Abstract
Interleukin-1β (IL-1β) is a key regulator of the body’s inflammatory response and is produced after infection, injury, and an antigenic challenge. Cloned in 1984, the single polypeptide IL-1β has been shown to exert numerous biological effects. It plays a role in various diseases, including autoimmune diseases such as rheumatoid arthritis, inflammatory bowel diseases, and Type 1 diabetes, as well as in diseases associated with metabolic syndrome such as atherosclerosis, chronic heart failure, and Type 2 diabetes. The macrophage is the primary source of IL-1β, but epidermal, epithelial, lymphoid, and vascular tissues also synthesize IL-1. Recently, IL-1β production and secretion have also been reported from pancreatic islets. Insulin-producing β-cells within the pancreatic islets are specifically prone to IL-β-induced destruction and loss of function. Macrophage-derived IL-1β production in insulin-sensitive organs leads to the progression of inflammation and induction of insulin resistance in obesity. This chapter explains the mechanisms involved in the inflammatory response during diabetes progression with specific attention to the IL-1β signal effects influencing insulin action and insulin secretion . We highlight recent clinical studies, rodent and in vitro experiments with isolated islets using IL-1β as a potential target for the therapy of Type 2 diabetes.
The online version of the erratum chapter can be found under DOI http://dx.doi.org/10.1007/978-3-642-17214-4_16
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction: The IL-1 Family
Twenty-five years ago, IL-1β was cloned in the lab of Charles Dinarello (Auron et al. 1984). Meanwhile, 11 ligands and 10 receptors of the IL-1 family have been discovered. The proinflammatory and agonistic ligands are IL-1α, IL-1β, IL-18, FIL-1ε, IL-1H2, IL-1ε, and IL-33; and the anti-inflammatory and antagonistic ligands are IL-1Ra, FIL-1δ, IL-1H4, and IL-1Hy2 (Dinarello 2009). IL-1α, IL-1β, and IL-1Ra bind to IL-1R1; IL-1β and the IL-1β precursors bind to IL-1R2; IL-33 binds to IL-1R4; IL-18 and IL-1H4 to IL-1R5; FIL-1ε, IL-1H2, and IL-1ε to IL-1R6; and IL-1R8, IL-1R9 and TIR8 remain orphan receptors (Boraschi and Tagliabue 2006). IL-1β is mainly produced by activated macrophages. Production and secretion of IL-1β have been linked not only to various autoimmune and autoinflammatory diseases, but also to metabolic dysregulation (Dinarello 2009). Signaling pathways of IL-1β have been shown to result in impaired insulin secretion and action (Maedler et al. 2009). Clearly, other cytokines and chemokines are involved in the inflammatory responses; however, this chapter focuses on the possibility of blocking only IL-1β as a target for improving glycemia in T2DM.
A recent paper showing that genetic variation in the IL-1 gene family is associated with hyperglycemia and insulin resistance provides another proof for the involvement of IL-1β in the pathogenesis of diabetes (Luotola et al. 2009).
2 IL-1β Links Obesity and Diabetes
Chronic subclinical inflammation is present in obesity, insulin resistance, and T2DM. The diseases related to metabolic syndrome are characterized by abnormal cytokine production, including elevated circulating IL-1β, increased acute-phase proteins, e.g., CRP (Koenig et al. 2006), and activation of inflammatory signaling pathways (Wellen and Hotamisligil 2005).
Proinflammatory cytokines can cause insulin resistance in adipose tissue, skeletal muscle, and liver by inhibiting insulin signal transduction. The sources of cytokines in insulin-resistant states are the insulin target tissues themselves, primarily fat and liver, but to a larger extent the activated tissue resident macrophages (de Luca and Olefsky 2008).
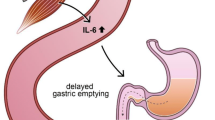
While macrophage infiltration in adipose and brain tissue has been shown in many studies (Schenk et al. 2008), increased islet macrophage infiltration has only recently been observed in pancreatic sections from patients with T2DM (Ehses et al. 2007; Richardson et al. 2009) and in T2DM animal models, such as the GK rat (Homo-Delarche et al. 2006), the HFD and db/db mouse (Ehses et al. 2007), and the hyperglycemic Cohen diabetic rat (Weksler-Zangen et al. 2008). While IL-1β signals induce destruction and impaired insulin secretion in the β-cells, insulin signaling is disturbed in the insulin target tissues (Fig. 1).
The inflammatory axis in metabolic diseases and interplay between macrophage-derived IL-1β and its action in adipose tissue, brain, pancreas, and liver. Macrophages migrate into insulin-sensitive organs and produce proinflammatory signals, which change the cell fate. In adipose tissue, this leads to increased production of cholesterol, triglycerides, cytokines, and the adipokines lepin and resistin, while adiponectin is decreased. Insulin sensitivity is impaired and glucose uptake disturbed. Mediated through intracellular signaling cascades, NF-κB and c-Jun are activated and insulin resistance in the liver and brain and impaired insulin secretion in the β-cells develop [adapted from Maedler et al. (2009)]
Insulin receptor signaling is complex. To summarize shortly, signaling downstream of the insulin receptor involves phosphorylation of IRS1/2 and the activation of the PI3K–AKT pathway (responsible for insulin action on glucose uptake) and the Ras-mitogen-activated protein kinase (MAPK) pathway (responsible for suppression of gluconeogenesis, reviewed in (Taniguchi et al. 2006)). Due to inflammation, IRS1 can be alternatively phosphorylated on serine 307, which leads to downstream activation of the NF-κB pathway, phosphorylation of C-jun N-terminal kinase 1 (JNK1), and activation of the JNK/AP-1 pathway and thus disturbed insulin signaling. Furthermore, IL-1β induces suppressor of cytokine signaling (SOCS), which leads to degradation of insulin receptor substrate (IRS) proteins (Rui et al. 2002).
2.1 IL-1β in Adipocytes
Infiltration of macrophages in adipose tissue is tightly correlated with obesity in mice and humans (Weisberg et al. 2003; Xu et al. 2003). Important modulators of inflammation are the adipocytokines, i.e., leptin, resistin, and adiponectin, which play a central role in the regulation of insulin resistance and β-cell function (Koerner et al. 2005; Tilg and Moschen 2006).
In obesity, not only circulating free fatty acids (FFA) and lipids but also leptin and resistin are increased; whereas adiponectin, which is known to prevent inflammation (Tilg and Moschen 2006) and is negatively correlated with insulin resistance, is decreased (Rasouli and Kern 2008). Leptin has been shown to exert pro- as well as anti-inflammatory properties, probably dependent on its dose and exposure time. While in vivo, leptin overexpression normalizes glycemia in the diabetic NOD mice as well as in STZ- and alloxan-induced diabetes (Yu et al. 2008), chronic leptin incubation in vitro leads to impaired β-cell function and survival (Maedler et al. 2004; Roduit and Thorens 1997; Seufert et al. 1999). Leptin has been shown to manipulate levels of IL-1β and IL-1Ra. While leptin acutely induces IL-1Ra expression in islets and monocytes (Gabay et al. 2001; Maedler et al. 2004), there is a chronic reduction of IL-1Ra and induction of IL-1β secretion.
IL-1Ra expression is increased in white adipose tissue in obese individuals with increased circulating FFA and lipids (Juge-Aubry et al. 2003). In contrast, daily IL-1Ra injections in HFD-fed mice normalize circulating FFA, lipids, as well as adipokines. Although the percentage of macrophages in a given adipose tissue depot is positively correlated with adiposity and adipocyte size (Weisberg et al. 2003), the normalization of lipids and adipokines by IL-1Ra seems to be independent of fat mass, since IL-1Ra treatment neither influences fat mass nor adipocyte size. In contrast, mRNA levels of the inflammatory cytokines IL-1β and TNF-α, the macrophage marker F4/80, and the proinflammatory macrophage marker CD11c are increased by the HFD in wild-type mice but reduced by IL-1Ra overexpression (Sauter et al. 2008). Interestingly, specifically the marker of the “classically activated” macrophages M1 (Lumeng et al. 2007) is highly induced by the HFD and normalized by IL-1Ra. Thus, the HFD-induced proinflammatory state of adipocytes may be the reason for the increased adipokines (resistin and leptin) and lipid production.
Undoubtedly, the effect of IL-1Ra on adipocyte-derived factors plays a protective role at the level of the β-cell.
2.2 IL-1β in the Liver
The bone marrow-derived macrophage cells in the liver are the Kupffer cells. Kupffer cells secrete cytokines, among them IL-1β, NO, and free radicals, which could, per se, induce β-cell failure (Barshes et al. 2005). This is specifically deleterious in the environment of transplanted islets in the liver. Cytokines (IL-1β, IFN-γ, and TNF-α) are particularly elevated after islet transplantation (Bottino et al. 1998), and liver tissue macrophages participate in cell injury and graft failure (Kaufman et al. 1990, 1994). Strategies to inhibit IL-1β-induced β-cell failure, e.g., by salicylate treatment of the islets (Tran et al. 2002; Zeender et al. 2004) may therefore improve graft survival.
