Abstract
Morinda pubescens var.pubescens (Rubiaceae) is a tropical plant whose leaves, bark, roots and fruits have been used as traditional remedy for various diseases. In our study we identified four Known compounds as two Pentacyclictriterpenoids Ursolic acid and Taraxerol‚ two sterols β-Sitosterol and Stigmasterol from Morinda pubescens var. pubescens (Rubiaceae). Solvent extraction‚column chromatography were major techniques used for isolation of compounds, while structures were elucidated by integration of data from IR,UV, 1H NMR AND 13C NMR analysis. We are reporting presence of Taraxerol a pentacyclictriterpenoid first time from the leaves of Morinda pubescens var. pubescens of Rubiaceae family.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The history of medicinal plants goes back to the history of human civilization. The ancient medical men through their trial and error mechanism identified herbs which are useful in healing various diseases and the same knowledge was passed on to the generations [1]. Even in recent times the plant products play very vital role in all the forms of medicine like Ayurveda, Sidda and Unnani. As the time proceeded the use of herbs transformed from raw form to purified form and extraction of chemical compounds leads to the synthesis of modern drugs [2]. The present study focuses on the Chemical Examination of Morinda pubescens var. pubsescens of Rubiaceae family and isolation of crystalline constituents.
Identification of the plant material
The plant was collected from Acharya Nagarjuna University Campus, Guntur. Andhra Pradesh, India. Based on Morphological and the Anatomical data [3] the plant was identified by B.S.I. Coimbatore as Morinda pubescens (J.E. Smith) var. pubescens of Rubiaceae family.
Chemical composition of Morinda species
The various chemical compounds were extracted from Morinda species like Anthraquinones [4, 10], Flavonoids [5], Iridoids [6, 9], Steroids [7] and Terpenoids [8].The molecular formula, Structure was elucidated based on melting point; I.R,U.V and 1HNMR data in the present work.
Extraction procedure adopted
The leaves were dried at room temperature. Dried and powdered leaves are weighed. The weight of the material is 690 Grams. The powdered leaves are taken in a 5-liter Soxhlet apparatus and 4 liter of acetone is added. The mixture is kept for 24 hours. The first 3 liters cold fraction is collected into 5 liters round bottom flask. The acetone extract-I was kept for distillation. The remaining solvent about 2.5 liters collected after distillation of cold extract-I is again added to the Soxhlet jar. The cold acetone fraction–I after dryness under reduced pressure yielded a residue of 7.770 grams. The acetone cold extract-II was collected next day about 3 liters in round bottomed flask and kept for distillation under reduced pressure yield around 3 grams of residue.
The remaining material after cold extraction, taken into the Soxhlet apparatus under controlled temperature the hot acetone extract –I was collected after successive 12 siphons. The methanol hot extract also collected after 6 siphons. The hot acetone extract yield 1.200 gms and the hot methanol extract give 3gms residue.
The cold acetone extract I, II and hot acetone extract-I mixed up; the substance weighted around 11.97 grams. The column is setup with 200 grams column silica gel (acme) 100 – 200 mesh in 1250ml of n-Hexane and Benzene in 9:1 ratio. The residue (around 12 grams) is taken and dissolved in acetone and impregnated in silica gel and the acetone is evaporated on water bath. The powdered substance with silica gel is further fine powdered (without granules). The fine powder is mixed with 9:1 Hexane: Benzene solvent. The column thus set is run with n-hexane, Benzene, ethyl acetate, ethyl alcohol (9:1 to 1:1) and finally with ethyl acetate.
Materials and Methods
General procedure: – TLC was carried out on percolated silica gel 60 F254 plates (Acme). Spots were detected under UV (254 and 366nm) before and after spraying with 1% methanol sulfuric acid spray reagent, followed by heating the plate at 1100C for 5 min. Preparative TLC was performed on percolated silica gel 60 F254 plates, layer thickness 0.5mm (Merck) unless indicated otherwise. Column chromatography was carried out on silica gel 60 (acme 100–200mesh) and sephadex LH20.
Chemical Examination of Morinda pubescens var. pubescens leaves:
The air-dried leaves powder was soxheleted with, n-hexane, Acetone and methanol. The hot and cold extracts of Acetone and n-hexane were found to be similar on TLC these extracts were combined and chromatographed extensively to yield eight compounds which are designated as AMP-1 to AMP-8 (Table 1) only four of them were isolated in a pure form for this present study. They are AMP-1 (Fig 1), AMP-4 (Fig 2), AMP-5 (Fig 3) and AMP-8 (Fig 4). The methanolic extract was in the form of greenish gummy substance. Hence the work was not pursued further.
