Abstract
Ground granulated blast furnace slag (GGBS) is a by-product from the blast-furnaces used to make iron. Blast-furnaces are fed with controlled mixture of iron-ore, coke and limestone, and operated at a temperature of about 1,500°C. When iron-ore, coke and limestone melt in the blast furnace, two products are produced—molten iron, and molten slag. The molten slag is lighter and floats on the top of the molten iron. The molten slag comprises mostly silicates and alumina from the original iron ore, combined with some oxides from the limestone. The process of granulating the slag involves cooling of molten slag through high-pressure water jets. This rapidly quenches the slag and forms granular particles generally not bigger than 5 mm. The rapid cooling prevents the formation of larger crystals, and the resulting granular material comprises around 95% non-crystalline calcium-aluminosilicates. The granulated slag is further processed by drying and then grinding in a rotating ball mill to a very fine powder, which is GGBS.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
3.1 Introduction
Ground granulated blast furnace slag (GGBS) is a by-product from the blast-furnaces used to make iron. Blast-furnaces are fed with controlled mixture of iron-ore, coke and limestone, and operated at a temperature of about 1,500°C. When iron-ore, coke and limestone melt in the blast furnace, two products are produced—molten iron, and molten slag. The molten slag is lighter and floats on the top of the molten iron. The molten slag comprises mostly silicates and alumina from the original iron ore, combined with some oxides from the limestone. The process of granulating the slag involves cooling of molten slag through high-pressure water jets. This rapidly quenches the slag and forms granular particles generally not bigger than 5 mm. The rapid cooling prevents the formation of larger crystals, and the resulting granular material comprises around 95% non-crystalline calcium-aluminosilicates. The granulated slag is further processed by drying and then grinding in a rotating ball mill to a very fine powder, which is GGBS.
GGBS can be used as a direct replacement for ordinary cement on one-to-one basis by weight. Replacement rates for GGBS vary from 30% to up to 85%. Generally 50% is used in most applications. Higher replacement rates up to 85% are used in specialist applications such as in aggressive environments and to reduce heat of hydration. GGBS can be used at replacement levels of 70% in lean mix concrete.
3.1.1 Storage and Handling of GGBS
Bulk GGBS is stored and handled in conditions identical to that of Portland cement. Bulk storage is in watertight silos. Transportation is by bulk tankers, as for Portland cement. GGBS can also be moved by air-slides, cement screws and bucket elevators. Dust control is the same as that required for Portland cement. GGBS dust does not present any fire or explosion hazard.
3.1.2 Environmental Benefits of Using GGBS
The use of GGBS as partial cement replacements with lower environmental burdens offers opportunities for significant reductions in energy use and carbon dioxide emissions. Proportions of up to 70 or even 80% can be used with advantage in suitable situations. The use of GGBS in concrete results in following environmental benefits:
-
Saves energy.
-
Reduces emission of carbon dioxide.
-
Conserves natural resources.
3.2 Characteristics of GGBS
3.2.1 Physical Properties
Ground granulated blast-furnace slag is a glassy material. The colour of GGBS varies from beige to dark to off-white depending on moisture content, chemistry and efficiency of granulation. When it is ground it has usually white colour. It imparts a lighter, brighter color to concrete. Figure 3.1 shows the colour of GGBS.
GGBS is a fine glassy material. Its specific gravity is less than that of cement, but has more fineness. Its physical properties, reported by some authors are given in Table 3.1.
3.2.2 Particle Morphology
Wan et al. [75] reported the diameter and surface area of GGBS samples (A, B, C, and D) prepared by using four processing approaches, a ball mill, steel balls as grinding medium, an airflow mill and a vibro-mill, respectively. Surface area and diameter of GGBS are given in Table 3.2. It can be seen that diameter of GGBS particles varied from 9.12 to 13.69 μm whereas surface area was between 510 and 685 m2/kg.
The shape of GGBS processed by a vibro-mill was predominantly spherical with a smooth surface, while that by a ball mill and an airflow mill appeared to have similar edges.
Wan et al. [75] observed the morphology of four GGBS samples (A, B, C, and D) prepared by using four techniques a ball mill, steel balls as grinding medium, an airflow mill and a vibro-mill, respectively. SEM images of samples A–D are shown in Fig. 3.2. It can be seen that the shape of GGBS is not spherical; it varies according to different grinding techniques. GGBS is mainly composed of glassy phases, which are in a continuous network structure, and there is no stress concentration in the interfacial area; thus, grinding GGBS leads to the disconnection of bonds between molecules and atoms. When slag particles are broken, the shape of the broken surface is not fixed. Fig. 3.2a and d is the appearance of GGBS processed by a ball mill, which is predominately in anomalous shape with clear edges and angles. This is due to inter-impacting and inter-rubbing between steel balls in the ball mill. Figure 3.2b shows that the shape of GGBS is mostly in sphericity and its surface is relatively smooth. The reason for this phenomenon is that GBBS is crushed by the interaction between a steel cylinder and a steel ring and that between a steel ring and a vessel wall; in addition, this increases the probability of inter-rubbing between particles and leads to smooth edges and angles of particles. Figure 3.2c shows that the GGBS sample has the most uniform particle size, and the particle appearance is similar to sample A. This has much to do with the working mechanism of airflow mills. When an airflow mill is working with a high-speed air current, particles collide severely with fixed manganese-steel boards, and this leads to pulverization and thus decreases the probability of contacts between particles.
SEM of granulated blast furnace slag samples: a SEM of sample A (Ball-mill), b SEM of sample B (Vibromill), c SEM of sample C (Airflow mill) d SEM of sample D (Ball-mill) [75]
3.2.3 Chemical Composition
Blast furnace slag is a non-metallic product, consisting essentially of silicates and alumino-silicates of calcium and other bases. Slag is made up of both glassy and crystalline phases. The glassy nature is responsible for its cementitious properties. In GGBS, glass content is between 85 and 90%. GGBS comprises mainly of CaO, SiO2, Al2O3, MgO. It has the same main chemical constituents as ordinary Portland cement, but in different proportions. Typical Chemical Composition of GGBS, reported by some authors is given in Table 3.3.
3.2.4 Reactivity
ASTM C 989 [6] is the standard for evaluating the cementitious potential of a slag. The factors that determine the cementitious properties of a slag are:
-
Chemical composition of the slag.
-
Alkali concentration of the reacting system.
-
Glass content of the slag.
-
Fineness of both slag and cement.
-
Temperature variations during the early phases of hydration process.
Daube and Bakker [22] defined two basic parameters that determine the hydraulic properties of a slag (1) chemical composition; and (2) vitreous state. Cheron and Lardinois [19] have defined the hydraulic activity index of the slag as below. Slag indices between 1.65 and 1.85 are considered normal:
3.2.4.1 Hydration Reaction
The hydration mechanism of a combination of GGBS and Portland cement is slightly more complex than that of a Portland cement. This reaction involves activation of the GGBS by alkalis and sulfates to form its own hydration products. Some of these combine with the Portland cement products to form further hydrates which have a pore blocking effect. The result is a hardened cement paste with more of very small gel pores and fewer of the much larger capillary pores for the same total pore volume. Generally, the rate of strength development is slower than for a Portland cement mortar.
The resulting hardened cement paste using GGBS is also more chemically stable. It contains much less free lime, which in concrete made with Portland cement leads to the formation of further reaction products such as ettringite or efflorescence. In addition, GGBS contains no C3A, making GGBS concrete much less reactive to sulfates.
When GGBS is used in concrete, resulting hardened cement paste has more smaller gel pores and fewer larger capillary pores than is the case with concrete made with normal Portland cement. This finer pore structure gives GGBS concrete a much lower permeability, and makes an important contribution to the greater durability of this concrete.
Ballim and Graham [15] studied the effect of GGBS on the heat of hydration of concrete. Concretes were prepared with GGBS, blended with Portland cement in proportions ranging from 20 to 80%. These concretes were subjected to heat of hydration tests under adiabatic conditions. Heat rate performances of materials are expressed in terms of maturity or t20 (h), which refer to the equivalent time of hydration at 20°C. This form of expression of heat rate function and the justification for its use is described by [14]. Table 3.4 shows that the time to reach this peak hydration rate is marginally reduced as the proportion of GGBS is increased to 60%. However, the reduction in time to peak heat rate is more significant as the proportion of GGBS was increased from 60 to 80%. It is also evident that normalized peak heat rates for the GGBS concretes are higher than that of the plain CEM I concrete. This indicates that the hydration of the GGBS is contributing to the generation of heat in the concrete, even at these relatively early ages.
Gao et al. [28] studied the hydration of concrete made with GGBS. Specific surface area of cement and GGBS were 361, 425, 600 m2/kg, respectively. Cement was replaced with 40% GGBS. The self-hydration of cement and GGBS produces Ca(OH)2. In a saturated solution of Ca(OH)2, the pozzolanic reaction of GGBS consumes Ca(OH)2. Therefore, the quantity of Ca(OH)2 crystals depends on its formation and reaction rates in a Ca(OH)2 saturated solution. When the formation rate is faster than reaction rate, then, amount of Ca(OH)2 crystal and height of the XRD peak increases.
Figure 3.3 shows the XRD diagram of paste containing 40% GGBS (specific surface area 425 m2/kg) as partial replacement of Portland cement. The peak height in the XRD curve, corresponding to Ca(OH)2 increased when the self-hydration rate of the Ca(OH)2 crystal XRD peak height. The above analysis demonstrates that XRD diagrams can indicate the pozzolanic reaction rate of GGBS indirectly.
XRD diagram of paste with GGBS (specific surface area 425 m2/kg) replacing 40% Portland cement [28]
Figure 3.4 is an XRD diagram of paste containing GGBS (specific surface area 600 m2/kg) partially replacing (40%) of Portland cement. It was indicated in Fig. 3.4 that the CH peak height at 7 days was not as high as that in Fig. 3.3; this means that the pozzolanic reaction of GGBS in the sample starts to consume CH at 7 days. It means that the pozzolanic reaction of GGBS (specific surface area 600 m2/kg and a weight fraction of 40%) starts earlier and proceeds at a fast rate.
XRD diagram of paste with GGBS (specific surface area 600 m2/kg) replacing 40% Portland cement [28]
Zang et al. [80] studied the hydration mechanism and mineral phase structures by waterglass activation of granulated blast furnace slag (GBFS). The GBFS used for synthesis of geopolymer had Blaine specific surface area of 701 m2/kg. Chemical reagent (sodium metasilicate Na2SiO3· 9H2O) was used as alkali activator with waterglass modulus as 1.0. Raw material was blended in the ratio of waterglass:GBFS:water = 1:9:2.5 (in weight). The paste was cast into 50 × 31.6 × 31.6 mm3 metal model. After demolding, a triplicate set of samples were put in a curing box at 20°C with 99% relative humidity and 0.5 h initial curing time, and final setting times of 1-day (1d), 3 days (3d), 7 days (7d), and 28 days (28d), respectively. The X-ray diffraction (XRD) patterns of geopolymers were carried out on an X’Pert PRO MPD diffractometer.
Figure 3.5 shows the XRD patterns of samples by waterglass activation of GBFS. From the pattern of GBFS it can be observed that there is a broad diffuse hump peak in the region 20–38o 2θ suggesting that the GBFS predominantly consists of glassy phases. Besides, there are four kinds of mineral phases, akermanite, gehlenite, calcium silicate, and merwinite. In comparison to the pattern of GBFS, the relative intensities of diffraction peaks had no distinct changes and have no new peak appearance from setting time for 0.5 h to final setting time for 4.2 h, implying that sodium hydroxide deriving from the hydrolysis of waterglass promotes some vitreous dissolution in the periods of curing ages.
XRD patterns of samples by waterglass activation of GBFS. The curve of GBFS, setting time for 0.5 h, initial time for 1.3 h and final setting time for 4.2 h, respectively [80]
The XRD patterns of samples for curing ages 1d, 3d, 7d, 28d and steam curing time of 28d are displayed in Fig. 3.6. After the curing time of 1d, the intensities of some diffraction peaks remarkably decreased and some peaks disappeared in comparison to final setting time in Fig. 3.3 (a) demonstrating that those crystal phases, either partial or whole, dissolved into aqueous sodium hydroxide solution derived from the hydrolysis of waterglass. Some new phases, tobermolite and calcium silicate hydrate (CSH) gel, were generated during curing time of 1d.
XRD patterns of samples by waterglass activation of GBFS in the period of curing times 1–28 days [80]
3.2.5 Specifications of GGBS
ASTM C 989 [6] provides for three strength grades of slag, depending upon their respective mortar strengths when blended with an equal mass of Portland cement. The classifications are; Grades 120, 100, and 80, based on the slag-activity index expressed as SP/P × 100, where SP is the average compressive strength of slag mortar cubes and P is the compressive strength of reference cement mortar cubes (without slag). Grades 100 and 120 are the most commonly used as admixtures in concrete. Table 3.5 gives criteria for ASTM C 989 classification.
3.2.6 Advantages of Using GGBS
Use of GGBS in cement and concrete results in
-
Improved workability and compaction characteristics.
