Abstract
Sulfide minerals are common minor constituents of the Earth’s crust. In some geological environments, sulfides constitute a major proportion of rocks. In particular, metallic ore deposits (Cu, Pb, Zn, Au, Ni, U, Fe), phosphate ores , coal seam s, oil shales , and mineral sands may contain abundant sulfides. Mining of these resources can expose the sulfides to an oxygenated environment. In fact, large volumes of sulfide minerals can be exposed in: tailings dams ; waste rock dumps; coal spoil heaps; heap leach piles ; run-of-mine and low-grade ore stockpiles; waste repository embankments; open pit floors and faces; underground workings ; haul roads; road cuts; quarries; and other rock excavations. When the sulfides are exposed to the atmosphere or oxygenated ground water, the sulfides will oxidize to produce an acid water laden with sulfate, heavy metal s and metalloids. The mineral pyrite (FeS2) tends to be the most common sulfide mineral present. The weathering of this mineral at mine sites causes the largest, and most testing, environmental problem facing the industry today – acid mine drainage (AMD ) (Scientific Issue 2.1).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
2.1 Introduction
Sulfide minerals are common minor constituents of the Earth’s crust. In some geological environments, sulfides constitute a major proportion of rocks. In particular, metallic ore deposits (Cu, Pb, Zn, Au, Ni, U, Fe), phosphate ores , coal seam s, oil shales , and mineral sands may contain abundant sulfides. Mining of these resources can expose the sulfides to an oxygenated environment. In fact, large volumes of sulfide minerals can be exposed in: tailings dams ; waste rock dumps; coal spoil heaps; heap leach piles ; run-of-mine and low-grade ore stockpiles; waste repository embankments; open pit floors and faces; underground workings ; haul roads; road cuts; quarries; and other rock excavations. When the sulfides are exposed to the atmosphere or oxygenated ground water, the sulfides will oxidize to produce an acid water laden with sulfate, heavy metal s and metalloids. The mineral pyrite (FeS2) tends to be the most common sulfide mineral present. The weathering of this mineral at mine sites causes the largest, and most testing, environmental problem facing the industry today – acid mine drainage (AMD ) (Scientific Issue 2.1).
2.3 Weathering of Sulfidic Mine Wastes
Sulfidic mine wastes are in most cases polymineralic aggregates. The aggregates contain, apart from sulfides, a wide range of possible minerals including silicates, oxides, hydroxides, phosphates, halides, and carbonates. Silicates are the most common gangue mineral s , and the sulfides may represent ore or gangue phases. Thus, the mineralogy of sulfidic wastes and ores is highly heterogeneous and deposit specific.
When mining exposes sulfidic materials to an oxidizing environment, the materials become chemically unstable. A series of complex chemical weathering reactions are spontaneously initiated. This occurs because the mineral assemblages contained in the waste are not in equilibrium with the surface environment. Weathering of the minerals proceeds with the help of atmospheric gases, meteoric water and microorganisms .
The chemical weathering of an individual mineral within a polymineralic aggregate can be classified as an acid producing (i.e. generation of H+), acid buffering (i.e. consumption of H+), or non-acid generating or consuming reaction (i.e. no generation or consumption of H+). For example, the degradation of pyrite is an acid producing reaction, whereas the weathering of calcite is acid buffering, and the dissolution of quartz does not consume or generate any acid. The balance of all chemical reactions, occurring within a particular waste at any time, will determine whether the material will turn acid and produce AMD .
2.4 Acid Producing Reactions
2.4.1 Pyrite
Sulfides are stable under strongly reducing conditions. Exposure of these minerals to oxidizing conditions will destabilize them, and the sulfides will be destroyed via various oxidation mechanisms. Pyrite is the most abundant of the sulfide minerals, occurs in nearly all types of geological environments, and is commonly associated with coal and metal ore deposits. Thus, pyrite oxidation has been studied extensively from all scientific angles, and there is a vast literature on the subject (e.g. Evangelou 1995; Evangelou and Zhang 1995; Keith and Vaughan 2000; Liu et al. 2008c, 2009; Luther 1987). In contrast, the oxidation of other sulfides such as galena , sphalerite and chalcopyrite has received in comparison only limited attention.