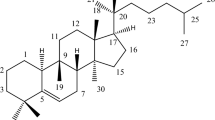
Examination of AMP – 1 :- (Urosolic acid XCI)
AMP-1 was colorless solid and recrystallized from ethylacetate gave a colorless compound. Indicated it is as triterpene. It give effervescences with NaHCO3 and Na2CO3, [α]24D+ 72.20C (methanol) soluble in hot CHCl3, MeOH, ethyl acetate. Melting point of the compound is 285.50C. L.B. Test gave +ve result with pink to violet coloration. Molecular formula C30 H48 O3.Element analysis Found C, 83.72%, H, 11.78% Requires: C, 83.82%; H, 11.82%. I.R.: 3421, 1620 Cm-1 . 1H (400, MHz, CDCl3): δ0.8 (3H, S 24-Me), 0.84 (3H, S, 28-Me), 0.90 (6H, S,25, 29-Me), 0.95 (3H, S, 30-Me), 0.98 (3H, s, 23-Me), 1.20 (6H, S, 26, 27-M2), 3.15 (1H, M,H-3), 5.2 (1H dd, 15-H).
Examination of AMP – 4 :- (β-Sitosterol LXXXVI)
AMP-4 isolated as white colored compound and recrystallized from methanol as colorless needles (150mg) melting point is 135–1360C, [α]27D–350, (C, 1.0, CHCl3) positive L.B. test for sterols (violet → blue → green)
Molecular formula C29 H50 O. Element analysis Found : C, 83.86%, H, 11.90%Requires: C, 83.98%; H, 12.16% Rf : 0.67 (n-hexane, ethyl acetate-4:1).U.V.: No prominent absorption was noticed. I.R.:νNujol: 3436, 1690, 1607, 1460, 1366, 985 Cm-1.1H NMR (400, MHz, CDCl3) : δ0.6 (3H, S 18-Me), 0.81 (6H, d, J = 7Hz, 26, 27-Me), 0.84 (3H, t, 29-Me), 0.89 (3H, d, J=6.3Hz, 21-Me), 0.99 (3H, s, 19-Me), 3.2 (1H, m, 3α-H], 5.3 (1H, m, 6-H),13C NMR (125.70, MHz, CDCl3) : δ36.1(C-1), 39.7 (C-2), 71.8 (C-3), 56.8 (C-4), 140.7 (C-5), 121.1 (C-6), 29.1 (C-7), 28.9 (C-8), 50.0 (C-9), 37.2 (C-10), 31.8 (C-11), 36.4 (C-12), 42.2 (C-13), 42.1 (C-14), 11.7 (C-15), 28.2 (C-16), 56.8 (C-17), 11.8 (C-18), 18.9 (C-19), 29.6 (C-20), 0.98 (C-21), 21.1 (C-22), 28.2 (C-23), 51.2 (C-24), 24.2 (C-25), 21.0 (C-26), 31.6 (C-27_, 19.4 (C-28), 19.3 (C-29).
Examination of AMP – 5 :- (Stigmasterol XCVII)
AMP-5 was crystallized from chloroform-methanol as needles (120mg). Melting point was 168 – 1700C, [α]D-450 (C, 1.2 CHCl3). It showed pink color, which finally turned to green. It gave positive L.B. test for steroids. Molecular formula:C29 H48O.Elementanalysis:Found:C,83.78%,H,11.60%Requires:C,84,40%;H, 11.72% Rf: 0.65 (n-hexane, ethyl acetate-4:1)U.V.: No prominent absorption was noticed. I.R.: νNujol 3442, 2928, 1693, 1457, 1387, 1031, 996 Cm-1.1H NMR (400, MHz, CDCl3) : δ0.70, 0.80, 0.90, 1.00, 1.05, 1.30, (18H, 6xMe), 3.25 (1H, br, 3α-H), 5.1 (2H, m, 22, 23-H), 5.3 (1H, m,6-H).13C NMR (125.70 MHZ, CDCl3):δ37.4 (C-1), 31.7 (C-2), 71.8 (C-3) 42.4 (C-4), 140.9 (C-5), 121.7 (C-6), 31.9 (C-7), 31.9 (C-8), 50.3 (C-9), 38.6 (C-10), 21.1 (C-11), 39.8 (C-12), 42.4 (C-13), 57.0 (C-14), 24.4 (C-15), 28.9 (C-16), 56.0 (C-17), 12.2 (C-18), 19.4 (C-19), 40.5 (C-20), 21.1 (C-21), 138.4 (C-22), 129.4 (C-23), 51.3 (C-24), 31.9 (C-25), 19.0 (C-26), 21.1 (C-27), 25.4 (C-28), 12.0 (C-29).