Similar to the role of macrophages in obese adipose tissue, secretion of IL-1β by the Kupffer cells could be central to hepatic insulin resistance in obesity. Cytokine-induced JNK phosphorylation and activation of the NF-κB pathway are indicative of insulin resistance in the liver, e.g., depletion of JNK in myeloid cells (including Kupffer cells) in mice leads to HFD-induced hepatic steatosis without an increase in inflammatory markers in the liver and no development of insulin resistance (Solinas et al. 2007). Furthermore, hepatocyte-specific inhibition of NF-κB (Cai et al. 2005) or of IKK-β (Arkan et al. 2005) in myeloid cells improves hepatic insulin sensitivity. These studies show that independent of obesity, the inflammatory status in the liver primarily regulates insulin sensitivity.
2.3 IL-1β in the Brain
In the healthy brain, members of the IL-1 family are expressed at low or undetectable levels (Allan et al. 2005). During neuro-inflammation, IL-1β is dramatically upregulated by various local and systemic brain insults including ischemia, trauma, hypoxia, and neurotoxic inflammatory stimuli (Allan et al. 2005).
IL-1β in the brain is produced primarily by microglia, which also express caspase-1 (Touzani et al. 1999). To a lesser extent, astrocytes, oligodendroglia, neurons, cerebrovascular cells, and circulating immune cells after infiltrating the brain under inflammatory conditions produce IL-1β (Rothwell and Luheshi 2000).
IL-1β has a number of diverse actions in the CNS to modify feeding behavior, fever (Dinarello and Wolff 1982), central pain modulation (Wolf et al. 2003), stress responses (Goshen et al. 2003) memory (Schneider et al. 1998), and neuroendocrine responses, mainly through actions in the hypothalamus (Sims and Dower 1994).
There is evidence of a hypothalamic control of insulin sensitivity, which is disturbed when elevated levels of proinflammatory cytokines are circulating. Studies in mice show that HFD promotes hypothalamic resistance to the main anorexigenic hormones, leptin and insulin, leading to the progressive loss of the balance between food intake and thermogenesis and, therefore, resulting in body mass gain (De Souza et al. 2005; Milanski et al. 2009; Munzberg et al. 2004). HFD feeding of rats resulted in hypothalamic induction of IL-1β, TNF-α, IL-6, and IL-10. Activation of the toll-like receptor 4 signaling induces local cytokine expression in the hypothalamus and promotes endoplasmic reticulum stress and insulin resistance (Milanski et al. 2009).
The structural and metabolic damage found in Alzheimer’s disease is in part due to sustained elevation of IL-1β (Holden and Mooney 1995; Vandenabeele and Fiers 1991; Zuliani et al. 2007). It upregulates expression of β-amyloid precursor protein (β-APP) and stimulates the processing of β-APP, resulting in amyloidogenic fragments in neurons (Goldgaber et al. 1989). Similarly, the β-APP deposits found in the Alzheimer brain share the same molecular structure as the amylin oligomer deposits found in the pancreatic β-cells in T2DM and are equally neurotoxic (Haataja et al. 2008). On the basis of the observations in the human islet amyloid polypeptide transgenic rat (Butler et al. 2004), there is evidence that IL-1β is expressed within the islets after the induction of severe hyperglycemia (unpublished observation), indicating that IL-1β expression can only be observed at high glucose levels. It remains to be elucidated if the toxicity of amylin oligomers on the β-cell involves IL-1β signals.
Possibly, the activation of cytokine-induced proinflammatory pathways (e.g., JNK) plays a major role in the modulation of neurodegeneration (Borsello and Forloni 2007). In line with this hypothesis, JNKs are negatively regulating insulin sensitivity in the obese state.
Four different pathways are shown in the brain as a consequence of diet-induced activation of inflammatory signaling: (1) induction of suppressor of cytokine signaling-3 (SOCS-3) expression (Howard et al. 2004), (2) activation of c-Jun N-terminal kinase (JNK) and I-kappa kinase (IKK) (De Souza et al. 2005), (3) induction of protein tyrosine phosphatase 1B (PTP1B) (Bence et al. 2006), and (4) activation of TLR4 signaling (Milanski et al. 2009). Thus, obesity and HFD induce activation of proinflammatory pathways in the brain, which may directly develop insulin resistance and lead to diminished glucose regulation by the insulin target tissues.
3 IL-1β Signaling in the β-Cell
Only when the β-cell compensates for the higher insulin demand during insulin resistance, normoglycemia can be maintained. A relative insulin deficiency leads to diabetes. From numerous in vitro studies from isolated islets and β-cell lines, we know that the β-cell is especially sensitive to cytokines. Consequently, circulating cytokines are likely to rapidly affect β-cell function and survival.
Soon after the cloning of IL-1β, Mandrup-Poulsen and colleagues observed that IL-1β impairs β-cell function (Mandrup-Poulsen et al. 1985, 1986). In addition to impaired insulin secretion, IL-1β was found to induce β-cell death, which was potentiated by the cytokines IFN-γ and TNF-α (Eizirik 1988; Pukel et al. 1988). In the pancreatic islet, IL-1R1 is present in the β-cells (Deyerle et al. 1992) and not in the α-cells (Scarim et al. 1997), and thus the β-cells are a target for IL-1a, IL-1β, and IL-1Ra.
Surprisingly, IL-1R1 is highly expressed in the β-cell; more than tenfold higher expression of IL-1RI mRNA was observed in isolated islets than in total pancreas, which is attributed to the expression in the β-cell. Furthermore, β-cell IL-1R1 expression levels are higher than in any other tissue. (Boni-Schnetzler et al. 2009), which may explain the high sensitivity of the β-cell to IL-1. Blocking IL-1β with specific IL-1β-neutralizing antibodies protected from the cytotoxic effects induced by activated mononuclear cell conditioned medium (Bendtzen et al. 1986), indicating that IL-1β may play an important role in the molecular mechanisms underlying autoimmune β-cell destruction.
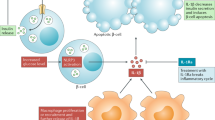
Since then, IL-1β signaling and the underlying mechanisms of IL-1β-induced β-cell destruction have been investigated. Importantly, IL-1β induces its own and the expression of other cytokines, e.g., IL-2, -3, -6, and interferons (Dinarello 1988). In turn, cells that produce IL-1β also respond to IL-1β (Warner et al. 1987). IL-1β initiates signal transduction by binding to IL-1R1 in the β-cell. This leads to docking of the IL-1RAcP to the IL-1/IL-1R1 complex, which is followed by recruitment of the adaptor protein MyD88. IRAK-4, Tollip, and IRAK-1 are then recruited, allowing IRAK-1 to activate TRAF6, which in turn triggers activation of TAK1. TAK1 is able to stimulate two main pathways: the IKK–NF-κB pathway and the mitogen-activated/stress-activated protein kinase (MAPK/SAPK) pathway (Frobose et al. 2006). In addition to TAK1, MEKK1 seems to participate in the activation of both NF-κB and SAPK in β-cells (Mokhtari et al. 2008). Phosphorylation of I-κB, a cytosolic inhibitor of NF-κB, by IKK leads to I-κB degradation and NF-κB translocation to the nucleus, thus regulating the transcription of many target genes, such as iNOS expression and NO production, a toxic reactive radical. Consistently, interfering with NF-κB activation decreases IL-1β-induced β-cell death (Giannoukakis et al. 2000; Kim et al. 2007).
IL-1β can also activate protein kinase C delta, which leads to β-cell apoptosis presumably through iNOS expression (Carpenter et al. 2001, 2002). Notably, IL-1β induces Fas expression on β-cells (Augstein et al. 2003; Stassi et al. 1995), increasing their sensitivity to FasL and accelerating apoptosis via cleavage of downstream caspases [see Fig. 2 and reviewed in Donath et al. (2003)]. A distal consequence of IL-1β signaling in β-cells is the induction of endoplasmic reticulum (ER) stress. IL-1β depletes ER Ca2+, leading to ER stress and induction of several ER stress markers including CHOP. The induction of ER stress by IL-1β can be prevented by inhibition of iNOS, suggesting that NO mediates ER stress (Cardozo et al. 2005). This is consistent with the notion that a chemical NO donor causes ER Ca2+ depletion and ER stress (Oyadomari et al. 2002). What is currently unclear is the importance of ER stress in IL-1β-induced β-cell impairment. Studies addressing the role of ER stress-induced CHOP so far indicate that ER stress and CHOP do not contribute to cytokine-induced β-cell death (Akerfeldt et al. 2008). Thus, while there is little doubt that ER stress is induced in β-cells by IL-1β, it is uncertain whether ER stress contributes to apoptosis or whether it may simply be a secondary effect and thus only plays a minor role, if any, in IL-1β-mediated apoptosis.