-
Increased pumpability.
-
Increased strength.
-
Enhanced durability.
-
Reduced permeability.
-
High resistance to chloride penetration.
-
High resistance to sulfate attack.
-
High resistance to ASR.
-
Low heat of hydration.
-
Improved surface finish.
-
Enhanced architectural appearance
-
Suppresses efflorescence
-
Enhancement of the life cycle of concrete structures.
-
Reduction in maintenance and repair costs.
-
Slashes lifetime construction costs.
-
Production of GGBS involves virtually zero CO2 emissions, and no emissions of SO2 and NOx.
3.3 Fresh Properties of Mortar/Paste/Concrete Containing GGBS
3.3.1 Bleeding Characteristics
Bleeding is the term used to describe the movement of water to the surface of the freshly placed concrete. All concrete bleeds to some extent but bleed water is only observed on the surface when the rate of bleeding exceeds the rate of evaporation. Immediately after compaction, there is a short dormant period which is followed by a period in which the rate of bleeding is almost uniform. Bleeding ends when either the movement of water is blocked by the growth of hydration products or by the solids effectively coming into contact with each other. Bleeding results in a variation in the effective water content throughout the concrete element which produces corresponding changes in the concrete properties.
Wainwright and Ait-Aider [73] investigated the influence of the addition of GGBS (40 and 70%) on the bleed characteristics of concrete. The bleed water was drawn at 10 min intervals during the first 40 min and at 30 min intervals thereafter until cessation of bleeding. Partial replacement of OPC with 40 and 70% of GGBS led to increase in the bleeding of the concretes, and increase were more pronounced at the higher replacement levels.
Olorunsogo [56] examined the effect of particle size distribution (PSD) of GGBS on the bleeding rate and bleeding capacity of mortar mixes containing 30 and 70% slag. Water retained on the surface of the mortar mixes was collected at intervals of 10 min within the first 40 min, and 30-min intervals thereafter until cessation of bleeding. They concluded that (1) on the basis of equal w/c, bleeding rate and capacity increased significantly with increase in slag content except for the 30% slag mortar mix, which had the lowest bled capacity at 0.45 w/c; (2) when PSD of slag was varied by altering slope (n) at constant position parameter (x 0) no specific relationship between bleeding characteristics and PSD was observed. However, the highest value of bleeding rate and capacity were exhibited by mixes in which the slag with intermediate value of slope was included. Also, the slag samples with similar size range distribution (having a constant slope, n) the bleeding rate increased with the increase in x 0, except the 30% slag mixes that were made to 0.35 w/c. Changing the w/c from 0.35 to 0.45 resulted in increase of 86, 83, and 71% in bleeding rate of the OPC, 30, and 70% slag mixes, respectively.
Wainwright and Rey [74] studied the influence of GGBS on the bleeding of concrete. GGBS from four different sources (S1–S4) and Portland cement from one source were used. One control mix with cement content of 300 kg/m3 was used in mix proportion of 1:2.5:3.6, having water/binder ratio of 0.56. Slag replacement levels were 55 and 85% by weight of cement. Bleed tests were performed in accordance with ASTM C232 [4], starting 30, 75 and 120 min after completion of mixing for each of the mixes. Bleeding rate is defined as the volume of water collected per second during the first 40 min of the test. The volume is expressed in ml/cm2 of exposed surface. Bleed capacity is defined as the fraction of the initial volume of the concrete that has separated out as bleed water during the entire course of the test. Bleed capacity and bleed rate of concrete mixes are given in Table 3.6. They concluded that (1) addition of 55% slag increased the bleed capacity by 30% (compared to the plain Portland cement (OPC mix) but had little effect on bleed rate; (2) Increasing slag content to 85% had no further significant effect on bleeding; (3) source of slag was also found to have little effect on the bleeding; (4) delaying the start of the bleed tests from 30 to 120 min reduced the bleed capacity of the OPC mix by more than 55% compared with 32% for the slag mixes. The reduction in bleed rate was similar for all mixes at about 45%.
3.3.2 Workability
As per ACI Committee [1], the greater solid volume and higher fineness of slag allow more coarse aggregate to be used without the loss of workability. This often reduces the stickiness of the mix.
Meusel and Rose [52] experimented with highly active slag at contents of 30–50% in concrete. They observed that inclusion of slag improved the workability of concrete mixes, but greater improvement was achieved with higher slag content, and higher fineness of slag did not had significant effect on the workability. Stutterheim [68] concluded that slag concretes have appreciably better workability than Portland cement concretes, allowing for reduction in water quantity.
Wainwright and Rey [74] reported the influence of GGBS additions on the slump of concrete. GGBS from four different sources (S1–S4) and Portland cement from one source were used. One control mix proportion of 1:2.5:3.6 was used having w/b ratio of 0.56. Slag replacement levels were 55 and 85% by weight of cement. Mixture proportions and slump results are given in Table 3.7.
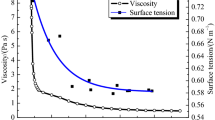
Wan et al. [75] concluded that workability of mortar increased with the increase in the surface area of GGBS. Four samples (A, B, C, and D) of GGBS were used. Fluidity ratio was determined of the samples. The influence of GGBS on the fluidity of mortar is shown in Table 3.8. The fluidity of sample B and D was much better than that of sample A. The fluidity of mortar was associated with the morphological characteristics of the GGBS particles. The smoother the surface of particles, the better was the fluidity of mortar.
3.3.3 Setting Times
The setting time of concrete with GGBS is generally greater than that of similar concrete with Portland cement only. Setting time increased with the increase in GGBS content as shown in Fig. 3.7.
Effect of GGBS on setting time [76]
Wainwright and Ait-Aider [73] investigated the effect of GGBS on the setting times and consistency of cements. Cement from three different sources and GGBS from one source was used. Cements were partially replaced with 40 and 70% of GGBS. Table 3.9 gives the results of setting times and consistency of OPC and blended cements with GGBS. They concluded that (1) consistency and setting times were almost similar for all three sources of cements; (2) inclusion of GGBS affected the consistency of cements, and it deceased with the increase in GGBS content; and (3) setting time of cements was increased with the increase in GGBS content.
3.4 Properties of Hardened Concrete Containing GGBS
3.4.1 Water Absorption
Pavía and Condren [60] studied the effect of GGBS on the water absorption of mortar subjected to immersion in silage effluent solution and magnesium sulfate solution. Cement was replaced with 0, 30, and 50% GGBS. The chemical composition of synthetic silage effluent was lactic acid (15 g/kg), acetic acid (5 g/kg), formalin (3 g/kg), KOH (3.67 g/kg), NaOH (0.78 g/kg), Ca (OH)2 (1.39 g/kg), and Mg (OH)2 (0.44 g/kg). Three 28-day cycles of effluent exposure were repeated over a 4-month period. It was observed that with the inclusion of GGBS, final-water absorption of mortar decreased. OPC mortar specimens absorbed 18.65% water whereas it was 12.65 and 8.26% with 30 and 50% GGBS.
Elahi et al. [25] reported the water absorption (sorptivity) results of concrete containing GGBS (50 and 70%) at the age of 44 and 91 days. The sorptivity values of control concrete were 100 m3 × 10−7/min½ at 44 and 91 days, respectively. The mix with 50% GGBS yielded lower value of sorptivity (77.1 m3 × 10−7/min½) at 44 days compared to that at 91 days (96.9 m3 × 10−7/min½). Increasing the GGBS content to 70% significantly increased the sorptivity (120.1 m3 × 10−7/min½) at 44 days but considerably reduced the sorptivity (103.8 m3 × 10−7/min½) at 91 days.
3.4.2 Microstructure
GGBS can be effectively used in concrete to reduce the pore size [16]. Higher GGBS replacement percentage has denser structure and prevents concrete from water penetration. The GGBS reacts with water in alkali environment and then with calcium hydroxide to form cement hydration product through pozzolanic reaction to form extra C–S–H gel in the paste and slow down the strength development at early age. Denser microstructure or lower porosity results from higher C–S–H content that represents higher GGBS replacement percentage and higher durability of concrete.
SEM micrographs (Fig. 3.8) by Daube and Bakker [22] indicated that the addition of GGBFS modifies the products and the pore structure in a hardened cementitious material. It was found that a great number of calcium hydroxide and large capillary pores (0.05–60 μm) were found in OPC specimens. But few needle shape ettringite existed in GGBFS concrete specimens and the capillary pores were less than (10–50 μm) which could be filled up with pozzolanic reaction product such as low density C–S–H gel. Hydration product; Ca(OH)2 activates the slag hydration to from a mixture of low CaO/SiO2 (C/S) ratio CaO–SiO2–H2O (C–S–H) and AFm (cementitious product from the reaction of reactive alumina and calcium hydroxide) phases. Pozzolanic reaction is also found to increase the C/S ratio to a value of about 1.7 in slag-cement blends due to unstable low calcium C–S–H and Ca(OH)2 mixture. When supplementary cementitious material like GGBFS are used in concrete, they do not only reduce the porosity but also the pores become finer and the change in mineralogy of the cement hydrates leads to the reduction in mobility of chloride ions.
SEM micrograph of: a ordinary Portland cement concrete and b GGBFS (60%) concrete [22]
Luo et al. [47] studied the pore structure of three types of concretes made with OPC, OPC/70% GGBS and OPC/65% GGBS/5% gypsum. The mix ratio for three concretes was 1:1.7:3.29 with water–binder ratio of 0.34. It was reported that coarse pores of GGBS concrete were much less and the pore structure of OPC concrete was improved greatly when 70% GGBS was added, especially for the pore structure for the time of 60 days. But sulfates did not improve the pore structure of GGBS as expected, and it showed that some other causes, except the expanding reaction between sulfates and C3A, influence the pore structure of concretes.
Li and Zhao [45] investigated the effect of combination of GGBS and fly ash (FA) on the microstructure of concretes. Three types of concretes viz GGFAC (concrete incorporating GGBS and FA), HFAC (high-volume FA concrete), and PCC (control Portland cement concrete) were made. PCC had 500 kg/m3 of cement, HFAC had 300 kg/m3 of cement and 200 kg/m3 FA, GGFAC had 300 kg/m3 of cement and 125 kg/m3 FA and 75 kg/m3 of GGBS. Microstructure of concretes was studied at the age of 7 and 360 days using scanning electron microscope.
The image characteristics (SEM) of concretes at 7 days are shown in Figs. 3.9a, b, 3.10a, b and 3.11a. As can be seen from Fig. 3.9a, there were great deals of needle-shaped ettringite and plated-shaped calcium hydroxide in PCC, and large pores could also be observed. Figure 3.11a shows numerous un-hydrated FA particles in HFAC. However, SEM observation of GGFAC (Fig. 3.10a) shows that microstructure changed greatly with the incorporation of GGBS, and no FA particles can be observed. The main hydration products were cotton-shaped C–S–H gel and a certain amount of needle-shaped ettringite. The characteristics of concretes at 360 days are shown in Figs. 3.9b, 3.10a, b and 3.11a, b. It is seen from Fig. 3.9b that though the microstructure of PCC was very compact; there were great deals of plated-shaped calcium hydroxide in it. The microstructures of HFAC and GGFAC were also very compact, and no needle-shaped ettringite or plated shaped calcium hydroxide was observed.
SEM micrograph of PCC; a at the age of 7 days, b at the age of 360 days [45]
SEM micrograph of GGFAC; a at the age of 7 days, b at the age of 360 days [45]
SEM micrograph of HFAC; a at the age of 7 days, b at the age of 360 days [45]
Gao et al. [28] studied the morphology (Fig. 3.12) of the hydration products in concrete made with (GGBS) using SEM. Specific surface area of cement and GGBS were 361, 600 m2/kg, respectively. Cement was replaced with 40% GGBS. At curing age of 7 days, the GGBS particles’ surface was covered with hydration product.
SEM picture of concrete containing GGBS at 7 days of curing [28]
Binici et al. [12] made the micro-structural investigations of blended cements prepared with clinker (4%), and made with basaltic pumice and GGBS. Different amounts of additives (10 and 30%) were incorporated into these blends. Cement paste and mortars were prepared using OPC, two types of grinding systems (inter-grinding and separate grinding) at two Blaine values (2,800 ± 30 and 4,800 ± 30 cm2/g). For micro-structural studies, SEM photos were taken at the end of 7-day testing (Fig. 3.13). A large amount of needle-shaped ettringite and plate-shaped calcium hydroxide can be observed in the blended cement. These figures show the formation of Portlandite (P) and CSH in OPC. The main hydration products are cotton-shaped CSH gel and a certain amount of needle-shaped ettringite.