Pyrite oxidation takes place when the mineral is exposed to oxygen (Rimstidt and Vaughan 2003). Oxidation which occurs in the presence of microorganisms is known as biotic. Pyrite oxidation may also occur without microorganisms as an abiotic or inorganic chemical oxidation process. Biotic and abiotic degradation can be caused by oxygen (i.e. direct oxidation ) or by oxygen and iron (i.e. indirect oxidation ) (Evangelou and Zhang 1995). Iron, both in its divalent and trivalent state, plays a central role in the indirect oxidation of pyrite. These different pyrite oxidation mechanisms can be summarized as:
-
1.
Oxidation by oxygen (abiotic direct oxidation );
-
2.
Oxidation by oxygen in the presence of microorganisms (biotic direct oxidation );
-
3.
Oxidation by oxygen and iron (abiotic indirect oxidation );
-
4.
Oxidation by oxygen and iron in the presence of microorganisms (biotic indirect oxidation).
Stoichiometric chemical reactions are commonly used to describe these different oxidation mechanisms. In the abiotic and biotic direct oxidation processes (mechanisms 1 and 2), oxygen directly oxidizes pyrite:
It is generally accepted, however, that pyrite oxidation is primarily accomplished by indirect oxidation (mechanisms 3 and 4). The indirect oxidation of pyrite involves the chemical oxidation of pyrite by oxygen and ferric iron (Fe3+), which occurs in three interconnected steps. The following chemical equations show the generally accepted sequence for such indirect oxidation of pyrite:
Oxidation of pyrite by oxygen (Step 1):
or,
Oxidation of ferrous iron to ferric iron (Step 2):
or,
Oxidation of pyrite by ferric iron (Step 3):
or,
Reactions 2.2, 2.3, and 2.4 release energy. Indirect pyrite oxidation is exothermic . In the initial step (Reaction 2.2), pyrite is oxidized by oxygen to produce dissolved ferrous iron (Fe2+), sulfate and hydrogen ions. The dissolved iron sulfate ions cause an increase in the total dissolved solids of the water. The release of hydrogen ions with the sulfate anions results in an acidic solution unless other reactions occur to neutralize the hydrogen ions. The second step (Reaction 2.3) represents the oxidation of ferrous iron (Fe2+) to ferric iron (Fe3+) by oxygen and occurs at a low pH. In the third reaction (Reaction 2.4) pyrite is oxidized with the help of Fe3+ generated in Reaction 2.3. Thus, Fe3+ acts as the oxidizing agent of pyrite. The oxidation of pyrite by Fe3+ in turn generates more Fe2+. This Fe2+ can then be oxidized to Fe3+ by oxygen via Reaction 2.3. The Fe3+ in turn oxidizes pyrite via Reaction 2.4, which in turn produces more Fe2+, and so on. Reactions 2.3 and 2.4 form a continuing cycle of Fe2+ conversion to Fe3+ and subsequent oxidation of pyrite by Fe3+ to produce Fe2+ (Fig. 2.1). This cyclic propagation of pyrite oxidation by Fe3+ continues until the supply of pyrite or Fe3+ to the reaction system is exhausted. While oxygen is not required for the Reaction 2.4 to occur, it is still needed to convert Fe2+ to Fe3+.
Simplified diagram illustrating the reaction pathways for pyrite oxidation (after Banks et al. 1997). Numbers 2.2 to 2.6 refer to Reactions 2.2, 2.3, 2.4, 2.5, and 2.6 in the text
The abundance of the oxidizing agent Fe3+ is influenced by the pH of the weathering solution. The solubility of Fe3+ is very low in neutral and alkaline waters. Hence, the concentrations of Fe3+ are very low in these solutions, and pyrite oxidation by Fe3+ in neutral to alkaline waters is slow and insignificant. Also, the concentration of dissolved Fe3+ decreases with increasing pH as Fe3+ solubility is limited by the precipitation of ferric hydroxide s (Fe(OH)3) and oxyhydroxides (FeOOH). In other words, if the pH increases to more than approximately 3 because of partial neutralization , for example, by carbonate minerals, then the following reactions will occur:
The precipitation of dissolved Fe3+ (Reactions 2.5 and 2.6) provides significant acidity to the solution by the release of hydrogen ions into water. This reaction lowers the pH and allows more Fe3+ to stay in solution. The Fe3+ is then involved in the oxidation of pyrite (Reaction 2.4) which results in a further reduction in pH.
