 3 respectively of 12.5%, 2%, and 0%. Two recurrences proven by biopsy were reported. In Toronto (Tang et al. 2008), 30 patients were treated at dose of 35 Gy in 5 fractions of 7 Gy with IMRT and under a conventional accelerator. At 6 months for all patients, no toxicity superior to grade 2 was observed. At Naples (Friedland et al. 2009), 112 patients with prostate cancer of favorable stage were treated at a dose of 35–36 Gy in 5 fractions. With a median follow-up of 24 months, two patients experienced a local recurrence, histologically proven. The average PSA value was 0.78 ng/ml. Only one patient presented a rectal toxicity of grade 3. At Dallas, a phase I study of dose escalation per fraction in 3 levels of 45, 47.5, and 50 Gy in 5 fractions was conducted (Boike et al. 2011). The irradiation technique combined an image-guided radiotherapy, an IMRT technique, and an endorectal balloon. In total, 45 patients were included (15 to different dose levels) without reaching the limiting toxicity dose. With a median follow-up of 30 months, only 18% of patients had a late rectal toxicity of grade ≥2 and 2% grade 3 toxicity. The rates of late urinary toxicities of grades ≥2 and ≥3 were respectively 31% and 4%. The PSA control was 100%. A phase II study is currently undergoing at a dose of 50 Gy in 5 fractions of 10 Gy. Finally in 2010, Katz et al. reported a series of 304 patients treated in 5 fractions of 7–7.25 Gy (Katz et al. 2010). After 17 months, only one urinary toxicity of grade 3 was described, but four biochemical recurrences were reported.
To summarize, four important informations can be taken from these studies: (1) stereotactic radiotherapy is technically feasible in prostate cancer; (2) with an experience still low, the rectal and urinary toxicities of grade 3 or greater are less frequent; (3) with the same experience, the local control is excellent (between 90% and 100%); and (4) in many of these studies, conventional accelerators were used, suggesting the possibility of development in a greater number of radiotherapy departments. However, to date, the literature data are not sufficient to allow the development of stereotactic irradiation outside studies. Phase III studies are needed to compare this new approach to conventional irradiation.
References
Acher P, Puttagunta S, Rhode K et al (2010) An analysis of intraoperative versus post-operative dosimetry with CT, CT-MRI fusion and XMR for the evaluation of permanent prostate brachytherapy implants. Radiother Oncol 96:166–171
Akimoto T, Muramatsu H, Takahashi M et al (2004) Rectal bleeding after hypofractionated radiotherapy for prostate cancer: correlation between clinical and dosimetric parameters and the incidence of grade 2 or worse rectal bleeding. Int J Radiat Oncol Biol Phys 60(4):1033–1039
Alicikus ZA, Yamada Y, Zhang Z et al (2011) Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer 117(7):1429–1437
Arcangeli G, Saracino B, Gomellini S et al (2010) A prospective phase III randomized trial of hypofractionation versus conventional fractionation in patients with high-risk prostate cancer. Int J Radiat Oncol Biol Phys 78(1):11–18
Ashman JB, Zelefsky MJ, Hunt MS et al (2005) Whole pelvic radiotherapy for prostate cancer using 3D conformal and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 63(3):765–771
Azria D, Aillieres N, Llacer Moscardo C et al (2009) Conformal intensity modulated radiation therapy for localized prostate cancer: toward a new standard. Cancer Radiother 13:409–415
Balter JM, Sandler HM, Lam K et al (1995a) Measurement of prostate movement over the course of routine radiotherapy using implanted markers. Int J Radiat Oncol Biol Phys 31:113–118
Balter JM, Lam KL, Sandler HM et al (1995b) Automated localization of the prostate at the time of treatment using implanted radiopaque markers: technical feasibility. Int J Radiat Oncol Biol Phys 33:1281–1286
Barney BM, Lee RJ, Handrahan D, Welsh KT et al (2011) Image-guided radiotherapy (IGRT) for prostate cancer comparing kV imaging of fiducial markers with cone-beam computed tomography (CBCT). Int J Radiat Oncol Biol Phys 80:301–305
