Abstract
Four novel sorbents were prepared by introducing functional groups as –NH2 -SH and –COOH into MCM-41 in various positions. The sorbents were characterized for BET surface area, pore size distribution, pore volume, surface functionality and surface group density. Multicomponent studies were also conducted. After sorption the metal-coated sorbents were tested to investigate the bonding mechanism of the metal ion using FTIR and XRD. The capacity was tested by batch process for Cadmium, Chromium, Lead and Zinc to study the effects of the different functional groups and their stereo specificity. The data were analyzed using various isotherms, kinetic and thermodynamic equations for the design of adsorption systems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Porous inorganic materials have been studied extensively for many years due to their utility as high surface area catalyst and sorption media. A significant breakthrough in the chemistry of material was accomplished with the synthesis of MCM-41. This new family of mesoporous material possesses uniform and large porous (2–10nm), a large area over 1000m2/g and a large adsorption capacity1. As a silica-based mesoporous material, M41S would be attractive as adsorbents, supports for catalysts, and hosts in inclusion chemistry, however it has several limitations due to the lattice of silica. From this view point, a number of groups have successfully incorporated various metal ions such as Al, Ti, V, Ga and B into the silica frame work of MCM-41. Metal oxides with porous structure have been synthesized by using surface directing agent similar to the templates used in the synthesis of M41S2. Some efforts have been made to obtain porous heteropoly acids, but none was accomplished due to the low thermal stability of the heteropoly anion3, 4. Layered mesostructured materials of (C19H42N)6(H2W12O40)[C12H25N(CH3)3]6(H2W12O40) are of great interested because their walls are made of cluster anion in contrast to the amorphous walls of the other materials. The oxides of W, V, Nb and Mo precursors react with cationic surfactants, often forms similar cluster ion salts with lamellar structures. The aim of this work was to synthesis surfactant salts of phosphomolybdic acids (PMA) and to treat them with tetraethyl orthosilicate (TEOS) in a “sol-gel” reaction in order to obtain a three dimensional tunnel structure.
Material and Methods
The solution of surfactant was prepared by dissolving CH3(CH2)15N(CH3)3Br (CTAB=cetyltrimethyl ammonium bromide) in the distilled water. An appropriate amount of H7 [P( Mo2O7)6].xH2O (PMA) was added with vigorous stirring. After being stirred at room temperature for 1 h, the resulting mixture was allowed to react at 100°C for 24 h. The resultant yellow solid product was filtered, washed with distilled water, and dried in air. Silicate was introduced into the salt by reacting 1g of the cluster/surfactant salt with excess TEOS at 150°C for 24 h in a Teflon-lined autoclave. The product was collected by filtration, washed with 95% ethanol, and dried in air. CTAB was removed by stirring in 150ml of 1M HCL/EtOH at room temperature for 4 h. The product was collected by filtration, washed with ethanol, dried in air and modified by using chemical moiety to introduce special selective surface functional groups,5–7 in particular nonfunctionalized, -NH2, -SH and -COOH to get four novel adsorbents A, B, C and D respectively.
The sorbents were characterized8 for BET surface area, pore size distribution, pore volume, surface functionality and surface group density. After sorption the metal-coated sorbents were tested to investigate the bonding mechanism of the metal ion using FTIR and X-ray diffraction (XRD). The capacity was tested by batch process for Cadmium, Chromium, Lead and Zinc to study the effects of the different functional groups and their stereospecificity.9 Single and multicomponent studies were also conducted. The data were analyzed using various isotherms, kinetic and thermodynamic equations for the design of adsorption systems.10
Results and Discussion
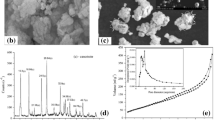
The IR spectrum of (C19H42N)3(PMo12O40) as compared with those of PMA and the TEOS treated sample as shown in Fig. 1. The bands in the IR spectrum (C19H42N)3(PMo12O40) correspond well to those of PMA, showing the typical features of Keggin anions. The four adsorption bands at 1062, 957, 881, 800cm-1 are assigned to the v(P-O), v(Mo-Ot) (Ot refers to the terminal oxygens), v(Mo-Oc-Mo) (Oc refers to the corner oxygens) and v(Mo-Oe-Mo) (Oe refers to be edge oxygens) respectively. The red shift of the bands of (C19H42N)3(PMo12O40) compared with those of PMA suggests some interaction between the cluster anions and the cationic headgroups of the surfactant. The IR spectrum of the TEOS-treated sample exhibits five absorption bands in the region between 1100 and 500cm-1.
It is different from the IR spectra of the PMA and (C19H42N)3(PMo12O40).The peaks of heteropoly acid near 800 and 880cm-1 are overlapped by the strong absorption peaks of silicate xerogel, which are near 800cm-1. Only the peak near 960cm-1 can show the existence of the heteropoly acid. After the extraction of the template with HCL/EtOH, the IR spectrum corresponds well to the product before the extraction, showing the structure remains intact.