Examination of AMP – 8:- (Taraxerol XCVIII)
AMP-8 was crystallized from chloroform-methanol, as crystalline solid (75mg). Melting point 279 – 2800C, [α]20D+ 0.72 (C, 0.97, CHCl3). It showed pink color in L.B. test for triterpenes. Molecular formula:C30 H50 O. Element analysis: Found: C, 87.32%, H, 11.63% Requires: C, 84.45%; H, 11.82% Rf : 0.59 (n-hexane, ethyl acetate-9:1) I.R.: νCHCL3 3442, 2933, 1694. 1458, 1376, 1033 Cm-1.1H NMR (500, MHz, CDCl3) : δ0.80 (3H, S, 24-Me), 0.84 (3H, S, 28-Me), 0.90 (6H, S, 25, 29 – Me), 0.95 (3H, S, 30 – Me), 0.98 (3H, S, 23-Me), 1.05 (6H, S, 26, 27 – Me), 3.15 (1H, m, H-3), 5.33 (1H, dd, J = 8.1 and 4.0Hz, 15-H).13C NMR (150.8MHz, CDCl3): δ39.97 (C-1), 27.11 (C-2), 79.07 (C-3), 38.96 (C-4), 55.49 (C-5), 18.77 (C-6), 35.07 (C-7), 38.96 (C-8), 48.70 (C-9), 37.97 (C-10), 17.49 (C-11), 35.78 (C-12), 38.74 (C-13), 158.05 (C-14), 116.85 (C-15), 36.65 (C-16), 37.70 (C-17), 49.25 (C-18), 41.29 (C-19), 29.36 (C-20), 33.67 (C-21), 33.07 (C-22), 27.98 (C-23), 15.45 (C-24), 15.45 (C-25), 29.91 (C-26), 25.89 (C-27), 29.81 (C-28), 33.34 (C-29), 21.30 (C-30).
Acknowledgements
A special thanks to Prof. Y.L.N. Murthy, Dept. of Chemistry, Andhra university for his guidance and patience. Thanks to the IICT for providing spectral data.
References
P. Ranjan, Ethnobot. Studies in India, (1994) 5 148.
The wealth of India Raw materials. CSIR (1962), 423–425.
A.K.R. Kumari, D. Narasimhan, C. Livingstone and P.J; Raman, Phytomorphology, (2002) 52 (2,3) 207–215.
T. Akihisa, K. Matsumoto, H. Tokuda, K. Yasukawa, K. Seino, K. Nakamoto, H. Kuninaga, T. Suzuki, Y. Kimura, Jour. of Natu. Pro. (2007) 70 (5) 754–757.
T. Robak and R.J. Gryglewski, Bio chem. pharmacol. 37 (1988) 837 – 841.
S. Song, G. Lin Bioorg. Med. Chem. (2003) 11 2499 – 2502.
G. Mistra and N. Gupta, J. Inst. Chem. 54 (1982) 22.
S.M. Kupchan, H. Meshulam and A.T. Sneden, Phytochemistry 17 (1978) 767.
Bao-Ning Su, A.D. Pawlus, Hyun-Ah Jung, W.J. Keller, J.L. McLaughlin, A. Douglas Kinghorn, Jour. of Natu. Prod. 68 (4) (2005) 592–595.
Ye Deng, Y. Won Chin, H. Chai, W.J. Keller, A.D. Kinghorn .Jour. of Natu. Prod. 70 (2007) (12) 2049–2052.
Author information
Authors and Affiliations
Editor information
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Viplava Prasad, U., Syamasunder, B., Anuradha, G., Sree Kanth Kumar, J. (2012). Chemical Examination of Morinda Pubescens var. pubescens. (Rubiaceae) and isolation of crystalline constituents. In: Khemani, L., Srivastava, M., Srivastava, S. (eds) Chemistry of Phytopotentials: Health, Energy and Environmental Perspectives. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-23394-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-642-23394-4_15
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-23393-7
Online ISBN: 978-3-642-23394-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)