Abstract
Our understanding of halovirus diversity is limited since viral isolation is traditionally based on the ability to culture the host species. Here, we review current knowledge of viruses in hypersaline environments. Many of these studies, however, do not reveal the true level of diversity in the microbial community. We describe a study of the hypersaline region of Great Salt Lake, Utah, USA, which utilizes a non-cultivation approach, which combines fractionation and negative staining transmission electron microscopy, to analyze viral diversity. Examination of the haloviruses pelleted from brine revealed haloviruses of the three major morphological types previously reported in other hypersaline waters: fusiform, spherical, and head–tail species. Partial success was accomplished in separating the different forms by cesium chloride density banding. We also discovered abundant filamentous viruses suggesting that “filamentous” should be considered a major new category of halovirus forms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
9.1 Introduction
9.1.1 The Setting
Great Salt Lake, Utah, USA, is a large body of water, segmented by a railroad causeway into two general systems. The south arm is a moderate saline bay (9–15% sodium chloride content), while the north arm is a hypersaline bay (24–30% sodium chloride). As a terminal lake in an elevated biome, the lake elevation (average 1,280 m) fluctuates as does the temperature, affecting the salt content at any given time (reviewed in Baxter et al. 2005).
The north arm, virtually cut off from fresh water supply save precipitation, is an appropriate model for hypersaline studies. It is an ecosystem of extremes beyond the salt content, as the lake elevation, desert environment, and saline waters also result in desiccation and high ultraviolet light penetration (Baxter et al. 2007). While studies on halophiles from such environments are numerous, we know much less about the viruses that also survive these multiple extremes and infect halophile hosts.
9.1.2 Halovirus Isolation Studies
In this chapter, our use of the term “halovirus” is to imply a virus that infects a halophile, the host being either a bacterial or archaeal species. While in isolation studies the host may be known, in other diversity studies discussed below, the host is unknown.
With the goal of examining halovirus diversity, a number of various strategies have been employed by other investigators. While all of the following techniques have been successful to a certain extent in isolating and visualizing hypersaline viruses, there are limitations that have resulted in an incomplete analysis of the total halovirus morphotypes that may exist. Some techniques outlined here, in particular the cultivation methods, are given in more detail in a recent review (Porter and Dyall-Smith 2006).
9.1.2.1 Isolation by Cultivation
A number of haloviruses have been isolated from hypersaline lakes using traditional cultivation methodology. The advantage of an isolated phage/host pair is the ability to replicate the virus and produce particles in sufficient number for more in-depth study. From this type of work, we can assess similarities and differences of structure, genomics, and biochemistry. Some reviews have addressed the question of diversity primarily summarizing the work to date on various characterized strains (e.g., Dyall-Smith et al. 2003; Prangishvili et al. 2006).
However, there are caveats to working with laboratory strains as hosts. First, direct plating only results in lytic viruses, which means that temperate viruses go undetected and unisolated. Second, optimal lytic conditions for each halovirus can be variable and also may be different than the optimal culture conditions of the host (Porter and Dyall-Smith 2006). In addition, if cultivation of environmental microorganisms is as limited as estimated, then only a small fraction of host strains may be lab-friendly. The haloviruses that infect them, then, are uncultivatable as well. Also, as other authors have noted, virus isolations are biased by use of standard lab host strains that might grow well in culture but be underrepresented in the environment (Dyall-Smith et al. 2003).
9.1.2.2 Isolation of Genome by Pulsed-Field Gel Electrophoresis
Recently, the complete genome of EHP-1, an uncultivated environmental halovirus, was sequenced (Santos et al. 2007). The whole genome of this virus was cloned and subsequently sequenced from a 37-kb band detected via pulsed-field gel electrophoresis (PFGE) analysis of an environmental brine sample. This strategy seems to be promising, yet there are caveats here as well. In this case, the halovirus is known, but not its host. So, although valuable genome sequence information can still be utilized, both virus morphology and host-virus dynamics remain a mystery until the host is discovered. Furthermore, while some uncultured viruses may be discovered through the direct cloning of whole environmental genomes, there is no guarantee that the same procedure will be successful for all viruses. Other, earlier groups attempted but failed to clone and sequence complete viral genomes from the environment, presumably due to the presence of viral genes that are toxic to cells (Forest Rohwer, personal communication). Ultimately, diversity of haloviruses is therefore not well understood from such standard and nonstandard methods as those described above.
9.1.2.3 Isolation by Phase Partition
A more detailed physical and biochemical characterization of individual phage species present in the hypersaline water would necessitate steps that provide larger amounts of phage, concentrated and at least partially purified based on parameters such as size or buoyant density. The phase partition method of Per Åke Albertsson (1960, 1986) employs a solution of polyethylene glycol in moderate salt (0.5–1.0 M NaCl) to selectively precipitate phage from solution. Precipitation can be carried out at 1 × g and thus large initial volumes can be processed for subsequent concentration and analysis. In the past, one of us (J.D.G.) used this procedure to concentrate M13 phage from 200 L of broth in which M13 infected Escherichia coli cells had been removed by slow speed centrifugation. This large-scale concentration can then be coupled with additional methods, including cesium chloride density banding, to further purify and separate different phage species.