Beckendorf V, Guerif S, Le Prisé E et al (2011) 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys 80(4):1056–1063
Boda-Heggemann J, Kohler FM, Kupper B et al (2008) Accuracy of ultrasound- based (BAT) prostate-repositioning: a three dimensional on-line fiducial-based assessment with cone-beam computed tomography. Int J Radiat Oncol Biol Phys 70:1247–1255
Boike TP, Lotan Y, Cho LC et al (2011) Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol 29(15):2020–2026
Brenner DJ, Hall EJ (1999) Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 43:1095–1101
Brenner DJ, Martinez AA, Edmundson GK (2002) Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 52:6–13
Cahlon O, Zelefsky MJ, Shippy A et al (2008) Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys 71(2):330–337
Carlson DJ, Stewart RD, Li XA et al (2004) Comparison of in vitro and in vivo α/β ratios for prostate cancer. Phys Med Biol 49:4477–4491
Chen L, Price RA, Wang L et al (2004) MRI-based treatment planning for radiotherapy: dosimetric verification for prostate IMRT. Int J Radiat Oncol Biol Phys 60:636–647
Clancy PE, Schuller BW, Sroczinski LM, Hirsch AE (2009) Assessment of patient setup error in prostate radiation therapy using fiducial-based image guided radiation therapy with kV onboard imaging and conebeam CT [abstract]. Int J Radiat Oncol Biol Phys 75(suppl 1):S579–S580
Coote JH, Wylie JP, Cowan RA et al (2009) Hypofractionated intensity-modulated radiotherapy for carcinoma of the prostate: analysis of toxicity. Int J Radiat Oncol Biol Phys 74(4):1121–1127
de Crevoisier R, Tucker SL, Dong L et al (2005) Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys 62:965–973
Dearnaley DP, Sydes MR, Graham JD et al (2007) Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol 8(6):475–487
Faria SL, Souhami L, Joshua B et al (2008) Reporting late rectal toxicity in prostate cancer patients treated with curative radiation treatment. Int J Radiat Oncol Biol Phys 72(3):777–781
Franiel T, Ludemann L, Taupitz M et al (2009) MRI before and after external beam intensity-modulated radiotherapy of patients with prostate cancer: the feasibility of monitoring of radiation-induced tissue changes using dynamic contrast-enhanced inversion-prepared dual-contrast gradient echo sequence. Radiother Oncol 93:241–245
Freedman GM, Price RA Jr, Mah D et al (2001) Routine use of MRI and CT simulation for treatment planning of intensity modulated radiation therapy (IMRT) in prostate cancer. Int J Radiat Oncol Biol Phys 51:301
Friedland JL, Freeman DE, Masterson-McGary ME et al (2009) Stereotactic body radiotherapy: an emerging treatment approach for localized prostate cancer. Technol Cancer Res Treat 8(5):387–392
Gao Z, Wilkins D, Eapen L et al (2007) A study of prostate delineation referenced against a gold standard created from the visible human data. Radiother Oncol 85:239–246
Ghadjar P, Gwerder N, Manser P et al (2010) High-dose (80 Gy) intensity modulated radiation therapy with daily image-guidance as primary treatment for localized prostate cancer. Strahlenther Onkol 186(12):687–692
Groenendaal G, Moman MR, Korporaal JG et al (2010a) Validation of functional imaging with pathology for tumor delineation in the prostate. Radiother Oncol 94:145–150
Groenendaal G, van den Berg Cornelis AT, Korporaal JG et al (2010b) Simultaneous MRI diffusion and perfusion imaging for tumor delineation in prostate cancer patients. Radiother Oncol 95:185–190
Haverkort MAD, van der Kamer JB, Pieters BR et al (2011) Position verification for the prostate: effect on rectal wall dose. Int J Radiat Oncol Biol Phys 80:462–468
Heemsbergen WD, Hoogeman MS, Witte MG et al (2007) Increased risk of biochemical and clinical failure for prostate patients with a large rectum at radiotherapy planning: results from the Dutch trial of 68 GY versus 78 Gy. Int J Radiat Oncol Biol Phys 67:1418–1424
