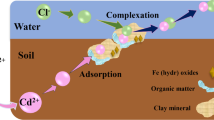
Abstract
Among the heavy metals, cadmium (Cd) and its fate in the soil have received a great attention because it is easily taken up by plants, making it more toxic than other heavy metals and consequently imposing harmful effects on human and other animals by entering food chain. This article reviews recent papers showing the behavior of Cd in calcareous soils specially under the salinity conditions. First, the chemistry of calcareous and saline soils is briefly discussed. A discussion on the application of fractionation and speciation analysis as important tools for investigating the mobility and environmental ecotoxicity of this element in calcareous soils is also being given. Finally, some examples on Cd detoxification in carbonate-rich soils along with applied techniques by different workers are also being outlined.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
13.1 Introduction
Soils containing free Ca or Mg carbonates are termed calcareous and usually have a pH above 7 (McBride 1994). The alkalinity in Ca2+- and Mg2+-rich soils is precipitated in the form of calcite (CaCO3), dolomite (Ca, Mg) (CO3)2, magnesium calcite (Ca1−x Mg x CO3, x < 0.2), and aragonite (CaCO3, polymorph). Calcite and aragonite are two common calcium carbonate minerals in which calcite is in more stable phase, but aragonite is often in the phase deposited biologically, and the conversion to calcite occurs slowly. Generally, the solubility of carbonates in soil is the function of [H+] and of the partial pressure of CO2. Assuming the constant CO2 partial pressure of 10−3.5 atmosphere, the soils containing carbonates have pH values near 8.25. The presence of CaCO3 in the calcareous soils can greatly increase the capacity of a carbonate solution to resist changes in the soil pH (Butler 1982) and from the principle of solubility products, these carbonates must be completely dissolved out of the soil before the pH can drop. Under these alkalinity conditions, micronutrients such as iron, manganese, and zinc are rendered unavailable to the plants growing in these soils as well as other metal elements. Indeed, the solubility of these elements is regulated by formation of their solid carbonate forms in calcareous soils. The prevalent example for nutrient metal deficiency in these soils is lime-induced chlorosis. The equilibrium concentration of Fe3+ in calcareous soil solution at pH 8.3 is 10−19 mM (Julian et al. 1983), which gives noticeable iron deficiency in plants not adapted to these conditions. Calcareous soils are distributed in arid and semiarid regions where soil salinization may occur as a consequence of exceeded evaporation.
13.2 Soil Salinity
Soil salinization has emerged as an environmental crisis of global proportions and threatening the quality and sustainability of arable lands. Around 25% of all lands are salt-affected to some degree mostly in arid and semiarid regions. Salinity affects physico-chemical and mineralogical properties, and also microbial communities and activities of the soil (Zahran 1997; Sardinha et al. 2003; Rietz and Haynes 2003). Salinity has a deleterious impact on crop production via osmotic effects and imbalance ion composition which results in some plant nutrient toxicity. Also results of experimental studies have demonstrated an increasing heavy metal mobilization with increasing salinities. The major cations of concern in saline soils and waters are Na+, Ca2+, Mg2+, and K+, and the primary anions are Cl–, SO 2–4 , HCO –3 , CO 2−3 , and NO −3 . Among these ions, chloride has been known as an anion which forms stable complexes with heavy metals especially the case for cadmium (Cd) and can determine Cd availability (Li et al. 1994; McLaughlin et al. 1994, 1997b; Smith and Martell 1981). In the next section, we will focus on the fate of Cd in calcareous soils.
13.3 Fate of Cadmium in Calcareous Soils
Cadmium is a transition element in periodic table of elements with an atomic weight of 112.41 and a melting point of 321°C. This metal is malleable and combines with other heavy metals to form alloys. Cd is the byproduct of the zinc, lead and copper smelting plants and mainly found as impurity in smithsonite (ZnCO3). Zn and Cd have similar electric configuration, but former is an essential element and later is nonessential, even very toxic, to nearly all living organisms (Lindsay 1979). Studies indicate that Cd inhibits seed germination and root elongation of plants (Fargasova 1994). Cd has deleterious impact on photosynthetic pigments as well as inhibition of cellular functions such as photophosphorylation, ATP synthesis, mitochondrial NADH oxidation, and electron-transport system. Adverse renal, kidney, liver, and lung effects are more commonly seen with exposure to Cd by human (Nordberg 1996).