Mechanisms of IL-1β signaling in the β-cell. Details are described in the text [adapted from Maedler et al. (2009)]
The MAPK/SAPK pathways consist of ERK1/2, p38, and JNK1/2, all of which are activated by IL-1β in β-cells (Larsen et al. 1998; Welsh 1996). Using both pharmacological and molecular inhibitor approaches, NF-κB, ERK1/2, p38, and JNK1/2 have been demonstrated to be involved in IL-1β-induced β-cell apoptosis (Abdelli et al. 2007; Bonny et al. 2001; Larsen et al. 1998; Pavlovic et al. 2000; Saldeen et al. 2001).
Another target of IL-1β signaling in β-cells is the survival kinase pathway PI3K–Akt. IL-1β reduces both PI3K (Emanuelli et al. 2004) and Akt (Storling et al. 2005) activation. Since Akt is a negative regulator of JNK/SAPK in β-cells (Aikin et al. 2004), reduced Akt signaling may allow increased and sustained proapoptotic JNK activation.
In general, signal transduction initiated by a ligand binding to membrane receptors leads to activation or induction of negative feedback mechanisms to ensure only transient signaling. This is also true for signal transduction evoked by proinflammatory cytokines such as IL-1β. IL-1β induces expression of SOCS-3 in β-cells (Emanuelli et al. 2004; Karlsen et al. 2001). SOCS-3 is a member of a family of proteins that function to terminate cytokine signaling, thereby constituting a negative feedback loop (Ronn et al. 2007). Although IL-1β induces SOCS-3 expression in β-cells, this induction seems to be insufficient to completely terminate IL-1β signal transduction, since prolonged NF-κB and MAPK/SAPK signaling is observed in β-cells exposed to IL-1β (Aikin et al. 2004; Larsen et al. 1998; Ortis et al. 2006). Putatively, either the amount of SOCS-3 induced by IL-1β in β-cells is too low to effectively block signaling or the kinetics of SOCS-3 induction by IL-1β may be abnormally slow in β-cells. In any case, forced SOCS-3 overexpression effectively inhibits IL-1β signaling at the level of TRAF6, leading to dampening of both the NF-κB and MAPK/SAPK pathways, thus protecting against apoptosis (Frobose et al. 2006; Ronn et al. 2008). Interestingly, IL-1β-induced endogenous SOCS-3 targets insulin signaling in the β-cell by associating with the insulin receptor (IR), thereby preventing activation of IRS and PI3K (Emanuelli et al. 2004). By this mechanism, SOCS-3 induction is likely to contribute to IL-1β-induced desensitization of insulin signaling, which is important for optimal β-cell function. One may speculate whether IL-1β-induced SOCS-3 expression is preferentially directed toward IR signals while leaving the IL-1β signaling cascade unaffected. The IL-1β signaling pathways are shown in Fig. 2.
4 IL-1β Secretion
The primary sources of IL-1β are blood monocytes, tissue macrophages, and dendritic cells. B lymphocytes and NK cells also produce IL-1β (Dinarello 2009). The release of the leaderless cytokine, IL-1β, cannot be initiated through the Golgi apparatus. Inactive pro-IL-1β precursor accumulates in the cytosol and is processed by caspase-1 (also named Interleukin-converting enzyme, ICE) into the mature secreted IL-1β. The maturation occurs in a large multiprotein complex. ATP activates the P2X7 receptor, which forms a pore in response to ligand stimulation and regulates cell permeability and cytokine release (Narcisse et al. 2005).
Resident islet macrophages are fundamental in the development of autoimmune diabetes (Arnush et al. 1998; Lacy 1994) and it is postulated that IL-1β secreted from such intra-islet macrophages results in β-cell destruction (Arnush et al. 1998). Recent studies show that the β-cells themselves are able to secrete IL-1β, which is induced by double-stranded RNA, a mechanism by which viral infection may mediate β-cell damage (Heitmeier et al. 2001) by elevated glucose concentrations (Boni-Schnetzler et al. 2008; Maedler et al. 2002) and by free fatty acids (Boni-Schnetzler et al. 2009).
A recent study shows that glucose-induced IL-1β secretion involves Caspase-1 activation mediated by the NALP3 inflammasome. The inflammasone is activated by bacterial toxins and endogenous stress signals (e.g., ATP and β-amyloid) through the formation of reactive oxygen species (Schroder et al. 2010; Zhou et al. 2009). Glucose-induced IL-1β secretion is prevented in NALP3 −/− mice, indicating that IL-1β is generated through glucose-induced ROS production and oxidative stress (Zhou et al. 2009). The thioredoxin (TRX)-interacting protein (TXNIP), which has been linked to insulin resistance (Parikh et al. 2007), functions as an activator of NALP3. In line with this data, another recent study shows that TXNIP is highly increased by elevated glucose in β-cells and that TXNIP-deficient islets are protected against glucose toxicity (Chen et al. 2009).
Despite the high expression of IL-1R1 in β-cells, expression of the NALP3 inflammasone components NALP3, ASC, and Caspase-1 show relatively low expression levels (Zhou et al. 2009), which may explain the modest release of IL-1β from islets.
Upregulation of the Fas receptor plays a central role in the mediation of β-cell death (Cnop et al. 2005; Donath et al. 2005). IL-1β rapidly induces Fas upregulation, whereas glucose only induces Fas in chronic conditions (Elouil et al. 2005). The dual role of glucose on β-cell turnover is illustrated in Fig. 3. While glucose promotes insulin secretion and β-cell survival in the short term, chronic glucose induces Fas upregulation, IL-1β secretion, which leads downstream to caspase cleavage, β-cell death, and loss of insulin secretion.
Dual role of glucose on β-cell turnover. Stimulation of β-cells with glucose induces insulin secretion and β-cell proliferation. In contrast, chronic glucose exposure leads to upregulation of the Fas receptor and ligation with FasL to caspase activation, apoptosis, and impaired function, which contributes to β-cell failure in diabetes. Under such conditions, IL-1β is produced and secreted by the β-cell. This is mediated through ROS-induced induction of the NALP3 inflammasome, which activates Caspase-1 and maturation of active IL-1β from pro-IL-1β. Preincubation of the islets with the naturally occurring IL-1 antagonist interleukin-1 receptor antagonist (IL-1Ra) inhibits glucose-induced apoptosis and improves β-cell function and could therefore be a valuable tool for diabetes therapy
In two animal models, Psammomys obesus and Goto-Kakizaki (GK) rat , pancreatic β-cells express IL-1β under hyperglycemic conditions (Maedler et al. 2002; Mine et al. 2004). In P. obesus, normalizing hyperglycemia with phlorizin, an inhibitor of the renal tubular glucose reuptake, inhibited intra-islet IL-1β expression (Maedler et al. 2002). In contrast, Jorns et al. found no IL-1β expression within the islets (Jorns et al. 2006). IL-1β production by islet cells was confirmed in several studies (Boni-Schnetzler et al. 2008; Venieratos et al. 2010; Welsh et al. 2005; Zhou et al. 2009). While glucose-induced IL-1β mRNA production was not found in human islets that had been preincubated in suspension for 3–5 days (Welsh et al. 2005), Boni-Schnetzler et al. show that glucose response in islets is negatively correlated with basal IL-1β expression levels (Boni-Schnetzler et al. 2008). These studies show that IL-β may also mediate β-cell destruction in Type 2 diabetes [T2DM, reviewed in Donath et al. (2005)]. It is tempting to suggest IL-1β as a target for the treatment of diabetes. However, whether changes in circulating cytokines are physiologically relevant in the face of locally produced inflammatory mediators remains unknown.
5 Blocking IL-1β Signals Protects the β-Cell
As described above, IL-1β has been shown to impair insulin release, to induce Fas expression, thus enabling Fas-triggered apoptosis in rodent and human islets (Corbett et al. 1993; Giannoukakis et al. 1999, 2000; Loweth et al. 1998, 2000; Maedler et al. 2001; Mandrup-Poulsen et al. 1985, 1986, 1993; Rabinovitch et al. 1990; Stassi et al. 1997), and to share similarities with glucose-induced apoptosis (see Fig. 3). In parallel to the essential role of glucose in mediating insulin secretion and proliferation, a low concentration of IL-1β also stimulates insulin release and proliferation in rat and human islets (Maedler et al. 2006; Schumann et al. 2005; Spinas et al. 1986, 1987, 1988). The beneficial IL-1β effects seem to be partly mediated by the increased secretion of the naturally occurring anti-inflammatory cytokine and antagonist of IL-1α and IL-1β, the interleukin-1 receptor antagonist (IL-1Ra). Since it was discovered in 1987 (Dinarello 2000; Seckinger et al. 1987a, b), four forms of IL-1Ra have been described, of which three are intracellular proteins (icIL-1Ra I, II and III) and one is secreted (sIL-1Ra) (Arend and Guthridge 2000). Similar to IL-1β, IL-1Ra binds to type 1 and 2 IL-1 receptors but lacks a second binding domain. Therefore, IL-1Ra does not recruit the IL-1 receptor accessory protein, the second component of the receptor complex.
