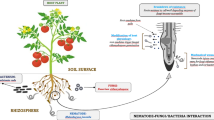
Abstract
The number of complex associations between plant-parasitic nematodes and pathogenic bacteria in causing plant diseases has been demonstrated in last seven decades. The role of nematodes in these interactions is complex, and each disease complex is distinct from another and largely dependent on the type of nematode parasitism involved. Nematodes greatly increase development of plant disease caused by bacteria. Nematodes participate in disease complexes in different ways such as (1) predisposing agent, (2) increasing susceptibility of host by modifying physiology of host tissues, (3) breaking host resistance to bacterial pathogens, (4) acting as vectors of bacterial pathogens, and (5) changing the rhizosphere microflora. Generally, in nematode-bacterium disease complexes, the nematode becomes established more readily in the presence of bacterium, but at the later stage of infection, nematode suffers some inhibition. Moreover, prior occupancy of host tissues by nematode or bacterium has different influence on the disease severity. Sometimes, the presence of both the nematode and the bacterial pathogen is necessary for production of certain types of symptoms; for example, interaction of Aphelenchoides fragariae, or A. fragariae, and Corynebacterium fascians is necessary to produce “cauliflower” disease of strawberry while neither pathogen inoculated separately reproduced the disease. The disease complexes involving plant-parasitic nematodes and pathogenic bacteria have major economic hazards. Therefore, improved understanding of relationship between nematodes and bacteria is needed to control plant disease complexes and the damage caused by them.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
14.1 Introduction
Plant-parasitic nematodes are cosmopolitan parasites, exploit all parts of the host plant, and affect virtually every crop. Plant-parasitic nematodes are devastating parasites of crop plants, reducing the overall yield or lowering the market value of crops (Sasser and Freckman 1987; Barker et al. 1994). It has been estimated that overall yield loss averages 12.3% annually; this figure approaches 20% for some crops (Sasser and Freckman 1987; Koenning et al. 1999). Plant-parasitic nematodes range from 250 μm to 12 mm in length, averaging 1 mm, to about 15–35 μm in width. There are two main types of plant-parasitic nematodes: ectoparasitic and endoparasitic. The ectoparasitic type lives outside the plant, feeding on roots with the ability to move about 3 ft to find a host, depending on the soil and species. Endoparasitic types penetrate the root, then enter and live inside it. Each type goes through development stages: starting from an egg, then four juvenile stages (molting after each one), and an adult stage. In addition to the more well-known root-knot nematode, there are many others, most of them named for physical characteristics. They include ring, dagger, sheath, stubby-root, spiral, pin, lesion, stem and bulb, and foliar nematodes. In fact, nematodes occupy all parts of vascular plants including leaves (Aphelenchoides spp.), stems (Bursaphelenchus xylophilus), tubers (Globodera rostochiensis), corms (Radopholus similis), and roots (Heterodera and Meloidogyne). To date, most attention has been focused on the root-parasitic species, and various classification schemes based on the site of feeding within the root have been developed (Dropkin 1969; Hussey and Grundler 1998; Wyss 1997). Nematodes deploy a broad spectrum of feeding strategies, ranging from simple grazing to establishment of complex cellular structures including galls in host tissues (Bird and Koltai 2000). Various models of feeding site formation have been proposed, and a role for phytohormones has long been speculated, although whether they perform a primary or secondary function is unclear (Bird and Koltai 2000). Sedentary endoparasitic nematodes are root parasites that interact with their hosts in a remarkable way. These obligate biotrophic pathogens establish an intimate relationship with their host plants, inducing the redifferentiation of root cells into specialized feeding cells. The successful establishment of feeding cells is essential for nematode development. Root-knot nematodes, of the genus Meloidogyne, have evolved strategies enabling them to induce feeding cell formation in thousands of plant species, probably by manipulating fundamental elements of plant cell development (Caillaud et al. 2008).
Many of the bacteria that are associated with plants are actually saprotrophic and do no harm to the plant itself. However, a small number, around 100 species, are able to cause diseases (Jackson 2009). Bacteria pathogenic for plants are responsible for devastating losses in agriculture and are a major problem worldwide for agriculture. There are 21 phyla within the domain Bacteria. Plant-pathogenic bacteria are found in three phyla: the Firmicutes, the Actinobacteria, and the Proteobacteria. The important genera include Clavibacter, Curtobacterium, Rathayibacter, Leifsonia, Nocardia, Rhodococcus, Streptomyces, Bacillus, Clostridium, Spiroplasma, Agrobacterium, Sphingomonas, Acidovorax, Burkholderia, Ralstonia, Xylophilus, Erwinia, Pseudomonas, Xanthomonas, and Xylella. List of plant-pathogenic bacteria is maintained by the International Society for Plant Pathology Committee on the Taxonomy of Plant Pathogenic Bacteria (Bull et al. 2008; ISPP-CTPPB; http://www.isppweb.org/about_tppb.asp).