13.3.1 Cadmium in Soil
Soils formed on igneous, metamorphic, and sedimentary rocks averagely contain 0.1–0.3, 0.1–1, and 0.3–11 mg kg−1 Cd, respectively (Alloway 1995). Cd tends to accumulate into the soil environment by burning of fossil fuels, mining and smelting of metalliferous ores, metallurgical industries, municipal wastes, fertilizers, pesticides, and sewage (Alloway 1990). The bioavailability of Cd in soil is controlled by the total concentration of Cd as well as the soil factors such as pH, organic matter and clay content (McBride et al. 1997; Yong 2001; Kabata-Pendias and Pendias 2001), soil salinity (McLaughlin et al. 1994), concentration of chloride (Weggler et al. 2004) and carbonate (Renella et al. 2004).
Two main factors which determine the solubility of heavy metals are pH and redox conditions (Sims and Patrick 1978). High mobility of heavy metals usually occurred in acid and reduction conditions, while the effect of pH is well pronounced. Indeed, not only the activity of heavy metals directly and indirectly depends on pH, but also pH controls the adsorption sites. At pH values above 7, cadmium is retained by replacement of calcium and magnesium on clay and organic surfaces making it inaccessible by plants. Precipitation of Cd largely occurred in calcareous soils in the form of CdO, CdCO3, and Cd3(PO4)2. Cd can be effectively retained by calcium carbonate surfaces at very low activities of solution Cd2+ (McBride 1980; Papadopoulos and Rowell 1988), and the adsorption reactions of Cd on calcite surfaces seemed to be very selective for Cd when Ca was a competing cation (Papadopoulos and Rowell 1988). Maftoun et al.(2004) studied the adsorption isotherms of Cd in 20 highly calcareous soils of Southern Iran, with CCE (calcium carbonate equivalent) being from 25.9 to 63.4%, and proved that the Cd sorption data well fits to two-surface Langmuir adsorption isotherm. They found that CCE is the main factor controlling the adsorption capacity of Cd in these soils as the following:
where b (mg kg−1) is the maximum adsorption capacity and CEC (me/100 g soil) is the cation exchange capacity of soil. Similar results have been obtained by Jalali and Moharrami (2007) in ten calcareous soils of Hamadan, Iran. They found a significant correlation of Langmuir b parameter with CCE (r 2 = 0.69; p ≤ 0.05) and CEC (r 2 = 0.61; p ≤ 0.05) in the case of Cd adsorption in these calcareous soils. In this viewpoint, calcareous soils have a natural ability to reduce Cd toxicity via chemiosorption or precipitation of Cd by soil components.
13.3.2 Cadmium Fractionation in Calcareous Soils
Sequential extraction procedures have been widely used for examining the physico-chemical forms of heavy metals, which has been described as “fractionation,” and are important tools for investigating the mobility and environmental ecotoxicity of these elements in soils. Recently for the assessment of the contamination levels of heavy metals the total concentration is considered, although it provides no insight into metal mobility and bioavailability. The bioavailability of metals in soil is affected by various physico-chemical forms of metals which are associated with soil constituents (Shuman 1991). In a sequential extraction procedure, a sample is treated with a series of progressively harsher reagents to dissolve increasingly refractory forms. Ideally, the reagents are chosen to selectively attack a specific soil compartment with minimal dissolution of nontargeted phases (Ahnstrom and Parker 1999).
Heavy metals including cadmium were partitioned into five operationally defined fractions: (1) Water soluble + Exchangeable fraction (EXCH); (2) Sorbed-Carbonate bound fraction (CARB); (3) Oxidizable or organically bound fraction (OM); (4) Reducible or associated with Mn and Fe oxides fraction (MNFEO); and (5) Residual fraction (RES). Water soluble and exchangeable forms of metals are the most mobile and bioavailable forms, so that closely related to plant uptake (Shuman 1979), whereas other metal fractions are considered immobile and tightly bound and may not be expected to be released under natural conditions. Renella et al. (2004) found that in a calcareous soil under various management, most of the native Cd of the soil was only slightly available, being mostly bound to CARB and RES Cd.
