Abstract
The synthesis of some new thiosemicarbazides derived from N-(substituted) phenyl malonamic acid hydrazide with 4-nitro phenyl isothiocyanate. All the new derivatives have been characterized by elemental analysis, IR, & NMR. The IR and NMR spectral data suggest the involvement of C=S, N-H, CH2, N-N, CONH, N-C=O. Compounds have been synthesized in an open vessel under microwave irradiation (MWI) using a domestic microwave oven. The reaction time decreases from hours to minutes with improved yield as compared to conventional heating. The thiosemicarbazides have been tested in vitro against a number of microorganisms in order to assess their antimicrobial properties. The results indicate that the thiosemicarbazides possess antimicrobial properties.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aspergillus Niger
- Microwave Irradiation
- Domestic Microwave Oven
- Microwave Irradiation Method
- Medium Intensity Band
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
In the field of medicine the importance of thiosemicarbazides is well known. Thiosemicarbazides (—N=C=S group) have been known to show pronounced biological activities[1]. Thiosemicarbazides have shown activity against protozoa[2], small pox[3] and certain kinds of tumor[4]. The anticonvulsant activity of thiosemicarbazides has been reported in the isolated cerebral cortex preparation[5]. The influence of the thiosemicarbazides has also been on the electrical activity in the interior brain stem of the cat[6]. The anti-viral activity was tested of some thiosemicarbazides against the influenza virus (strain PR-8, type)[7,8]. Thiosemicarbazides have also been reported to posses hypoglycemic activity and usefulness in agriculture. Such types of compounds have been found to be useful as a large number of anticonvulsant, insecticides, rodenticides, anti-tubercular activity against M. Tuberculosis (H37Rv), anti-viral, hypoglycemic, hypotensive as well as metabolic convulsants. The increasing application of microwave irradiation (MWI) in the synthesis of organic compounds has been receiving attention during recent years. Microwave heating has proved to be very useful tool to carry out certain organic transformations which not only excludes the use of hazardous non-eco friendly solvents but also enhances the reaction rates greatly. A much faster reaction under microwave makes it less expensive in terms of energy, yield and time compared to its thermal analogue. Also, reactions under this condition are very clean and no byproduct form even at high power irradiation. These features make microwave approach very compatible with the upcoming concept of “Green Chemistry”.
Materials and methodology
N-(substituted) phenyl malonamic acid hydrazide was prepared from N-(substituted) phenyl malonamate ester of various substituted aromatic amines. 4-nitro phenyl isothiocyanate used were of Sigma-Aldrich. Ethanol and other solvents of A.R. grade were used as received.
Synthesis of thiosemicarbazides
Classical heating based synthesis (Method A)
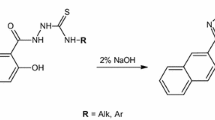
A mixture of N-(substituted) phenyl malonamic acid hydrazide (0.01mol) and 4-nitro phenyl isothiocyanate (0.01mol), dissolved in 10ml ethanol was refluxed for two hours. The solid obtained on cooling was recrystallized with hot absolute ethanol and was found to be N-(malon substituted anilic)-4-(4’-nitro phenyl) thiosemicarbazides.
Microwave “Jump Start” synthesis (Method B)
A mixture of N-(substituted) phenyl malonamic acid hydrazide (0.01mol) and 4-nitro phenyl isothiocyanate (0.01mol), dissolved in 4ml ethanol and were exposed to microwave irradiation for 4–6 minutes. The solid obtained on cooling was recrystallized with hot absolute ethanol and was found to be N-(malon substituted anilic)-4-(4’-nitro phenyl) thiosemicarbazides.
Physical measurements and analytical data
Melting points were determined in open capillary tubes and are uncorrected (Table 1). The purity of the compound was checked by on TLC. The structures of the compounds are confirmed on the basis of their IR and 1H NMR. All the compounds gave satisfactory microanalysis. Microwave irradiations were carried out in an unmodified IFB domestic microwave oven. All the chemicals were of analytical grade.
Antibacterial activity
Antibacterial activity was evaluated by the paper disc method. The Müller-Hinton agar (beef infusion, casein hydrolyzate, starch, agar) and 5mm diameter paper discs of whatman No. 1 were used. The compound was dissolved in DMSO. The filter paper discs were soaked in different solutions of the compounds, dried and then placed in the petriplates previously seeded with the test organisms E. coli and S. aureus. The plates were incubated for 24–30 hours at 28±2°C and the inhibition zone around each disc was measured[9].