9.1.3 Halovirus Community Studies
Population studies of hypersaline virioplankton have employed various methods, including filtration, PFGE, TEM, and metagenomic analyses. While such methods are limited in the type of biological information acquired about haloviruses, they nonetheless contribute to providing an overall view of what exists in the halovirus community.
9.1.3.1 Filtration
Large-scale filtration and concentration techniques, such as tangential flow filtration (TFF), have been employed to collect haloviruses from environmental water samples. The obvious advantage of TFF is the ability to simultaneously filter and concentrate relatively large volumes (e.g., 20 L), resulting in a high virus concentration. However, reports of TFF with hypersaline water samples without a prior pre-filtration step show high viral loss compared to other filtration methods, such as positive pressure filtration (Diez et al. 2000). While the authors of this study concluded that viral diversity was not compromised through their use of TFF, the reduction in virus number as a result of direct and exclusive TFF use could presumably also reduce representation of the halovirus community.
9.1.3.2 Pulsed-Field Gel Electrophoresis
Diez and co-workers (2000) reported observing fusiform viruses in their Spanish saltern samples. In addition, PFGE and DNA hybridization analyses of the viral community’s nucleic acid showed that different viral assemblages existed in different hypersaline ponds at the same field site, and that there was less diversity than in marine or haloalkaliphile environments, with a maximum number of eight bands observed via PFGE (Diez et al. 2000). PFGE and DNA hybridization analyses of the moderately hypersaline Mono Lake virioplankton showed that viral genome sizes were similar to those in marine and estuarine environments with the most common-sized genome being ~35 kbp, and that approximately 27 bands could be discerned via PFGE (Jiang et al. 2004). Furthermore, there were temporal and spatial differences in the viral assemblages as some viral genomes were only present at certain depths and at certain times of the year (Jiang et al. 2004; Sabet et al. 2006).
9.1.3.3 Transmission Electron Microscopy
Water samples can also be ultracentrifuged first to pellet the hypersaline viruses before adsorption onto transmission electron microscopic (TEM) grids (e.g., Guixa-Boixareu et al. 1996; Oren et al. 1997). This method seems to be the most logical strategy to investigate halovirus diversity, as it is culture-independent and does not involve time-consuming filtration protocols.
Using this method, the first filamentous virus-like particles (VLPs) were reported from Spanish salterns, along with icosahedral, tailed, and lemon-shaped viruses; furthermore, the observation was made that the number of lemon-shaped haloviruses increased with salinity (Santos et al. 2007). To visualize VLPs adsorbed to the TEM supports, concentrations in the range of 108–109/ml are required and it has been argued that different viruses have different adsorptive and diffusive properties (Grieg Steward, personal communication; Wommack and Colwell 2000). Furthermore, some viruses, especially those that are not tailed capsids, might be too fragile for direct ultracentrifugation (Porter and Dyall-Smith 2006). Collectively, these limitations could result in an incomplete representation of the hypersaline virioplankton.
Halovirus populations have been successfully visualized from water samples by directly pelleting them onto TEM grids via the airfuge, a specialized ultracentrifuge. This method is ideal as it is cultivation-independent, does not require filtration, and does not depend on a high concentration of viruses for successful adsorption. This technique has recently yielded TEM images of very diverse halovirus morphotypes discovered in Lake Retba, Senegal (Sime-Ngando et al. 2010). According to the authors, 46% of the VLPs viewed by TEM were spindle-shaped (4% with a tail and 42% without a tail); 35% were spherical (7% icosahedral and 28% non-icosahedral); 13% were linear; only 1% were head-tailed (i.e., tailed capsids such as the Siphoviridae and Myoviridae); and, surprisingly, 5% of the total viruses from the environment were unidentified or of unknown shapes including hairpin-shaped particles, bacilliform particles with and without an appendage, chains of small globules, hook-shaped particles, tadpole-shaped particles, reed-shaped particles, and complex particles with branched filaments and spherical units. These exciting new forms indicate our current categories for haloviruses are too narrow.
Another study using TEM to evaluate the morphotype diversity in the stratified water column of Mono Lake showed that “exotic” shapes did not exist, only VLPs with hexagonal capsids, prolate capsids, and capsids with single-axis symmetry. There were three different viral assemblages in three different layers. The abundance of tailed viruses was highest in the oxic layer (epilimnion) and decreased with increasing depth. Large untailed capsids, >150 nm, were most numerous in the middle layer (metalimnion) and least numerous in the oxic layer. Furthermore, tail length increased with depth, with Podoviruses existing exclusively at the epilimnion and absent in the metalimnion and hypolimnion depths; Myoviruses being most abundant in the epilimnion, then in the metalimnion, and least abundant in the hypolimnion; and Siphoviruses existing in the epilimnion, but then increasing with depth being most numerous in the hypolimnion (Brum and Steward 2010).