The XRD pattern of the (C19H42N)3(PMo12O40) is shown in Fig. 2, and the (hk1) indexes are also indicated with the corresponding diffraction peaks. There are several sharp peaks at low angles (less than 10°), while the XRD pattern only shows broad lumps at angles greater than 10°. These features are again reminiscent of MCM-41 which shows sharp low-angle lines and diffuse high-angle lines, indicative of long-range ordering of channels but a locally glassy aluminosilicate framework. However, the peaks are different from those for MCM-41 but similar to those for the layer compounds at low angles which indicates that the salt has the layer structure. The interlamellar distance can be calculated from the 001 index as d 001=3.00nm. On the basis of CPK modeling, a fully extended CTA ion has a length of 30A°; completely coiled up it extends~15 A°. As implies that the hydrocarbon tails penetrate each other, as might be expected.
The XRD patterns of the TEOS-treated sample and the solvent-extracted sample indicate that the peak near 2.9° change to 3.4° for the TEOS-treated sample, which means the interlamellar distance decreases when treated with TEOS. The strength of the peaks at low angles becomes less sharp than the salt. It is in contrast to the increase in the surface area of the material. We can deduce that the addition of Si leads to the bending of the layers, and at last results in the transformation of the lamellar salt into a microporous structured compound through the interaction between the Surfactant/heteropoly-acid and the silicate.
With the presence of the surfactant, the TEOS- treated sample is nonporous (0.05m2/g). After partial removal of the template, the surface area increases to 140m2/g. The bands around 3000 and 1500cm-1 assigned to the stretching and bending vibrations of C-H of the surfactants are observed in the IR spectrum, indicating that some surfactant is still present and the extraction is uncompleted. Even in the elementary analysis the component of the element N decreases from 1.02% to 0.77%. If HCL/EtOH extraction is complete, the BET surface area may be more than 140m2/g.
The effects of various parameters affecting the adsorption such as initial toxic concentration, sorbent dose, contact time, pH and temperature were determined in the batch process. A, B, C and D could remove toxic ions in the order Pb(II)> Zn(II)> Cd(II)> Cr(VI). The order of toxic removal capacities for these adsorbents was found to be: D>C>B>A as per removal % and isotherm data. Adsorption decreased with rise in toxic concentration but increased with increase in sorbent dose and contact time. The optimum pH and contact time were different for different toxics. Cr (VI) as anion showed much lower removal than other metals because most of these sorbents including D has more negative or cation exchanging sites than positive or anion exchanging ones.
Conclusions
The porous structures with high surface area are obtained after the surfactant template is partially removed from TEOS-treated phosphomolybdate salts by solvent extraction. IR and XRD data indicate that silicate is indeed incorporated in the salt structures and the heteropoly structure remained intact. The hydrolysis product of TEOS is the SiO2 xerogel. These highly porous MCM-41 based sorbents can be used as sorbents for heavy metals. They are also expected to act as catalysts, ion exchangers, demulsifiers etc. Their new applications need further research.
References
X.Bao, X.S. Zhao, X. Li and J. Li; Applied Surface Sc.; 237 (2004) 380–386.
X. Bao, X.S. Zhao, X. Li, P.A. Chia and J. Li; J Phys Chem B.; 108 (2004) 4684–4689.
A. Lapkin, B. Bozkaya, T. Mays, L. Borello, K. Edler and B. Crittenden; Catalysis Today. 81(2003) 611–621.
X.Y. Bao and X.S. Zhao; J Phys Chem B. 109 (2005) 10727–10736.
A.S.M. Chong, X.S. Zhao, A.T. Kustedjo and S.Z. Qiao; Microporous and Mesoporous Materials. 72(2004) 33–42.
R. Compostrini, M. Isehia, G. Carturan and L. Armelao; J Sol-Gel Sc Tech. 23(2002) 107–117.
C. Yang; Chinese Chem Lett. 14 (2003)96–99.
P. Delmelle, F. Villieras and M. Pelletier; Bull Volcanol. 67(2005)160 -169.
A. Bibby and L. Mercier. Chem. Mater. 14(2002) 1591–1597.
L. Bois, A. Bonhomme, A. Ribes, B. Pais G. Raffin and F. Tessier; Colloids and Surface A: Physicochemical and Engineering Aspects. 221(2003) 221–230.
Author information
Authors and Affiliations
Editor information
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Vashishtha, S., Singh, R., Kulshreshtha, H. (2012). Functionalized MCM-41 Type Sorbents for Heavy Metals in Water: Preparation and Characterization. In: Khemani, L., Srivastava, M., Srivastava, S. (eds) Chemistry of Phytopotentials: Health, Energy and Environmental Perspectives. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-23394-4_72
Download citation
DOI: https://doi.org/10.1007/978-3-642-23394-4_72
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-23393-7
Online ISBN: 978-3-642-23394-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)