Abstract
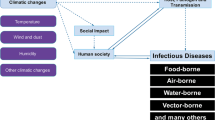
Changing environmental conditions are not only the result of the direct impact of climate change, but represent also the consequences of migration, urbanisation and the globalisation of trade and human mobility. In consequence, changes in the occurrence of infectious diseases in humans and animals ensue. Several infectious diseases which were hitherto considered “exotic” in Europe have largely lost this feature and can now occur nearly everywhere. These include a number of arthropod-borne diseases such as Bluetongue, which mainly affects ruminants, West Nile Fever in humans, horses and birds, as well as Chikungunya Fever in humans. There is a trend towards a global spread of Dengue Fever and Japanese Encephalitis, associated with extension of the habitats of the respective arthropod vectors. In addition, transportation of animals and products of animal origin has caused the spread of animal diseases, notably of Rift Valley Fever from Africa to the Arabic peninsula and of African Swine Fever from East Africa into the Caucasus region where it shows a clear tendency of spreading in a northerly and westerly direction. Therefore, we propose to stop using the term “exotic” for these diseases, because infections that are today considered “exotic”, may tomorrow be part of our daily life.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Humans and animals have always been exposed to infectious diseases. Environmental factors and anthropogenic activities have influenced their distribution in the past and continue to do so. Areas in southern Europe, for example, were endemic for malaria and yellow fever until the early twentieth century. Animal diseases, which are today considered “exotic”, such as Rinderpest, now close to global extinction, led two centuries ago to the introduction of veterinary measures which are still valid today, like veterinary certificates and movement restrictions. Because of the implementation of these regulations and further measures including herd hygiene and immune prophylaxis, control of infectious animal diseases improved. However, the debate over future influenza pandemics, irrespective of their cause (H5N1‚ “bird flu” or H1N1‚ ”swine flu” or any other influenza virus), clearly illustrates the threat which infectious diseases still pose today. Two-thirds of the infectious diseases that emerged in the past 20 years had their origin in animals, i.e. represent zoonotic infections (Jones et al. 2008). Obviously, also the “new” pandemic influenza virus A/H1N1 originated in an animal host reservoir. However, the role of wildlife as a reservoir for particular pathogens is often largely unknown and the risk of transmission of the infections into the human population difficult to assess.
Increasing urbanisation in the absence of appropriate infrastructure, inadequate medical care and veterinary surveillance in particular in crisis regions, flight, migration and the progressive destruction of habitats lead to alterations in the exposure of humans and animals to several pathogens (Woodhouse 2008). Globalisation, including world-wide trade and travel, facilitates the introduction of pathogens from remote countries over large distances within short periods of time (Mettenleiter and Böhle 2008).
Changing environmental factors also modulate the exposure to pathogens. The increasing number of cases of tick-born encephalitis in Germany until 2007 can be attributed equally to the expansion of tick vector habitats, to an increase in tick activity due to climate change and to the altered leisure behaviour of humans, in particular outdoor activities. The outbreaks of infections with highly pathogenic avian influenza virus of the subtype H5N1, which was first described in Hong Kong in 1997, in Europe in spring 2006, showed how fast “exotic” infections can spread and establish themselves under favourable conditions. This also applies to Bluetongue disease, which was originally endemic in Africa, but occurred in central Europe for the first time in history in August 2006, and was rapidly transmitted by indigenous Culicoides spp. (Mehlhorn et al. 2007; Hoffmann et al. 2009). In the following, several examples of the recent past are presented.
2 Bluetongue Disease
Bluetongue is mainly a disease of ruminants, but affects also camelids. It is caused by an orbivirus (bluetongue virus [BTV], family Reoviridae), which is transmitted by biting midges of the genus Culicoides. At least 24 serotypes of BTV have been described. Bluetongue has spread along with its main “African” vector Culicoides imicola into the European part of the Mediterranean basin since the mid 1990s, possibly due to the expansion of the habitat range of C. imicola caused by long-term climatic changes (Purse et al. 2005). Nevertheless, the occurrence of BTV in Belgium, Germany and the Netherlands in August 2006 was unexpected. Not only had Bluetongue never before occurred in central Europe, but the serotype 8, which had caused the infections, had never been detected in Europe at all. C. imicola, the prime vector of Bluetongue in Africa and the Mediterranean, was not found in Central Europe despite intensive monitoring. This suggested that indigenous biting midges were capable of transmitting BTV-8 effectively. Comprehensive entomological studies showed that biting midges with a Palaearctic distribution, in particular members of the C. obsoletus complex, represent competent vectors for BTV-8 (Hoffmann et al. 2009). It remains unclear, however, whether the extreme weather conditions observed in summer 2007, when temperatures exceeded the long-term averages considerably, had an essential effect on establishment and spread of the disease in a region of temperate climate. The available data suggest, however, that increased temperatures may have triggered the Bluetongue epidemic after initial introduction of BTV, and led to a lasting, perhaps irreversible, establishment of the disease in central Europe (Conraths et al. 2010).