14.2 Interactions of Plant-Parasitic Nematodes with Bacteria
Since the first report of an interaction of Meloidogyne sp. with Fusarium oxysporum on cotton (Atkinson 1892), numerous interactions of plant-parasitic nematodes with the plant-pathogenic fungi, viruses, bacteria, and nematodes have been described. Hunger (1901) first reported the possible association between plant-parasitic nematodes and plant-pathogenic bacteria. He noted that tomato plants cultivated in nematode-infested soil were severely attacked by Pseudomonas solanacearum, in comparison to those cultivated in nematode-free soil remained healthy. Carne (1926) established that Anguina tritici is a carrier of Corynebacterium tritici, the causal agent of yellow slime bacteriosis of wheat.
Plant-parasitic nematodes alone can sap the vitality of a plant, but they can also facilitate infection of additional pathogens. Plant-parasitic nematodes as primary pathogens favor establishment of secondary pathogens which alone cannot infect plant under normal condition. Primary pathogens induce changes in the host whereas secondary pathogens after infection by primary pathogen participate actively and alter the process of pathogenesis. Secondary pathogens generally colonize dead cells induced by primary pathogens (Mayol and Bergeson 1969). Nematodes are of tremendous important as a component of disease complexes because when plant is infected by one pathogen, its response to additional invaders is altered. These alterations exert significant influence upon disease development, etiology of pathogens involved, and ultimately on disease control. It is therefore important to consider the role of primary pathogen and its relationship with secondary pathogen and their ultimate effect on host plant. Reviews and book chapters on the interactions of plant-parasitic nematodes with bacteria (Pitcher 1963, 1965; Sitaramaiah and Pathak 1993), other plant pathogens (Riedel 1988; Taylor 1990), and root-nodule bacteria (Siddiqui and Mahmood 1995) have appeared in last few decades. Ways in which nematodes participate in disease complexes include serving as vectors or agents of pathogen transmission, providing portals of entry, inducing necrotic infection courts, modifying the physiology of host, breaking of host resistance to other pathogens, etc. Disease development in complex diseases may also be controlled by changes in rhizosphere microflora mediated by the nutritional quality and quantity of exudates from nematode-parasitized roots which enhance or suppress growth of other organisms. By limiting host root development, nematodes may induce drought stress in the host, a factor thought to influence development of some plant diseases. Interactions between plant-parasitic nematodes and bacteria on different plants have been summarized in Table 14.1. Plant disease complexes involving nematodes and bacteria have two types of relationships:
-
(a)
The expression of disease symptoms occurs only when both nematodes and bacteria are present together; neither pathogen inoculated separately reproduced the disease.
-
(b)
Each pathogen acts independently and not directly influenced by others; generally, nematodes enhance the incidence of disease.
14.3 The Role of Nematodes in Interactions with Bacterial Pathogens
Interactions of plant-parasitic nematodes with host plants exhibit most elaborate feeding sites and evolutionary most advance form of parasitism (Bird and Koltai 2000). All parasitic nematodes should be considered to be equally evolved, and differences between parasitic strategies reflect adaptations to exploit different ecological niches within the host. Root parasites (Meloidogyne and Heterodera spp.) hatch in soil as L2 larva which penetrates and migrates within a host root to establish permanent feeding sites that are characterized by extensive modifications to host cells. The nematode undergoes dramatic developmental and morphological changes and adopts a sedentary life style. Eggs are either released in masses on the surface of the root gall or encased in the body of the female forming cyst. Depending on the particular nematode and host as well as environmental conditions, there are between one to four generations per year (Bird and Koltai 2000). Nematodes participate in disease complexes in following ways.
14.3.1 Nematodes as Vector
Phytoparasitic nematodes transmit certain bacteria which can incite diseases. Nematodes mainly carry pathogens from soil to plant or from plant organs to meristematic tissues. Kalinenko (1936) has proved that various nematodes such as Pratylenchus pratensis, Helicotylenchus multicinctus, and Aphelenchus avenae are vectors of bacteria. Nematodes extracted from the roots of Scorzonera tau-saghyz were washed in distilled water and transferred to an agar culture; the resultant bacterial growth was identified. The same species of bacteria were found in the culture medium as were found in the roots of the plant, namely, Erwinia carotovora, Xanthomonas phaseoli, X. necrosis, Pseudomonas fluorescens, and Bacillus mesentericus. Fungus Dilophospora alopecuri is introduced into apical meristem of wheat by Anguina tritici. Attempts to produce the disease in the absence of the nematode have been unsuccessful (Atanasoff 1925; Leukel 1948). Lordello and Joly (1961) investigated simultaneous attack of artichoke by a nematode Protorhabditis oxyuris and four bacterial species and concluded that nematodes are not primary pathogens but are carriers of bacteria. Similarly, Ditylenchus dipsaci sometimes transmits the causal agent of bacterial wilt of alfalfa, Corynebacterium insidiosum, and feeding by nematode results in greater wilt severity than when the bacterium occurs alone (Hawn 1971). In general, nematodes parasitizing the roots, stems, leaves, and seeds of plants facilitate the penetration and transmission of bacteria. More often, the bacteria are first transmitted from the soil to plant tissues, where they spread throughout the infested plant; they are less often transmitted from plant to plant.