9.1.3.4 Metagenomics
A metagenomics approach has only very recently been applied to hypersaline aquatic habitats. One group invented a metagenomics tool called Genome relative Abundance and Average Size, or GAAS, which delivers more accurate results when undertaking genome database searches (Angly et al. 2009). By analyzing ten different hypersaline viral metagenomes, this methodology revealed that the average genome length of haloviruses was between 51 and –263 kbp. In addition, the size range of hypersaline viral genomes was relatively narrow compared to the size range of hypersaline microbial genomes (Angly et al. 2009), indicating that there is no trend between viral and microbial genome lengths and that they vary independently of each other. Another study showed that, while dominant microbial and viral communities in low-, medium-, and high-salinity aquatic environments maintained stability over time, thereby confirming that a stable geochemistry leads to a stable biology, a “fine-grained” analysis of viral genotypes and microbial strains actually reflected temporal fluctuations in both the microbiome (metagenome of microbes) and virome (metagenome of viruses) from samples collected between 1 day and 1 year of each other from a San Diego, USA, saltern system (Rodriguez-Brito et al. 2010). These data are interpreted to support the “kill-the-winner” theory that explains how dominant microbial strains in an environment grow in abundance until a certain threshold is reached when viruses then act as predators and very quickly reduce the dominant, virus-sensitive strain allowing less dominant, virus-resistant strains to predominate (Wommack and Colwell 2000).
A metagenomics analysis of a Spanish saltern showed that 75–80% of the metavirome contained hypothetical proteins, many conserved, while there were some matches to known halovirus isolates, as well as some surprising hits to the San Diego, USA, salterns metavirome (Santos et al. 2010). Single-nucleotide polymorphisms (SNPs) existed along this metavirome, the majority of which were neutral, signifying the presence of variants. There was also a detection of high mutation rates that reflected high intra-species diversity, meaning that similar hosts are infected by closely related viruses. One especially interesting result of this metagenomics study was the observation that there were low numbers of integrases within the metavirome, implying that lysogeny may not be a prevalent form of infection for haloviruses (Santos et al. 2010). This result seems to agree with the observation made that cultured haloviruses typically cause chronic infections in their host, more so than strictly lytic or strictly lysogenic infections (Porter et al. 2007). In other words, chronic infection may be the preferred choice of haloviruses.
In conjunction with more standard methodologies, metagenomics is able to contribute to our understanding of halovirus ecology. Valuable insight is being provided by such analyses that allow us to know the halovirus community more generally; however, metagenomics is unable to address the question of morphology at this time.
9.1.4 Halovirus Diversity
So what do we know about halovirus diversity? If it is possible to look at viruses with a “macro” lens, we can look at the shape and structure of a virus and begin some level of classification. When we get down to the genome level, we may find that our classification was ineffective, but it is a place to start. Of course, the more comprehensive our approach in addressing a question, the better our answer will be. A true picture of diversity will come from coupling methods and from exploring new hypersaline systems.
From the published studies that utilize EM, at least three major halovirus morphotypes are known to exist in significant numbers and they are described below.
9.1.4.1 Fusiform Haloviruses
Spindle-shaped viruses have been observed exclusively in the Archaea, including methanogens, thermophiles, and halophiles (reviewed in Prangishvili et al. 2006). In a number of studies of hypersaline ecosystems, fusiforms appear to be the dominant virus type (e.g., Guixa-Boixareu et al. 1996; Oren et al. 1997). Two well-characterized fusiform haloviruses are His1 and His2, both of which infect Haloarcula hispanica. These have been classified in the Salterprovirus genus (Bath et al., 2006). These virions have their lemon shape and size range (44 × 67–77 nm) in common, and they are sometimes seen with short tails, but they are composed of dissimilar structural proteins. Both viruses are lytic and have no integrase activity for integration into the genome of the host. They are distantly related genetically in that they have similar genome structure, linear dsDNA with terminal proteins, and related DNA polymerase genes; however, they do not show homology to the other fusiform genera. For this reason, Dyall-Smith and colleagues suggest that they should be classified in a distinct family (Dyall-Smith et al. 2003).
9.1.4.2 Head–Tail Haloviruses
The members of this broad category of haloviruses are characterized by icosahedral heads and a variety of tail structures. Families are grouped by their tail structures: Siphoviridae viruses have long, non-contractile tails, Myoviridae have contractile tails, and Podoviridae which have very short tails (Ackermann 2007). Several hypersaline-dwelling viruses make good models for this morphotype including ΦH (Gropp et al. 1992), ΦCh1 (Klein et al. 2002; Witte et al. 1997), HF1 and HF2 (Nuttall and Dyall-Smith 1993, 1995; Tang et al. 2002). HF1/2 have been especially well-described; both are lytic phage which can infect a range of genera including Haloferax, Halobacterium, Haloarcula, Natrialba, and Halorubrum.