The chemical precipitation of iron hydroxides in Reactions 2.5 and 2.6 is termed hydrolysis . Hydrolysis is the chemical process whereby water molecules react with dissolved cations; the cations become bonded to the hydroxy group and hydrogen ions are released. Consequently, hydrolysis results in the production of hydrogen ions, thereby causing the pH to fall. As mentioned above, the hydrolysis reaction of iron is controlled by pH. Under acid conditions of less than about pH 3, Fe3+ remains in solution. At higher pH values, precipitation of Fe3+ hydroxides occurs. Such a precipitate is commonly observed as the familiar reddish-yellow to yellowish-brown stain, coating, slimy sludge, gelatinous flocculant and precipitate in AMD affected streams and seepage areas (Chap. 3.5.7).
The Reactions 2.2, 2.3, 2.4, 2.5, and 2.6 show that in the presence of molecular oxygen, Fe2+ and S2– in pyrite are oxidized by oxygen to produce solid iron hydroxides and oxyhydroxides as well as dissolved sulfate and hydrogen ions. Clearly, oxygen and Fe3+ are the major oxidants of pyrite (Evangelou 1998; Singer and Stumm 1970). The oxidation of pyrite continues indefinitely unless one of the vital ingredients of pyrite oxidation is removed (i.e. Fe3+, oxygen or pyrite), or the pH of the weathering solution is significantly raised.
The reaction pathways of pyrite (Reactions 2.2, 2.3, 2.4, 2.5, and 2.6) have also been referred to as the AMD engine (Fig. 2.2). Pyrite , Fe3+ and oxygen represent the fuel, oxygen is also the starter engine, and Fe3+ hydroxides, sulfuric acid and heat come out of the exhaust pipe of the sulfidic waste. Such a simplified model of indirect oxidation of pyrite (Reactions 2.2, 2.3, 2.4, 2.5, and 2.6) can be summarized by one overall chemical reaction:
The self-sustaining, cyclic destruction of pyrite simplified as the “AMD engine”. The oxidation of pyrite is initiated through oxygen (“starter switch”). Pyrite , oxygen and iron (“fuel”) combust in the waste (“engine room”), and release Fe3+ hydroxides, sulfuric acid and heat into mine waters (“exhaust pipe”)
The above reaction describes the weathering of pyrite, highlights the need for water and oxygen, and illustrates the production of acid and iron hydroxide . However, there is little consensus in the literature on the precise reaction mechanisms describing the chemical oxidation of pyrite. Also, the chemical equations 2.2, 2.3, 2.4, 2.5, 2.6, and 2.7 are gross oversimplifications since: (a) the reactions do not explain that the Fe3+ hydroxides and sulfates are fictious, idealized solid phases; (b) they do not illustrate the range of iron hydroxide, oxyhydroxide and oxyhydroxysulfate minerals formed during pyrite oxidation; (c) they do not reflect the slow oxidation of Fe2+ in acid waters; (d) they disregard adsorption, desorption and neutralization reactions; (e) they disregard super-saturation of waters with iron and sulfate; (f) they do not consider the precipitation of elemental sulfur (S0) and the formation of polysulfide (\( \textrm{S}_{\textrm{n}}^{2-}\)), sulfite \( {\rm SO}_{3}^{2-}\); S: 4+), thiosulfate (\( {\rm S}_{3}{\rm O}_{3}^{2-}\); S: 2+), and polythionates (\( {\rm S}_n{\rm O}_{6}^{2-}\)) ions; and (g) they do not describe the rate or speed (i.e. kinetics) of pyrite oxidation (Nordstrom and Alpers 1999a; Ritchie 1994b). Hence, the above reaction paths (Reactions 2.1, 2.2, 2.3, 2.4, 2.5, 2.6, and 2.7) represent only approximations for actual field conditions.