Han and Banin (1999) studied the transformation of added soluble Cd in a loessial calcareous soil incubated at the field capacity regime under controlled conditions. The soil had a loamy texture, intermediate carbonate content (23.2%) and intermediate CEC, and Cd(NO3)2 salt was added in the rates of 0, 0.09, 0.54 and 0.9 mg Cd kg−1 soil. Then after Cd fractions were determined for 1 h, 1 day, 1, 3, 6, 12, 24, and 48 weeks of incubation time, respectively. They reported that within the first 1 h from the start of incubation time, under the field capacity regime, added soluble Cd was transformed from the Exchangeable + Soluble fraction mainly into the carbonate fraction (60–75%). Jalali and Khanlari (2008) have found that the proportion of Cd associated with the most weakly bound fraction (EXCH) tended to decrease, with corresponding increases in the other five more strongly binding fractions during the incubation. In this study, Cd was added to five calcareous soils at the rate of 8 mg kg−1 of Cd as chloride. The samples were incubated for 3 h and 1, 3, 7, 14, 21, and 28 days at 25 C and constant moisture. After incubation, Cd in amended and control soils was fractionated by the sequential extraction procedure. Most of Cd added to soils appeared in the EXCH fraction after 3 h which decreased markedly within the first 3–24 h following Cd addition.
Rajaie et al. (2006) studied the effect of incubation time, soil texture, and application of enriched compost on chemical forms of Cd. In this study, a clay loam calcareous soil [Fine, mixed (calcareous), mesic Typic Calcixerepts] was converted to sandy loam by adding acid-washed pure quartz sand and both the original clay loam and the produced sandy loam were treated with 30 g kg−1 of municipal waste compost. The compost had been enriched with different amounts of CdSO4 to obtain Cd concentrations ranging from 5 to 60 mg Cd kg−1 in treated soils. After 0, 1, 2, 4, 8, and 16 weeks of incubation, a sequential extraction scheme was used to fractionate Cd of incubated samples into soluble + exchangeable, carbonate-bound, organically bound, Mn-oxide-bound, amorphous Fe-oxide-bound, crystalline Fe-oxide-bound, and residual forms. They found that Cd mostly was converted to soluble + exchangeable, carbonate-bound, and organically bound Cd forms (82–88%) and those for carbonate fraction were dominant and showed the highest capacity for retention. The proportion of soluble + exchangeable fraction in the sandy soil was higher than those for the clay loam after the incubation period suggesting the role of the fine fractions on immobilizing added metals. The study showed that more than 80% of applied Cd immediately converted to carbonate and organic forms and did not change during the remained incubation time. Although the presence of a large amount of cadmium in the first three steps of extraction procedure shows a relatively high Cd bioavailability in soils under study, it can be concluded that the calcareous nature of these soils plays a key role in cadmium retention because a major portion of the soluble Cd entered carbonate fraction immediately after addition to the soils.
13.3.3 Salinity and Cadmium Fractionation
Very limited information is available on the effect of salinity on the bioavailability and fractionation of cadmium in calcareous soils. In an experiment, Abbaspour et al. (2007) evaluated the effects of salinity on distribution of added Cd among soil fractions in a calcareous soil from Central Iran. In this work, soil was treated with 20 mg Cd kg−1 as Cd(NO3)2·4H2O and 50 mmol kg−1 of NaCl, and then incubated at 60% water-holding capacity (60% WHC) and constant temperature (25°C) for 12 weeks. Various fractions of Cd were extracted from the soil after 2 and 12 weeks of incubation using a sequential extraction technique. Results showed that the overall distribution pattern of the unspiked Cd in the calcareous soil was RES > SS > OM > EXC > MnO > AFeO. This may show the positive effect of CaCO3 on the specifically sorbed (SS) Cd. The applied Cd was mainly associated with the SS and MnO fractions, nonetheless addition of NaCl increased the EXC Cd fraction in unspiked and spiked soils, when the time passed a decrease of Cd in the SS fraction and a partial increase of Cd in the MnO, AFeO, RES and OM fractions were observed in the calcareous soil. The study showed slowly transformation and partitionation of Cd applied to the soil in soluble form among the solid-phase component of the soil. EXC forms of Cd are the most mobile and bioavailable form, so that closely related to plant uptake (Shuman 1979).