14.3.2 As Wounding Agent
Nematodes feeding cause physical damage to host plant and provide direct passage for pathogenic bacteria especially when the pathogen is not strong enough to break mechanical barriers of the host. Stewart and Schindler (1956) concluded that endoparasitic and ectoparasitic nematodes aggravated bacterial wilt by wounding the roots and allow bacteria to enter the plant. Wilt inducing bacteria depends mainly on wounds for penetration and establishment of an infection court (Goodman et al. 1967). The stylet opening of plant-parasitic tylenchid nematodes ranges from 0.2 to 1 μm in diameter and restricts the passage of pathogenic bacteria into intact plant cell. Number of bacterial pathogens normally inhabit soil and may become pathogenic on roots. At low nematode population levels, crown gall symptoms were no more severe than those occurring in plants inoculated with bacterium alone after wounding (Nigh 1966). Similarly, initial damage by Rotylenchulus reniformis facilitates entry and establishment of A. tumefaciens and disease development. Bookbinder et al. (1982) reported that M. hapla, Pratylenchus penetrans, and Helicotylenchus dihystera produced wounds in alfalfa roots which were invaded by Pseudomonas spp. Numbers of studies explain that mechanical root injury or root wounding of plant cell by nematodes is important factor for introduction of bacterial pathogen in host (Libman et al. 1964; Johnson 1966; Johnson and Powell 1969; Jatala and Martin 1977a, b; Sitaramaiah and Sinha 1984a, b). Pitcher (1965) noted that wounds created by nematodes apparently favor bacteria more than fungi because bacteria are less adapted for penetrating the host’s epidermis. Disease symptoms similar to those which occur in nematode-bacterium wilt interactions were simulated by substituting mechanical injury for nematode feeding (Libman et al. 1964; Lucas et al. 1955). Predisposing effect of nematodes has been attributed to the creation of wounds which leak nutrients and allow soil bacteria to multiply both in the lesions and in the rhizosphere (Kurppa and Vrain 1985). Lucas et al. (1955) demonstrated that wounding of roots by penetration of M. incognita larvae facilitates infection of tobacco roots by Pseudomonas solanacearum. There are strong indications that nematodes, especially root-knot nematodes, may induce physiological and/or biochemical changes in their hosts which enhance the development of pathogenic bacteria and/or predispose their host to bacterial pathogens. Griffin et al. (1968) demonstrated that M. hapla was necessary for establishment of A. tumefaciens in raspberry tissue. However, these authors referred to other works that wounding of roots by other agents permits the infection of the bacteria. Nematodes improve bacterial growth (Weischer 1968) but mostly measured by plant symptoms and not by qualitative analyses of bacteria.
14.3.3 Nematode Infection Causes Necrosis
Wounding of a host, by some species of nematodes, results in decay of root tissues, which may favor ingress of certain additional pathogens (Baldwin 1977). These pathogens are often unspecialized and may be facultative parasites, i.e., they generally survive on dead plant tissues, but are also capable of invading living tissue. Generally, lesion nematodes produce characteristic necrotic lesions (darkened areas of dead tissue) on the surface and throughout the cortex of infected roots. The lesions turn from reddish-brown to black and are initially spotty along the root surface. As the nematodes continue to migrate and feed within the roots, the lesions can coalesce to become large necrotic areas of tissue that may eventually girdle the root. Tissue distal to the lesion is frequently sloughed off. Severe damage from high populations of lesion nematodes can result in a stunted and necrotic plant root system. The extent of lesion formation can be accelerated during concomitant root invasion by other soil-borne plant pathogens, and sometimes, these interactions can develop into synergistic disease complexes. The wounds inflicted on plant roots and other belowground plant parts by lesion nematodes can serve as infection courts for pathogenic soil microbes (Davis and MacGuidwin 2000). This appears to be particularly true in disease complexes that involve lesion nematodes and wilt inducing bacteria.
14.3.4 Nematodes Act as Modifier of Substrate
All parasitic nematodes have extensive stylet that is connected to a well-developed pharynx containing three or five gland cells. Marked changes in the shape and volume of the pharyngeal glands were observed that appeared to correlate with key events in establishment of the parasitic interaction. In root-knot and cyst nematodes, the subventral glands seem to be more active before host penetration, with the reduction of secretary activity coordinated with onset of parasitism (Endo 1987; Endo and Wegin 1988) at which time activity of the dorsal gland increases (Bird 1983). Similarly, phytohormones play a role in feeding site formation and, indeed, may be the key factors in modulating the host-parasite interaction. Direct biochemical methods have shown that root-knot nematode-induced galls have elevated levels of auxin and its precursors (Balasubrama and Rangaswami 1962; Viglierchio and Yu 1968). In addition, cytokinin levels were found to be increased in nematode-infected roots (Bird and Loveys 1980). Root-knot nematodes have been shown to produce biologically active cytokinin (Bird and Loveys 1980).