9.1.4.3 Spherical Haloviruses
Icosahedral-shaped particles without tails comprise another group of observed haloviruses. The isolated SH1, a lytic virus which infects the genera Haloarcula and Halorubrum, has an internal lipid layer beneath the protein coat (Porter et al. 2005). Though similar in appearance, the SH1 linear genome has no relationship to that of another spherical archaeal virus SSIV (Prangishvili et al. 2006), which infects Sulfulobus (Porter et al. 2005). The structure of SH1 is well-described in a cryo-EM study which reveals several unique structural features, especially unusual capsid proteins and protruding spikes (Jäälinoja et al. 2008). This category of viruses is difficult to assess with TEM studies in that it may be representative of head–tail species that have lost their tails or of pleomorphic viruses that may assume a spherical shape (Porter et al. 2005; Dyall-Smith, personal communication).
9.2 Results
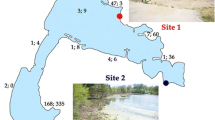
The hypersaline north arm of Great Salt Lake and its accompanying salt production ponds, provide a vast resource for halovirus diversity exploration. There are a number of microniches, including petroleum seep areas, freshwater spring inputs, and desiccated regions. Since the lake is in an elevated desert biome, the seasonal variation is extreme, and annual precipitation is also variable (Baxter et al. 2005). In fact, Bacteria/Archaea diversity along this north meander line varies by location, by season and by year (Litchfield and Baxter, unpublished). One would then anticipate that the halovirus diversity also fluctuates. Studies here should take both a temporal and a spatial approach.
9.2.1 Cell and Viral Counts
In sampling twice a season over a 3-year period (2007–2010), the cell counts representing bacteria and archaea were relatively stable, a range of 4–10 × 107 cells/ml in the spring/summer months, and a range of 1–3 × 106 cells/ml in fall/winter months.
In July of 2009, we counted the phage with epifluorescence microscopy (EFM) using a standard protocol that stains the viral nucleic acid to allow counting of the particles (Patel et al. 2007) (Table 9.1). EFM is currently the most widely used approach for estimating the total abundance of virus particles in aquatic systems (Suttle 2007). Unfixed water samples were processed within several hours after collection to prevent loss of viral counts (Wen et al. 2004). Counts of haloviruses indicated values at all four locations are in the range of 3–6 × 109 particles/ml. Cell counts were also done for site 4, which revealed 6.2 × 107 cells/ml. Therefore, the viral load on the ecosystem was observed to be approximately 100-fold greater than that of the cells in Great Salt Lake. We have not repeated the viral counts in fall/winter sampling as of yet; temporal data will be forthcoming.
9.2.2 Purification of the Virus Community from Great Salt Lake Brine
In June 2007, we began a project to examine the diversity of the lake’s haloviruses. Our goal was to eliminate the bias of cultivatable hosts and to find a method that would produce an overall picture of the viruses present at the gross morphology level. We envisioned a procedure that would allow one to more quickly assess the population and to potentially isolate individual haloviruses for which the host was unknown. Unlike the airfuge study (Sime-Ngando et al. 2010), this would require no specialized equipment beyond an ordinary ultracentrifuge. The use of phase partition provided a means of processing large volumes of hypersaline brine.
Forty liters of hypersaline brine were collected from the north arm of the lake. Cells were removed by slow speed centrifugation, and the supernatant was diluted from approximately 5 M to 1 M NaCl with water and then mixed with polyethylene glycol 8000 to 10%. This slurry was left in the cold room to precipitate over several days and then the supernatant was siphoned off and the flocculent precipitate fraction was spun down at 10,000 × g for 10 min and finally resuspended in a small volume of a buffer of 1 M NaCl, 10 mM Tris, and 2 mM MgCl2. No VLPs were present in the supernatant. This precipitate thus represented the entire viral community present in the brine.
For electron microscopy analysis, 10 μl of this concentrated halovirus suspension was adsorbed to a carbon-covered copper grid (glow-charged just before application) and then stained with a 2% uranyl acetate solution for 2–3 min. After letting the grid air-dry, it was analyzed in a Tecnai 12 instrument. The analysis revealed the presence of expected morphotypes as described above (Fig. 9.1), and in addition, large amounts of a morphotype which should constitute a new halovirus category, as described below.
9.2.2.1 Fusiform Viruses
Though not the most numerous of VLP forms in our Great Salt Lake samples, many lemon-shaped particles were observed (Fig. 9.2). These virus forms were ~50–120 nm in length, appear to have a membrane enclosure, and have a small tail structure at one end. The size diversity may indicate the presence of more than a single species of fusiform halovirus present in GSL brine. However, it is important to note that purified His1 also showed a broad size distribution (Bath and Dyall-Smith 1998).