13.3.4 Salinity and Cadmium Speciation
Cd2+ is the predominant ionic species in soil solution, while it can form ionic complexes such as CdCl+, CdOH+, CdHCO +3 , CdCl −3 , CdCl 2−4 , Cd(OH) −3 and Cd(OH) 2−4 as well as complexes with organic substances. Studies have shown that chloride-induced salinity, mainly NaCl, increased Cd concentration in cultivated plants (McLaughlin et al. 1994; Weggler-Beaton et al. 2000; Mühling and Läuchli 2003). In a large-scale study, on wheat fields of northeastern North Dakota, Norvell et al. (2000) found that accumulation of Cd in grain was strongly and positively associated with soil salinity. In a greenhouse study, Khoshgoftar et al.(2004) applied different levels of NaCl (0, 60, 120, and 180 mM) and 120 mM NaNO3, via irrigation water, on a Cd-contaminated soil under cultivation of different genotypes of wheat. They found that increasing the NaCl concentration of irrigation water was associated with a significant (p < 0.05) increase in Cd concentrations in shoots, whereas application of NaNO3 had no significant effect on Cd concentrations in shoots. They implied that three probable mechanisms are responsible to elevated Cd solution concentration including competition of Na with other cations for sorption sites, complex of cations with added Cl−, and increased ionic strength in soil solution. Khoshgoftar et al. (2006) found that the major Cd species present in MINTEQA2-calculated soil solution, treated with NaCl-containing irrigation water, were free Cd2+ ion, CdCl1+, CdSO 04 , and CdHCO3+ species. McLaughlin et al. (1997b) reported that tuber Cd concentrations of potato (Solanun tuberosum L.) were related to activities of the chloro-complexes rather than to Cd2+ activities in saline/sodic soil solutions which occurs under high chloride concentration in soil solution. Weggler-Beaton et al. (2003) found that with increases in Cl concentrations in soil solution, Cd–chloro complexes became the dominant species in solution. They found that shoot Cd concentration was most closely correlated with the CdCl+ activity in solution while the activity of free Cd2+ was only weakly correlated. The effect of Cl on Cd uptake could be explained by the fact that CdCl n 2−n complexes in soil solution are also available for plant uptake (Smolders et al. 1997).
Ghallab and Usman (2007) conducted a greenhouse experiment with two levels of Cd (0.5 and 10 mg Cd kg−1, in the form of CdCl2), and five salinity levels of irrigation water (0, 8.6, 17.1, 34.2, and 68.4 mM NaCl) to determine the effect of NaCl-induced salinity on the solution speciation and availability of Cd in two, clay loam and sandy, calcareous soils. In this study, a chemical equilibrium modeling system (MINEQL+) was applied to predict solution speciation of Cd, which predicted that Cd was present mainly as free Cd2+ ions followed by CdCl+ and CdSO 04 in the soils irrigated with deionized water. However, Cd species in the soil solution were significantly altered by increasing chloride concentration, with Cd–chloro complexes becoming the dominant Cd species in the soil solution. They showed that added NaCl resulted in a large decrease in the ratios of Cd2/CdT and CdSO4/CdT, and increase in the ratios of CdCl2 2−n/CdT. For example, under the highest level of Cd (10 mg Cd kg−1) and NaCl (68.4 mM), the predicted percentage of free Cd2+ decreased from 71.3 to 19.8% and from 50.4 to 17.8%, respectively, in solutions of clay loam and sandy calcareous soils. In addition, the predicted percentage of CdSO4 decreased from 10.3 to 1.1% in clay loam soil and from 16.2 to 1.3% in sandy calcareous soil. In contrast, the predicted percentage of CdCl2 2−n increased from 18.4 to 78.2% in clay loam soil and from 33.0 to 80.8% in sandy calcareous soil. It was observed that CdCl2 2−n species were present mainly as CdCl+ and, with increasing chloride concentrations in soil solution, followed by the uncharged CdCl 02 . But, the proportion of the negatively charged CdCl −3 was very small (<1%), even at very high Cl concentrations.
13.4 Cadmium Detoxification
Calcareous soils represent potential adsorptive surfaces for heavy metals by means of carbonate and phosphate components. The presence of carbonate minerals in soils directly affects the mobility and reactivity of heavy metals through the surface interaction and indirectly through their affects on soil pH (McBride 1980). Phosphatic components are the important reaction products of added phosphorus with calcium carbonate as well as in calcareous soils, depending upon Ca:P ratio (Griffin and Jurinak 1973; Chand et al. 1991).