Powell and Nusbaum (1960) first demonstrated the modification in the substrate due to nematode infestation provide an advantage to pathogen. Creation of an infection court is one way in which nematodes modify a host to enhance infection by additional pathogens. However, there is increasing evidence that nematodes modify host substrates in more subtle ways. Changes in biochemistry of the host are probably the most important factors favoring disease complexes involving nematodes (Slack 1963). Nematodes may induce production of host metabolites which are favorable to other pathogens, or they may destroy host metabolites that provide resistance to potential pathogens (Pitcher 1965). Johnson and Powell (1969) reported that root-knot nematodes act as modifiers of infested tissues so that infected tissue and surrounding cells become more suitable for bacterial colonization. The plants inoculated with the nematodes 3 to 4 weeks prior to bacterial inoculation develop bacterial wilt symptoms to a greater extent than plants inoculated with nematodes and bacteria simultaneously. Meloidogyne sp. induces gross physiological changes in a host. Thus, infection with root-knot nematodes prior to inoculation with bacterial pathogen is more likely to result in a synergistic disease complex, than when inoculations are simultaneous. The nematodes substantially alter host physiology so that a subsequently introduced pathogen is favored (Powell 1971; Yang et al. 1976).
14.3.5 Nematodes as Breakers of Disease Resistance
It is observed that a resistant cultivar to bacterial pathogen becomes susceptible in the presence of plant-parasitic nematodes as nematodes bring about physiological changes favoring the bacterial pathogen. Using tobacco variety Dixie Bright 101 which is resistant to bacterial wilt, Lucas et al. (1954) obtained similar results in experiments on infestation by gall nematodes M. incognita acrita and infection with bacteria P. solanacearum. Three variants of these causative agents were added to experimental pots of cultivated tobacco plants: a suspension of bacteria, soil infested with gall nematodes, and lastly both components. Within 21 days, 10%, 0%, and 100% of the tobacco plants were infested with bacterial wilt, respectively. Alfalfa cultivars with high resistance to wilt by Corynebacterium insidiosum may be diseased by this bacterium when Ditylenchus dipsaci is present (Hawn and Hanna 1967). Field resistance in potato to P. solanacearum was broken down when plants were infected with M. incognita acrita (Jatala and Martins 1977a, b). Similarly, Reddy et al. (1979) observed that when eggplant cultivar “Pusa purple cluster” highly resistant to P. solanacearum was inoculated together with M. incognita a greater number of plants wilted. Nematodes may alter hosts to such an extent that such plants may become susceptible to organisms to which they are otherwise resistant.
14.3.6 Nematode Infection Changes Rhizosphere Microflora
Nematodes seem to favor all stages of bacterial infection and development by modifying the composition of the root leachates. They can promote the growth of microorganisms in the rhizosphere. Moreover, these modifications of the rhizospheric environment may limit the development of organisms antagonistic to the pathogenic bacteria. Their feeding sites and the cells they modify, especially the giant cells induced by root-knot nematodes, may serve as a favorable substrate which helps the bacteria to establish within the plant and promote their development. Nematode-induced or nematode-produced factors appear to be translocated from the nematode feeding sites to other parts of their host, especially in the above ground parts. These factors seem to modify the resistance of the host to the bacteria and/or directly stimulate bacterial growth. The balance between the rhizosphere microflora and plant pathogens and soil microflora and plant pathogens is important in host-pathogenic relationship. The biochemical qualities of root exudates and the presence of antagonistic microorganisms play an important role in the proliferation and survival of root infecting pathogens in soil either through soil fungistasis, inhibition, or antibiosis of pathogens in the rhizosphere. Disease development in complex diseases may be controlled by changes in rhizosphere flora mediated by the nutritional quality and quantity of exudates from nematode-parasitized roots which enhance or suppress growth of organisms antagonistic to plant pathogens (Riedel 1988). Such exudates may also overcome fungistasis. By these mechanisms, nematodes also exert influence on their own reproduction and cohabitation in host plants.
14.4 Effect of Bacterial Pathogens on Plant-Parasitic Nematode
Relatively few interactions involving nematodes and bacteria have been investigated as because bacterial pathogens are less in number as compared to fungi and viruses. Agrobacterium, Clavibacter (Corynebacterium), Ralstonia, Pseudomonas, and Xanthomonas are the most common genera of bacteria commonly associated with nematodes in disease complexes. Effect of bacteria on disease complexes may be of following types.
14.4.1 Toxin Production by Bacteria
Limited information is available on the production of toxins by bacteria. The association between Anguina funesta (Anguina agrostis) and Clavibacter sp. (Corynebacterium rathayi) infesting Lolium rigidum produces toxin. Galls produced by A. funesta in annual ryegrass become toxic to nematodes when colonized by the bacterium (Stynes et al. 1979). Pitcher (1963) in his studies on the interaction of Aphelenchoides fragariae and Corynebacterium fascians found that bacteria at first increase but then decrease the rate of population growth of nematodes. The mechanism of this interaction is unknown, and the possible production by toxins by bacteria had adverse effect on nematodes. Infection of tobacco roots by P. solanacearum caused decrease of M. incognita in roots (Lucas et al. 1955; Johnson and Powell 1969). The contents of giant cells degenerated following bacterial invasion, leaving virtually empty cells resulting into the death of root-knot nematodes. The strong antagonistic effect of A. tumefaciens on the reproduction of P. penetrans was observed on raspberry (Vrain and Copeman 1987). Similar result has been observed in another interaction study (Pitcher and Crosse 1958). Bird et al. (1980) concluded that toxin production is associated with an interaction between nematode-infected plant cells and the bacterium.