Antifungal screening
The antifungal activity of the compounds was evaluated against Aspergillus niger by the agar plate technique. The Sabouraud dextrose agar (dextrose, peptone, agar) and 5mm diameter paper discs of whatman No. 1 were used. The compounds were dissolved in DMSO and then were mixed with in the medium. These petriplates were wrapped in the polythene bags containing a few drops of alcohol and were placed in an incubator at 25±2°C. The activity was determined after 96 hours of incubation at room temperature (25°C)[10].
Results and Discussion
Infrared spectra
Infrared spectra of the substituted thiosemicarbazides show medium intensity bands at 3455–3168cm-1 due to υ NH vibrations. A sharp bands found at 1245–1025cm-1 due to υ C=S. υ N-N stretching bands in the thiosemicarbazides appeared at 980–1219cm-1. In the IR spectra of the substituted thiosemicarbazides the band appeared at 2997–1330cm-1 due to the υ CH2. υ CONH band appeared at 1620–1488cm-1 in the compounds. A sharp and medium bands of υ N-C=O showed at 1529–1718cm-1.
1H NMR
The bonding patterns of these compounds are further supported by the proton magnetic resonance spectral studies in DMSO-d6. The compounds exhibit a singlet at δ 4.9–3.22 ppm due to NH. This compound shows multiplet in the region at δ 7.98–6.49 ppm attributable to the aromatic protons. Another singlet appearing at δ 4.34–3.33 due to the CH2. A singlet due to the –CONH group appears around δ 11.20–8.61 ppm.
Antimicrobial activity
The data in Table 2, showing zone of inhibition against the bacterium S. aureus, E. coli and fungus Aspergillus niger due to the different substituted thiosemicarbazides. G & H compound of thiosemicarbazides were found to be weak in activity against E. coli and compound D & F against S. aureus. Highest antimicrobial potential was observed with compound B & D against E. coli and compound C & G against S. aureus.
Compound A showed highest antifungal potential against Aspergillus niger.
Conclusion
In conclusion, from our point of view, microwave irradiation method has been proved here as a better method for the synthesis of thiosemicarbazides and increase in percentage (%) yield is in following order: Method-1(Classical heating synthesis) < Method-2 (Microwave “jump start” synthesis). N-(substituted) phenyl malonamic acid hydrazide with 4-nitro phenyl isothiocyanate were proved to have some antibacterial activity against Gram-negative E. coli & Gram-positive Staphylococcus aureus bacteria and these compound also showed highly antifungal activity against Aspergillus niger.
Acknowledgements
We are thankful to Central Drug Research Institute (CDRI), Lucknow for spectral and elemental analysis. We are also very grateful to Department Of Microbiology, R.B.S. College, Agra for antimicrobial screening.
References
G. Mazzone, F. Bonia, A.R. Reeina, G. Blandino, Farmaco, Ed. Sci., (1981) 36,181.
K. Butler, U.S. Pat., (1968) 3, 382, 266.
J.D. Bauer, L. St. Vincent, H.C. Kampe, W.A. Dowine, Lancet (1963) 494.
G.H. Peterling, H.H. Buskirk, E.G. Underwood, Cancer Res., (1964) 64, 367.
B.J. Preston, (Univ. Illinois, Coll. Med. Chicago) J. Pharmacol. Exptl. Therap. (1955) 115, 28–39.
Idem., Ibid, (1955) 115, 39–45.
P.N. Buu-Hoi, A. Bouffanais, P. Gley, D.N. Xuong, H.N. Nam, Experimentia (1956) 12, 73.
N.N. Orlova, A.V. Aksenova, A.D. Selidoukin, S.N. Bogdanova, N.G. Pershin, Farmakologiya. i. Toksikologiya (1968) 31, 725.
C. Saxena, D.K. Sharma, and R.V. Singh; Phosphorus, Sulfur and Silicon 85 (1993).
M. Jain, S. Nehra, P.C. Trivedi, and R.V. Singh; Heterocyclic Communications 9 (2003) 1.
Author information
Authors and Affiliations
Editor information
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Shukla, M., Dubey, M., Kulshrashtha, H., Seth, D. (2012). Synthesis of Bioactive Thiosemicarbazides: Antimicrobial Agents Against Drug Resistant Microbial Pathogens. In: Khemani, L., Srivastava, M., Srivastava, S. (eds) Chemistry of Phytopotentials: Health, Energy and Environmental Perspectives. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-23394-4_2
Download citation
DOI: https://doi.org/10.1007/978-3-642-23394-4_2
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-23393-7
Online ISBN: 978-3-642-23394-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)