Thakur et al. (2006) conducted a laboratory investigation to determine the mechanism of cadmium sorption by pure calcium carbonate and to examine whether reaction products of phosphate with calcium carbonate serve as a sink for sorption of toxic heavy metal cations like cadmium. Calcium carbonates were treated with orthophosphoric acid in Ca:P ratio of 5:3, 3:2, 4:3 and 1:1, representing the Ca:P ratios of carbonate apatite, tricalcium phosphate, octacalcium phosphate and dicalcium phosphate and then equilibrated for 2 months. They found that cadmium was effectively retained on CaCO3 by the mechanism of chemisorption at lower Cd2+ concentrations as the pH of the equilibrium system remained constant (8.6) up to initial Cd2+ concentrations of 10−4 mol, coinciding to 100% sorption of Cd2+ from the solution. At higher concentrations, precipitation of CdCO3 on CaCO3 surface or as a separate phase predominated. Enrichment of CaCO3 with P reduced the Cd-sorption. The chemisorption of Cd probably involved the exchange of Ca2+ by Cd2+ from CaCO3 surface.
Brown et al. (2004) showed that soil amendments containing phosphorus reduce Cd availability in soils near a former Zn and Pb smelter in Joplin, Missouri, USA. Soil collected from the field was amended in the laboratory with phosphorus added in different ways, including as 1% P-H3PO4, a high-Fe by-product + P-triple superphosphate (TSP) (2.5% Fe + 1% P-TSP), 1% P-TSP, 3.2% P-TSP, 1% P-phosphate rock, sludge compost at 10% + 0.32% P-TSP, and sludge compost at 10% + 1% P-TSP. After treatment of the plots, Ca(OH)2 (71% purity) was applied evenly and rototilled into each plot to bring the pH to 7.0. The amount of lime required ranged from 200 ton ha−1 (3.2% P-TSP) to 50 ton ha−1 (10% compost + 0.32% P-TSP) and no lime for the compost-alone treatment. The indicator plant was tall fescue (Festuca arundinaceae), which grew in the field. They found that P-TSP and 1% P-H3PO4 were the most effective treatments for reducing plant concentrations of Pb, Zn, and Cd. In this study, they applied high amounts of Ca(OH)2 to maintain soil pH about 7.0 and this would be an important factor controlling solubility of heavy metals and the formation of carbonate components for these metals should be addressed.
13.5 Remediation Techniques
Soil remediation is one of the permanent alternatives to remove metal contaminants from soils. The remediation of metal-contaminated soils involves physical, chemical, and biological techniques. Physical techniques are based on approaches generally applied in mining and the mineral processing industry to extract the desired metal-bearing particles from mineral ores (Dermont et al. 2008). These approaches involve mechanical screening, hydrodynamic classification, gravity concentration, froth flotation, magnetic separation, electrostatic separation, and attrition scrubbing (Dermont et al. 2008). Chemical techniques are based on application of leaching solutions containing chemical reagents to enhance the solubility of metals and to transfer the metals from the soils into extractant solution. Depending on the metal type, degree of contamination, and soil characteristics different chemical reagent including acids, salts and high-concentration chloride solutions, chelating agents, surfactants, and reducing or oxidizing agents can be used in chemical extractions. The metal partitioning in the soil fractions also has a key role in reagent selection. For example, the carbonate-bound fraction is the predominant metal fraction in calcareous soils so that application of a chelating agent such as ethylenediaminetetraacetic acid (EDTA) causes co-dissolution of CaCO3 and Ca2+ interferences in target heavy metal complexation by EDTA in high Ca2+ solution (Di Palma and Ferrantelli 2005; Papassiopi et al. 1999). Papassiopi et al. (1999) reported that 90% of applied EDTA was consumed to dissolve CaCO3 and less than 10% of remained EDTA was utilized to complex heavy metals in a leaching experiment of a calcareous soil.