How quickly pyrite weathers is influenced by its mineralogical properties and by external chemical, physical and biological factors. Mineralogical properties include the particle size , porosity , surface area , crystallography, and trace element content of pyrite. External factors are the presence of other sulfides, the presence or absence of microorganisms , as well as the oxygen and carbon dioxide concentration, temperature, pH and Fe2+/Fe3+ ratio of the weathering solution. Therefore, the rate of pyrite oxidation (i.e. the weathering kinetics of pyrite) is influenced by the following factors:
-
Pyrite particle size , porosity and surface area . The oxidation reactions occur on the surfaces of pyrite particles. Small particle sizes and large surface areas increase the reactivity of pyrite, and maximum oxidation of the pyrite surface occurs along pits, cracks, pores, and solid and liquid inclusions. For example, pyrite grains are exceptionally small in diameter in so-called framboidal pyrite . Framboidal pyrite refers to small-grained pyrite crystals with a grain size less than one micron. The grains are dispersed in the matrix or agglomerated to form a small spherical mass, typically several tens of micron in diameter. Such framboidal pyrite is more reactive than other pyrite morphologies – cubic pyrite crystals or coarse pyrite nodules – because of the greater surface area and porosity per volume of framboidal pyrite. Thus, pyrite oxidation is a surface controlled reaction (Evangelou 1995; Rose and Cravotta 1998). The quantity of a particle’s surface area is most decisive in determining reaction rates and its dissolution (Kuechler and Noack 2007; Liu et al. 2008d).
-
Mining , crushing and milling of pyrite-bearing rock to fine particle size s, for the purpose of metal extraction, vastly increase the pyrite surface area and potentially expose more pyrite to oxidation and weathering. However, crushing and milling of pyritic materials do not necessarily increase the oxidation rate of pyrite in waste rock dumps. This is because coarse-grained pyritic wastes have more pore space and allow greater oxygen movement into the wastes. Consequently, acid generation in coarse-grained wastes may occur to a greater depth than in fine-grained wastes.
-
Pyrite crystallography. Poorly crystalline pyrites or pyrites with structural defects have an imperfect or distorted crystal lattice. This leads to physical stress in the crystal structure which makes the mineral more susceptible to chemical attack (Hutchison and Ellison 1992; Rose and Cravotta 1998).
-
Trace element substitution . Trace elements can be present in pyrite in the form of minute mineral inclusions and as chemical impurities in the crystal lattice (Table 2.1). This puts strain on the crystal structure and diminishes the sulfide’s resistance to oxidation. For instance, the occurrence of arsenic in pyrite greatly decreases the resistance of pyrite to oxidation (Hutchison and Ellison 1992; Plumlee 1999). Pyrite with arsenic is thereby more reactive to oxidation than pyrite with either nickel or cobalt (Lehner and Savage 2008; Lehner et al. 2007).
-
Presence of other sulfides. Sulfidic wastes commonly contain sulfides other than pyrite. If there is direct physical contact between at least two different sulfide minerals, electrons move between the sulfides and a galvanic cell is formed. During weathering the sulfide mineral with the highest electrode potential is galvanically protected from oxidation, while the mineral with the lowest electrode potential is weathered more strongly. Selective oxidation of sulfide minerals occurs as one sulfide mineral is preferentially leached over another (Abraitis et al. 2004; Evangelou 1995; Evangelou and Zhang 1995; Hita et al. 2006; Kwong et al. 2003; Liu et al. 2008b; Nordstrom and Alpers 1999a; You et al. 2007). This galvanic protection process is the same as that for galvanized iron. The more electroconductive sulfide oxidizes at a slower rate than it would when not in contact with another sulfide. For example, among the three common sulfide minerals – pyrite, galena and sphalerite – pyrite has the highest electrode potential followed by galena and then sphalerite (Sato 1992). If these minerals are in contact with each other, sphalerite will be preferentially weathered and oxidation of pyrite is reduced. Hence, pyrite in direct contact with other sulfides does not react as vigorously as it does in isolation (Cruz et al. 2001a). Also, the oxidative dissolution of pyrite can be delayed, while other sulfides are preferentially oxidized (Hita et al. 2006; Kwong et al. 2003; You et al. 2007).
-
Temperature of the waste. The oxidation of pyrite is exothermic and generates heat as shown by the above equations. Such elevated temperatures are also advantageous to the growth of thermophilic bacteria . These bacteria use some of the released energy for their metabolic processes. However, most of the energy is released as heat and within the physical confines of waste dumps and tailings dams , there is little dissipation of the heat due to the abundance of gangue mineral s with poor heat conductivity. Thus, the pyritic waste gets warmer. Pyrite oxidation occurs faster as its oxidation rate nearly doubles with each 10°C increase in temperature (Smith et al. 1992) (Scientific Issue 2.2).