SEM images of blended cement samples:a OPC sample (clinker 96%, gypsum 4%, fineness 2800 cm2/g), b Blended cement sample—separate grinding (clinker 66%, gypsum 4%, GGBS 15%, blast pumice 15%, fineness 2800 cm2/g), c Blended cement sample (clinker 96%, gypsum 4%, fineness 4800 cm2/g)[12]
Bouikni et al. [17] studied the microstructure of concrete made with 50 and 65% slag. A high alkali Portland cement with 1% sodium oxide and slag with 0.53% sodium oxide equivalent were used. Two concrete mixtures having high workability and low water–binder ratios were used. The mix proportions (by mass) were 1:1.75:2.53:0.43 and 1:1.67:2.41:0.40 (cement + slag:sand:aggregate:water), respectively, for the 50 and 65% slag content, respectively. Scanning electron microscopy (SEM) was used to study the internal microstructure. The fractured sections of concrete prisms were examined by SEM at about 6 months. Concrete cured continuously in a wet environment, irrespective of the slag replacement level, showed development with time of a compact and dense texture. Concretes exposed to a prolonged drying environment invariably showed the presence of pores and micro cracks. Figure 3.14 shows the microstructure of the two concretes subjected to 7 days initial water curing prior to drying in ambient conditions. These pictures confirm the drying environment to be the major cause of increased porosity in slag concretes as the replacement level and the drying exposure period both increase.
SEM micrographs of a concrete with 50% and b concrete with 65% slag subjected to 7 days fog + Lab curing [17]
3.4.3 Compressive Strength
3.4.3.1 Compressive Strength of Mortar
According to Neville [55], slag cements tend to be finer than Portland cement, but the strength gain in first 28 days is somewhat slower. At later-ages, the strength is similar. The low heat of hydration of slag cements make them vulnerable to frost damage in cold weather concreting.
Hogan and Meusel [35] reported strength of slag-blended cements made with using a slag with a target fineness of 55 cm2/g. They found that (1) mortar strengths of cements developed more slowly than the controls for the first 3 days, after which the rate of strength development increased sharply. The 7-day and later-age strengths were greater than for the controls; (2) slag cement mortars subjected to elevated temperature-curing were stronger than the controls at all ages (1, 7, and 28 days); (3) strengths of slag-cement mortars subjected to low temperature curing were lower than for the controls at 1, 7, and 28 days. Similar results were also found for concrete; and (4) the optimum dosage of slag for strength development was 40%.
Gee [30] reported that early strength development in slag cement is affected by the chemistry of the clinker, since the manner in which it releases calcium and alkali cations affects the rate of hydration of the slag. Clinker can be formulated with high lime content for use in blends with high slag contents.
Wan et al. [75] reported the effect of GGBS on compressive strength and activity index of mortars up to the age of 28 days. Four samples (A, B, C, and D) of GGBS were used. The weight proportion of reference sample (with out GGBS) was: Portland cement/standard sand/water = 540:1,350:238, whereas for samples containing GGBS, it was: Portland cement/GGBS/standard sand/water = 270:270:1,350:238. Results of compressive strength and activity index are given in Table 3.10. They concluded that strength of mortar incorporating GGBS is both related to the surface area and PSD of GGBS. When GGBS had the same surface area, the more the mortar contained fine particles (<3 μm) of GGBS, the higher its early strength was. The mortars containing 3–20 μm particles of GGBS, the higher was its long-term strength; and (2) compressive strengths increased with the increase of the surface area of GGBS.
Cakir and Aköz [21] studied the effect of curing conditions on the compressive strength of mortars with and without GGBS. In mortar mixes, cement was replaced with 0, 30, and 60% GGBS by weight. One group of mortar mixes (W) was kept in water at 20°C standard conditions, and the other group of mortar mixes (H) was kept in moisture cabinet at 40°C and 100% relative humidity (RH). They concluded that (1) at the age of 7 days, compressive strength of mortars (W) were 33.7, 31.0, and 26.3 MPa at 0, 30 and 60% GGBFS content whereas compressive strength of mortar mixes (H) were 36.9, 34.1, and 32.6 MPa with 0, 30 and 60% GGBFS; (2) compressive strength of mortars increased with time for both curing conditions. In comparison with 7-day strength, strength of control mortars cured in water (0 W) at 20°C temperature increased by 54 and 109% at 28 and 180 days, respectively. Slag replaced mortars 3 W group’s (30% GGBS) compressive strength increased by 60 and 117%, 6 W group’s (60% GGBS) strength increased by 72 and 140% at 28 and 180 days; and (3) compressive strength of control specimens, cured, in moisture, increased by 34 and 82% at 28 and 180 days, respectively, in comparison with 7-day strength. Compressive strength of slag replaced mortars 3H group (30% GGBS) increased by 26 and 68%, and 6H group (60%) increased by 27 and 69% at 28 and 180 days.
Pavía and Condren [60] investigated the effect of GGBS on the compressive strength of mortar specimens subjected to immersion in silage effluent solution and magnesium sulfate solution. Cement was replaced with 0, 30, and 50% GGBS. The chemical composition of synthetic silage effluent was lactic acid (15 g/kg), acetic acid (5 g/kg), formalin (3 g/kg), KOH (3.67 g/kg), NaOH (0.78 g/kg), Ca(OH)2 (1.39 g/kg), and Mg (OH)2 (0.44 g/kg). Three 28-day cycles of effluent exposure were repeated over a 4-month period. The initial compressive strength of the samples prior to effluent exposure was 13.83, 19.76, and 24.79 N/mm2 for the OPC, 30% GGBS and 50% GGBS samples, respectively. They concluded that there was 46.7, 32.74, and 21.82% loss in compressive strength for OPC, 30 and 50% GGBS.
Roy and Idorn [64] also reported similar results. However, it was reported that the benefit in strength of concrete containing 20–60% GGBS did not occur until after 28 days of curing, where similar or higher long term strength was obtained as compared with that of normal PC concrete [2, 39, 53].
3.4.3.2 Compressive Strength of Concrete
Douglas et al. [24] investigated the compressive strength development of alkali activated ground granulated blast-furnace slag concretes. These concretes incorporated sodium silicate as an activator but did not contain any Portland cement. Five alkali activated slag concrete mixtures were made. The first three mixtures were made using a sodium silicate solution having a silicate modulus (Ms = 1.47) and low water-to-binder ratios ranging from 0.34 to 0.39. Two other mixtures incorporated a sodium silicate solution of silicate modulus (Ms = 1.36) and higher water-to-binder ratios of 0.50 and 0.46. One of these mixtures was air-entrained. Cylinders of size 102 × 203 mm were cast to determine compressive strengths up to one year. Compressive strength test results are given in Table 3.11. They reported that (1) strength of all the mixtures increased with age; (2) 28-day strength ranged from 46.3 to 59.6 MPa, where as 91-day strength was between 48.5 and 62.8 MPa; (3) ground granulated blast-furnace slag activated with sodium silicate can be used to make slag concretes with satisfactory workability and strength up to 91 days.
Ujhelyi and Ibrahim [72] studied the effect of hot weather on the compressive strength of concrete made with various percentages of GBFS and ground rhyolite tuff as partial replacement of cement. The cement in control mix was 350 kg/m3 with water-to-cement ratio 0.531. Two series of mixes were made: the materials of the first were stored in laboratory (room temperature 20°C ± 3°C, relative humidity 20–35%), that of the second in room (temperature 42°C ± 1°C, relative humidity 20%). Compressive strength was determined at 7, 28 and 90 days. Based on the investigation, it was concluded that (1) concretes containing slag as a partial replacement of cement (up to 40%) achieved higher compressive strength than that of concretes made with Portland cement alone when casting and curing at 42°C; (2) 7-day compressive strength of concretes made with additives were less than that of concretes made with Portland cement; (3) 28- and 90-day compressive strength of concretes made with slag or pozzolana reached that of concretes made with pure Portland cement; and (4) in-spite of reducing Portland cement content with increasing ratio of additive, compressive strengths remain unchanged after casting and curing at high air temperature comparing with that of Portland cement concretes because the active SiO2 of additives reacts at high temperature with Ca(OH)2 of cement in the early period.
Wainwright and Rey [74] studied the influence of GGBS on the compressive strength of concrete. GGBS from four different sources (S1–S4) and Portland cement from one source were used. Control mix had 1:2.5:3.6 proportion with water/binder ratio of 0.56. Cement was replaced with 55 and 85% of slag by weight. Compressive strength of all mixes up to 28 days is given in Table 3.12. It can be seen from the table that source of the slag appeared to have little influence on strength development, and strength of OPC concrete and those containing GGBS increased with age.
Babu and Kumar [13] determined the cementitious efficiency of GGBS in concrete at various replacement percentages (10–80%) through the efficiency concept by establishing variation of strength to w/cm ratio relations of the GGBS concretes from the normal concretes at the age of 28 days. The 28-day compressive strength of concretes containing GGBS up to 30% replacement were all slightly above that of normal concretes, and at all other percentages, the relationships were below that of normal concretes. It was also observed that variations due to different percentages of slag replacement were smaller than the corresponding variations in case of FA. The result showed that the slag concretes based on overall efficiency factor (k), will need an increase of 8.6 for 50% replacement and 19.5 for 65% replacement in the total cementitious materials for achieving strength equivalent to that of normal concrete at 28 days.
Quillin et al. [62] studied the early strength development of ‘BRECEM’ concretes, made from 50:50 mixtures of calcium aluminate cement (CAC) and GGBS, using 100-mm cubes at w/c ratios of 0.35 and 0.45. Concretes were both air-cured and water-cured. Tests were conducted up to the age of 90 days. Compressive strength results are shown in Fig. 3.15. They concluded that (1) BRECEM concrete with w/c ratio of 0.35 developed strength more rapidly than those made with w/c ratio of 0.45. The 90-day strength was also higher than those made with w/c ratio of 0.45, although for water stored concretes, strengths were still increasing in both cases; (2) BRECEM concretes stored in air initially developed strength more rapidly than equivalent concretes stored in water. The initial strength development was predominantly due to the hydration of the more reactive CAC component. However, the compressive strength of air-stored concretes rapidly leveled out after 14 days and at later test age’s water-stored concretes had a higher compressive strength than air stored ones. The continuing increase in compressive strength with time for water-stored samples after 14 days will have risen due to the ongoing hydration of the GGBS and any remaining CAC.
Compressive strength development in BRECEM concretes [62]
Jianyong and Yan [41] reported the 28-day compressive strength of three high-performance concrete (HPC) mixtures. Mix proportions and compressive strength results are given in Table 3.13. Concretes B and C acquired much higher compressive strength than Concrete A at each testing age. At the age of 3 days, the compressive strengths of concrete A, B and C were 63.8, 69.3 and 69.3 MPa, respectively. At 28 days, compressive strengths of Concrete B and C increased greatly to 100.4 and 104.0 MPa, respectively, compared with 81.1 MPa of Concrete A. The development of compressive strength reflected the strengthening effect of ultra-fine GGBS and silica fume (SF) on mechanical properties of concrete.
Li and Zhao [45] studied the influence of combination of GGBS and FA on the compressive strength of high-strength concrete. Three types of concretes; GGFAC (concrete incorporating GGBS and FA), HFAC (high-volume FA concrete), and PCC (control Portland cement concrete) were made and their strength was determined up to the age of 360 days. PCC had 500 kg/m3 of cement content, HFAC had 300 kg/m3 of cement and 200 kg/m3 FA, GGFAC had 300 kg/m3 of cement and 125 kg/m3 FA and 75 kg/m3 of GGBS. Table 3.14 gives the strength development of PCC, HFAC (containing 40% of FA) and the concrete (GGFAC) incorporating a combination of 25% FA and 15% GGBS. It can be seen that there is a general trend of increasing strength with age up to 1 year for all concretes. As expected, the behavior of HFAC at early ages is different from that of PCC and GGFAC. Though it had the highest strength at the end test age, its strength was the lowest before 56 days. The strength development of GGFAC is similar to that of PCC, only with slightly lower values before 28 days. This indicates that GGFAC can achieve adequate early compressive strength, while maintaining a high long-term strength.
Cheng et al. [20] reported the effect of GGBS on the compressive strength of concrete. They made three concrete mixes (A, B, and C). Mix A was control mix with mixture proportion 1:1.82:1.97 with water–binder ratio of 0.55. Mixes B and C were made by replacing cement with 40 and 60% of GGBS, respectively. Compressive strength was determined according to ASTM C39 [10]. At 91 days, compressive strength of specimens was 42.4, 45.3 and 48.6 MPa for mix A, B and C, respectively. Compressive strength developments depend upon the GGBS percentage and testing age. The glassy compounds in GGBS react slowly with water and it takes time to obtain hydroxyl ions from the hydration product of Portland cement to breakdown the glassy slag parcels at early age. However, GGBS concrete had higher compressive strength than ordinary Portland cement concrete after GGBS hydration and pozzolanic reaction was almost accomplished. Higher GGBS replacement percentage had higher ultimate strength.
Khatib and Hibbert [42] investigated the influence of GGBS on the compressive strength of concrete. Portland cement (PC) was partially replaced with 0–80% GGBS. First mix was control (M1) having a proportion of 1 (PC): 2 (fine aggregate): 4 (coarse aggregate). In the next three mixes, PC was partially replaced with 40, 60 and 80% GGBS (by mass).