13.6 Phytoremediation
Most of the physical and chemical approaches are economically prohibitive due to their modern engineering-type techniques. On the other hand, more ecologically friendly approaches such as phytoremediation had been suggested widely. The use of green plants for reclamation of contaminated land can be a low-cost and ecologically sustainable alternative to other techniques. The effectiveness of phytoremediation of heavy metal-polluted soil depends on (1) the soil properties, (2) the metal content, (3) climatic factors, and (4) the plant. Among the soil properties, pH is closely related to heavy metal bonding in soils (Sauvé et al. 2000) with weak or negligible bonding at acidic pH (Jensen et al. 2000) and strong bonding at neutral to alkaline pH (Sukreeyapongse et al. 2002). Two characteristics of the selected plant to be considered in phytoremediation approaches include their ability to accumulate large concentrations of heavy metals in their tissue which is the so-called “hyper accumulator” and the high biomass producers that accumulate lower metal concentrations in their tissue, but compensates for this by a large production of biomass. Jensen et al. (2009) conducted a field and growth chamber experiment to assess the suitability of willow (Salix viminalis) for remediation of two, strongly and moderately, polluted calcareous soils. They stated that although the concentration of Cd in willow leaves grown on strongly polluted calcareous soils was high (80 mg kg−1), it is unsuited on this strongly polluted soil because of poor growth. For a tree species to be suitable for phytostabilization, the root system should be able to both retain and tolerate high concentrations of available metals (Domínguez et al. 2009). Domínguez et al. (2009) investigated the performance of Holm oak (Quercus ilex subsp. ballota) seedlings in Cd stabilization in another greenhouse study. They found that seedlings had a high Cd retention capacity in fine roots (up to 7 g kg−1) and low rates of Cd translocation to the leaves (transfer coefficients below 0.03). Also the chlorophyll fluorescence measurements (an indicator of plant stress) only differed slightly from the control treatment and showed that this species has a relatively high tolerance to Cd; therefore, may be useful for the phytostabilization of soils contaminated by Cd. Thlaspi caerulescens is one of the hyper accumulators known to accumulate Cd (McGrath 1998; Wang et al. 2006). These species are able to uptake and translocate high amounts of Cd to above-ground parts as a defense mechanism minimizing metal phytotoxicity. Nevertheless, in a critical review Kirkham (2006) stated that plants are limited in the amount of Cd that they can accumulate, even when chelates are used to solubilize the Cd for uptake. Consequently, phytoremediation will be of limited use in removing Cd from the soil.
13.7 Conclusion
This chapter has shown that calcareous soils have a natural ability to retain Cd in insoluble forms with limited treatment on living organism compared to neutral and acidic soils. Nevertheless, under the salinity condition the presence of chloride anions are known to reduce soil sorption of Cd, and an increase in Cl concentration in the soil or soil solution has been shown to increase Cd concentration in plants. Three probable mechanisms are responsible for elevated Cd solution concentration: competition of Na with other cations for sorption sites, complex of cations with added Cl−, and increased ionic strength in soil solution.
References
Abbaspour A, Kalbasi M, Hajrasuliha S, Golchin A (2007) Effects of plant residue and salinity on fractions of cadmium and lead in three soils. Soil Sediment Contam Int J 16:539–555
Ahnstrom ZS, Parker DR (1999) Development and assessment of a sequential extraction procedure for the fractionation of soil cadmium. Soil Sci Soc Am J 63:1650–1658
Alloway BJ (1990) Heavy metals in soils. Blackie and Sons, UK
Alloway BJ (1995) Heavy metals in soils. Blackie Academic and Professional, London, England
Brown S, Chaney R, Hallfrisch J, Ryan JA, Berti WR (2004) In situ soil treatments to reduce the phyto- and bioavailability of lead, zinc, and cadmium. J Environ Qual 33:522–531