Endogenous production and secretion of IL-1Ra limits inflammation and tissue damage (Dinarello 2009). In vivo, exogenous IL-1Ra counteracts low-dose streptozotocin-induced diabetes (Sandberg et al. 1994) and autoimmune diabetes (Nicoletti et al. 1994) and promotes graft survival (Nicoletti et al. 1994; Sandberg et al. 1997; Stoffels et al. 2002; Tellez et al. 2007) and islet survival after transplantation (Satoh et al. 2007).
We have recently shown that IL-1Ra is secreted from the β-cell and expressed in β-cell granules (Maedler et al. 2004). IL-1Ra protects cultured human islets from the deleterious effects of glucose (Maedler et al. 2002) as well as IL-1β (Mandrup-Poulsen et al. 1993; Sandberg et al. 1997, 1993; Stoffels et al. 2002; Tellez et al. 2005). Inhibition of IL-1Ra with small interfering RNAs or long-term treatment with leptin leads to β-cell apoptosis and impaired function, which may provide a further link between obesity and diabetes.
The definite secretion and regulation mechanisms of IL-1Ra are unknown. Like IL-1β, IL-1Ra may also be secreted by a leaderless pathway via activation of the P2X7 receptor ( Glas et al. 2009; Wilson et al. 2004). In pancreatic islets from obese individuals, P2X7 receptors are highly expressed and these receptors were almost undetectable in T2DM (Glas et al. 2009). In accordance with the P2X7 receptor expression levels, increased IL-1Ra serum levels correlate with obesity and insulin resistance (Abbatecola et al. 2004; Meier et al. 2002; Ruotsalainen et al. 2006; Salmenniemi et al. 2004), but IL-1Ra is decreased in T2DM (Marculescu et al. 2002). Recent results from the Whitehall Study show that IL-1Ra levels are increased before the onset of T2DM (Herder et al. 2008), which are consistent with findings in mice fed with a high fat/high sucrose diet (HFD). IL-1Ra levels were increased after 4 and 8 weeks of diet together with an increase in β-cell mass and body weight. Serum concentrations of IL-1Ra are influenced by adipose tissue, which is a major source of IL-1Ra (Juge-Aubry et al. 2003). After 16 weeks, when the HFD-fed mice displayed glucose intolerance and β-cell apoptosis, IL-1Ra levels were lower than in the normal diet-fed mice. Mice deficient for the P2X7 receptor were unable to compensatorily increase β-cell mass in response to the HFD feeding and had no adaptive increase in IL-1Ra levels (Glas et al. 2009).
The increased IL-1Ra could be an attempt of the body to counteract the deleterious effects of IL-1β and to preserve β-cell survival, insulin secretion, and insulin sensitivity. It is hypothesized that IL-1Ra could have an additional metabolic effect that leads to insulin resistance. However, when we treated mice daily for 12 weeks with IL-1Ra, we did not observe changes in insulin sensitivity at any time point (Sauter et al. 2008).
Whether serum IL-1Ra levels would explain the progression of diabetes in obese individuals and whether serum IL-1Ra affects IL-1Ra expression in the β-cell is not known. We hypothesize that a decreased β-cell IL-1Ra expression could trigger the progression from obesity to diabetes and high IL-1Ra expression could possibly protect the β-cell and enable it to adapt to conditions of higher insulin demand; this is illustrated in the cartoon shown in Fig. 4.
Our hypothetical model illustrating the consequence of obesity on the development of Type 2 diabetes. (1) When IL-1Ra is highly expressed in the β-cell and the IL-1Ra/IL-1β balance is toward the protective IL-1Ra, β-cell mass and insulin secretion increase. The β-cell is able to adapt to a situation of higher insulin demand. (2) On the other hand, decreased β-cell expression of IL-1Ra, together with hyperglycemia-induced β-cell production of IL-1β, shifts the balance toward the proapoptotic IL-1β, leading to decreased β-cell mass, impaired β-cell function, and increased β-cell apoptosis. Glucose levels can no longer be regulated. This results in a vicious cycle and Type 2 diabetes develops. (3) But overexpression of IL-1Ra could reverse the process and protect from hyperglycemia-induced β-cell apoptosis [adapted from Maedler et al. (2009)]
5.1 Lessons from IL-1 Mouse Models
Having shown the deleterious effects of IL-1β on the β-cell, one would hypothesize that the IL-1β-knockout mouse would be the ideal model for improved β-cell survival and function. Conversely, IL-1β-KO mice show impaired glucose tolerance, decreased β-cell mass, and decreased expression of β-cell transcription factors (e.g., PDX-1 and Pax-4) (Maedler et al. 2006), indicating that IL-1β has a dual role in the β-cell and activated pathways, e.g., FLIP, Fas, and NF-κB might be needed for insulin secretion and survival (Maedler et al. 2006; Liadis et al. 2007; Schumann et al. 2007). In line with these data, Caspase-8-knockout (Liadis et al. 2007) and Fas-deficient mice (Schumann et al. 2007) show impaired glucose tolerance. NF-κB is for a long time known to be responsible for IL-1β-induced β-cell destruction (Flodstrom et al. 1996). In contrast, NF-κB also induces activation of the antiapoptotic gene A20, which protects against cell death (Liuwantara et al. 2006) and promotes insulin secretion (Hammar et al. 2004). β-Cell-specific NF-κB depletion accelerates diabetes in the NOD mouse (Kim et al. 2007).
Despite their basally impaired glucose tolerance, IL-1β-KO mice are protected against the diabetogenic effects of the HFD as well as against glucotoxicity (Maedler et al. 2006), which supports the concept that IL-β mediates nutrient-induced β-cell dysfunction during the development of T2DM.
In NOD mice, IL-1R deficiency slows but does not prevent diabetes progression (Thomas et al. 2004), and caspase-1 (interleukin-converting enzyme) deficiency has no effect on diabetes progression (Schott et al. 2004), although both IL-1R subtype 1 and caspase-1 are highly expressed in islets from wild-type NOD mice (Jafarian-Tehrani et al. 1995). It is possible that pathways other than IL-1β signals are involved in diabetes in NOD mice since it was shown that IL-10 promotes diabetes in NOD mice independent of Fas, perforin, TNFR 1, and TNFR 2 (Balasa et al. 2000).
5.2 Blocking IL-1β Signals In Vivo Inhibits Diabetes Progression
Recently, the hypothesis that blocking IL-1β as a successful strategy for the therapy of T2DM has been proved by several studies. Daily injection of IL-1Ra in mice fed an HFD improved glycemia, glucose-stimulated insulin secretion, and survival (Sauter et al. 2008), reduced hyperglycaemia, and reversed the islet inflammatory phenotype in the GK rat (Ehses et al. 2008). Treatment with an IL-1β antibody also improved glycemic control in diet-induced obesity in mice (Owyang et al. 2010; Osborn et al. 2008).
Importantly, results from a recent clinical study in patients with T2DM showed that IL-1Ra improved glycemic control and β-cell function (Larsen et al. 2007). After 13 weeks of treatment, C-peptide secretion was increased and inflammatory markers, e.g., interleukin-6 and C-reactive protein were reduced in the IL-1Ra group. HbA1c was significantly lower in the IL-1Ra compared to the placebo group, which correlated with the body surface area in the IL-1Ra group. The dose of 100 mg IL-1Ra was given daily to the patients without weight adjustment. Currently, ongoing trials that include dose adjustment to the body weight may result in better glycemic control in the higher body surface area group. The effect of interleukin-1 antagonism on β-cell function is currently tested in patients with recent onset of T1DM (Pickersgill and Mandrup-Poulsen 2009). Both IL-1Ra and anti-IL-1β antibody Xoma 052 do not completely block IL-1β signaling. While IL-1Ra is a competitive antagonist to IL-1β, XOMA 052 has a novel mechanism of action that reduces IL-1β activity by 40- to 50-fold rather than completely blocking it (Donath et al. 2008; Owyang et al. 2010). Given the dual role of IL-1β on β-cell survival and insulin secretion, this may be an important characteristic of both drugs.
As shown by these recent studies, blocking IL-1β signaling may be a powerful new treatment for T2DM, which does not rely on replacing insulin exogenously but acts at the level of the β-cell to improve β-cell survival and to improve endogenous insulin secretion and action. Moreover, blocking IL-1β may also improve insulin sensitivity. Further studies will be necessary to clarify the contradiction of IL-1Ra’s modulation of insulin sensitivity and the impact of IL-β on β-cell survival in T2DM.