The compressive strength development for concretes containing 0, 40, 60 and 80% GGBS (mixes M1–M4) is shown in Fig. 3.16. There was a systematic decrease in compressive strength with the increase in GGBS content during the early stages of hydration. Beyond 28 days and up to at least 90 days, the presence of GGBS was highly beneficial at 40 and 60% replacement with a strength exceeding that of the control. A noticeable strength reduction at all ages is observed at 80% GGBS.
Effect of GGBS on compressive strength development [42]
Atis and Bilim [11] investigated the compressive strength of ground granulated blast-furnace slag concrete under dry and wet curing conditions. Total of 45 concretes, including control normal Portland cement (NPC) concrete and GGBS concrete, were produced with three different water–cement ratios (0.3, 0.4, 0.5), three different cement dosages (350, 400 and 450 kg/m3) and four partial GGBS replacement ratios (20, 40, 60, and 80%). Twelve cubic samples produced from fresh concrete were de-moulded after a day, then, six cubic samples were cured at 22 ± 2°C with 65% relative humidity (RH), and the remaining six cubic samples were cured at 22 ± 2°C with 100%RH until the samples were used for compressive strength measurement at 28 days and 3 months. Average 28-day compressive strength of control and GGBS concrete are given in Table 3.15 for dry- and wet-curing conditions. It can be seen from this table that, in general, wet-cured compressive strength of GGBFS was higher than that of control NPC concrete for 20 and 40% replacement ratios at 28 days. Compressive strength of GGBS was found to be equivalent to that of control NPC concrete for 60% replacement ratio. However, compressive strength of GGBS was found to be satisfactory when compared to control NPC concrete for 80% replacement ratio. It is also evident that, for dry curing conditions, compressive strength of GGBS concrete is found to be equivalent to that of control NPC concrete for 20 and 40% replacement ratio at 28 days. Compressive strength of GGBFS is found to be satisfactory when compared to control NPC concrete for 60% replacement ratio. However, concrete containing 80% GGBS developed lower strength than that of control NPC concrete.
Hui-sheng et al. [38] studied the influence of GGBS on the compressive strength of HPC up to the age of 180 days. Cement, fine aggregate, and coarse aggregates contents were 550, 687, and 1,030 kg/m3, respectively. GGBS contents were 0, 15, 30, 45 and 60% by weight of cement, with water–binder ratio of 0.30 and 0.35. It was observed that (1) HPC showed significantly higher compressive strength at lower w/b than that at higher w/b. As expected, prolonging curing period evidently benefits compressive strength development, which is attributed to the fact that the pozzolanic reaction between GGBS and cementitious system is slow; hence, the beneficial effects were exhibited at a later curing age; and (2) for HPC with GGBS at w/b of 0.30, compressive strength reached highest value at optimum replacement of 15%. However, at higher w/b 0.35, compressive strength evidently decreased with the increase of replacement at each curing age.
3.4.4 Tensile and Flexural Strength
Ujhelyi and Ibrahim [72] investigated the effect of hot weather on the flexural strength of concrete containing various percentages of GBFS and ground rhyolite tuff as partial replacement of cement. The cement content in control mix was 350 kg/m3 with water-to-cement ratio 0.531. Two series of mixes were made: the materials of the first were stored in laboratory room (temperature: +20°C ± 3°C, relative humidity: 20–35%), that of the second in special room (temperature 42°C ± 1°C, relative humidity 20%). Following conclusions were drawn: (1) Concretes containing slag as a partial replacement of cement (up to 40%) had higher flexural strengths casting and curing at +42°C than that of concretes made with Portland cement alone; (2) flexural strength of concretes made with slag was equal (or higher) comparing to Portland cement concrete cast and cured in high temperature; and (3) flexural strengths remain unchanged despite reduction in cement content and with increasing ratio of additive, curing at high air temperature comparing with that of Portland cement concretes because the active SiO2 of additives reacts at high temperature with Ca(OH)2 of cement in the early period.
Jianyong and Yan [41] reported the 28-day compressive strength of three HPC mixtures. Concrete A was made of OPC (600 kg/m3). Concrete B had (420 kg/m3 of OPC and 180 kg/m3 GGBS), and concrete C had (360 kg/m3 of OPC and 180 kg/m3 GGBS, 60 kg/m3 of SF). Splitting tensile strength results are given in Table 3.16. It is evident that Concretes B and C acquired more splitting tensile strength than Concrete A at each age. The development of splitting tensile strength showed the same tendency as that of compressive strength. Such a tendency reflects the strengthening effect of ultra-fine GGBS and SF on mechanical properties of concrete.
Khatib and Hibbert [42] studied the effect of GGBS on the flexural strength of concrete. Portland cement (PC) was partially replaced with 0–80% GGBS. Control mix had a proportion of 1 (PC): 2 (fine aggregate): 4 (coarse aggregate). In the other three mixes, cement was partially replaced with 40, 60 and 80% GGBS (by mass). Figure 3.17 shows 90-day flexural strength values for mixes containing 0, 40, 60, and 80% GGBS. The flexural strength of concrete containing 60% GGBS was noticeably higher than the control, whereas a slight decrease at 40% and marked decrease at 80% replacement were observed.
Effect of GGBS on flexural strength [42]
Guo et al. [29] investigated the flexural fatigue performance of concretes with 50 and 80% proportions of ground granulated blast-furnace slag by mass of total cementitious materials. Control concrete had 460 kg/m3 of cement, aggregate-binder ratios and water–binder ratios were 2.4 and 0.35, respectively. The flexure fatigue tests were carried at cyclic loading frequency of 10 Hz. Six nominal stress levels (0.90, 0.85, 0.80, 0.75, 0.70 and 0.65) were chosen. Flexural strength of concrete was 7.65, 7.14 and 5.87 MPa with 0, 50, and 80% GGBS content. The flexural fatigue life of concretes is given in Table 3.17. It is clear that the fatigue life of 50% GGBS concrete is the longest and that of 0% GGBS concrete is the shortest with stress level of 0.80 or more. However, when stress level was lower than 0.80, the fatigue life of 80% GGBS concrete was the longest among three mixes. When stress level is 0.65, 0% GGBS concrete was at about 2 × 106 circles, whereas the fatigue life of concretes with GGBS was more than 2 × 106 circles. This is because of the bond strength of interface transition zone (ITZ) between coarse aggregate and matrix is weakened by incorporation of GGBS with a specific surface area of 372 m2/kg. The potential reason is that the grain size of GGBS (372 m2/kg) was similar to those of Portland cement (309 m2/kg) and the elastic modulus of GGBS grain was lower than those of cement grain. Moreover, the effect of GGBS on bond strength of ITZ in concrete is more negative as increasing the mass fraction of GGBS in matrix.
Cakir and Aköz [21] investigated the influence of curing conditions on the flexural strength of mortars with and without GGBS. In mortar mixes, cement was replaced with 0, 30, and 60% GGBS by weight. One group of mortar mixes (W) was kept in water at 20°C standard conditions, and the other group of mortar mixes (H) was kept in moisture cabinet at 40°C and 100% relative humidity (RH). They reported that (1) at the age of 7 days, flexural strength of mortars (W) were 6.8, 6.5, and 6.0 MPa at 0, 30 and 60% GGBS whereas flexural strength of mortar mixes (H) were 7.0, 6.3, and 6.1 MPa with 0, 30 and 60% GGBS; (2) Flexural strength of mortars increased with time for both curing conditions. In comparison with 7-day strength, strength of control mortars cured in water (0 W) at 20°C temperature increased by 7 and 21% at 28 and 180 days, respectively. Slag replaced mortars 3W group’s (30% GGBS) flexural strength increased by 8 and 23%, 6 W group’s (60% GGBS) strength increased by 13 and 25% at 28 and 180 days; (3) flexural strength of control specimens which cured in moisture cabinet increased by 3 and 7% at 28 and 180 days, respectively, in comparison with 7-day strength. Flexural strength of slag replaced mortars 3H group (30% GGBS) increased by 8 and 13%, and 6H group (60%) increased by 7 and 15% at 28 and 180 days; and (4) slag replaced mortars’ flexural strengths were negatively affected at elevated temperature and lower humidity condition. The flexural strength of mortars produced with Portland cement and cured in water at 40°C was lower than the flexural strength of mortars cured in water at 20°C.
3.5 Durability Properties of Concrete Containing GGBS
3.5.1 Creep and Shrinkage
The creep of concrete is the deformation of hardened concrete caused by a long-lasting constant load applied on it. Drying shrinkage of concrete is the shrinkage caused by evaporation of internal water in hardened concrete. Creep and drying shrinkage are very important time-dependent properties of HPC.
Jianyong and Yan [41] studied the creep and drying shrinkage of three HPC mixtures. Concrete A was made of OPC (600 kg/m3). Concrete B had (420 kg/m3 of OPC and 180 kg/m3 GGBS), and concrete C had (360 kg/m3 of OPC and 180 kg/m3 GGBS, 60 kg/m3 of SF). Specimens for creep and shrinkage were of size 100 × 100 × 300 and 100 × 100 × 500 mm, respectively. The creep and shrinkage tests were conducted up to the age of 180 and 210 days, respectively. Creep and shrinkage results are shown in Figs. 3.18 and 3.19, respectively.
Development of creep of concrete [41]
Test results of drying shrinkage of concrete [41]
It is evident that up to 180-day of testing, Concretes B and C always obtained much smaller creep than Portland cement concrete A. When ultra-fine GGBS and SF were used at the same time (Concrete C), the creep value was the lowest, which is in accordance with the development trend of mechanical strengths. At age of 180 days, creep for Concretes A, B and C were 1,293 × 10−6, 623 × 10−6 and 450 × 10−6, respectively. According to creep rate, three groups of concrete were subject to a faster development of creep at early ages than at late ages, and the age of 60 days was the turning point. For the individual concrete, the value of creep rate was different. Before 60 days, Concrete A was much greater than B and C, and C was the lowest. After 60 days, the creep rates of A, B and C became similar while Concretes B and C can be thought to have equal creep rate.
Figure 3.19 shows the drying shrinkage of concretes. It can be seen that at early ages, the difference between drying shrinkage of Concrete A, B and C was small. After 28 days, Concrete A obtained substantially greater drying shrinkage than Concretes B and C, while the latter two always had similar shrinkage amount. At age of 180 days, the amount of shrinkage for Concretes A, B and C was 220 × 10−6, 96 × 10−6 and 127 × 10−6, respectively.
3.5.2 Chloride Binding Capacity/Resistance
Dhir et al. [23] determined the chloride binding capacity of paste samples containing Portland cement (PC), and blends of PC and GGBS. GGBS was added as 0, 33.3, 50 and 66.7% of the total binder. The water–binder ratio was kept constant at 0.55. They concluded that (1) with the increase in GGBS content, the chloride binding capacity increased for all chloride concentrations. For a GGBS replacement level of 66.7%, the chloride binding capacity was around five times that of the PC control for the case of 5 mol/l exposure concentrations; and (2) as the chloride concentration increased, the binding capacity increased for all GGBS contents. The chloride binding capacity appeared to be directly proportional to the exposure concentration. This could be attributed to the sensitivity of the chloride binding capacity to the ratio of Cl−/OH−ions in the cement pore solutions (in this case, the chloride solutions to which the pastes were exposed). As the Cl−/OH− ratio increased chloride binding capacity increased.
Dhir et al. [23] determined the strength and intrinsic permeability of concrete mixes. Four concrete mixes were made. All the mixes had the same total binder content of 300 kg/m3 with GGBS replacement levels of 0, 33.3, 50 and 66.7%. An air permeability test was conducted at ages of 28 and 90 days, whilst chloride diffusion testing was carried out at 28 days. Strength and intrinsic permeability results of PC and GGBS concrete are given in Table 3.18. It is evident from this table that (1) 280-day cube strength decreased with the increase in GGBS content; (2) however, at 90 days, strength of GGBS and PC concrete mixes were much closer; (3) at 28 days, despite the lower compressive strengths of the GGBS concrete, compared to the control, the intrinsic permeabilities were similar. At 90 days, the intrinsic permeability of the GGBS became much better; (4) GGBS enhanced the protection against chloride ingress and with the increase in GGBS replacement level, the coefficient of chloride diffusion steeply decreased.
Xu [77] investigated the chloride binding properties and the concentrations of chloride and hydroxyl ions in the pore solutions of Ordinary Portland cement (OPC) and blended (35% OPC, 65% GGBS) cements, containing 2.0 to 9.0% sulfates derived from sodium sulfate and calcium sulfate. Chlorides derived from sodium and calcium chlorides were introduced at the time of mixing. The results indicated that calcium sulfate had a different effect on chloride binding and the pore solution chemistry than sodium sulfate. The slag cement had higher chloride binding capacities as a result of simple replacement for OPC, but at the same sulfate contents, the slag cement did not give the expected higher binding capacities, suggesting that the difference in sulfate content between the two cements may be the main reason for their different chloride binding behavior.
Huang and Yeih [37] studied the diffusivity of chloride ions through high strength concrete containing slag. Numerical methods were used to compute the diffusion coefficients of chloride ions in concrete, and were found to be 2.53 × 10−14m2/s to 9.84 × 10−4m2/s for compressive strengths between 62.5 and 91.1 MPa.