Butler JN (1982) Carbon dioxide equilibria and their applications. Harvard university, USA
Chand T, Tomar NK, Singh JP (1991) Effect of soil properties on the forms inorganic phosphorus in alkaline calcareous soils of different agroclimatic zones. Arid Soil Res Rehabil 5:199–210
Dermont G, Bergeron M, Mercier G, Richer-Laflèche M (2008) Soil washing for metal removal: a review of physical/chemical technologies and field applications. J Hazard Mater 152:1–31
Di Palma L, Ferrantelli P (2005) Copper leaching from a sandy soil: mechanism and parameters affecting EDTA extraction. J Hazard Mater 122:85–90
Domínguez MT, Madrid F, Marañón T, Murillo JM (2009) Cadmium availability in soil and retention in oak roots: potential for phytostabilization. Chemosphere 76:480–486
Fargasova A (1994) Effect of Pb, Cd, Hg, As, and Cr on germination and root growth of Sinapis alba seeds. Bull Environ Contam Toxicol 52:452–462
Ghallab A, Usman ARA (2007) Effect of sodium chloride-induced salinity on phyto- availability and speciation of Cd in soil solution. Water Air Soil Pollut 185:43–51
Griffin RA, Jurinak JJ (1973) The interaction of phosphate with calcite. Soil Sci Soc Am J 37:847–850
Han FX, Banin A (1999) Long-term transformation and redistribution of potentially toxic heavy metals in arid-zone soils: II. Incubation at the field capacity moisture content. Water Air Soil Pollut 114:221–250
Jalali M, Khanlari ZV (2008) Cadmium availability in calcareous soils of agricultural lands in Hamadan, Western Iran. Soil Sediment Contam Int J 17(3):256–268
Jalali M, Moharrami S (2007) Competitive adsorption of trace elements in calcareous soils of western Iran. Geoderma 140:156–163
Jensen DL, Holm PE, Christensen TH (2000) Soil and groundwater contamination with heavy metals at two scrap iron and metal recycling facilities. Waste Manag Res 18:52–63
Jensen JK, Holm PE, Nejrup J, Larsen MB, Borggaard OK (2009) The potential of willow for remediation of heavy metal polluted calcareous urban soils. Environ Pollut 157:931–937
Julian G, Cameron HJ, Olsen RA (1983) Role of chelation by ortho dihydroxy phenols in iron absorption by plant roots. J Plant Nutr 6:163–175
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants, 2nd edn. CRC, Boca Raton, FL
Khoshgoftar AH, Shariatmadari H, Karimian N, Kalbasi M, Van der Zee SEATM, Parker DR (2004) Salinity and zinc application effects on phytoavailability of cadmium and zinc. Soil Sci Soc Am J 68:1885–1889
Khoshgoftar AH, Shariatmadari H, Karimian N, Kalbasi M, van der Zee SEATM (2006) Cadmium and zinc in saline soil solutions and their concentrations in wheat. Soil Sci Soc Am J 70:582–589
Kirkham MB (2006) Cadmium in plants on polluted soils: effects of soil factors, hyperaccumulation, and amendments. Geoderma 137:19–32
Li Y, Chanet RL, Schneiter AA (1994) Effect of soil chloride level on cadmium concentration in sunflower kernels. Plant Soil 167:275–280
Lindsay WL (1979) Chemical equilibria in soils. Wiley, New York, USA
Maftoun M, Rassooli F, Alinejad Z, Karimian N (2004) Cadmium sorption behavior in some highly calcareous soils of Iran. Commun Soil Sci Plant Anal 35:1271–1282
McBride MB (1980) Chemisorption of Cd2+ on calcite surfaces. Soil Sci Soc Am J 44:26–28
McBride MB (1994) Environmental chemistry of soils. Oxford University Press, New York
McBride MB, Richards BK, Steenhuis T, Russo JJ, Sauve S (1997) Mobility and solubility of toxic metals and nutrients in soil fifteen years after sludge application. Soil Sci 162:487–500
McGrath SP (1998) Phytoextraction for soil remediation. In: Brooks RR (ed) Plants that hyperaccumulate heavy metals. CAB International, Wallingford, Oxon, United Kingdom, pp 261–287
McLaughlin MJ, Palmer LT, Tiller KG, Beech TA, Smart MK (1994) Increased soil salinity causes elevated cadmium concentrations in field grown potato tubers. J Environ Qual 23:1013–1018
McLaughlin MJ, Maier NA, Rayment GE, Sparrow LABG, McKay A, Milham P, Merry RH, Smart MK (1997a) Cadmium in Australian potato tubers and soils. J Environ Qual 26:1644–1649
McLaughlin MJ, Tiller KG, Smart MK (1997b) Speciation of cadmium in soil solution of saline/sodic soils and relationship with cadmium concentrations in potato tubers (Solanum tuberosum L.). Aust J Soil Res 35:183–198