The fact, that vaccines against BTV-8 became available just before or at the beginning of the Bluetongue transmission season in 2008, reduced the incidence and the economic losses caused by the disease considerably (Conraths et al. 2008). However, a few infections with BTV serotypes 6 and 11 were also detected, possibly due to illegal use of life vaccines. By contrast, the northward spread of BTV-1 from southern France to regions bordering Germany raised concern that this serotype might also establish in Germany. In conclusion, it has become obvious that a disease which had never occurred in central Europe until 2006 seemed suddenly to be in the process of establishing itself permanently in this region.
3 African Horse Sickness
African Horse Sickness (AHS) is transmitted in Africa by the same vectors as Bluetongue. AHS virus, of which nine different serotypes are known, is a close relative of BTV. Zebras represent the prime reservoir for AHS. Normally, infections in zebras remain asymptomatic or subclinical, or lead to only mild symptoms. By contrast, horses develop a severe, acute or peracute disease associated with high mortality. Historically, AHS has been endemic mainly in central Africa from where it spread to northern Africa and Europe. At least some introductions of AHS were due to the import of infected zebras into European zoos. Fortunately, the virus has not established itself in Europe so far. It cannot be excluded, however, that biting midges transmitting BT in central Europe can also spread AHS, which could lead to an epidemic of AHS similar to the one that occurred with BTV-8. Consequently, the European Commission has established a vaccine bank for AHS for rapid use to contain any primary AHS outbreak. It remains to be seen if this strategy is successful in the case of an emergency.
4 Chikungunya Fever
Chikungunya infections are caused by an alphavirus from the family Togaviridae. The virus was first described in East Africa in 1952 and remained confined to the African continent for a long time. Since 2005 it spread eastwards into countries around the Indian Ocean and to Indonesia. In the meantime, almost the entire territory of Southeast Asia is endemically affected (Enserink 2008). The virus is transmitted by mosquitoes of the genus Aedes. It is important to note that the Asian tiger mosquito or forest day mosquito, Aedes albopictus, has been spreading massively in recent years. It has reached Africa, America and southern Europe (Enserink 2008). Consequently, the first Chikungunya epidemic in Europe occurred in Italy in the region of Rimini-Ravenna during summer 2007. The virus had been introduced by an infected tourist from India and was spread by A. albopictus, which had already established itself in the region. Although there were no further cases in 2008, it cannot be excluded that the infection may re-occur in this region as the virus is transmitted transovarially. It is also possible, however, that vector control programmes, which were started immediately, could have diminished the vector population under the critical limit.
5 African Swine Fever
African Swine Fever (ASF) is caused by a large double-stranded DNA virus of the family Asfarviridae. It occurs primarily in sub-Saharan Africa, where it has its reservoir in warthogs and bush pigs. For these animals, the ASF virus is harmless, while domestic pigs are highly susceptible to ASF. Leather (soft) ticks of the genus Ornithodoros can transmit ASF, but the disease is also spread by direct contact. Ornithodoros moubata, the prime vector in south-eastern Africa (Madagascar, Mozambique, Zambia), usually lives in burrows made by the warthog, where it relies on this animal for its blood meals and can transmit ASF virus.
Until recently, the only existing focus in Europe was on the Italian Island of Sardinia. In 2007, the disease was first detected in domestic pigs in Georgia, spread rapidly in the Caucasus region and into southern Russia, where wild boar contracted the infection with the result of the potential formation of a reservoir in this wildlife species.
The virus was probably introduced via the harbour of Poti on the Black Sea by ship from East Africa, since the virus found in the Caucasus region shows the highest degree of homology with isolates from East Africa. Transmission to domestic pigs may have occurred through food waste of porcine origin deposited on dumps outside Poti, where the contaminated material was apparently accessible to free-ranging pigs. In the Caucasus, ASF seems to be primarily transmitted by direct contact. It appears that ticks do not play a role in the transmission of the disease in this region.
Although domestic pigs and wild boar are usually highly susceptible to ASF and die with haemorrhagic symptoms, the persistence of ASF virus in the swine population cannot be excluded, as the disease situation on the island of Sardinia has shown. In the meantime, the infection has spread westwards towards the Crimean peninsula and northwards towards Siberia. In October 2009, ASF was detected close to St. Petersburg in northern Russia, approximately 2,000 km away from the region where the disease had occurred before in the Russian Federation.
Rapid eradication of the disease in these regions is unlikely, since there is no vaccine against ASF, and only the systematic culling of potentially infected animals remains to control the disease. However, due to the rural farming conditions in the Caucasus, a systematic culling strategy is difficult to implement, with the consequence that a new endemic focus of ASF may have been established in this region.