References
Abbatecola AM, Ferrucci L, Grella R, Bandinelli S, Bonafe M, Barbieri M, Corsi AM, Lauretani F, Franceschi C, Paolisso G (2004) Diverse effect of inflammatory markers on insulin resistance and insulin-resistance syndrome in the elderly. J Am Geriatr Soc 52(3):399–404
Abdelli S, Abderrahmani A, Hering BJ, Beckmann JS, Bonny C (2007) The c-jun n-terminal kinase jnk participates in cytokine- and isolation stress-induced rat pancreatic islet apoptosis. Diabetologia 50(8):1660–1669
Aikin R, Maysinger D, Rosenberg L (2004) Cross-talk between phosphatidylinositol 3-kinase/akt and c-jun nh2-terminal kinase mediates survival of isolated human islets. Endocrinology 145(10):4522–4531
Akerfeldt MC, Howes J, Chan JY, Stevens VA, Boubenna N, McGuire HM, King C, Biden TJ, Laybutt DR (2008) Cytokine-induced beta-cell death is independent of endoplasmic reticulum stress signaling. Diabetes 57(11):3034–3044
Allan SM, Tyrrell PJ, Rothwell NJ (2005) Interleukin-1 and neuronal injury. Nat Rev Immunol 5(8):629–640. doi:nri1664 [pii] 10.1038/nri1664
Arend WP, Guthridge CJ (2000) Biological role of interleukin 1 receptor antagonist isoforms. Ann Rheum Dis 59(Suppl 1):i60–i64
Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M (2005) Ikk-beta links inflammation to obesity-induced insulin resistance. Nat Med 11(2):191–198
Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT (1998) Corbett JA Il-1 produced and released endogenously within human islets inhibits beta cell function. J Clin Invest 102(3):516–526, (3):26
Augstein P, Dunger A, Heinke P, Wachlin G, Berg S, Hehmke B, Salzsieder E (2003) Prevention of autoimmune diabetes in nod mice by troglitazone is associated with modulation of icam-1 expression on pancreatic islet cells and ifn-gamma expression in splenic t cells. Biochem Biophys Res Commun 304(2):378–384
Auron PE, Webb AC, Rosenwasser LJ, Mucci SF, Rich A, Wolff SM, Dinarello CA (1984) Nucleotide sequence of human monocyte interleukin 1 precursor cdna. Proc Natl Acad Sci USA 81(24):7907–7911
Balasa B, La Cava A, Van Gunst K, Mocnik L, Balakrishna D, Nguyen N, Tucker L, Sarvetnick N (2000) A mechanism for il-10-mediated diabetes in the nonobese diabetic (nod) mouse: Icam-1 deficiency blocks accelerated diabetes. J Immunol 165(12):7330–7337
Barshes NR, Wyllie S, Goss JA (2005) Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol 77(5):587–597
Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB (2006) Neuronal ptp1b regulates body weight, adiposity and leptin action. Nat Med 12(8):917–924. doi:nm1435 [pii] 10.1038/nm1435
Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, Svenson M (1986) Cytotoxicity of human pi 7 interleukin-1 for pancreatic islets of langerhans. Science 232(4757):1545–1547
Boni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, Pattou F, Halban PA, Weir GC, Donath MY (2008) Increased interleukin (il)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of il-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab 93(10):4065–4074
Boni-Schnetzler M, Boller S, Debray S, Bouzakri K, Meier DT, Prazak R, Kerr-Conte J, Pattou F, Ehses JA, Schuit FC, Donath MY (2009) Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor i. Endocrinology 150(12):5218–5229
Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF (2001) Cell-permeable peptide inhibitors of jnk: novel blockers of beta-cell death. Diabetes 50(1):77–82
Boraschi D, Tagliabue A (2006) The interleukin-1 receptor family. Vitam Horm 74:229–254
Borsello T, Forloni G (2007) Jnk signalling: a possible target to prevent neurodegeneration. Curr Pharm Des 13(18):1875–1886
Bottino R, Fernandez LA, Ricordi C, Lehmann R, Tsan MF, Oliver R, Inverardi L (1998) Transplantation of allogeneic islets of langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes 47(3):316–323
Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC (2004) Diabetes due to a progressive defect in beta-cell mass in rats transgenic for human islet amyloid polypeptide (hip rat): a new model for type 2 diabetes. Diabetes 53(6):1509–1516
Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE (2005) Local and systemic insulin resistance resulting from hepatic activation of ikk-beta and nf-kappab. Nat Med 11(2):183–190
Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL (2005) Cytokines downregulate the sarcoendoplasmic reticulum pump ca2+ atpase 2b and deplete endoplasmic reticulum ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes 54(2):452–461
Carpenter L, Cordery D, Biden TJ (2001) Protein kinase cdelta activation by interleukin-1beta stabilizes inducible nitric-oxide synthase mrna in pancreatic beta-cells. J Biol Chem 276(7):5368–5374
Carpenter L, Cordery D, Biden TJ (2002) Inhibition of protein kinase c delta protects rat ins-1 cells against interleukin-1beta and streptozotocin-induced apoptosis. Diabetes 51(2):317–324
Chen J, Fontes G, Saxena G, Poitout V, Shalev A (2009) Lack of txnip protects against mitochondria-mediated apoptosis, but not against fatty acid-induced, er-stress-mediated beta cell death. Diabetes. doi:db09-0949 [pii] 10.2337/db09-0949
Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL (2005) Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54(Suppl 2):S97–107
Corbett JA, Sweetland MA, Wang JL, Lancaster JR Jr, McDaniel ML (1993) Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of langerhans. Proc Natl Acad Sci USA 90(5):1731–1735
de Luca C, Olefsky JM (2008) Inflammation and insulin resistance. FEBS Lett 582(1):97–105
De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA (2005) Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146(10):4192–4199. doi:en.2004-1520 [pii] 10.1210/en.2004-1520
Deyerle KL, Sims JE, Dower SK, Bothwell MA (1992) Pattern of il-1 receptor gene expression suggests role in noninflammatory processes. J Immunol 149(5):1657–1665
Dinarello CA (1988) Biology of interleukin 1. FASEB J 2(2):108–115
Dinarello CA (2000) The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med 343(10):732–734
Dinarello CA (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550
Dinarello CA, Wolff SM (1982) Molecular basis of fever in humans. Am J Med 72(5):799–819
Donath MY, Storling J, Maedler K, Mandrup-Poulsen T (2003) Inflammatory mediators and islet beta-cell failure: a link between type 1 and type 2 diabetes. J Mol Med 81(8):455–470
Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, Reinecke M (2005) Mechanisms of {beta}-cell death in type 2 diabetes. Diabetes 54(Suppl 2):S108–113
Donath MY, Weder C, Brunner A, Keller C, Whitmore J, Der K, Scannon PJ, Dinarello CA, Solinger AM (2008) Xoma 052, a potential disease modifying anti-il-10 antibody shows sustained hba1c reductions 3 months after single injection with no increases in safety parameters in subjects with t2dm. Diabetes 58(S1):A30
Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY (2007) Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56(9):2356–2370
Ehses JA, Giroix M-H, Coulaud J, Akira S, Homo-Delarche F, Donath MY (2008) Il-1β-myd88 signaling is central to islet chemokine secretion in response to metabolic stress: evidence from a spontaneous model of type 2 diabetes, the gk rat. Diabetologia 50(Suppl 1):S177
Eizirik DL (1988) Interleukin-1 induced impairment in pancreatic islet oxidative metabolism of glucose is potentiated by tumor necrosis factor. Acta Endocrinol (Copenh) 119(3):321–325
Elouil H, Cardozo AK, Eizirik DL, Henquin JC, Jonas JC (2005) High glucose and hydrogen peroxide increase c-myc and haeme-oxygenase 1 mrna levels in rat pancreatic islets without activating nfkappab. Diabetologia 48(3):496–505
Emanuelli B, Glondu M, Filloux C, Peraldi P, Van Obberghen E (2004) The potential role of socs-3 in the interleukin-1{beta}-induced desensitization of insulin signaling in pancreatic beta-cells. Diabetes 53(Suppl 3):S97–S103
Flodstrom M, Welsh N, Eizirik DL (1996) Cytokines activate the nuclear factor kappa b (nf-kappa b) and induce nitric oxide production in human pancreatic islets. FEBS Lett 385(1–2):4–6