Intensity Modulated Radiation Therapy Collaborative Working Group (2001) Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys 51(4):880–914
Jackson ASN, Reinsberg SA, Moore EM et al (2004) Distortion-corrected T2-weighted MRI: a comparison with CT for prostate contouring in radiotherapy planning. Radiother Oncol 73:S457
Junius S, Haustermans K, Bussels B et al (2007) Hypofractionated intensity modulated irradiation for localized prostate cancer, results from a phase I/II feasibility study. Radiat Oncol 2:29
Kajihara H, Hayashida Y, Murakami R et al (2009) Usefulness of diffusion-weighted imaging in the localization of prostate cancer. Int J Radiat Oncol Biol Phys 74:399–403
Katz AJ, Santoro M, Ashley R et al (2010) Stereotactic body radiotherapy for organ-confined prostate cancer. BMC Urol 10:1
King CR, Brooks JD, Gill H et al (2009) Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys 73(4):1043–1048
King CR, Brooks JD, Gill H et al (2012) Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 82(2):877–882
Kitamura K, Shirato H, Seppenwoolde Y et al (2002) Three-dimensional intrafractional movement of prostate measured during real-time tumor-tracking radiotherapy in supine and prone treatment positions. Int J Radiat Oncol Biol Phys 53:1117–1123
Kuban DA, Tucker SL, Dong L et al (2008) Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 70(1):67–74
Kupelian PA, Reddy CA, Carlson TP et al (2002) Preliminary observations on biochemical relapse-free survival rates after short-course intensity-modulated radiotherapy (70 Gy at 2.5 Gy/fraction) for localized prostate cancer. Int J Radiat Oncol Biol Phys 53(4):904–912
Kupelian PA, Willoughby TR, Meeks SL et al (2005) Intraprostatic fiducials for localization of the prostate gland: monitoring intermarker distances during radiation therapy to test for marker stability. Int J Radiat Oncol Biol Phys 62:1291–1296
Kupelian PA, Willoughby TR, Reddy CA et al (2007) Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland clinic experience. Int J Radiat Oncol Biol Phys 68(5):1424–1430
Kupelian PA, Willoughby TR, Reddy CA et al (2008) Impact of image-guided on outcomes after external beam radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 70:1146–1150
Langen KM, Willoughby TR, Meeks SL et al (2008) Observations on real-time prostate gland motion using electromagnetic tracking. Int J Radiat Oncol Biol Phys 71:1084–1090
Leborgne F, Fowler J (2009) Late outcomes following hypofractionated conformal radiotherapy vs standard fractionation for localized prostate cancer: a nonrandomized contemporary comparison. Int J Radiat Oncol Biol Phys 74:1441–1446
Leborgne F, Fowler J, Leborgne JH, Mezzera J. (2012) Later outcomes and alpha/beta estimate from hypofractionated conformal three-dimensional radiotherapy versus standard fractionation for localized prostate cancer. Int J Radiat Oncol Biol Phys 82(3):1200–1207
Lips I, Dehnad H, Kruger AB et al (2007) Health-related quality of life in patients with locally advanced prostate cancer after 76 Gy intensity-modulated radiotherapy vs. 70 Gy conformal radiotherapy in a prospective and longitudinal study. Int J Radiat Oncol Biol Phys 69(3):656–661
Lock M, Best L, Wong E et al (2011) A phase II trial of Arc-based hypofractionated intensity-modulated radiotherapy in localized prostate cancer. Int J Radiat Oncol Biol Phys 80(5):1306–1315
Madsen BL, Hsi RA, Pham HT et al (2007) Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: first clinical trial results. Int J Radiat Oncol Biol Phys 67(4):1099–1105
Martin JM, Rosewall T, Bayley A et al (2007) Phase II trial of hypofractionated image-guided intensity-modulated radiotherapy for localized prostate adenocarcinoma. Int J Radiat Oncol Biol Phys 69(4):1084–1089
Martin JM, Frantzis J, Eade T, Chung P (2010) Clinician’s guide to prostate IMRT plan assessment and optimisation. J Med Imaging Radiat Oncol 54(6):569–575
McLaughlin PW, Evans C, Feng M, Narayana V (2010) Radiographic and anatomic basis for prostate contouring errors and methods to improve prostate contouring accuracy. Int J Radiat Oncol Biol Phys 76:369–378