6 West Nile Fever
The family Flaviviridae contains a number of viruses causing important diseases in humans or animals, such as Yellow Fever and Dengue as human infections, and zoonotic viruses, causing for example Japanese Encephalitis, Tick-borne encephalitis and West Nile Fever (WNF). West Nile Fever virus (WNV) was first detected in the West Nile district of Uganda, Africa, in 1937. WNV is an arbovirus (‘arthropod-borne’) as it is transmitted by blood-feeding arthropods. It has long been present in large areas of Asia, Eastern Europe, Africa and Australia, but gained centre stage in 1999, when it suddenly occurred in New York, USA, causing widespread deaths among corvids, in particular American crows (Corvus brachyrhynchos), and birds of prey as well as infections in humans and horses, some of which were lethal. In the meantime, the virus has spread over the entire North-American continent and is continuing to expand into South America. In Europe, cases of WNF were recorded in birds, horses and humans in France, Romania, Hungary and Italy in the past decade. Infections detected in wild birds (mainly raptors) in Austria in 2008 may suggest that the virus is spreading northwards.
Wild birds represent the natural reservoir of the virus which is mainly transmitted by mosquitoes of the genus Culex. Several Culex spp. occurring in central Europe, such as C. pipiens, may be capable of transmitting WNV. Infections in wild birds normally remain asymptomatic, although some bird species, for instance crows and some birds of prey, can develop severe disease and die in large numbers upon infection with WNV. Horses and humans represent dead-end hosts. They become infected by mosquitoes which blood-feed on birds and mammals. The spread of WNF via Hungary to Western Austria suggests that WNV may also invade Germany. While a vaccine for horses has been registered in Europe, vaccines for humans are not available yet. Thus, WNF may have become established as another arbovirus infection in Europe. However, our knowledge on the factors that determine the spread of WNF in Europe is still sparse.
7 Conclusions
Alterations in environmental conditions such as climate change, globalisation in trade and human mobility, urbanisation on the one hand, and increasing contact with wildlife on the other hand have a major impact on the spread of infectious diseases. The term “exotic disease” has therefore become inappropriate as virtually every disease can be rapidly introduced from anywhere on the globe into our region. To improve risk assessments, a better knowledge of the impact of the different parameters mentioned above on the spatial distribution of infectious agents and their vectors, where applicable, is required. Our understanding of the reservoir function of wildlife for various pathogens needs to be improved with research focussing on the biology and epidemiology of infectious agents and their vectors, including the causative agents of those diseases which have so far been regarded as “exotic”. It is necessary to establish science-based prevention and control strategies, preferably prior to the arrival of the respective pathogens and their vectors in our region.
References
Conraths FJ, Gethmann J, Staubach C, Mettenleiter TC, Beer M, Hoffmann B (2008) Epidemiology of bluetongue virus serotype 8, Germany. Emerg Infect Dis 15:433–435
Conraths FJ, Gethmann JM, Hoffmann B, Beer M, Kramer M, Staubach C (2010) Impact of climate change on orbivirus infection. Nova Acta Leopoldina 381:93–98
Enserink M (2008) A mosquito goes global. Science 320:864–866
Hoffmann B, Bauer B, Bauer C, Bätza H-J, Beer M, Clausen P-H, Geier M, Gethmann J, Kiel E, Liebisch G, Liebisch A, Mehlhorn H, Schaub G, Werner D, Conraths FJ (2009) Large scale monitoring of putative vectors of BTV-8 in Germany. Emerg Infect Dis 15:1481–1484
Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman J, Daszak P (2008) Global trends in emerging infectious diseases. Nature 451:990–993
Mehlhorn H, Walldorf V, Klimpel S, Jahn B, Jaeger F, Eschweiler J, Hoffmann B, Beer M (2007) First occurrence of Culicoides obsoletus-transmitted Bluetongue virus epidemic in Central Europe. Parasitol Res 101:219–228, Erratum in: Parasitol Res 101:833–834
Mettenleiter TC, Böhle W (2008) Erregerbedingte Erkrankungen unter veränderten Umweltbedingungen. Arch Tierz (Dummerstorf) 51:49–56
Purse BV, Mellor PS, Rogers DJ, Samuel AR, Mertens PP, Baylis M (2005) Climate change and the recent emergence of bluetongue in Europe. Nat Rev Microbiol 3:171–181
Woodhouse MEJ (2008) Emerging diseases go global. Nature 451:898–899
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Conraths, F.J., Mettenleiter, T.C. (2011). Infectious Diseases Under the Influence of Changing Environmental Factors. In: Mehlhorn, H. (eds) Progress in Parasitology. Parasitology Research Monographs, vol 2. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-21396-0_13
Download citation
DOI: https://doi.org/10.1007/978-3-642-21396-0_13
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-21395-3
Online ISBN: 978-3-642-21396-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)