Frobose H, Ronn SG, Heding PE, Mendoza H, Cohen P, Mandrup-Poulsen T, Billestrup N (2006) Suppressor of cytokine signaling-3 inhibits interleukin-1 signaling by targeting the traf-6/tak1 complex. Mol Endocrinol 20(7):1587–1596
Gabay C, Dreyer M, Pellegrinelli N, Chicheportiche R, Meier CA (2001) Leptin directly induces the secretion of interleukin 1 receptor antagonist in human monocytes. J Clin Endocrinol Metab 86(2):783–791
Giannoukakis N, Rudert WA, Ghivizzani SC, Gambotto A, Ricordi C, Trucco M, Robbins PD (1999) Adenoviral gene transfer of the interleukin-1 receptor antagonist protein to human islets prevents il-1beta-induced beta-cell impairment and activation of islet cell apoptosis in vitro. Diabetes 48(9):1730–1736
Giannoukakis N, Mi Z, Rudert WA, Gambotto A, Trucco M, Robbins P (2000) Prevention of beta cell dysfunction and apoptosis activation in human islets by adenoviral gene transfer of the insulin-like growth factor i. Gene Ther 7(23):2015–2022
Glas R, Sauter NS, Schulthess FT, Shu L, Oberholzer J, Maedler K (2009) Purinergic p2x(7) receptors regulate secretion of interleukin-1 receptor antagonist and beta cell function and survival. Diabetologia 52(8):1579–1588
Goldgaber D, Harris HW, Hla T, Maciag T, Donnelly RJ, Jacobsen JS, Vitek MP, Gajdusek DC (1989) Interleukin 1 regulates synthesis of amyloid beta-protein precursor mrna in human endothelial cells. Proc Natl Acad Sci USA 86(19):7606–7610
Goshen I, Yirmiya R, Iverfeldt K, Weidenfeld J (2003) The role of endogenous interleukin-1 in stress-induced adrenal activation and adrenalectomy-induced adrenocorticotropic hormone hypersecretion. Endocrinology 144(10):4453–4458. doi:10.1210/en.2003-0338 en.2003-0338 [pii]
Haataja L, Gurlo T, Huang CJ, Butler PC (2008) Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev 29(3):303–316
Hammar E, Parnaud G, Bosco D, Perriraz N, Maedler K, Donath M, Rouiller DG, Halban PA (2004) Extracellular matrix protects pancreatic {beta}-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes 53(8):2034–2041
Heitmeier MR, Arnush M, Scarim AL, Corbett JA (2001) Pancreatic {beta}-cell damage mediated by {beta}-cell production of il-1: a novel mechanism for virus-induced diabetes. J Biol Chem 276:11151–11158
Herder C, Brunner EJ, Rathmann W, Strassburger K, Tabak AG, Schloot NC, Witte DR (2008) Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist (il-1ra) precede the onset of type 2 diabetes (whitehall ii study). Diab Care 32(3):421–423
Holden RJ, Mooney PA (1995) Interleukin-1 beta: a common cause of Alzheimer’s disease and diabetes mellitus. Med Hypotheses 45(6):559–571
Homo-Delarche F, Calderari S, Irminger JC, Gangnerau MN, Coulaud J, Rickenbach K, Dolz M, Halban P, Portha B, Serradas P (2006) Islet inflammation and fibrosis in a spontaneous model of type 2 diabetes, the gk rat. Diabetes 55(6):1625–1633
Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS (2004) Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of socs3. Nat Med 10(7):734–738. doi:10.1038/nm1072 nm1072 [pii]
Jafarian-Tehrani M, Amrani A, Homo-Delarche F, Marquette C, Dardenne M, Haour F (1995) Localization and characterization of interleukin-1 receptors in the islets of langerhans from control and nonobese diabetic mice. Endocrinology 136(2):609–613
Jorns A, Rath KJ, Bock O, Lenzen S (2006) Beta cell death in hyperglycaemic psammomys obesus is not cytokine-mediated. Diabetologia 49(11):2704–2712
Juge-Aubry CE, Somm E, Giusti V, Pernin A, Chicheportiche R, Verdumo C, Rohner-Jeanrenaud F, Burger D, Dayer JM, Meier CA (2003) Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes 52(5):1104–1110
Karlsen AE, Ronn SG, Lindberg K, Johannesen J, Galsgaard ED, Pociot F, Nielsen JH, Mandrup-Poulsen T, Nerup J, Billestrup N (2001) Suppressor of cytokine signaling 3 (socs-3) protects beta-cells against interleukin-1beta and interferon-gamma -mediated toxicity. Proc Natl Acad Sci USA 98:12191–12196
Kaufman DB, Platt JL, Rabe FL, Dunn DL, Bach FH, Sutherland DE (1990) Differential roles of mac-1+ cells, and cd4+ and cd8+ t lymphocytes in primary nonfunction and classic rejection of islet allografts. J Exp Med 172(1):291–302
Kaufman DB, Gores PF, Field MJ, Farney AC, Gruber SA, Stephanian E, Sutherland DE (1994) Effect of 15-deoxyspergualin on immediate function and long-term survival of transplanted islets in murine recipients of a marginal islet mass. Diabetes 43(6):778–783
Kim S, Millet I, Kim HS, Kim JY, Han MS, Lee MK, Kim KW, Sherwin RS, Karin M, Lee MS (2007) Nf-kappa b prevents beta cell death and autoimmune diabetes in nod mice. Proc Natl Acad Sci USA 104(6):1913–1918
Koenig W, Khuseyinova N, Baumert J, Thorand B, Loewel H, Chambless L, Meisinger C, Schneider A, Martin S, Kolb H, Herder C (2006) Increased concentrations of c-reactive protein and il-6 but not il-18 are independently associated with incident coronary events in middle-aged men and women: results from the monica/kora augsburg case-cohort study, 1984–2002. Arterioscler Thromb Vasc Biol 26(12):2745–2751
Koerner A, Kratzsch J, Kiess W (2005) Adipocytokines: leptin – the classical, resistin – the controversical, adiponectin – the promising, and more to come. Best Pract Res Clin Endocrinol Metab 19(4):525–546
Lacy PE (1994) The intraislet macrophage and type i diabetes. Mt Sinai J Med 61(2):170–174
Larsen CM, Wadt KA, Juhl LF, Andersen HU, Karlsen AE, Su MS, Seedorf K, Shapiro L, Dinarello CA, Mandrup-Poulsen T (1998) Interleukin-1beta-induced rat pancreatic islet nitric oxide synthesis requires both the p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Biol Chem 273(24):15294–15300
Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY (2007) Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356(15):1517–1526
Liadis N, Salmena L, Kwan E, Tajmir P, Schroer SA, Radziszewska A, Li X, Sheu L, Eweida M, Xu S, Gaisano HY, Hakem R, Woo M (2007) Distinct in vivo roles of caspase-8 in beta-cells in physiological and diabetes models. Diabetes 56(9):2302–2311
Liuwantara D, Elliot M, Smith MW, Yam AO, Walters SN, Marino E, McShea A, Grey ST (2006) Nuclear factor-kappab regulates beta-cell death: a critical role for a20 in beta-cell protection. Diabetes 55(9):2491–2501
Loweth AC, Williams GT, James RF, Scarpello JH, Morgan NG (1998) Human islets of langerhans express fas ligand and undergo apoptosis in response to interleukin-1beta and fas ligation. Diabetes 47(5):727–732
Loweth AC, Watts K, McBain SC, Williams GT, Scarpello JH, Morgan NG (2000) Dissociation between fas expression and induction of apoptosis in human islets of langerhans. Diabetes Obes Metab 2(1):57–60
Lumeng CN, Bodzin JL, Saltiel AR (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117(1):175–184
Luotola K, Paakkonen R, Alanne M, Lanki T, Moilanen L, Surakka I, Pietila A, Kahonen M, Nieminen MS, Kesaniemi YA, Peters A, Jula A, Perola M, Salomaa V (2009) Association of variation in the interleukin-1 gene family with diabetes and glucose homeostasis. J Clin Endocrinol Metab 94(11):4575–4583. doi:jc.2009-0666 [pii] 10.1210/jc.2009-0666
Maedler K, Spinas GA, Lehmann R, Sergeev P, Weber M, Fontana A, Kaiser N, Donath MY (2001) Glucose induces beta-cell apoptosis via upregulation of the fas-receptor in human islets. Diabetes 50:1683–1690
Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY (2002) Glucose-induced beta-cell production of interleukin-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110:851–860
Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, Dayer JM, Reinecke M, Halban PA, Donath MY (2004) Leptin modulates beta cell expression of il-1 receptor antagonist and release of il-1beta in human islets. Proc Natl Acad Sci USA 101(21):8138–8143
Maedler K, Schumann DM, Sauter N, Ellingsgaard H, Bosco D, Baertschiger R, Iwakura Y, Oberholzer J, Wollheim CB, Gauthier BR, Donath MY (2006) Low concentration of interleukin-1{beta} induces flice-inhibitory protein-mediated {beta}-cell proliferation in human pancreatic islets. Diabetes 55(10):2713–2722
Maedler K, Dharmadhikari G, Schumann DM, Størling J (2009) Interleukin-1 beta targeted therapy for type 2 diabetes. Expert Opin Biol Ther Sep 9(9):1177–1188