Luo et al. [47] measured the chloride diffusion coefficients and chloride-binding capacity of the three kinds of concretes made with OPC, OPC/70% GGBS and OPC/65% GGBS/5% gypsum. The mix ratio for the three kinds of concretes was 1:1.7:3.29 with water–binder ratio of 0.34. Both the total chloride diffusion coefficient and the free chloride diffusion coefficient were determined. The total chloride diffusion coefficient is the coefficient for all the diffused chlorides including the bound chloride by the concrete, and the free chloride diffusion coefficient is only the unbound chloride diffused to the concrete. Results of chloride diffusion coefficients are given in Table 3.19. They concluded that (1) GGBS deceased the chloride diffusion coefficient greatly; (2) both the total chloride diffusion coefficient and the free chloride diffusion coefficient decreased substantially with 70% GGBS; (3) but chloride diffusion coefficient increased greatly with 5% gypsum; (4) GGBS increased the chloride-binding capability greatly, especially the chemical chloride-binding capability, but sulfates and alkalinity decreased the chloride-binding capability greatly.
Cheng et al. [20] studied the influence of GGBS on the rapid chloride permeability (RCPT) and water permeability of concrete. Three were three concrete mixes A, B, and C. Mix A was control one with mixture proportion 1:1.82:1.97 and water–binder ratio of 0.55. Mixes B and C were made by replacing cement with 40 and 60% of GGBS, respectively. ASTM C1202 [7] procedure was followed for carrying out RCPT test. For each mix, cylindrical specimens (100 × 200 mm) were cast and moist-cured for 91 days. 50-mm-thick samples were cut from the middle portion of each cylinder. For measuring water permeability 100 × 200 mm cylinders were cast. Based on the results obtained they reported that (1) water permeability was 2.56 × 10−13, 1.52 × 10−13, and 1.32 × 10−13 m/s for mixes A, B, and C, respectively. It appeared that higher GGBS percentage made structure denser and prevented concrete from water penetration; (2) RCPT results indicated the highest total charge-passed (10,271 C) obtained in mix A specimen and the lowest total charge passed (1,864 C) in mix C specimen, which represented highest chloride-ion penetration resistance. The reaction products were very efficient to fill up the large capillary pores and refine the pore system in concrete thereby enhancing its permeability resistance.
Hootan and Titherington [36] investigated the strength and chloride penetration resistance of HPC after curing either at 23ºC or accelerated by heating to 65ºC. Six air-entrained concrete mixtures with different cement replacement levels of slag (25–27%) and SF (4–8%) (by mass) were made. The water to cementitious materials ratio (w/cm) was kept constant, at 0.30. The total cementing materials content was 460 kg/m3 for all mixtures. Chloride penetration resistance of HPC was measured as per ASTM C 1202 [7]. They concluded that (1) concretes containing SF or ternary blends of SF and GGBS exhibited improved chloride penetration resistance compared to those of plain Portland cement concretes; (2) all concretes recorded are below 4,000 C, which was also possible because of water/cement ratio, 0.30. The 100% OPC mixture, whether accelerated or ambient cured, was in the moderate permeability category (between 2,000 and 4,000 C). All mixtures containing supplementary cementing materials, for both accelerated and ambient curing conditions, were in the very low permeability category of <1,000 C; and (4) chloride penetration resistance of Portland cement concrete was adversely affected by accelerated curing.
Roy et al. [65] studied the characteristics and performance of alkali-activated cementitious materials (AAC) whose properties equal or exceed those of normal Portland cement-based materials. The materials activated included: GGBS, FA, SF, and other pozzolans whether alone or when combined with Portland cement. The activators used were NaOH, KOH, Na2SO4, Na2CO3, CaSO4, and soluble silicates of sodium and potassium. Steady state chloride diffusion studies were conducted of pastes of Type I Portland cement, and its blends with different proportions of ground granulated blast-furnace slag. Very substantial reductions in diffusion rates have been found with the increased proportion of slag. In addition, alkali activation has been shown to reduce the diffusion rate by at least a factor or two.
Hui-sheng et al. [38] investigated the effect of GGBFS on the nitrogen gas permeability of HPC up to the age of 180 days. Control mixture contained cement (550 kg/m3), fine aggregate (687 kg/m3), and coarse aggregates (1,030 kg/m3). Cement was replaced with 0, 15, 30, 45 and 60% GGBFS by weight of cement, with water–binder ratio of 0.30 and 0.35. Gas permeability of control HPC mix with water–binder ratio of 0.30 and 0.35 ranged between 29 and 31 × 10−17 m2. Influencing trends of GGBFS on gas permeability of HPC differed little for different w/b ratios. At w/b of 0.30, gas permeability coefficient slightly increased with the increase in GGBFS replacement up to 60%. At w/b of 0.35, gas permeability coefficient at an optimum replacement of 30% is slightly lower than that of the control HPC.
Elahi et al. [25] reported the air-permeability of concrete containing GGBS (50 and 70%) at the age of 44 and 91 days. The air-permeability indices of control concrete were 100 ln (pressure)/min at 44 and 91 days. Binary mixes, obtained after inclusion of GGBS, resulted in an increase in air permeability at both 44 and 91 days. However, the increase in air permeability was more pronounced at 91 days. The mix with 50% GGBS yielded air-permeability indices of 116 ln (pressure)/min at 44 days and 421.7 ln (pressure)/min at 91 days. Increasing the GGBS content to 70% significantly increased the air-permeability indices of 268.1 ln (pressure)/min at 44 days and 673.9 ln (pressure)/min at 91 days.
3.5.3 Sulfate Resistance
Portland cement mortar/concrete is attacked by the solutions containing sulfate (sodium or magnesium sulfate) in natural or polluted ground water. Sulfate attack can lead to expansion, cracking, strength loss, and disintegration. The constituents of hydrated cement paste taking part in the expansive reactions are monosulfoaluminate hydrate, calcium aluminate hydrate, and calcium aluminate hydroxide.
In Portland cement mortar and concrete, sulfate attack can be minimized by reducing the presence of monosulfoaluminate and calcium aluminate hydrates. The addition of supplementary cementing materials (GGBS) to Portland cement minimizes sulfate attack in three ways: (1) GGBS does not contain any C3A, and its inclusion in concrete reduces the overall proportion of C3A in the mix; (2) GGBS reacts with Ca(OH)2 to substantially reduce its presence in the concrete, leaving significantly less Ca(OH)2 to react to form ettringite; and (3) greatly reduced permeability of GGBS concrete.
The rate and extent of sulfate attack depends upon the ease with which sulfate ions are able to penetrate the concrete and upon the chemical resistance of the cement paste. The effect of GGBS in limiting the impact of sulfate attack, as measured from the expansion it produces in concrete, is shown in Fig. 3.20.
Effect of GGBS on sulfate resistance [76]
Fearson [27] observed that by increasing the slag contents in Portland cement-slag mortars increased the sulfate resistance substantially. The sulfate resistance of blended cements made with slag was less influenced by the water-to-cement ratio than by the amount of cement replaced by slag.
Mangat and El-Khatib [49] investigated the effect of initial curing conditions on the sulfate resistance of concrete made with ordinary Portland cement and using pulverized fuel ash (PFA), SF and GGBS as partial replacement of cement. Five different concrete mixes were used. First was control mix having proportions 1:2:3.4 with water/cement ratio of 0.45. The cement content was 350 kg/m3. In the remaining mixes, cement was partially replaced by 22% PFA, 9% SF, 40 and 80% GGBS by weight, respectively. Three different initial curing conditions were adopted, namely: AI (air 45°C, 25% RH); CH (wet/air 45°C, 25% RH); AL (air 20°C, 55% RH). After the initial curing period of 28 days under the three curing regimes CH, AI and AL, specimens were immersed in fresh water for another 28 days. The temperature of the fresh water was 20°C for specimens cured initially at 20°C and 45°C for specimens cured initially at 45°C. After soaking the specimens for 28 days, extensometer measurements were taken for the specimens across each face. These initial measurements were considered as datum for sulfate attack studies. After that, specimens were immersed in a sulfate solution (7% Na2SO4 + 3% MgSO4 by weight). The temperature of the sulfate solution was 20°C for specimens cured initially at 20°C and 45°C for specimens cured initially at 45°C. Expansion measurements across the demec points to measure the change in length were taken after 47, 83, 152, 207, 337, 502 and 660 days of immersion in the sulfate solution. Based on the results, following conclusions were drawn (1) initial moist curing of concrete (at 45°C) followed by dry curing at 45°C, 25% RH resulted in lower sulfate resistance than initial air curing at low relative humidity (25 and 55% RH); (2) sulfate resistance of concrete increased with the replacement of cement with 22% PFA, 9% SF, 80% GGBF slag. The sulfate resistance also increased due to drying out of concrete during early curing at low relative humidity and due to carbonation; (3) the use of PFA (22%), SF (9%) and GGBS (80%), for partial replacement of OPC, resulted in higher sulfate resistance than for plain concrete under curing conditions.
Gollop and Taylor [31] examined the cubes of a blended cement paste stored for 6 months in solutions of Na2SO4 or MgSO4 by scanning electron microscopy. The blend of cement contained 31% of PC and 69% of blast furnace slag (GGBS). It was observed that (1) tendency of the materials made with slag cements to soften and disintegrate as a result of sulfate attack rather than to expand; (2) when a blend containing 69% of blast furnace slag was stored in 0.25 mol l−l Na2SO4 solution, the principal chemical reaction resulted in partial decalcification of the C–S–H and replacement of an AFm phase, loosely described as mono-sulfate, by ettringite. The results suggested that neither Al+3 substituted in the C–S–H, nor that present in hydrotalcite, was available for reaction with sulfate; (3) storage in 0.25 mol l−l MgSO4, solution produced the same reaction, but the dominant effects in this case were decomposition of the C–S–H and formation of brucite and gypsum in the surface regions below the cube faces and of magnesium silicate hydrate and gypsum at the cube edges; (4) SO4 2− ions penetrated to a depth of about 1 mm below the cube faces and probably more deeply at the edges. These results were similar to those observed with a sulfate-resisting PC under similar conditions; (5) the micro-structural features of the deposits of gypsum, and of the brucite formed on reaction with MgSO4 differed from those observed with the plain PC. The quantity of gypsum formed in the paste stored in Na2SO4 solution was probably less for the slag blend; (6) damage from sulfate attack was attributed partly to decalcification, which weakened the C–S–H matrix, and partly to ettringite formation, which caused expansion and cracking; (7) In typical cases of blends high in slag, at least as much sulfur entered the hydration products in sulfide as in sulfate.
El-Darwish et al. [26] studied the replacement of OPC by ground steel slag up to 30% volume replacement in concrete. Concrete specimens exposed to sodium and magnesium sulfate solutions containing 3.5% SO3 and seawater were monitored for expansion, strength loss and stiffness up to one year. Samples in seawater and sodium sulfate solutions performed satisfactorily. However, C–S–H gel transformed into M–S–H in magnesium sulfate solution
Cao et al. [18] investigated the sulfate resistance of Portland cements and blended cements. Four Portland cements of different characteristics and blended cements containing FA, GGBS and SF were used. Mortar cubes were made with water-to-binder ratio (w/b) of 0.6, sand-to-binder ratio of 2.75 and cured for 7 days prior to immersion in sulfate solutions. Three sulfate solutions were (1) 5% Na2SO4 solution having pH 12; (2) 5% Na2SO4 and maintained at nominal pH of 7 and 3 by automatic titration with 10% H2SO4 at regular set intervals. ASTM C 1012 [9] procedure was followed to evaluate the performances of binders in sulfate solutions maintained at different pH levels ranging from 3 to 12 using expansion of mortar prisms (ASTM C 1012) and strength development of mortar cubes. The results indicated that sulfate resistance of cementitious materials was dependent on its composition and on the pH of the environment. Portland cement with low C3A and low C2S performed well in all sulfate solutions. Blended cements containing SF and FA (particularly at 40% replacement) showed a much superior performance than any of the Portland cements used. For slag blended cement, this can be achieved when the replacement percentage was higher than 60%. The selection of the mineral admixture and its replacement dosage became more critical as the pH of the sulfate solution decreased. It was found that 40% FA blend, 5% SF blend or a high slag blend (80%) provided a good overall performance related to resistance to sulfate attack over a wide pH range.
Higgins [33] studied the influence of addition of a small percentage of calcium carbonate or calcium sulfate on the sulfate resistance of concrete containing GGBS. Six concrete mixtures were made having water/cementitious ratio of 0.5 and total cementitious content of 350 kg/m3. Three test methods were employed to assess sulfate resistance at 20°C (1) 100-m cubes were immersed in magnesium (containing 1.5% SO3) and sodium sulfate solutions (containing 1.5 and 2.4% SO3) and monitored for corner-loss and strength-loss, over 6 years; (2) 20 × 20 × 160-mm prisms made of mortar were immersed in magnesium and sodium sulfate solutions and their expansions monitored for up to 6 years.