9.2.2.2 Head–Tail Viruses
Hypersaline VLPs exhibiting an icosahedral head and filamentous tail were the most numerous forms that we encountered in the TEM analysis. Perhaps this group also exhibited the most variety since the head size and tail size varied as well as the morphology of the components. Attached tails varied widely, from simple filaments ending in a point to stiff rods with a complex attachment structure at the end (Fig. 9.3). In some of the phage, the tail sheaths had contracted resulting in a thick segment closest to the head and then a long protruding thin filament extending from the thick segment (Fig. 9.3c). Some tails were long and sometimes curved (Fig. 9.3d). Examples of these forms are prevalent in the published literature, the best-described species being E. coli bacteriophage such as T4, which has an icosahedral head, stiff tail and complex attachment structure at its end, and phage lambda, whose tail is long, often curved and comes to a point.
Since these VLPs were so diverse, we measured the head structures observed on the micrographs to evaluate not only the range but also the distribution (Fig. 9.4). There is a clear cluster between 50 and 64 nm in head diameter. For comparison, the Senegal study reported head size ranges of 50–130 nm (Sime-Ngando et al. 2010).
9.2.2.3 Spherical Viruses
Spherical VLPs with icosahedral structure, electron dense centers, and no tail were observed throughout the Great Salt Lake samples (Fig. 9.5). These forms varied in size from ~40 to ~70 nm in diameter. Some others may have a short tail structure not easily visualized. These would mostly resemble coli phage T7. Smaller homogeneous-looking spherical particles were frequently observed in the background ranging from 20 to 40 nm in diameter. However, their small size, lack of a clear icosahedral shape, and range in sizes precluded a clear identification of them as phage in contrast to other biological structures that could have been present. Indeed, E. coli bacteriophage such as ΦX174 would have fallen into this category of scoring due to their small size and homogenous appearance in the EM.
9.2.2.4 A New Major Category of Haloviruses: Filamentous and Rod-Shaped Viruses
Though there are a few scattered reports of filamentous VLPs in hypersaline waters (e.g., Diez et al. 2000; Sime-Ngando et al. 2010), there has been too little data to create another category. In the negative stained images, we frequently observed many different filamentous forms. Some appeared very similar to bacterial fimbriae, pili, and flagella, being extremely stiff, variable in length, and having diameters in the range of 20 nm. In addition, however, there were also thinner, more flexible filaments and they frequently measured ~1 μm in length (Fig. 9.6a). These resemble both in dimensions and appearance, the well-known E. coli bacteriophages M13 and fd which are 5 nm in width and ~1 μm in length. Occasionally, wider, shorter filamentous particles with a clear central channel were observed (Fig. 9.6b) and these were strikingly similar to the contracted forms of M13 reported previously (Manning et al. 1981).
9.2.3 Coupled Fractionation and TEM
To further purify the viral precipitate, we designed a cesium chloride density gradient that would enable separation of species or morphotypes. We selected the density range (1.2–1.6 g/ml) based on published viral density data, with a focus on haloviruses (Table 9.2).
The precipitate was resuspended in a buffer of 1 M NaCl, 10 mM Tris, 2 mM MgCl2, and 1.35 g/ml cesium chloride to form a density gradient over 24 h ultracentrifugation at 60,000 rpm in a Beckman 70Ti rotor. The fractions formed by the cesium chloride gradient were collected at various densities as indicated in Fig. 9.7. This procedure yielded VLP-rich fractions that could be examined for structure, size, and diversity using TEM.
Analysis of VLP-rich fractions provided some intriguing results. Though there were no fractions that contained exclusively a single morphotype, there were fractions that showed significant enrichment.
For example, fraction 6 (Fig. 9.7), at a cesium chloride density of 1.28 g/ml, was enriched with fusiform VLPs. This is consistent with the density of purified haloviruses, His1, at 1.28 g/ml, and His 2, at 1.30 g/ml (see Table 9.2). However, the measurements of the Great Salt Lake fusiforms demonstrated that they were on average larger than the mean size for His1: Bath and colleagues report a 74-nm length for His1 (Bath et al. 2006) while the haloviruses in Great Salt Lake were larger, measuring 100–138 nm with a mean value of 118 nm. Another example, fractions 12–15 (Fig. 9.7), at a cesium chloride density of 1.33–1.38 g/ml, were enriched with head–tail phage, consistent with the density and size of the well-studied bacteriophages such as T4 (see Table 9.2).
Attempts at cultivation from the cesium chloride fractions with lab-isolated Great Salt Lake halophiles were unsuccessful, underscoring the fact that many host strains may be uncultivatable. However, this approach demonstrates the feasibility of large-scale phage purification from hypersaline brine and the possibility in the future of further purification of single phage species at a scale that would allow nucleic acid sequencing.