Mandrup-Poulsen T, Bendtzen K, Nielsen JH, Bendixen G, Nerup J (1985) Cytokines cause functional and structural damage to isolated islets of langerhans. Allergy 40(6):424–429
Mandrup-Poulsen T, Bendtzen K, Nerup J, Dinarello CA, Svenson M, Nielsen JH (1986) Affinity-purified human interleukin i is cytotoxic to isolated islets of langerhans. Diabetologia 29(1):63–67
Mandrup-Poulsen T, Zumsteg U, Reimers J, Pociot F, Morch L, Helqvist S, Dinarello CA, Nerup J (1993) Involvement of interleukin 1 and interleukin 1 antagonist in pancreatic beta-cell destruction in insulin-dependent diabetes mellitus. Cytokine 5(3):185–191
Marculescu R, Endler G, Schillinger M, Iordanova N, Exner M, Hayden E, Huber K, Wagner O, Mannhalter C (2002) Interleukin-1 receptor antagonist genotype is associated with coronary atherosclerosis in patients with type 2 diabetes. Diabetes 51(12):3582–3585
Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM (2002) Il-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J Clin Endocrinol Metab 87(3):1184–1188
Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA (2009) Saturated fatty acids produce an inflammatory response predominantly through the activation of tlr4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci 29(2):359–370. doi:29/2/359 [pii] 10.1523/JNEUROSCI.2760-08.2009
Mine T, Miura K, Okutsu T, Mitsui A, Kitahara Y (2004) Gene expression profile in the pancreatic islets of goto-kakizaki (gk) rats with repeated postprandial hyperglycemia. Diabetes 53(Suppl 2):2475A
Mokhtari D, Myers JW, Welsh N (2008) The mapk kinase kinase-1 is essential for stress-induced pancreatic islet cell death. Endocrinology 149(6):3046–3053
Munzberg H, Flier JS, Bjorbaek C (2004) Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145(11):4880–4889. doi:10.1210/en.2004-0726 en.2004-0726 [pii]
Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF (2005) The cytokine il-1beta transiently enhances p2x7 receptor expression and function in human astrocytes. Glia 49(2):245–258
Nicoletti F, Di Marco R, Barcellini W, Magro G, Schorlemmer HU, Kurrle R, Lunetta M, Grasso S, Zaccone P, Meroni P (1994) Protection from experimental autoimmune diabetes in the non-obese diabetic mouse with soluble interleukin-1 receptor. Eur J Immunol 24(8):1843–1847
Ortis F, Cardozo AK, Crispim D, Storling J, Mandrup-Poulsen T, Eizirik DL (2006) Cytokine-induced proapoptotic gene expression in insulin-producing cells is related to rapid, sustained, and nonoscillatory nuclear factor-kappab activation. Mol Endocrinol 20(8):1867–1879
Osborn O, Brownell SE, Sanchez-Alavez M, Salomon D, Gram H, Bartfai T (2008) Treatment with an interleukin 1 beta antibody improves glycemic control in diet-induced obesity. Cytokine 44(1):141–148
Owyang A, Maedler K, Gross L, Yin J, Esposito L, Shu L, Jadhav J, Domsgen E, Bergemann J, Lee S, Kantak S (2010) Xoma 052, an anti-IL-1β monoclonal antibody, improves glucose control and β-cell function in the diet-induced obesity mouse model. Endocrinology 151(6):2515–2527
Oyadomari S, Araki E, Mori M (2002) Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta- cells. Apoptosis 7(4):335–345
Parikh H, Carlsson E, Chutkow WA, Johansson LE, Storgaard H, Poulsen P, Saxena R, Ladd C, Schulze PC, Mazzini MJ, Jensen CB, Krook A, Bjornholm M, Tornqvist H, Zierath JR, Ridderstrale M, Altshuler D, Lee RT, Vaag A, Groop LC, Mootha VK (2007) Txnip regulates peripheral glucose metabolism in humans. PLoS Med 4(5):e158. doi:06-PLME-RA-0918R1 [pii] 10.1371/journal.pmed.0040158
Pavlovic D, Andersen NA, Mandrup-Poulsen T, Eizirik DL (2000) Activation of extracellular signal-regulated kinase (erk)1/2 contributes to cytokine-induced apoptosis in purified rat pancreatic beta-cells. Eur Cytokine Netw 11(2):267–274
Pickersgill LM, Mandrup-Poulsen TR (2009) The anti-interleukin-1 in type 1 diabetes action trial – background and rationale. Diabetes Metab Res Rev 25(4):321–324
Pukel C, Baquerizo H, Rabinovitch A (1988) Destruction of rat islet cell monolayers by cytokines. Synergistic interactions of interferon-gamma, tumor necrosis factor, lymphotoxin, and interleukin 1. Diabetes 37(1):133–136
Rabinovitch A, Sumoski W, Rajotte RV, Warnock GL (1990) Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J Clin Endocrinol Metab 71(1):152–156
Rasouli N, Kern PA (2008) Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab 93(11 Suppl 1):S64–S73
Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG (2009) Islet-associated macrophages in type 2 diabetes. Diabetologia 52(8):1686–1688. doi:10.1007/s00125-009-1410-z
Roduit R, Thorens B (1997) Inhibition of glucose-induced insulin secretion by long-term preexposure of pancreatic islets to leptin. FEBS Lett 415(2):179–182
Ronn SG, Billestrup N, Mandrup-Poulsen T (2007) Diabetes and suppressors of cytokine signaling proteins. Diabetes 56(2):541–548
Ronn SG, Borjesson A, Bruun C, Heding PE, Frobose H, Mandrup-Poulsen T, Karlsen AE, Rasschaert J, Sandler S, Billestrup N (2008) Suppressor of cytokine signalling-3 expression inhibits cytokine-mediated destruction of primary mouse and rat pancreatic islets and delays allograft rejection. Diabetologia 51(10):1873–1882
Rothwell NJ, Luheshi GN (2000) Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci 23(12):618–625
Rui L, Yuan M, Frantz D, Shoelson S, White MF (2002) Socs-1 and socs-3 block insulin signaling by ubiquitin-mediated degradation of irs1 and irs2. J Biol Chem 277(44):42394–42398
Ruotsalainen E, Salmenniemi U, Vauhkonen I, Pihlajamaki J, Punnonen K, Kainulainen S, Laakso M (2006) Changes in inflammatory cytokines are related to impaired glucose tolerance in offspring of type 2 diabetic subjects. Diab Care 29(12):2714–2720
Saldeen J, Lee JC, Welsh N (2001) Role of p38 mitogen-activated protein kinase (p38 mapk) in cytokine- induced rat islet cell apoptosis. Biochem Pharmacol 61(12):1561–1569
Salmenniemi U, Ruotsalainen E, Pihlajamaki J, Vauhkonen I, Kainulainen S, Punnonen K, Vanninen E, Laakso M (2004) Multiple abnormalities in glucose and energy metabolism and coordinated changes in levels of adiponectin, cytokines, and adhesion molecules in subjects with metabolic syndrome. Circulation 110(25):3842–3848
Sandberg JO, Eizirik DL, Sandler S, Tracey DE, Andersson A (1993) Treatment with an interleukin-1 receptor antagonist protein prolongs mouse islet allograft survival. Diabetes 42(12):1845–1851
Sandberg JO, Andersson A, Eizirik DL, Sandler S (1994) Interleukin-1 receptor antagonist prevents low dose streptozotocin induced diabetes in mice. Biochem Biophys Res Commun 202(1):543–548
Sandberg JO, Eizirik DL, Sandler S (1997) IL-1 receptor antagonist inhibits recurrence of disease after syngeneic pancreatic islet transplantation to spontaneously diabetic non-obese diabetic (nod) mice. Clin Exp Immunol 108(2):314–317
Satoh M, Yasunami Y, Matsuoka N, Nakano M, Itoh T, Nitta T, Anzai K, Ono J, Taniguchi M, Ikeda S (2007) Successful islet transplantation to two recipients from a single donor by targeting proinflammatory cytokines in mice. Transplantation 83(8):1085–1092
Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K (2008) The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology 149(5):2208–2218
Scarim AL, Arnush M, Hill JR, Marshall CA, Baldwin A, McDaniel ML, Corbett JA (1997) Evidence for the presence of type i il-1 receptors on beta-cells of islets of langerhans. Biochim Biophys Acta 1361(3):313–320
Schenk S, Saberi M, Olefsky JM (2008) Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 118(9):2992–3002
Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO (1998) A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci USA 95(13):7778–7783
Schott WH, Haskell BD, Tse HM, Milton MJ, Piganelli JD, Choisy-Rossi CM, Reifsnyder PC, Chervonsky AV, Leiter EH (2004) Caspase-1 is not required for type 1 diabetes in the nod mouse. Diabetes 53(1):99–104
Schroder K, Zhou R, Tschopp J (2010) The nlrp3 inflammasome: a sensor for metabolic danger? Science 327(5963):296–300. doi:327/5963/296 [pii] 10.1126/science.1184003