Compressive strength of the cubes stored in water and in the sulfate solutions are given in Table 3.20. The strength in the sulfate solutions is expressed as a percentage of the strength of equivalent concretes stored in water for the same age. Table 3.21 shows the ‘wear ratings’ of the cubes, measured annually. ‘Wear rating’ is taken as the measure of the attack on the corners of a cube. It is the average depth of erosion or damage for one corner (in mm).
In sodium sulfate solution, the PC concrete had almost completely disintegrated by 6 years, while the GGBS concretes showed minor strength- and corner-loss. As would be expected, 70% GGBS concrete was more resistant to attack than 60% GGBS concrete. The lower level of addition of calcium sulfate (to increase the SO3 level of the cementitious to 2%), significantly increased the degree of attack. However, at the higher level of addition of calcium sulfate (to increase the SO3 level of the cementitious to 3%) the degree of attack was reduced, as was also the case with the addition of calcium carbonate.
In magnesium sulfate solution, the PC concrete performed somewhat better, whereas the GGBS concretes performed distinctly less in the magnesium than the sodium solution. This is what is normally observed [58] with GGBS concrete generally more susceptible to attack by magnesium sulfate than sodium sulfate. Once again 70% GGBS concretes were more resistant than 60% and the lower level of addition of calcium sulfate gave no advantage. The higher level of addition of calcium sulfate and the addition of calcium carbonate both reduced the degree of attack.
For PC concrete, the expansion was rapid, reaching 1% after about 9 months in all solutions. In sulfate solutions, 60% GGBS specimens disintegrated at a relatively low expansion of less than 0.1%. The 70% GGBS and specimens with additions of calcium sulfate or carbonate, all survived with low expansions, although the lower level of calcium sulfate addition showed signs of more rapid expansion at 72 months. In magnesium sulfate, expansion is more rapid and severe. All the specimens had disintegrated by 48 months. Once again the higher level of addition of calcium sulfate and the addition of calcium carbonate, proved particularly beneficial.
Higgins and Crammond [34] investigated how cement-type, aggregate-type and curing, affect the susceptibility of concrete to the thaumasite form of sulfate attack (TSA). The cements were Portland cement (PC), sulfate-resisting Portland cement (SRPC) and a combination of 70% GGBS with 30% PC. These were combined with various carbonate aggregates or a non-carbonate control. Initial curing was either in water or in air. Concrete cubes were immersed in four strengths of sulfate solution at 5 and 20°C up to 6 years. They concluded that (1) deterioration, consistent with TSA was found to occur on all of the PC and SRPC concretes made with carbonate aggregate and stored in sulfate solutions at 5°C; (2) degree of TSA increased with the sulfate concentration of the test solution and with time; (3) at 5°C, there was no discernible difference between the performance of SRPC concretes containing carbonate aggregates and those made with PC; (4) concretes made with 70% GGBS/30% PC and normal quality carbonate aggregates performed extremely well and showed no evidence of TSA in any of the solutions, with either normal or high-alumina GGBS. However, concretes made with 70% GGBS/30% PC and inferior quality carbonate aggregates did not perform well at either 5°C or 20°C; (5) an initial air-cure proved beneficial against both conventional sulfate attack and TSA. After six years, the air-cured 70% GGBS concretes showed no evidence of attack, even in the strongest sulfate solution; (6) presence of carbonate in the mix substantially improved the resistance of 70% GGBS/30% PC concretes to conventional sulfate attack; (7) overall, the worst performers were concretes made with PC. However, the concretes made with PC, appeared slightly less susceptible to conventional sulfate attack than those made with 70% GGBS/30% PC and it would appear that the low C3A PC used in the present study, had some sulfate-resisting properties.
3.5.4 Alkali Silica Reaction
Alkali-Silica Reaction (ASR) is a chemical reaction which occurs in concrete between the highly alkaline cement paste and reactive non-crystalline (amorphous) silica, which is found in many common aggregates. The ASR reaction is the same as the Pozzolanic reaction which is a simple acid–base reaction between calcium hydroxide, also known as Portlandite, or (Ca(OH)2), and silicic acid (H4SiO4, or Si(OH)4). For the sake of simplicity, this reaction can be schematically represented as following:
This reaction causes the expansion of the altered aggregate by the formation of a swelling gel of CSH. This gel increases in volume with water and exerts an expansive pressure inside the material, causing spalling and loss of strength of the concrete, finally leading to its failure.
The mechanism of ASR can be detailed in following four steps; (1) alkaline solution attacks the siliceous aggregate to convert it to viscous alkali silicate gel; (2) consumption of alkali by the reaction induces the dissolution of Ca2+ ions into the cement pore water. Calcium ions then react with the gel to convert it to hard CSH; (3) penetrated alkaline solution converts the remaining siliceous minerals into bulky alkali silicate gel. The resultant expansive pressure is stored in the aggregate; and (4) accumulated pressure cracks the aggregate and the surrounding cement paste.
GGBS could be very effective in controlling ASR because (1) GGBS reduces the alkalinity of the concrete, and thus the alkali-silica ratio; (2) GGBS reduces mobility of alkalis in the concrete; and (3) GGBS reduces free lime in concrete which is regarded as an important factor for alkali silica reaction.
Hogan and Meusel [35] reported that partial replacement of high-alkali cement with slag dramatically reduces the likelihood of alkali aggregate reaction in concrete. Mehta [51] mentioned that even if the alkali content of the slag is high, its solubility in the high-pH environment of concrete may be low, so that even a high alkali content of a blended cement may not cause any problems. He further observed that depending on the characteristics of the slag combinations of high-alkali Portland cement with 40–65% slag are effective in limiting the alkali-aggregate expansions to acceptable limits.
Kwon [44] studied the effect of ground granulated blast-furnace slag on the alkali-aggregate reaction of high-strength concrete. High-strength concrete with a cement content of 450 kg/m3, and three types of GGBFS with a fineness of 4,000, 6,000, and 8,000 cm2/g were used. Percentage replacements of GGBFS were 0, 30, 45 and 60. They observed that (1) the expansion coefficients were 0.20, 0.105, 0.06, and 0.02% with GGBFS replacement ratio of 60, 45, 30, and 0%, indicating the AAR-inhibiting effect of GGBFS; and (2) fineness of GGBFS had a significant effect, and differences were most significant when the replacement ratio was 30%. The AAR-inhibiting effect was highest with a fineness of 8,000 cm2/g, followed by 6,000 and 4,000 cm2/g in this order.
Hester et al. [32] made a comparative assessment of the effects of alkali-level of GGBS on potential alkali–silica reactivity of concrete. Expansion tests were conducted on concrete mixes made with Portland cement (NPC), two slags of differing alkali content (<1 and >1%), three aggregates and two alkali loads of 5 and 6 kg Na2Oeq./m3. Cement was replaced with 50% slag. Expansion at 52 weeks is given in Table 3.22. It was reported that (1) partial replacement of Portland cement with slag (50%) significantly reduced the expansion of the concrete, and slag concretes had very low expansion levels. There was no significant difference in behavior irrespective of aggregate type or alkali load, indicating that the alkali level of the slag was is not a contributory factor at the 50% replacement level.
Bouikni et al. [17] investigated the alkali–silica reaction of concrete made with 50 and 65% slag. A high alkali Portland cement with 1% sodium oxide and slag with 0.53% sodium oxide equivalent were used. Two concrete mixtures having high workability and low water–binder ratios were used. The mix proportions, by mass were 1:1.75:2.53:0.43 and 1:1.67:2.41:0.40 (cement + slag: sand: aggregate: water), respectively, for the 50 and 65% slag content, respectively. Table 3.23 shows that continued exposure to a hot and humid regime resulted in increasing expansion, but the concrete containing 65% slag replacement always exhibited less expansion than concrete incorporating 50% slag at corresponding ages. On removing the specimens from the hot room to dry ambient conditions, the specimens showed recovery and a reduction in expansion. Subsequent exposure to the hot and humid environment, again accelerated expansion, but this time, concretes with 65% slag replacement reached higher expansion than concretes with 50% slag. Further, concretes with 50% slag did not reach the same high expansion of 0.31% which it had reached in its previous exposure to the hot, humid regime. However, continued exposure to the hot, humid environment appeared to stabilize the subsequent progress of expansion in both concrete at about 6 months, and there was little increase in expansion with further exposure.
Puertas et al. [61] evaluated the alkali–silica reaction in waterglass-alkali-activated slag (waterglass-AAS) and ordinary Portland cement (OPC) mortars using three types of (siliceous and calcareous) aggregates. The slag was alkali-activated with a waterglass solution (Na2O. nSiO2. mH2O + NaOH) containing 4% Na2O by slag mass and a SiO2/Na2O ratio of 1.08. It was concluded that waterglass-AAS mortars are stronger and more resistant to alkali-aggregate reactions than OPC mortars. When the mortars were made with a reactive siliceous aggregate, expansion was four times greater in OPC than in AAS material. When a reactive calcareous (dolomite) aggregate was used, no expansion was detected in any of the mortars after 14 days, although the characterization results showed that the dolomite had reacted and calcareous-alkali products (brucite) had in fact formed in both mortars. These reactive processes were more intense in OPC than in AAS mortars, probably due to the absence of portlandite in the latter. When the calcareous aggregate was non-reactive, no expansions were observed in any of the mortars, although a substantial rise was recorded in the mechanical strength of AAS mortars exposed to the most aggressive conditions (1 M NaOH and 80ºC).
3.5.5 Freezing and Thawing Resistance
Hogan and Meusel [35] tested air-entrained concrete with and without slag for frost resistance. The specimens were moist-cured for 14 days before tested as per ASTM C666 [5] (Procedure A) for 300 cycles. The slag concrete did not perform as well as the Portland cement concrete, but both were of the standard of acceptable durability criteria.
Stark and Ludwig [67] mentioned that freezing and thawing resistance, deicing salt resistance of blast furnace cement concrete was related to the carbonation of the surface which increases the capillary porosity and leads to metastable calcium carbonates that are soluble in NaCl. The degree of hydration at the beginning of freezing determines the pore size distribution and the strength of the structure.
Stark [66] investigated the frost and deicing-salt resistance of slag cement and observed that frost resistance of slag concrete was highly dependent on the degree of hydration. Carbonation was more important than the degree of hydration on resistance to deicing salt.
Nanaumi et al. [54] studied the frost resistance of blast furnace slag concrete (with 30–60% per cement unit addition) and plastic micro-spheres using cellulose ether as anti-washout admixture. At 60% addition of slag and 2% (per cement unit) addition of micro-spheres, scaling resistance was improved.
Knaack and Stark [43] studied the super-sulfated cements (more than 75–85% of highly basic slag and alumina content >15%). They mentioned that in comparison to conventional slag cements, super-sulfated cements are mainly activated by a high amount of gypsum with only a small amount of Portland cement. These cements displayed lower performances in scaling and freeze–thaw due to a lower degree of hydration at 28 days.
Yuksel et al. [79] investigated the usage of GBFS, bottom ash (BA), and combination of both as partial replacement of fine aggregate on the concrete durability. Three series of concretes were produced, i.e. of GBFS, BA and GBFS + BA, which were coded as C, K, and CK, respectively. Five test groups were constituted with the replacement percentages as 10, 20, 30, 40 and 50% in each series. Freezing–thawing resistance was measured by freezing and thawing the specimens in water. Weight-loss results are shown in Fig. 3.21. For three series, resistance to freeze–thaw increased up to 20% replacement. However, it decreased again for the replacement level above 20%. C-series gave the best performance among the three series. For all the series, the loss in strength was below the loss in strength value for reference concrete.
Freezing–thawing test results [79]
3.5.6 Corrosion Resistance
Corrosion can be defined as the degradation of a material due to a reaction with its environment. Degradation implies deterioration of physical properties of the material. This can be a weakening of the material due to a loss of cross-sectional area; it can be the shattering of a metal due to hydrogen embrittlement.
Corrosion of reinforcements is the most common cause of deterioration of concrete structures. Corrosion of steel reinforcement leads to cracking and eventually spalling of concrete. Good quality concrete reduces the deterioration of concrete under adverse environmental conditions.
Li and Roy [46] have indicated that for making good quality durable concrete, use of GGBS is very advantageous. It not only reduces the porosity but also the pores become finer and the change in mineralogy of the cement hydrates leads to a reduction in the mobility of chloride ions.
Mangat and Molloy [50] studied the corrosion of steel reinforcement electrodes embedded in different matrices of concrete, exposed to simulated marine splash zone exposure for about 600 days (1,200 cycles) after initial curing in air for 14 days. The control mix had proportions of 1:2.5:1.2 with a water/cement ratio of 0.58. The cement content was 430 kg/m3, and GGBS replacement levels were 20, 40 and 60%. The results showed that (1) replacement of cement by up to 40% GGBFS had no significant influence on rebar corrosion. At 60% GGBFS, corrosion rate of reinforcement was significantly reduced; (2) corrosion process of reinforcement in plain concrete, and concrete with low contents of GGBS was similar and was anodically controlled. In GGBFS (60%) concrete, the cathodic polarization increased greatly although control of corrosion remained principally anodic; (3) chloride concentration of the pore fluid gave a more reliable indication of corrosivity of a matrix than Cl−/OH− ratio, especially when comparing concretes with different types and contents of replacement materials.