14.4.2 Inhibits Nematode Development
Pitcher (1963) suggested that the bacteria modify host tissues which do not favor nematode multiplication. Lucas et al. (1955) reported that infection of tobacco roots by the P. solanacearum caused a decrease of M. incognita in roots. The adverse effects on nematode are expected as these pathogens share and compete for same host substrate. The unfavorable effect of bacteria pathogen on nematode may also be due to the destruction of feeding sites, impaired nutrition, and harmful byproducts produced by bacterial colonization. Similarly, Swain et al. (1987) reported inhibitory effect of R. solanacearum on M. incognita. Inoculation of M. incognita alone produces more galls and egg masses compared to its association with R. solanacearum (Hussain and Bora 2009). It may be due to the reason that establishment of the bacteria induces certain changes in root system which are not favorable for nematodes. Bhagawati et al. (1996) and Hazarika (2003) reported significant poor galls and egg masses in jute when M. incognita was associated with R. solanacearum.
14.4.3 Nematodes and Bacteria Together May Result in a Different Disease
Symptoms of disease of the host plant usually appear much faster and are more pronounced when two pathogens are present, than when just one infested the host. The host reaction may or may not be synergistic. This is largely influenced by environmental factors; the effect of these factors on nematode injury to plants was reviewed by Smart (1964). Sometimes, the presence of both the nematode and the other pathogen is necessary for production of certain types of symptoms, as it has been shown by Pitcher and Crosse (1958) and Blinov (1969) in their work on the association of Aphelenchoides fragariae and Corynebacterium fascians in “cauliflower” disease of strawberries. The expression of “cauliflower” symptoms depends also upon the cultivar studied. Interaction of P. penetrans and A. tumefaciens on raspberry might be causing the sudden decline, which is not a symptom characteristic of either pathogen alone (McElroy 1977). The yellow ear rot or tundu disease requires both the nematodes A. tritici and Clavibacter tritici for the expression of complex disease. Surface-sterilized nematode larvae alone caused only ear-cockle disease; the bacterium alone was not capable of causing disease (Gupta and Swarup 1972).
14.5 No Effect of Nematodes on Disease Complexes
Despite rapid advances on certain aspects of plant-pathogenic bacteria, many economically important pathosystems are largely unexplored, and biologically relevant life stages of even familiar systems remain poorly understood. We know remarkably little about interactions between microbes in a plant, and the effects of quantitative virulence factors. Not all species of nematodes assist in the development of bacterial wilt; in few cases, no effect of nematodes in diseases complexes was observed. Experiments conducted by Lucas and Krusberg (1956) stated the ectoparasitic root nematode Tylenchorhynchus claytoni exerted no influence on the appearance of bacterial wilt in tobacco variety Dixie Bright 101. Neither did the ectoparasitic nematode Xiphinema diversicaudatum on the severity of bacterial wilt in carnation caused by Pseudomonas caryophylli (Stewart and Schindler 1956). Generally, plant age, cultivar (resistant or susceptible), nematode inoculums levels, type of nematode parasitism (ecto or endo), environmental conditions, and their interaction with the type of microorganism have significant effect in determining the role of nematode in disease complexes.
14.6 Conclusion
In nature, plants are rarely exposed to the influence of only a single pathogen, particularly in soil environment. Roots are constantly exposed to a wide range of microorganisms which are likely to influence one another because they occupy the same habitat. It is reasonable to expect the infection by one pathogen may alter the host response to subsequent infection by another. It is apparent that plant-parasitic nematodes are involved in disease complexes and play a major role in synergistic interactions. Disease complexes are major economic hazards posed by nematodes, and interaction studies involving nematodes and bacteria should receive more attention of plant pathologists. The understanding of nematode-induced physiological and biochemical changes induce in their hosts that are responsible for the predisposition of the host plants to bacterial pathogens could be the necessary bases to develop control strategies against these parasites. More multidisciplinary research between biochemists, geneticists, and pathologists is necessary to understand the interrelationships between nematodes, bacteria, and plants. The improved understanding of relationship among host plant, nematodes, and bacteria will enhance our ability to control these plant diseases and the damage caused by them.