Meirovitz A, Troyer S, Evans V et al (2003) Rectum and prostate separation by MRI vs CT in external beam and post-implant patients [abstract]. Int J Radiat Oncol Biol Phys 57(Suppl):S334
Milosevic M, Voruganti S, Blend R et al (1998) Magnetic resonance imaging (MRI) for localization of the prostatic apex: comparison to computed tomography (CT) and urethrography. Radiother Oncol 47:277–284
Miralbell R, Roberts SA, Zubizarreta E et al (2012) Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9–2.2) Gy. Int J Radiat Oncol Biol Phys 82:e17–e24
Mitchell DM, Perry L, Smith S et al (2009) Assessing the effect of a contouring protocol on postprostatectomy radiotherapy clinical target volumes and interphysician variation. Int J Radiat Oncol Biol Phys 75:990–993
Neicu T, Chetty IJ, Pradhan D et al (2009) A comparative study for daily localization with 3D ultrasound, cone beam CT, and implanted prostate fiducial markers for patients undergoing IGRT for prostate cancer. Int J Radiat Oncol Biol Phys 75(Suppl):S606
Owen R, Foroudi F, Kron T et al (2010) A comparison of in-room computerized tomography options for detection of fiducial markers in prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys 77:1248–1256
Parker CC, Damyanovich A, Haycocks T et al (2003) Magnetic resonance imaging in the radiation treatment planning of localized prostate cancer using intra-prostatic fiducial markers for computed tomography co-registration. Radiother Oncol 66:217–224
Peeters ST, Heemsbergen WD, Koper PC et al (2006) Dose–response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 24(13):1990–1996
Perna L, Fiorino C, Cozzarini C et al (2009) Sparing the penile bulb in the radical irradiation of clinically localised prostate carcinoma: a comparison between MRI and CT prostatic apex definition in 3DCRT, Linac-IMRT and helical tomotherapy. Radiother Oncol 93:57–63
Petersch B, Bogner J, Fransson A et al (2004) Effects of geometric distortion in 0.2 T MRI on radiotherapy treatment planning of prostate cancer. Radiother Oncol 71:55–64
Pinkawa M, Siluschek J, Gagel B et al (2006) Influence of the initial rectal distension on posterior margins in primary and postoperative radiotherapy for prostate cancer. Radiother Oncol 8:284–290
Pouliot J, Morin O, Aubin M et al (2006) Mégavoltage cone-beam CT: récents développements et applications cliniques pour la radiothérapie de modulation d’intensité. Cancer Radiother 10:258–268
Rasch C, Barillot I, Remeijer P et al (1999) Definition of the prostate in CT and MRI: a multi-observer study. Int J Radiat Oncol Biol Phys 43:57–66
Rene N, Faria S, Cury F et al (2010) Hypofractionated radiotherapy for favorable risk prostate cancer. Int J Radiat Oncol Biol Phys 77(3):805–810
Roach M III, Faillace-Akazawa P, Malfatti C et al (1996) Prostate volumes defined by magnetic imaging and computerized tomographic scans for three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 35:1011–1018
Rosewall T, Kong V, Vesprini D et al (2009) Prostate delineation using CT and MRI for radiotherapy patients with bilateral hip prostheses. Radiother Oncol 90:325–330
Sanguineti G, Cavey ML, Endres EJ et al (2006) Does treatment of the pelvic nodes with IMRT increase late rectal toxicity over conformal prostate-only radiotherapy to 76 Gy? Strahlenther Onkol 182(9):543–549
Sannazzari GL, Ragona R, Ruo Redda MG et al (2002) CT-MRI image fusion for delineation of volumes in three-dimensional conformal radiation therapy in the treatment of localized prostate cancer. Br J Radiol 75:603–607
Sathya JR, Davis IR, Julian JA, Guo Q (2005) Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol 23(6):1192–1199
Scarbrough TJ, Golden NM, Ting JY et al (2006) Comparison of ultrasound and implanted seed marker prostate localization methods: implications for image-guided radiotherapy. Int J Radiat Oncol Biol Phys 65:378–387
Sharma NK, Li T, Chen DY, Pollack A et al (2011) Intensity-modulated radiotherapy reduces gastrointestinal toxicity in patients treated with androgen deprivation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 80(2):437–444