Al-Amoudi et al. [3] studied the long-term corrosion resistance of steel rebars in 5% sodium chloride solution. Regression analyses indicated excellent correlation between corrosion resistance and porosity for both plain and blended cement concretes. It was found that the corrosion rate of the steel rebar in GGBFS containing concrete specimen was between one-half and one-twelfth of those in plain concrete specimens.
Torii et al. [71] reported that the resistance to chloride ion penetration of 50% GGBFS concrete was almost the same as that of 10% SF concrete. Tsay [40] evaluated the slag cement subjected to seawater corrosion at various ages and concluded that slag concrete with 20–30% substitution has the best corrosion resistance properties.
Pal et al. [59] studied the corrosion behavior of embedded reinforcement under different proportions of slag. Corrosion of steel was examined electrochemically and also by an accelerated carbonation test. It can be seen from Table 3.24 that with the increase in slag content, corrosion rate, weight loss, half cell potentials and the carbonation depth were found to be decreasing.
Yeau and Kim [78] examined the influence of GGBS on the corrosion resistance of concrete. Concrete mixtures were made with two types of cements (ASTM Type I or ASTM Type V) and varying percentages (0, 25, 40, and 55%) of GGBS. Concrete mixtures were proportioned to have the 28-day strength of 35 MPa. Concrete specimens of size 150 × 150 × 500 mm with three different covers, 10, 20, and 30 mm. were prepared. Accelerated steel corrosion (ASC) test was conducted to measure half-cell potential and the surface areas of corroded steel bars. Repeated wetting and drying method was used to accelerate steel corrosion. These tests were conducted up to 30 cycles after concrete specimens were cured in water for 28, 56, and 91 days. For each wetting–drying cycle, test specimens were wetted in 3% NaCl solution for 3 days and then dried at 60°C for 4 days. After 30 weeks, the specimens were crushed out and corroded surface area was calculated as a percentage to the total area of steel embedded in concrete. Figure 3.22 shows the results obtained from the measurements of surface area corroded from steel bars. They concluded that (1) the corroded surface area (%) depend on the thickness of the concrete cover, and GGBS content; (2) corroded areas of steel embedded in control concrete mixtures were about two and three times larger than those of steel involved in 40% GGBS concrete mixture and 55% GGBS concrete mixture, respectively; and (3) proportion of corroded areas in Type I cement was lower than that of the corroded areas in Type V cement. These results suggested that the resistance to steel corrosion can be superior, since not only Type I cement was used, but also the amount of GGBS was increased.
Surface area (%) corroded from steel bars in concrete specimens [78]
Cheng et al. [20] studied the influence of GGBS on corrosion behavior of reinforced concrete beams (150 × 150 × 900 mm) under various loading ratios (37 and 75% of the ultimate load) and exposed to 3.5% NaCl solution. Beams were made of three concrete mixes (A, B, and C). Mix A was control one with mixture proportion 1:1.82:1.97 with water–binder ratio of 0.55. Mixes B and C were made by replacing cement with 40 and 60% of GGBS, respectively. A direct current (1 mA cm−2) was applied to accelerate the corrosion process. Open circuit potential (OCP) and direct current polarization resistance were obtained to evaluate the rebar corrosion. As per ASTM C876 [8], when OCP is higher than −127 mV (SCE), there is only 10% probability that the reinforcement may corrode. If the potential is from −127 to −276 mV (SCE), corrosion probability is uncertain. Corrosion probability may be higher than 90% for OCP between −276 and −427 mV (SCE). Corrosion results indicated that (1) without sustained loading, mixes with 40 and 60% GGBS significantly increased the exposure time span to severe corrosion in comparison with control mix; (2) GGBS replacement for cement refined the pore structure and reduced the corrosion probability of reinforcing steel; (3) although, the pH value was 13.2 for control concrete, 12.8 for 40% GGBS replacement concrete and 12.4 for 60% GGBS replacement concrete, respectively. There seemed no prominent negative effect on the corrosion behavior due to decreasing alkalinity in pore solution; (4) with 37% loading ratio, the OCP of control specimen dropped from −75 to −427 mV (SCE) after 4-day accelerated exposure. Specimens containing 40 and 60% GGBS reached severe corrosion condition after 6–8-day exposures, which revealed that sustained loading or cracks plays paramount role in the corrosion process of reinforcing steel in concrete; (5) for the control specimen with 75% sustained loading, the potential dropped below −427 mV (SCE) even after 2-day accelerated corrosion process and GGBS concretes having cracks wider than 0.02 mm had no better corrosion resistance; (6) at higher sustained loading ratio, OCP did not significantly vary among the three mixes, and the corrosion process was controlled by the pre-determined cracks.
3.5.7 Carbonation
Carbonation occurs in concrete because the calcium bearing phases present are attacked by carbon dioxide of the air and converted to calcium carbonate. Cement paste contains 25–50 wt% calcium hydroxide (Ca(OH)2), which means that the pH of the fresh cement paste is at least 12.5. The pH of a fully carbonated paste is about 7.
The concrete will carbonate if CO2 from air or from water enters the concrete according to
When Ca(OH)2 is removed from the paste, hydrated CSH will liberate CaO which will also carbonate. The rate of carbonation depends on porosity and moisture content of the concrete.
The carbonation process requires the presence of water because CO2 dissolves in water forming H2CO3. If the concrete is too dry (RH < 40%) CO2 cannot dissolve and no carbonation occurs. If on the other hand it is too wet (RH > 90%) CO2 cannot enter the concrete and the concrete will not carbonate. Optimal conditions for carbonation occur at a RH of 50% (range 40–90%).
According to, the cement type affects the rate of carbonation with sulfate-resisting cement having a 50% higher rate than Portland cement. Slag cements can have carbonation rates up by 200% more than Portland cement.
Osborne investigated structures made with Portland cement with and without 50 or 70% slag. Total cement contents were in the range 360–380 kg/m3. They concluded that approximately after 5 years of exposure, carbonation depths were greatest for the 70% slag concretes. Portland cement concrete showed little or no sign of carbonation after 7–17 years exposure. Malhotra et al. [48] reported that after 10 years of outdoor exposure, the carbonation depths of concrete (water to total cementitious material ratios of 0.27–0.29), which included plain concrete and concrete with 28–35% slag, were small (in the range of less than 1–5 mm).
Sulapha et al. [69] studied the carbonation of concrete incorporating ground granulated blast-furnace slag. In the concrete mixtures, OPC was partially replaced with GGBS. The replacement percentages of GGBS (having 4,500 cm2/g fineness) were 30, 50, and 65%; the replacement level of GGBS (with 6,000 or 8,000 cm2/g fineness) was 65%. Prisms of size 100 × 100 × 400 mm were made. The carbonation depth of the specimens was monitored every 2 weeks in the first 2 months, and thereafter once a month up to 12 months. After a certain exposure period, the specimens were split and freshly broken surfaces were sprayed with a standard solution of 1% phenolphthalein in 70% ethyl alcohol. In the noncarbonated region with pH values above 9.2, the indicator turned purple-red; in the carbonated portion with pH less than 9.2, the solution remained colorless. The distance between the color change boundary and the concrete surface was measured as the carbonation depth. Based on the results, they concluded that (1) lower w/b (0.3–0.6) and a prolonged curing age in water (from 1 to 28 days) generally led to a slower rate of carbonation, possibly due to pore structure densification; (2) carbonation rate of low fineness GGBS (4,500 cm2/g) increased with an increase in OPC replacement level; (2) concrete containing low fineness GGBS (4,500 cm2/g) exhibited higher rates of carbonation than plain OPC concrete. This could be due to the reduction in Ca(OH)2 content, which seemed to have more influence over pore refinement, and hence led to faster rates of carbonation; and (3) concrete incorporating GGBS of higher fineness (6,000 and 8,000 cm2/g), the carbonation rates were lower than that of plain OPC concrete. The pore modification, being more dominant than the change in Ca(OH)2 content, appeared to control the carbonation rate.
Bouikni et al. [17] studied the carbonation of concrete made with 50 and 65% slag. A high alkali Portland cement with 1% sodium oxide and slag with 0.53% sodium oxide equivalent were used. Two concrete mixtures having high workability and low water–binder ratios were used. The mix proportions, by mass were 1:1.75:2.53:0.43 and 1:1.67:2.41:0.40 (cement + slag:sand:aggregate:water), respectively, for the 50 and 65% slag content, respectively. Carbonation tests were performed on the broken faces after flexural strength tests, by spraying with 2% phenolphthalein in 70% ethyl alcohol, as recommended by RILEM [63]. The carbonation depth measurements were taken at 3 and 6 months, and the results of these measurements for the 50 and 65% slag concretes subjected to the three curing regimes is given in Table 3.25. The results demonstrated that the carbonation depths, were at both ages, higher for the concrete with 65% slag replacement than for the concrete with 50% slag level, and that, of all the curing regimes, concrete exposed to an internal environment without any water curing permitted the highest pen-carbonation measurement. Compressive strength values showed small increase beyond the 28 days, and carbonation did not generally appear to reverse this trend with increased carbonation penetration and ageing.
Hui-sheng et al. [38] investigated the effect of GGBS on the carbonation of HPC up to the age of 56 days. Cement, fine aggregate, and coarse aggregates contents were 550, 687, and 1,030 kg/m3, respectively. GGBS levels were 0, 15, 30, 45 and 60% by weight of cement, with water–binder ratio of 0.30. Carbonation depth of HPC with GGBFS is shown in Fig. 3.23. It can be seen that the incorporation of GGBS decreased the carbonation resistance of HPC, but at significantly different levels. For HPC with GGBS at w/b of 0.30, carbonation depth significantly increased with the increase in GGBS content.