References
Atanasoff D (1925) Dilophospora disease of cereals. Phytopathology 15:11–40
Atkinson GF (1892) Some diseases of cotton. Bull Ala Agric Exp Stat 41:61–65
Balasubrama M, Rangaswami G (1962) Presence of indole compound in nematode galls. Nature 194:774–775
Baldwin JG (1977) The role of nematodes in disease complexes. Nematology circular No. 26. Fla. Department of Agriculture and consumer service. Division of Plant Industry, Gainesville Florida
Barker KR, Hussey RS, Krusberg LR, Bird GW, Dunn RA, Ferris H, Ferris PA, Schmitt DP (1994) Plant and soil nematodes: societal impact and focus for the future. J Nematol 26:127–137
Bhagawati B, Gogoi R, Phukan PN (1996) Interaction of Meloidogyne incognita and Pseudomonas solanacearum on jute. Ind J Nematol 26:259–261
Bird AF (1983) Changes in dimensions of the oesophageal glands in root-knot nematodes during the onset of parasitism. Int J Parasitol 13:343–348
Bird DM, Koltai H (2000) Plant parasitic nematodes: habitats, hormones and horizontally acquired genes. J Plant Growth Regul 19:183–194
Bird AF, Loveys BR (1980) The involvement of cytokinins in a host-parasite relationship between the tomato (Lycopersicon esculentum) in a nematode (Meloidogyne javanica). Parasitology 80:497–505
Bird AF, Stynes BA, Thomson WW (1980) A comparison of nematode and bacteria-colonized galls induced by Anguina agrostis in Lolium rigidum. Phytopathology 70:1104–1109
Blinov VA (1969) The disease of strawberry caused by bacteria and nematodes. Vestniks -kh nauki Moskva 7:127–129 (in Russian)
Bookbinder MG, Bloom JR, Lukezic FL (1982) Interactions among selected endoparasitic nematodes and three pseudomonads on alfalfa. J Nematol 14:105–109
Boubals D, Dalmasso A (1967) Resultats d'essais de desinfection de sol 'a vigne du sud de la France. Progr Agric Vitic 168(2–3):1–16
Bull CT, De Boer SH, Denny TP, Firrao G, Fischer-Le Saux M, Saddler GS, Scortichini M, Stead DE, Takikawa Y (2008) Demystifying the nomenclature of bacterial plant pathogens. J Plant Pathol 90:403–417
Caillaud M, Dubreuil G, Quentin M, Perfus-Barbeoch L, Lecomte P, Engler JA, Abad P, Rosso M, Favery B (2008) Root-knot nematodes manipulate plant cell functions during a compatible interaction. J Plant Physiol 165:104–113
Carne WM (1926) Earcockle (Tylenchus tritici) and bacterial disease (Pseudomonas tritici) of wheat. J Agric West Aust 3:508–512
Caubel G, Smason R (1984) Effect of stem nematode (Ditylenchus dipsaci) on the development of a bacterial disease of garlic (Allium sativum L.) caused by a biovar of Pseudomonas fluorescens. Agronomie 4:311–313
Charchar JM, Lopes CA, Oliveira VR, Moita AW (2007) Effects of fumigants nematicides and genotype resistance on the damages of Meloidogyne spp. and Ralstonia solanacearum in potato. Nematol Brasil 31:20–26
Chindo PS, Khan FA, Erinle ID (1991) Reaction of three tomato cultivars to two vascular diseases in presence of the root-knot nematode, Meloidogyne incognita race 1. Crop Protect 10:62–64
Crosse JE, Pitcher RS (1952) Studies in the relationship of eelworms and bacteria to certain plant disease I The etiology of strawberry cauliflower disease. Ann Appl Biol 39:475–484
Davis EL, MacGuidwin AE (2000) Lesion nematode disease. Plant Health instruct. doi:10.1094/PHI-I-2000-1030-02
De Moura RM, Echandi E, Powell NT (1975) Interaction of Corynebacterium michiganense and Meloidogyne incognita on tomato. Phytopathology 65:1332–1335
Deberdt P, Que´ne´herve P, Darrasse A, Prior P (1999) Increased susceptibility to bacterial wilt in tomatoes by nematode galling and the role of the Mi gene in resistance to nematodes and bacterial wilt. Plant Pathol 48:408–414
Dropkin VH (1969) Cellular responses of plants to nematode infections. Annu Rev Phytopathol 7:101–122
El-Goorani MA, Abo-El-Dahab MK, Mehiar FF (1974) Interaction between root knot nematode and Pseudomonas marginata on gladiolus corms. Phytopathology 64:271–271
El-Sherif AG, Elwakil MA (1991) Interaction between Meloidogyne incognita and Agrobacterium tumefaciens or Fusarium oxysporum f. sp. lycopersici on tomato. J Nematol 23:239–242
Endo BY (1987) Ultrastructure of the esophagus of larvae of the soybean cyst nematode, Heterodera glycines. J Nematol 19:469–483
Endo BY, Wegin WP (1988) Ultrastructure of the second stage juvenile of the root-knot nematode, Meloidogyne incognita. Proc Helminthol Soc Wash 55:286–316
Goodman RN, Kiraly Z, Zaitlin M (1967) The biochemistry and physiology of infectious plant disease. D. Van Nostrand Company, Princeton, NJ
Griffin GD, Anderson JL, Jorgenson CE (1968) Interaction of Meloidogyne hapla and Agrobacterium tumefasciens in relation to raspberry cultivars. P1. Dis Reptr 52:492–493
Gupta P, Swarup G (1972) Ear-Cockle and yellow ear-rot diseases of wheat: I Nematode bacterial association. Nematologica 18:320–324