Schumann DM, Maedler K, Franklin I, Konrad D, Storling J, Boni-Schnetzler M, Gjinovci A, Kurrer MO, Gauthier BR, Bosco D, Andres A, Berney T, Greter M, Becher B, Chervonsky AV, Halban PA, Mandrup-Poulsen T, Wollheim CB, Donath MY (2007) The fas pathway is involved in pancreatic beta cell secretory function. Proc Natl Acad Sci USA 104:2861–2866
Seckinger P, Lowenthal JW, Williamson K, Dayer JM, MacDonald HR (1987a) A urine inhibitor of interleukin 1 activity that blocks ligand binding. J Immunol 139(5):1546–1549
Seckinger P, Williamson K, Balavoine JF, Mach B, Mazzei G, Shaw A, Dayer JM (1987b) A urine inhibitor of interleukin 1 activity affects both interleukin 1 alpha and 1 beta but not tumor necrosis factor alpha. J Immunol 139(5):1541–1545
Seufert J, Kieffer TJ, Leech CA, Holz GG, Moritz W, Ricordi C, Habener JF (1999) Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus. J Clin Endocrinol Metab 84(2):670–676
Sims JE, Dower SK (1994) Interleukin-1 receptors. Eur Cytokine Netw 5(6):539–546
Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M (2007) Jnk1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab 6(5):386–397
Spinas GA, Mandrup-Poulsen T, Molvig J, Baek L, Bendtzen K, Dinarello CA, Nerup J (1986) Low concentrations of interleukin-1 stimulate and high concentrations inhibit insulin release from isolated rat islets of langerhans. Acta Endocrinol (Copenh) 113(4):551–558
Spinas GA, Hansen BS, Linde S, Kastern W, Molvig J, Mandrup-Poulsen T, Dinarello CA, Nielsen JH, Nerup J (1987) interleukin 1 dose-dependently affects the biosynthesis of (pro)insulin in isolated rat islets of langerhans. Diabetologia 30(7):474–480
Spinas GA, Palmer JP, Mandrup-Poulsen T, Andersen H, Nielsen JH, Nerup J (1988) The bimodal effect of interleukin 1 on rat pancreatic beta-cells – stimulation followed by inhibition – depends upon dose, duration of exposure, and ambient glucose concentration. Acta Endocrinol (Copenh) 119(2):307–311
Stassi G, Todaro M, Richiusa P, Giordano M, Mattina A, Sbriglia MS, Lo MA, Buscemi G, Galluzzo A, Giordano C (1995) Expression of apoptosis-inducing cd95 (fas/apo-1) on human beta-cells sorted by flow-cytometry and cultured in vitro. Transplant Proc 27(6):3271–3275
Stassi G, De Maria R, Trucco G, Rudert W, Testi R, Galluzzo A, Giordano C, Trucco M (1997) Nitric oxide primes pancreatic beta cells for fas-mediated destruction in insulin-dependent diabetes mellitus. J Exp Med 186(8):1193–1200
Stoffels K, Gysemans C, Waer M, Laureys J, Bouillon R, Mathieu C (2002) Interleukin-1 receptor antagonist inhibits primary non-function and prolongs graft survival time of xenogeneic islets transplanted in sponaeously diabetic autoimmune nod mice. Diabetologia 45(Suppl 2):424–424
Storling J, Binzer J, Andersson AK, Zullig RA, Tonnesen M, Lehmann R, Spinas GA, Sandler S, Billestrup N, Mandrup-Poulsen T (2005) Nitric oxide contributes to cytokine-induced apoptosis in pancreatic beta cells via potentiation of jnk activity and inhibition of akt. Diabetologia 48(10):2039–2050
Taniguchi CM, Emanuelli B, Kahn CR (2006) Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7(2):85–96
Tellez N, Montolio M, Biarnes M, Castano E, Soler J, Montanya E (2005) Adenoviral overexpression of interleukin-1 receptor antagonist protein increases beta-cell replication in rat pancreatic islets. Gene Ther 12(2):120–128
Tellez N, Montolio M, Estil-les E, Escoriza J, Soler J, Montanya E (2007) Adenoviral overproduction of interleukin-1 receptor antagonist increases beta cell replication and mass in syngeneically transplanted islets, and improves metabolic outcome. Diabetologia 50(3):602–611
Thomas HE, Irawaty W, Darwiche R, Brodnicki TC, Santamaria P, Allison J, Kay TW (2004) Il-1 receptor deficiency slows progression to diabetes in the nod mouse. Diabetes 53(1):113–121
Tilg H, Moschen AR (2006) Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6(10):772–783
Touzani O, Boutin H, Chuquet J, Rothwell N (1999) Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J Neuroimmunol 100(1–2):203–215
Tran PO, Gleason CE, Robertson RP (2002) Inhibition of interleukin-1beta-induced cox-2 and ep3 gene expression by sodium salicylate enhances pancreatic islet beta-cell function. Diabetes 51(6):1772–1778
Vandenabeele P, Fiers W (1991) Is amyloidogenesis during Alzheimer’s disease due to an il-1-/il-6-mediated ‘acute phase response’ in the brain? Immunol Today 12(7):217–219
Venieratos PD, Drossopoulou GI, Kapodistria KD, Tsilibary EC, Kitsiou PV (2010) High glucose induces suppression of insulin signalling and apoptosis via upregulation of endogenous il-1beta and suppressor of cytokine signalling-1 in mouse pancreatic beta cells. Cell Signal. doi:S0898-6568(10)00009-4 [pii] 10.1016/j.cellsig.2010.01.003
Warner SJ, Auger KR, Libby P (1987) Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J Exp Med 165(5):1316–1331
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112(12):1796–1808
Weksler-Zangen S, Raz I, Lenzen S, Jorns A, Ehrenfeld S, Amir G, Oprescu A, Yagil Y, Yagil C, Zangen DH, Kaiser N (2008) Impaired glucose-stimulated insulin secretion is coupled with exocrine pancreatic lesions in the cohen diabetic rat. Diabetes 57(2):279–287
Wellen KE, Hotamisligil GS (2005) Inflammation, stress, and diabetes. J Clin Invest 115(5):1111–1119
Welsh N (1996) Interleukin-1 beta-induced ceramide and diacylglycerol generation may lead to activation of the c-jun nh2-terminal kinase and the transcription factor atf2 in the insulin-producing cell line rinm5f. J Biol Chem 271(14):8307–8312
Welsh N, Cnop M, Kharroubi I, Bugliani M, Lupi R, Marchetti P, Eizirik DL (2005) Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes milieu to human pancreatic islets? Diabetes 54(11):3238–3244
Wilson HL, Francis SE, Dower SK, Crossman DC (2004) Secretion of intracellular il-1 receptor antagonist (type 1) is dependent on p2x7 receptor activation. J Immunol 173(2):1202–1208
Wolf G, Yirmiya R, Goshen I, Iverfeldt K, Holmlund L, Takeda K, Shavit Y (2003) Impairment of interleukin-1 (il-1) signaling reduces basal pain sensitivity in mice: genetic, pharmacological and developmental aspects. Pain 104(3):471–480. doi:S0304395903000678 [pii]
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112(12):1821–1830
Yu X, Park BH, Wang MY, Wang ZV, Unger RH (2008) Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA 105(37):14070–14075
Zeender E, Maedler K, Bosco D, Berney T, Donath MY, Halban PA (2004) Pioglitazone and sodium salicylate protect human {beta}-cells against apoptosis and impaired function induced by glucose and interleukin-1{beta}. J Clin Endocrinol Metab 89(10):5059–5066
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J (2009) Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. doi:ni.1831 [pii]10.1038/ni.1831
Zuliani G, Ranzini M, Guerra G, Rossi L, Munari MR, Zurlo A, Volpato S, Atti AR, Ble A, Fellin R (2007) Plasma cytokines profile in older subjects with late onset Alzheimer’s disease or vascular dementia. J Psychiatr Res 41(8):686–693
Acknowledgments
This work was supported by the German Research Foundation (DFG, Emmy Noether Programm, MA4172/1-1), the Juvenile Diabetes Research Foundation (JDRF 26-2008-861), and the European Foundation for the Study of Diabetes (EFSD)/Merck Sharp & Dohme (MSD) European Studies on Beta Cell Function and Survival. The authors thank the members of the Islet Biology laboratory in Bremen for critical discussion.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Maedler, K., Dharmadhikari, G., Schumann, D.M., Størling, J. (2011). Interleukin-Targeted Therapy for Metabolic Syndrome and Type 2 Diabetes. In: Schwanstecher, M. (eds) Diabetes - Perspectives in Drug Therapy. Handbook of Experimental Pharmacology, vol 203. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-17214-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-642-17214-4_11
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-17213-7
Online ISBN: 978-3-642-17214-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)