Influence of GGBS on carbonation depth of HPC at w/b ratio of 0.30 [38]
References
ACI Committee 226: Ground granulated blast furnace slag as a cementitious constituent in concrete. ACI Mater. J. 84(4), 327–342 (1987)
Aldea, C., Young, F., Wang, K., Shah, S.P.: Effect of curing conditions on properties of concrete using slag replacement. Cem. Concr. Res. 30(3), 465–472 (2000)
Al-Amoudi, O.S.B., Rasheeduzzafar, M.M., Al-Mana, A.I.: Prediction of long-term corrosion resistance of plain and blended cement concretes. ACI Mater. J. 90(6), 564–570 (1993)
ASTM C 232: Standard test methods for bleeding of concrete. ASTM Standards. 4.02:13942, Annual Book of ASTM Standards, American Society for Testing and Materials, West Conshohocken (1992)
ASTM C666-Part A: Standard test method for resistance of concrete to rapid freezing and thawing. Annual Book of ASTM Standards, vol. 03.02, 19428–2959, American Society for Testing and Materials, West Conshohocken (1997)
ASTM C 989: Standard specification for ground granulated blast-furnace slag for use in concrete and mortars. Annual Book of ASTM Standards, American Society for Testing and Materials, West Conshohocken (1999)
ASTM C1202: Standard test method for electrical indication of concrete’s ability to resist chloride ion penetration. Annual Book of ASTM Standards, vol. 4.02, American Society for Testing and Materials, West Conshohocken (1997)
ASTM C 876: Standard test method for half-cell potentials of uncoated reinforcing steel in concrete, Annual Book of ASTM Standards. American Society for Testing and Materials, West Conshohocken (1991)
ASTM C 1012-95a: Standard test method for length change of hydraulic cement mortars exposed to a sulfate solution. Annual book of ASTM Standards, Section 4—Construction, Cement; Lime; Gypsum. vol. 04.01, pp. 450–456. American Society for Testing and Materials, West Conshohocken (1995)
ASTM C39/C39 M: Standard test method for compressive strength of cylindrical concrete specimens, Annual Book of ASTM Standards, vol. 4.02, American Society for Testing and Materials, West Conshohocken (2004)
Atis, C.D., Bilim, C.: Wet and dry cured compressive strength of concrete containing ground granulated blast-furnace slag. Build. Environ. 42(8), 3060–3065 (2007)
Binici, H., Cagatay, I.H., Shah, T., Kapur, S.: Mineralogy of plain Portland and blended cement pastes. Build. Environ. 43(7), 1318–1325 (2008)
Babu, K.G., Kumar, V.S.R.: Efficiency of GGBS in concrete. Cem. Concr. Res. 30(7), 1031–1036 (2000)
Ballim, Y., Graham, P.C.: A maturity approach to the rate of heat evolution in concrete. Mag. Concr. Res. 55(3), 249–256 (2003)
Ballim, Y., Graham, P.C.: The effects of supplementary cementing materials in modifying the heat of hydration of concrete. Mater. Struct. 42, 803–811 (2009)
Basheer, P.A.M., Gilleece, P.R.V., Long, A.E., McCarter, W.J.: Monitoring electrical resistance of concretes containing alternative cementitious materials to assess their resistance to chloride penetration. Cem. Concr. Compos. 24(5), 437–449 (2002)
Bouikni, A., Swamy, R.N., Bali, A.: Durability properties of concrete containing 50% and 65% slag. Construct. Build. Mater. 23(8), 2836–2845 (2009)
Cao, H.T., Bucea, L., Ray, A., Yozghatlian, S.: The effect of cement composition and pH of environment on sulfate resistance of Portland cements and blended cements. Cem. Concr. Compos. 19(2), 161–171 (1997)
Cheron, M., Lardinois, C.: In: Proceedings of the 5th International Congress on the Chemistry of Cement, Tokyo, vol. IV, pp. 277–285 (1968)
Cheng, A., Huang, R., Wu, J.K., Chen, C.H.: Influence of GGBS on durability and corrosion behavior of reinforced concrete. Mater. Chem. Phys. 93, 404–411 (2005)
Cakir, Ö., Aköz, F.: Effect of curing conditions on the mortars with and without GGBFS. Construct. Build. Mater. 22(3), 308–314 (2008)
Daube, J., Bakker, R.: Portland blast-furnace slag cement: a review. Blended Cement, ASTM-STP 897 pp. 5–14 (1986)
Dhir, R.K., El-Mohr, M.A.K., Dyer, T.D.: Chloride binding in GGBS concrete. Cem. Concr. Res. 26(12), 1767–1773 (1996)
Douglas, E., Bilodeau, A., Brandstetr, J.: Alkali activated ground granulated blast-furnace slag concrete: preliminary investigation. Cem. Concr. Res. 21(1), 101–108 (1991)
Elahi, A., Basheer, P.A.M., Nanukuttan, S.V., Khan, O.U.Z.: Mechanical and durability properties of high performance concretes containing supplementary cementitious materials. Constr. Build. Mater. 24(3), 292–299 (2010)
El-Darwish, I., Kurdi, A., Mahmoud, H., El-Kair, H.A.: Mechanical properties and durability of Portland cement concrete incorporating ground steel making slag. AEJ Alex. Eng. J. 36, 1–14 (1997)
Fearson, J.P.H.: Sulfate resistance of combination of Portland cement and granulated blast furnace slag. vol. 2, pp. 1495–1524. ACI Special Publication SP-91, Dr. Farmington Hills (1986)
Gao, J.M., Qian, C.X., Liu, H.F., Wang, B., Li, L.: ITZ microstructure of concrete containing GGBS. Cem. Concr. Res. 35(7), 1299–1304 (2005)
Guo, L.P., Sun, W., Zheng, K.R., Chen, H.J., Liu, B.: Study on the flexural fatigue performance and fractal mechanism of concrete with high proportions of ground granulated blast-furnace slag. Cem. Concr. Res. 37(2), 242–250 (2007)
Gee, K.H.: The potential for slag in blended cements. In: Proceedings of the 14th International Cement Seminar, Rock Products, pp. 51–53 (1979)
Gollop, R.S., Taylor, H.F.W.: Micro structural and micro analytical studies of sulfate attack IV. Reactions of a slag cement paste with sodium and magnesium sulfate solutions. Cem. Concr. Res. 26(7), 1013–1028 (1996)
Hester, D., McNally, C., Richardson, M.: A study of the influence of slag alkali level on the alkali–silica reactivity of slag concrete. Construct. Build. Mater. 19(9), 661–665 (2005)
Higgins, D.D.: Increased sulfate resistance of GGBS concrete in the presence of carbonate. Cem. Concr. Compos. 25(8), 913–919 (2003)
Higgins, D.D., Crammond, N.J.: Resistance of concrete containing GGBS to the thaumasite form of sulfate attack. Cem. Concr. Compos. 25(8), 921–929 (2003)
Hogan, F.J., Meusel, J.W.: Evaluation for durability and strength development of a ground granulated blast furnace slag. Cem. Concr. Aggreg. 3(1), 40–52 (1981)
Hooton, R.D., Titherington, M.P.: Chloride resistance of high-performance concretes subjected to accelerated curing. Cem. Concr. Res. 34(9), 1561–1567 (2004)
Huang, R., Yeih, W.C.: Assessment of chloride diffusion in high strength concrete using the accelerated ionic migration test. J. Chin. Inst. Eng. 20, 39–45 (1997)
Hui-sheng, S., Bi-wan, X., Xiao-chen, Z.: Influence of mineral admixtures on compressive strength, gas permeability and carbonation of high performance concrete. Construct. Build. Mater. 23(5), 1980–1985 (2009)
Hwang, C.L., Lin, C.Y.: Strength development of blended blast furnace slag cement mortars. ACI SP 91, 1323–1340 (1986)
Jau, W.C., Tsay, D.S.: A study of the basic engineering properties of slag cement concrete and its resistance to sea water corrosion. Cem. Concr. Res. 28(10), 1363–1371 (1998)
Jianyong, L., Yan, Y.: A study on creep and drying shrinkage of high performance concrete. Cem. Concr. Res. 31(8), 1203–1206 (2001)
Khatib, J.M., Hibbert, J.J.: Selected engineering properties of concrete incorporating slag and metakaolin. Construct. Build. Mater. 19(6), 460–472 (2005)
Knaack, U., Stark, J.: Frost and frost-deicing salt resistance of super-sulfated cement concrete, frost resistance of concrete. In: Proceedings of the International RILEM Workshop on Resistance of Concrete to Freezing and Thawing with or Without De-icing Chemicals. pp. 139–146. Essen, Germany (1997)
Kwon, Y.: A study on the alkali-aggregate reaction in high-strength concrete with particular respect to the ground granulated blast-furnace slag effect. Cem. Concr. Res. 35(7), 1305–1313 (2005)
Li, G., Zhao, X.: Properties of concrete incorporating fly ash and ground granulated blast-furnace slag. Cem. Concr. Compos. 25(3), 293–299 (2003)
Li, S., Roy, D.M.: Investigation of relations between porosity, pore structure and chloride diffusion of fly ash blended cement pastes. Cem. Concr. Res. 16(5), 749–759 (1986)
Luo, R., Cai, Y., Wang, C., Huang, X.: Study of chloride binding and diffusion in GGBS concrete. Cem. Concr. Res. 33(1), 1–7 (2003)
Malhotra, V.M., Zhang, M.H., Read, P.H., Ryell, J.: Long-term mechanical properties and durability characteristics of high strength/high-performance concrete incorporating supplementary cementing materials under outdoor exposure conditions. ACI Mater. J. 97(5), 518–525 (2000)
Mangat, P.S., El-Khatib, J.M.: Influence of initial curing on sulfate resistance of blended cement concrete. Cem. Concr. Res. 22(6), 1089–1100 (1992)
Mangat, P.S., Molloy, B.T.: Influence of PFA, slag and microsilica on chloride induced corrosion of reinforcement in concrete. Cem. Concr. Res. 21(5), 819–834 (1991)
Mehta, P.K.: Concrete:Structure, Properties and Materials. Prentice Hall, Englewood Cliffs (1986)
Meusel, J.W., Rose, J,H.: Production of granulated blast furnace slag at sparrows point and the workability and strength potential of concrete incorporating the slag. vol. 79, pp. 867–890. ACI Special Publication SP, Dr. Farmington Hills (1983)
Miura, T., Iwaki, I.: Strength development of concrete incorporating high levels of ground granulated blast-furnace slag at low temperatures. ACI Mater. J. 97(1), 66–70 (2000)
Nanaumi, T., Ayuta, K., Yamakawa, T.: Frost resistance of antiwashout underwater concrete containing expanded plastic micro-spheres and blast-furnace slag. Semento Konkurito Ronbunshu 51, 90–95 (Chem. Abstr. 128, 208152) (1997)
Neville, A.M.: Properties of Concrete, 3rd edn. Pitman, London (1981)
Olorunsogo, F.T.: Particle size distribution of GGBS and bleeding characteristics of slag cement mortars. Cem. Concr. Res. 28(6), 907–919 (1998)
Oner, A., Akyuz, S.: An experimental study on optimum usage of GGBS for the compressive strength of concrete. Cem. Concr. Compos. 29(6), 505–514 (2007)
Osborne, G.J.: The effectiveness of a carbonated outer layer to concrete in the prevention of sulfate attack. In: Proceedings of International Conference on the Protection of Concrete, University of Dundee, UK (1990)
Pal, S.C., Mukherjee, A., Pathak, S.R.: Corrosion behavior of reinforcement in slag concrete. ACI Mater. J. 99(6), 1–7 (2002)
Pavía, S., Condren, E.: Study of the durability of OPC versus GGBS concrete on exposure to silage effluent. ASCE J. Mater. Civ. Eng. 20(4), 313–320 (2008)
Puertas, F., Palacios, M., Gil-Maroto, A., Vázquez, T.: Alkali-aggregate behaviour of alkali-activated slag mortars: effect of aggregate type. Cem. Concr. Compos. 31(5), 277–284 (2009)
Quillin, K., Osborne, G., Majumdar, A., Singh, B.: Effects of w/c ratio and curing conditions on strength development in BRECEM concretes. Cem. Concr. Res. 31(4), 627–632 (2001)
RILEM draft recommendation: Measurement of hardened concrete carbonation depth. Mater. Struct. 3(102), 435–90 (1984)
Roy, D.M., Idorn, G.M.: Hydration, structure, and properties of blast furnace slag cement, mortars, and concrete. Am. Concr. Inst. J. 79(6), 444–457 (1982)
Roy, D.M., Jiang, W., Silsbee, M.R.: Chloride diffusion in ordinary, blended, and alkali-activated cement pastes and its relation to other properties. Cem. Concr. Res. 30(12), 1879–1884 (2000)
Stark, J., Frost resistance with and without deicing salt—a purely physical problem, frost resistance of concrete. In: Proceedings of the International RILEM Workshop on Resistance of Concrete to Freezing and Thawing with or Without De-Icing Chemicals. pp. 83–99. Essen, Germany (1997)
Stark, J., Ludwig, H.M.: Freeze–thaw and freeze-deicing salt resistance of concretes containing cement rich in granulated blast furnace slag. ACI Mater. J. 94(1), 47–55 (1997)
Stutterheim, N.: Portland blast furnace cements—a case for separate grinding of slag. In: Fifth International Symposium on the Chemistry of Cement, Tokyo, pp. 270–276 (1968) (Supplementary Paper IV-113)
Sulapha, P., Wong, S.F., Wee, T.H., Swaddiwudhipong, S.: Carbonation of concrete containing mineral admixtures. J. Mater. Civ. Eng. 15(2), 134–143 (2003)
Tasong, W.A., Wild, S., Tilley, R.J.D.: Mechanism by which ground granulated blast furnace slag prevents sulfate attack of lime stabilized kaolinite. Cem. Concr. Res. 29(7), 975–982 (1999)
Torii, K., Sasatani, T., Kawamura, M.: Effects of fly ash, blast furnace slag, and silica fume on resistance of mortar to calcium chloride attack. In: Proceedings of Fifth International Conference on Fly Ash, Silica Fume, Slag, and Natural Pozzolans in Concrete, SP-153 vol. 2, pp. 931–949, American Concrete Institute (1995)
Ujhelyi, J.E., Ibrahim, A.J.: Hot weather concreting with hydraulic additives. Cem. Concr. Res. 21(2–3), 345–354 (1991)
Wainwright, P.J., Ait-Aider, H.: The influence of cement source and slag additions on the bleeding of concrete. Cem. Concr. Res. 25(7), 1445–1456 (1995)
Wainwright, P.J., Rey, N.: The influence of ground granulated blast furnace slag (GGBS) additions and time delay on the bleeding of concrete. Cem. Concr. Compos. 22(4), 253–257 (2000)
Wan, H., Shui, Z., Lin, Z.: Analysis of geometric characteristics of GGBS particles and their influences on cement properties. Cem. Concr. Res. 34(1), 133–137 (2004)
http://www.ecocem.ie Concrete mix design using GGBS. Ecocem Ireland Ltd. Dublin
Xu, Y.: The influence of sulfates on chloride binding and pore solution chemistry. Cem. Concr. Res. 27(12), 1841–1850 (1997)
Yeau, K.Y., Kim, E.K.: An experimental study on corrosion resistance of concrete with ground granulate blast-furnace slag. Cem. Concr. Res. 35(7), 1391–1399 (2005)
Yuksel, I., Bilir, T., Ozkan, O.: Durability of concrete incorporating non-ground blast furnace slag and bottom ash as fine aggregate. Build. Environ. 42(7), 2651–2659 (2007)
Zhang, Y.J., Zhao, Y.L., Li, H.H., Xu, D.L.: Structure characterization of hydration products generated by alkaline activation of granulated blast furnace slag. J. Mater. Sci. 43(22), 7141–7147 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Siddique, R., Iqbal Khan, M. (2011). Ground Granulated Blast Furnace Slag. In: Supplementary Cementing Materials. Engineering Materials, vol 37. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-17866-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-642-17866-5_3
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-17865-8
Online ISBN: 978-3-642-17866-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)