Mühling KH, Läuchli A (2003) Interaction of NaCl and Cd stress on compartmentation pattern of cations, antioxidant enzymes and protein in leaves of two wheat genotypes differing in salt tolerance. Plant Soil 253:219–231
Nordberg GF (1996) Current issues in low-dose cadmium toxicology: nephrotoxicity and carcinogenicity. Environ Sci 4:133–150
Norvell WA, Wu J, Hopkins DG, Welch RM (2000) Association of cadmium in durum wheat grain with soil chloride and chelate extractable soil cadmium. Soil Sci Soc Am J 64:2162–2168
Papadopoulos P, Rowell DL (1988) The reaction of cadmium with calcium carbonate surfaces. J Soil Sci 39:23–36
Papassiopi N, Tambouris S, Kontopoulos A (1999) Removal of heavy metals from calcareous contaminated soils by EDTA leaching. Water Air Soil Pollut 109:1–15
Rajaie M, Karimian N, Maftoun M, Yasrebi J, Assad MT (2006) Chemical forms of cadmium in two calcareous soil textural classes as affected by application of cadmium-enriched compost and incubation time. Geoderma 136:533–541
Renella G, Adamo P, Bianco MR, Landi L, Violante P, Nannipieri P (2004) Availability and speciation of cadmium added to a calcareous soil under various management. Eur J Soil Sci 55:123–133
Rietz DN, Haynes RJ (2003) Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol Biochem 35:845–854
Sardinha M, Müller T, Schmeisky H, Joergensen RG (2003) Microbial performance in soils along a salinity gradient under acidic conditions. Appl Soil Ecol 23(3):237–244
Sauvé S, Hendershot W, Allen HE (2000) Solid-solution partitioning of metals in contaminated soils. Dependence on pH, total metal burden, and organic matter. Environ Sci Technol 34:1125–1131
Shuman LM (1979) Zinc, manganese and copper in soil fractions. Soil Sci 127:10–17
Shuman LM (1991) Chemical forms of micronutrient in soils. In: Morvedt JJ et al (eds) Micronutrients in agriculture, vol 2, SSSA Book Series No. 4. Soil Science Society of America, Madison, WI, pp 113–144
Sims JL, Patrick JL (1978) The distribution of micronutrient cations in soil under conditions of varying redox potential and pH. Soil Sci Soc Am J 42:258
Smith RM, Martell AE (1981) Critical stability constants, vol 4. Plenum Press, New York
Smolders E, Lambrechts RM, McLaughlin MJ, Tiller KG (1997) Effect of soil solution chloride on Cd availability to Swiss chard. J Environ Qual 27:426–431
Sukreeyapongse O, Holm PE, Strobel BW, Panichsakpatana S, Magid J, Hansen HCB (2002) pH-dependent release of cadmium, copper, and lead from natural and sludge-amended soils. J Environ Qual 31:1901–1909
Thakur SK, Tomar NK, Pandeya SB (2006) Influence of phosphate on cadmium sorption by calcium carbonate. Geoderma 130:240–249
Wang AS, Angle JS, Chaney RL, Delorme TA, Reeves RD (2006) Soil pH effects on uptake of Cd and Zn by Thlaspi caerulescens. Plant Soil 281:325–337
Weggler K, McLaughlin MJ, Graham RD (2004) Effect of chloride in soil solution on the plant availability of biosolid-borne cadmium. J Environ Qual 33:496–504
Weggler-Beaton K, McLaughlin MJ, Graham RD (2000) Salinity increases cadmium uptake by wheat and Swiss chard from soil amended with biosolids. Aust J Soil Res 38:37–45
Weggler-Beaton K, Graham RD, McLaughlin MJ (2003) The influence of low rates of air-dried biosolids on yield and phosphorus and zinc nutrition of wheat (T. durum) and barley (H. vulgare). Aust J Soil Res 41:293–308
Yong R (2001) Geoenvironmental engineering: contaminated soils, pollutant fate and mitigation. CRC Press, Boca Raton, FL
Zahran HH (1997) Diversity, adaptation and activity of the bacterial flora in saline environments. Biol Fertil Soils 25:211–223
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Khanmirzaei, A. (2011). Fate of Cadmium in Calcareous Soils under Salinity Conditions. In: Sherameti, I., Varma, A. (eds) Detoxification of Heavy Metals. Soil Biology, vol 30. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-21408-0_13
Download citation
DOI: https://doi.org/10.1007/978-3-642-21408-0_13
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-21407-3
Online ISBN: 978-3-642-21408-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)