Smith WL, Lewis C, Bauman G et al (2007) Prostate volume contouring: a 3D analysis of segmentation using 3DTRUS, CT and MR. Int J Radiat Oncol Biol Phys 67:1238–1247
Sorcini B, Tilikidis A (2006) Clinical application of image-guided radiotherapy (IGRT) (on the Varian OBI platform). Cancer Radiother 10:252–257
Steenbakkers RJHM, Deurloo KEI, Nowak PJCM et al (2003) Reduction of dose delivered to the rectum and bulb of the penis using MRI delineation for radiotherapy of the prostate. Int J Radiat Oncol Biol Phys 57:1269–1279
Tanaka O, Hayashi S, Sakurai K et al (2006) Importance of the CT/MRI fusion method as a learning tool for CT-based postimplant dosimetry in prostate brachytherapy. Radiother Oncol 81:303–308
Tang CI, Loblaw DA, Cheung P et al (2008) Phase I/II study of a five-fraction hypofractionated accelerated radiotherapy treatment for Low-risk localised prostate cancer: early results of pHART3. Clin Oncol 20:729–737
Usmani N, Sloboda R, Kamal W et al (2011) Can images obtained with high field strength magnetic resonance imaging reduce contouring variability of the prostate? Int J Radiat Oncol Biol Phys 80:728–734
Valicenti RK, Sweet JW, Hauck WW et al (1999) Variation of clinical target volume definition in three-dimensional conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 44:931–935
Veldeman L, Madani I, Hulstaert F et al (2008) Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol 9(4):367–375
Vesprini D, Sia M, Lockwood G, Moseley D et al (2011) Role of principal component analysis in predicting toxicity in prostate cancer patients treated with hypofractionated intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 81:e415–e421
Villeirs GM, Verstraete KL, De Neve WJ, De Meerleer GO (2005) Magnetic resonance imaging anatomy of the prostate and periprostatic area: a guide for radiotherapists. Radiother Oncol 76:99–106
Vora SA, Wong WW, Schild SE et al (2007) Analysis of biochemical control and prognostic factors in patients treated with either low-dose three-dimensional conformal radiation therapy or high-dose intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 68(4):1053–1058
Wang JZ, Guerrero M, Li XA (2003) How low is the α/β ratio for prostate cancer? Int J Radiat Oncol Biol Phys 55:194–203
Yassa M, Fortin B, Fortin MA et al (2008) Combined hypofractionated radiation and hormone therapy for the treatment of intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 71(1):58–63
Yeoh EE, Botten RJ, Butters J et al (2010) Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. Int J Radiat Oncol Biol Phys 81:1271–1278
Zelefsky MJ, Fuks Z, Happersett L et al (2000) Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiother Oncol 55(3):241–249
Zelefsky MJ, Fuks Z, Hunt M et al (2002) High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in n772 patients. Int J Radiat Oncol Biol Phys 53(5):1111–1116
Zelefsky MJ, Yamada Y, Fucks Z et al (2008a) Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases free survival outcomes. Int J Radiat Oncol Biol Phys 71:1028–1033
Zelefsky MJ, Levin EJ, Hunt M et al (2008b) Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 70(4):1124–1129
Zerini D, Jereczek-Fossa BA, Vavassori A et al (2010) 3D-conformal hypofractionated radiotherapy for prostate cancer with daily transabdominal ultrasonography prostate localization: toxicity and outcome of a pilot study. Tumori 96(6):941–946
Zietman AL, DeSilvio ML, Slater JD et al (2005) Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 294(10):1233–1239
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Chapet, O., Udrescu, C., Enachescu, C. (2012). Intensity Modulated Radiotherapy for Prostate Cancer. In: Bolla, M., van Poppel, H. (eds) Management of Prostate Cancer. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-27597-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-642-27597-5_12
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-27596-8
Online ISBN: 978-3-642-27597-5
eBook Packages: MedicineMedicine (R0)