9.3 Discussion
9.3.1 Virus-Host Ratios
In hypersaline ecosytems like Great Salt Lake, it seems the predators (viruses) are in greater numbers than the prey (bacteria/archaea). We find a 100-fold difference in numbers, which is supported by other studies that show similar ratios, between 10 and 100-fold (Oren et al. 1997; Dyall-Smith et al. 2003; Jiang et al. 2004; Suttle 2007; Sime-Ngando et al. 2010). The literature suggests that as the number of bacteria/archaea increase or decrease along a salinity gradient, so do the viral counts (Guixa-Boixareu et al. 1996; Oren et al. 1997). The number of haloviruses relative to cells in the brine generally goes against the notion in ecology that predators are limited. That said, common laboratory bacteriophage have been found to be most stable in high salt solutions. Thus, these haloviruses may have a very long lifetime in the brine, and their population numbers may resist fluctuation.
Even if the Great Salt Lake virus-to-cell counts are representative of hypersaline systems, simple counts cannot reveal the actual ratio between a specific host/virus pair. Many viruses produce high particle to plaque-forming units (pfu) ratios, meaning that the majority of virions produced are non-infectious for one reason or another. Furthermore, some halophages have been shown to have a broad host range, infecting not just different species of the same genus, but even being able to infect different genera (e.g., Kauri et al. 1991; Nuttall and Dyall-Smith 1993; Porter et al. 2005). These factors and others may ultimately impact the observed high virus-to-host counts in brine.
9.3.2 Methodology
As we explore the various methods used to access viral load on a system, it seems clear that TEM is the only method that provides data on both abundance and morphology. This, combined with EfM data and fractionation by density gradient, can provide a broad overview of the morphotypes present and a way to enrich for these forms for future studies.
Most significant in our approach, fractionation of environmental samples can be used to enrich specific morphotypes. The assay presented here can be modified in that fractions can be sub-fractionated to increase the separation, for example. Though we did not succeed in isolating single species, perhaps this is because of the great diversity present, which suggests enrichment can be achieved for morphotypes, but not individual species, in an environment with such diversity.
9.3.3 A Call for New Categories of Haloviruses
Morphotypes may suggest similarities among haloviruses. As we obtain genomic data, however, we may find that shape means little. For example, His1 and His2 are fusiform, like SSV1 which infects a thermophile, Sulfolobus, but they do not share significant genetic homology with SSV1 (Porter et al. 2005). Nonetheless, it is a starting place to access diversity.
In the Senegal study (Sime-Ngando et al. 2010), 5% of the total VLPs are forms distinct from the categories of fusiform, spherical and head–tail shapes. The researchers observed hairpin-shaped, filamentous, chain-shaped, hook-shaped, tadpole-shaped, reed-shaped and other complex particles. Oren et al. (1997) reported various morphotypes including an unusual star-shaped morphology. Though these represent a minor component of the overall diversity, it suggests that we should broaden the halovirus morphotype categories. This suggestion comes with an understanding that until one such halovirus is isolated together with its host, we cannot truly distinguish a viral structure from a structure from another source (e.g., sub-cellular structures free in the brine).
Filamentous viruses may infect uncultivatable strains by and large, and so may be under-represented in the halophile literature. In Great Salt Lake, we see filamentous virus-like particles that appear distinct from pili or flagella. Pili and flagella have large diameters (~20 nm) and a clear helical repeat along their length as seen by EM. They are also very stiff. The classic filamentous phage on the other hand are thin, ~5 nm, and relatively flexible with a helical repeat that can be seen only under the most ideal conditions. They are also long, on the order of 1 μm. These features were present in the filamentous particles observed in the samples examined from the Great Salt Lake water. Linear or filamentous particles appear to be the predominant form of archaeal viruses in hyperthermophile communities (Rice et al. 2001; Rachel et al. 2002). However, according to the current literature, this morphotype appears to be missing from hypersaline communities save a few brief notations (e.g., Diez et al. 2000; Sime-Ngando et al. 2010).
In the history of the identification of E. coli phage, the filamentous phage (M13, fd) were some of the very last to be discovered using TEM. While T4 phage can be readily identified even with a TEM of minimal resolution and marginal staining methods, due to its large size and distinct appearance, the filamentous phage may be easily dismissed as background protein filaments and further, their very thin diameter requires higher resolution instruments and optimal negative staining methods. The one clear distinguishing characteristic is their uniform length (~1 μm). However, deletion mutants of M13 readily appear upon cultivation that generate shorter particles (Griffith and Kornberg 1974). Therefore, a clear proof that filamentous phage are present in these Great Salt Lake samples will ultimately require a high enough purification so that length determinations can be made and ideally single-stranded circular DNA purified from the phage. These studies are in progress.
Thus, there is strong evidence that the “filamentous” form should now be considered a new halovirus category. The diversity from a genetic and biochemical point of view within this category is yet to be determined and will depend on isolated haloviruses of this form. Findings like the ones presented here that underscore new categories point to the significance of coupled fractionation/TEM work.