Hawn EJ (1963) Transmission of bacterial wilt of alfalfa by Ditylenchus dipsaci. Nematologica 9:65–68
Hawn EJ (1965) Influence of stem nematode infestation on the development of bacterial wilt in irrigated alfalfa. Nematologica 11:39
Hawn EJ (1971) Mode of transmission of Clavibacter michiganense subsp. insidiosum by Ditylenchus dipsaci. J Nematol 3:420–421
Hawn EJ, Hanna MR (1967) Influence of stem nematode infestation on bacterial wilt reaction and forage yield of alfalfa varieties. Can J Plant Sci 47:203–208
Hazarika K (2003) Interrelationship of Meloidogyne incognita and Pseudomonas solanacearum on jute and management of the disease complex caused by them. Ph.D. (Nematology) Thesis (submitted to Assam Agricultural University Johrat-13)
Hunger FWT (1901) Een bacterie-ziekte der tomaat. G. Kolff & Company, Batavia, p 57, in Dutch
Hunt OJ, Griffin GD, Murray JJ, Pedersen MW, Peaden RN (1971) The effects of root knot nematode on bacterial wilt in alfalfa. Phytopathology 61:256–259
Hussain Z, Bora BC (2009) Interrelationship of Meloidogyne incognita and Ralstonia solanacearum complex in brinjal. Ind J Nematol 39:41–45
Hussey RS, Grundler FMW (1998) Nematode parasitism of plants. In: Perry RN, Wright DJ (eds) The physiology and biochemistry of free living and plant parasitic nematodes. CAB Publishing, Wallingford, CT, pp 213–243
Jackson RW (2009) Plant pathogenic bacteria: genomics and molecular biology. Caister Academic, Norfolk, UK. ISBN ISBN 978-1-904455-37-0
Jatala J, Martin C (1977a) Interactions of Meloidogyne incognita acrita and Pseudomonas solanacearum on field grown potatoes. Proc Am Phytopath Soc 4:177–178
Jatala P, Martin C (1977b) Interactions of Meloidogyne incognita acrita and Pseudomonas solanacearum on Solanum chacoense and Solanum sparsipilum. Proc Am Phytopath Soc 4:178
Johnson HA (1966) Studies on Granville wilt-root-knot interaction on flue-cured tobacco. MS thesis North Carolina State University, Raleigh
Johnson HA, Powell NT (1969) Influence of root knot nematodes on bacterial wilt development in flue-cured tobacco. Phytopathology 59:486–491
Kalinenko VO (1936) The inoculation of phytopathogenic microbes into rubber-bearing plants by nematodes. Phytopathol Z 9:407–416
Koenning SR, Overstreet C, Noling JW, Donald PA, Becker JO, Fortnum BA (1999) Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. J Nematol 31:587–618
Kurppa S, Vrain TC (1985) Penetration and feeding behavior of Pratylenchus penetrans in strawberry roots. Rev Nematol 8:273–276
Lele VC, Durgapal JC, Agrawal DK, Sethi CL (1978) Crown and root gall of grape (Vitis vinifera L.) in Andhra Pradesh. Curr Sci 47:280
Leukel RW (1948) Dilophospora and nematode disease in wheat in South Carolina. Plant Dis Rep 32:291–292
Libman G, Leach JG, Adams RE (1964) Role of certain plant-parasitic nematodes in infection of tomatoes by Pseudomonas solanacearum. Phytopathology 54:151–153
Lordello LGE, Joly S (1961) Nematodeos e bacterias em folhas de alcachofra Anais Escola Sup. Agric Luiz de Queiroz 18:243–250
Lucas GB, Krusberg LR (1956) The relationship of the stunt nematode to Granville wilt resistance in tobacco. Plant Dis Rep 40:150–152
Lucas GB, Sasser JN, Kelman A (1954) The effect of root-knot nematodes on the expression of Granville wilt resistance in tobacco. Phytopathology 44:497, abstr
Lucas GB, Sasser JN, Kelman A (1955) The relationship of root-knot nematodes to Granville wilt resistance in tobacco. Phytopathology 45:537–540
Mallesh SB, Lingraju S, Byadgi AS, Hegde YR, Mokashi AN, Krishnaraj PU (2009) Bioefficacy of rhizobacteria on root-knot/ wilt disease complex in coleus and ashwgandha. Karnataka J Agric Sci 22:1116–1120
Mayol PS, Bergeson GB (1969) The role of secondary invaders in premature breakdown of plant roots infected with Meloidogyne incognita. J Nematol 1:17
McClure MA, Spiegel Y (1991) Role of the nematode surface coat in the adhesion of Clavibacter sp. to Anguina funesta and Anguina tritici. Parasitology 103:421–427
McElroy FD (1977) Effect of two nematode species on establishment, growth and yield of raspberry. Plant Dis Rep 61:277–279
Mojtahedi H, Lownsbery BF, Moody EH (1975) Ring nematodes increase development of bacterial cankers in plums. Phytopathology 65:556–559
Munnecke DE, Chandler PA, Starr MP (1963) Hairy root (Agrobacterium rhizogenes) of field roses. Phytopathology 53:788–799
Napiere CM, Quinio AJ (1980) Influence of root knot nematode on bacterial wilt severity in tomato. Ann Trop Res 2:29–39
Nigh EL Jr (1966) Incidence of crown gall infection in peach as affected by Javenese root-knot nematode. Phytopathology 56:150, abstr
Orion D, Zutra D (1971) Effect of root-knot nematode on the penetration of crown gall bacteria into almond roots. Israel J Agric Res 21:27–29
Partridge JE (2008) Bacterial wilt of alfalfa. Department of plant pathology. University of Nebraska-Lincoln. Available at http://nudistance.unl.edu/homer/disease/agron/alfalfa/AlfBacWi.html