References
Ackermann HW (2007) 5500 phages examined in the electron microscope. Arch Virol 152:227–243
Albertsson PA (1960, 1986) Partition of cell particles and macromolecules. Wiley, New York
Angly FE, Willner D, Prieto-Davo A, Edwards RA, Schmieder R, Vega-Thurber R, Antonopoulos DA, Barott K, Cottrell MT, Desnues C, Dinsdale EA, Furlan M, Haynes M, Henn MR, Hu Y, Kirchman DL, McDole T, McPherson JD, Meyer F, Miller RM, Mundt E, Naviaux RK, Rodriguez-Mueller B, Stevens R, Wegley L, Zhang L, Zhu B, Rohwer F (2009) The GAAS metagenomic tool and its estimations of viral and microbial average genome size in four major biomes. PLoS Comput Biol 5:e1000593
Bath C, Dyall-Smith ML (1998) His1, an archaeal virus of the Fuselloviridae family that infects Haloarcula hispanica. J Virol 72:9392–9395
Bath C, Cukalac T, Porter K, Dyall-Smith ML (2006) His1 and His2 are distantly related, spindle-shaped haloviruses belonging to the novel virus group, Salterprovirus. Virology 350:228–239
Baxter BK, Litchfield CD, Sowers K, Griffith JD, DasSarma PA, DasSarma S (2005) Great Salt Lake microbial diversity. In: Gunde-Cimerman N, Oren A, Plemenitaš A (eds) Adaptation to life in high salt concentrations in archaea bacteria, and eukarya. Springer, Dordrecht, pp 11–25
Baxter BK, Eddington B, Riddle MR, Webster TN, Avery BJ (2007) Great Salt Lake halophilic microorganisms as models for astrobiology: evidence for desiccation tolerance and ultraviolet radiation resistance. In: Hoover RB, Levin GV, Rozanov AY, Davies PCW (eds) Instruments, methods, and missions for astrobiology X, 6694. SPIE, Bellingham WA, document ID 669415
Brum JR, Steward GF (2010) Morphological characterization of viruses in the stratified water column of alkaline, hypersaline Mono Lake. Microb Ecol 60:636–643
Diez B, Antón J, Guixa-Boixereu N, Pedrós-Alió C, Rodríguez-Valera F (2000) Pulsed-field gel electrophoresis analysis of virus assemblages present in a hypersaline environment. Int Microbiol 3:159–164
Dyall-Smith M, Tang SL, Bath C (2003) Haloarchaeal viruses: how diverse are they? Res Microbiol 154:309–313
Griffith J, Kornberg A (1974) Mini M13 bacteriophage: circular fragments of M13 DNA are replicated and packaged during normal infections. Virology 59:139–152
Gropp F, Grampp B, Stolt P, Palm P, Zillig W (1992) The immunity-conferring plasmid p ΦHL from the Halobacterium salinarium phage ΦH: nucleotide sequence and transcription. Virology 190:45–54
Guixa-Boixareu N, Calderon-Paz JI, Heldal M, Bratbak G, Pedrós-Alió C (1996) Viral lysis and bacterivory as prokaryotic loss factors along a salinity gradient. Aquat Microb Ecol 11:215–227
Jäälinoja HT, Roine E, Laurinmäki P, Kivelä HM, Bamford DH, Butcher SJ (2008) Structure and host-cell interaction of SH1, a membrane-containing, halophilic euryarchaeal virus. Proc Natl Acad Sci USA 105:8008–8013
Jiang S, Steward G, Jellison R, Chu W, Choi S (2004) Abundance, distribution, and diversity of viruses in alkaline, hypersaline Mono Lake, California. Microb Ecol 47:9–17
Kaiser AD (1966) On the internal structure of bacteriophage lambda. J Gen Physiol 1:171–178
Kauri T, Ackerman HW, Goel U, Kushner DJ (1991) A bacteriophage of a moderately halophilic bacterium. Arch Microbiol 156:435–438
Klein R, Baranyi U, Rössler N, Greineder B, Scholz H, Witte A (2002) Natrialba magadii virus ΦCh1: first complete nucleotide sequence and functional organization of a virus infecting a haloalkaliphilic archaeon. Mol Microbiol 45:851–863
Leibo SP, Mazur P (1966) Effect of osmotic shock and low salt concentration on survival and density of bacteriophages T4B and T4Bo1. Biophys J 6:747–772
Manning M, Chrysogelos S, Griffith J (1981) Mechanisms of phage M13 contraction: intermediate structures trapped at low temperature. Biophys J 37:28–31
Martin A, Yeats S, Janekovic D, Reiter WD, Aicher W, Zillig W (1984) SAV 1, a temperate U.V.-inducible DNA virus-like particle from the archaebacterium Sulfolobus acidocaldarius isolate B12. EMBO J 3:2165–2168
Nuttall SD, Dyall-Smith ML (1993) HF1 and HF2: novel bacteriophages of halophilic archaea. Virology 197:678–684
Nuttall SD, Dyall-Smith ML (1995) Haloviruses HF2: genome organization and replication strategy. J Virol 69:2322–2327