Pathak KN, Roy S, Ojha KL, Jha MM (1999) Influence of Meloidogyne incognita on fungal and bacteria wilt complex of banana. Ind J Nematol 29:39–43
Pitcher RS (1963) Role of plant parasitic nematodes in bacterial diseases. Phytopathology 53:35–39
Pitcher RS (1965) Interrelationships of nematodes and other pathogens of plants. Helm Abstr 34:1–17
Pitcher RS, Crosse JE (1958) Studies in the relationship of eelworm and bacteria to certain plant disease. I. Further analysis of the strawberry cauliflower disease complex. Nematologica 3:244–256
Powell NT (1971) Interactions between nematodes and fungi in disease complexes. Annu Rev Phytopathol 9:253–274
Powell NT, Nusbaum CJ (1960) The black shank-root knot complex in flue-cured tobacco. Phytopathology 50:899–906
Reddy PP, Singh DB, Ramkishun M (1979) Effect of root-knot nematodes on the susceptibility of Pusa Purple Cluster brinjal to bacterial wilt. Curr Sci 48:915–916
Riedel RM (1988) Interactions of plant-parasitic nematodes with soil-borne plant pathogens. Agric Ecos Environ 24:281–292
Rubio-Cabetas M, Minot J, Voisin R, Esmenjaud D (2001) Interaction of root-knot nematodes (RKN) and the bacterium Agrobacterium tumefaciens in roots of Prunus cerasifera: evidence of the protective effect of the Ma RKN resistance genes against expression of crown gall symptoms. Eur J Plant Path 107:433–441
Sasser JN, Freckman DW (1987) A world perspective on nematology: the role of the society. In: Dickson DW, Veech JA (eds) Vistas on nematology. Society of Nematologists, Hyatttsville, Md, pp 7–14
Siddiqui ZA, Husain SI (1991) Studies on the biological control of root-knot nematode. Curr Nematol 2:5–6
Siddiqui ZA, Mahmood I (1995) Role of plant symbionts in nematode management: a review. Bioresour Technol 54:217–226
Sitaramaiah K, Pathak KN (1993) Nematode bacterial disease interactions, pp 232–250. In: Khan MW (ed) Nematode interactions. Chapman & Hall, New York, p 377
Sitaramaiah K, Sinha SK (1984a) Histological aspects of Pseudomonas and root-knot nematode wilt complex in brinjal. Ind J Nematol 14:175–178
Sitaramaiah K, Sinha SK (1984b) Interaction between Meloidogyne javanica and Pseudomonas solanacearum on brinjal. Ind J Nematol 14:1–5
Slack DA (1963) Introduction. Symposium of interrelationships between nematodes and other agents causing plant diseases. Phytopathology 53:27–47
Smart GC (1964) Environmental factors affecting nematode injury. Proc Soil Crop Sci Soc Florida 24:294–302
Stansbury C, MCKirdy S, Mackie A, Power G (2001) Bacterial wilt Ralstonia solanacearum-race 3, exotic threat to western Australia. Fact Sheet ISSN 1443–7783 No.7
Stewart RN, Schindler AF (1956) The effect of some ectoparasitic and endoparasitic nematodes on the expression of bacterial wilt in carnations. Phytopathology 46:219–222
Stynes BA, Petterson DS, Lloyd J, Payne AL, Lanigan GW (1979) The production of toxin in annual ryegrass, Lolium rigidum, infected with a nematode, Anguina sp., and Corynebacterium rathayi. Aust J Agric Res 30:201–209
Sule S, Lehoczky J (1993) Agrobacterium-nematode interactions on grapevine root. Novenyvedelem 29:412–417
Swain PK, Rath JC, Mishra SK (1987) Interaction between Meloidogyne incognita and Pseudomonas solanacearum on brinjal. Ind J Nematol 17:61–71
Taylor CE (1990) Nematode interactions with other pathogens. Ann Appl Biol 116:405–416
Viglierchio DR, Yu PK (1968) Plant growth substances and plant parasitic nematodes. II. Host influence on auxin content. Exp Parasitol 23:88–95
Vrain TC, Copeman RJ (1987) Interactions between Agrobacterium tumefaciens and Pratylenchus penetrans in the roots of two raspberry cultivars. Can J Plant Pathol 9:236–240
Weischer B (1968) Wechselwirkungen zwischen Nematoden und andere Schaderregern au Nutzpflanzen. C.r. 8eme Symp. Intern. Nematologie, Antibes 8–14 Sept. 1965, 91–107
Wyss U (1997) Root parasitic nematodes: An overview. In: Fenoll C, Grundler FMW Ohl SA (eds) Cellular and molecular aspects of plant-nematode interactions. Kluwer, Dordrecht, pp 5–22
Yang H, Powell NT, Baker KR (1976) The influence of Trichoderma harzianum on the root-knot Fusarium wilt complex in cotton. J Nematol 8:81–86
Zutra D, Orion D (1982) Crown gall bacteria (Agrobacterium radiobacter var. tumefaciens) on cotton roots in Israel. Plant Dis 66:1200–1201
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Siddiqui, Z.A., Nesha, R., Singh, N., Alam, S. (2012). Interactions of Plant-Parasitic Nematodes and Plant-Pathogenic Bacteria. In: Maheshwari, D. (eds) Bacteria in Agrobiology: Plant Probiotics. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-27515-9_14
Download citation
DOI: https://doi.org/10.1007/978-3-642-27515-9_14
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-27514-2
Online ISBN: 978-3-642-27515-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)