Oren A, Bratbak G, Heldal M (1997) Occurrence of virus-like particles in the Dead Sea. Extremophiles 1:143–149
Patel A, Noble RT, Steele JA, Schwalbach MS, Hewson I, Fuhrman JA (2007) Virus and prokaryote enumeration from planktonic aquatic environments by epifluorescence microscopy with SYBR Green I. Nat Protoc 2:269–276
Porter K, Dyall-Smith M (2006) The isolation and study of viruses of halophilic microorganisms. In: Rainey F, Oren A (eds) Methods in microbiology, vol 35, Extremophiles. Elsevier/Academic, London, pp 669–690
Porter K, Kukkaro P, Bamford JKH, Bath C, Kivelä HM, Dyall-Smith ML, Bamford DH (2005) SH1: a novel, spherical halovirus isolated from an Australian hypersaline lake. Virology 335:22–33
Porter K, Russ BE, Dyall-Smith ML (2007) Virus-host interactions in salt lakes. Curr Opin Microbiol 10:418–424
Prangishvili D, Forterre P, Garrett RA (2006) Viruses of the Archea: a unifying view. Nat Rev Microbiol 4:837–848
Rachel R, Bettstetter M, Hedlund BP, Häring M, Kessler A, Stetter KO, Prangishvili D (2002) Remarkable morphological diversity of viruses and virus-like particles in hot terrestrial environments. Arch Virol 147:2419–2429
Rice G, Stedman K, Snyder J, Wiedenfeft B, Willits D, Brumfield S, McDermott T, Young MJ (2001) Viruses from extreme thermal environments. Proc Natl Acad Sci USA 98:13341–13345
Rodriguez-Brito B, Li L, Wegley L, Furlan M, Angly F, Breitbart M, Buchanan J, Desnues C, Dinsdale E, Edwards R, Felts B, Haynes M, Liu H, Lipson D, Mahaffy J, Martin-Cuadrado AB, Mira A, Nulton J, Pašić L, Rayhawk S, Rodriguez-Mueller J, Rodriguez-Valera F, Salamon P, Srinagesh S, Thingstad TF, Tran T, Thurber RV, Willner D, Youle M, Rohwer F (2010) Viral and microbial community dynamics in four aquatic environments. ISME J 4:739–751
Sabet S, Chu W, Jiang SC (2006) Isolation and genetic analysis of haloalkaliphilic bacteriophages in a North American soda lake. Microb Ecol 51:543–554
Salivar WO, Tzagoloff H, Pratt D (1964) Some physical-chemical and biological properties of the rod-shaped coliphage M13. Virology 24:359–371
Santos F, Meyerdierks A, Peña A, Rosselló-Móra R, Amann R, Antón J (2007) Metagenomic approach to the study of haloviruses: the environmental halophage 1. Environ Microbiol 9:1711–1723
Santos F, Yarza P, Parro V, Briones C, Antón J (2010) The metavirome of a hypersaline environment. Environ Microbiol 12:2965–2976
Schnabel H, Zillig W, Pfäffle M, Schnabel R, Michel H, Delius H (1982) Halobacterium halobium phage ΦH. EMBO J 1:87–92
Sime-Ngando T, Lucas S, Robin A, Tucker KP, Colombet J, Bettarel Y, Desmond E, Gribaldo S, Forterre P, Breitbart M, Prangishvili D (2010) Diversity of virus-host systems in hypersaline Lake Retba, Senegal. Environ Microbiol (in press)
Suttle CA (2007) Marine viruses – major players in the global ecosystem. Nat Rev Microbiol 5:801–812
Tang SL, Nuttall S, Ngui K, Fisher C, Lopez P, Dyall-Smith M (2002) HF2: a double-stranded DNA tailed haloarchaeal virus with a mosaic genome. Mol Microbiol 44:283–296
Vinograd J, Hearst JE (1962) Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst 20:372–422
Wais AC, Kon M, McDonald RE, Stollar BD (1975) Salt-dependent bacteriophage infecting Halobacterium cutirubrum and Halobacterium halobium. Nature 256:314–315
Wen K, Ortmann AC, Suttle CA (2004) Accurate estimation of viral abundance by epifluorescence microscopy. Appl Environ Microbiol 70:3862–3867
Witte A, Baranyi U, Klein R, Sulzner M, Luo C, Wanner G, Krüger DH, Lubitz W (1997) Characterization of Natronobacterium magadii phage ΦCh1, a unique archaeal phage containing DNA and RNA. Mol Microbiol 23:603–616
Wommack KE, Colwell RR (2000) Virioplankton: viruses in aquatic ecosystems. Microbiol Mol Biol Rev 64:69–114
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Baxter, B.K., Mangalea, M.R., Willcox, S., Sabet, S., Nagoulat, MN., Griffith, J.D. (2011). Haloviruses of Great Salt Lake: A Model for Understanding Viral Diversity. In: Ventosa, A., Oren, A., Ma, Y. (eds) Halophiles and Hypersaline Environments. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-20198-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-642-20198-1_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-20197-4
Online ISBN: 978-3-642-20198-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)