-
Microbiological activity ( Bacteria , Archaea, fungi, algae, yeasts, and protozoa). AMD environments commonly contain an abundance of microorganisms . Some of these microorganisms thrive under aerobic or anaerobic conditions and favour acid or neutral pH regimes. Archaea, Eukarya and Bacteria isolated from AMD environments are diverse and include Acidithiobacillus thiooxidans (previously Thiobacillus; Kelly and Wood 2000), Acidithiobacillus ferrooxidans (previously Thiobacillus), Leptospirillum ferrooxidans, Thiobacillus thioparus and Ferroplasma spp. (e.g. Bernier and Warren 2007; Blowes et al. 1998; Bond et al. 2000; Bryan et al. 2006; Fowler et al. 1999; Gleisner et al. 2006; Gould and Kapoor 2003; Gould et al. 1994; Hallberg and Johnson 2005; Johnson 1998a, b; Kock and Schippers 2006, 2008; Ledin and Pedersen 1996; Natarajan et al. 2006; Sánchez España et al. 2008a; Schippers and Sand 1999; Schippers et al. 2007; Schrenk et al. 1998). Certain bacteria grow particularly well in pH 2 –3 environments. These acidophilic (i.e. acid loving) bacteria and Archaea participate in the conversion of Fe2+ to Fe3+ and the oxidation of sulfur and sulfur compounds. They utilize the oxidation of the metal component (i.e. predominantly Fe) and sulfur compounds to obtain energy for their growth. Consequently, some bacteria and Archaea significantly accelerate the rate of Fe2+ oxidation to Fe3+. In fact, these bacteria and Archaea accelerate the rate of Fe2+ oxidation, which is relatively slow under abiotic, acid (pH < 4) conditions (Reaction 2.3), by a factor of hundreds to as much as one million times (Singer and Stumm 1970). In turn, the increased concentrations of Fe3+ oxidize the pyrite and accelerate acid formation. A so-called self-perpetuating or autocatalytic reaction develops whereby the microorganisms serve as a reaction catalyst for Fe2+ oxidation (Reaction 2.3). Iron oxidizing bacteria such as Acidithiobacillus ferrooxidans, Leptospirillum ferrooxidans and Ferroplasma spp. oxidize Fe2+ to Fe3+ whereas sulfur oxidizing thiobacteria such as Acidithiobacillus thiooxidans oxidize sulfides and other sulfur compounds. These aerobic bacteria and Archaea speed up the chemical oxidation rate of Fe2+ and sulfur compounds when molecular oxygen is present.
2.6 Acid Buffering Reactions
The oxidation of pyrite, the precipitation of iron and aluminium hydroxides, and the dissolution of some secondary minerals release hydrogen to solution. These processes increase the solution’s acidity unless the hydrogen is consumed through buffering reactions. Much of the buffering of the generated acidity is achieved through the reaction of the acid solution with rock-forming minerals in the sulfidic wastes. These gangue mineral s have the capacity to buffer acid; that is, the minerals will react with and consume the hydrogen ions. Acid buffering is largely caused by the weathering of silicates, carbonates and hydroxides.
The buffering reactions occur under the same oxidizing conditions, which cause the weathering of sulfide minerals. However, unlike sulfide oxidation reactions, acid buffering reactions are independent of the oxygen concentration of the gas phase or water in which the weathering reactions take place. The individual gangue mineral s dissolve at different pH values, and buffering of the solution pH by individual minerals occurs within certain pH regions (Fig. 2.3). As a consequence, depending on the type and abundance of gangue minerals within the waste (i.e. the buffering capacity of the material), not all sulfide wastes produce acidic leachates and the same environmental concerns.
Stepwise consumption of buffering capacity in a hypothetical sulfidic waste dump. (Reprinted from Salomons (1995) with permission from Elsevier Science)
2.6.1 Silicates
The major reservoir of buffering capacity in the environment are the silicate minerals which make up the majority of the minerals in the Earth’s crust. Chemical weathering of silicate minerals consumes hydrogen ions and occurs via congruent or incongruent weathering. Congruent weathering involves the complete dissolution of the silicate mineral and the production of only soluble components (Reaction 2.8). Incongruent weathering is the more common form of silicate weathering whereby the silicate mineral is altered to another phase (Reaction 2.9). The chemical composition of most silicates such as olivines, pyroxenes, amphiboles, garnets, feldspars, feldspathoids, clays and micas is restricted to a range of elements. Thus, the two types of silicate weathering can be represented by the following reactions:
Chemical weathering of silicates results in the consumption of hydrogen ions, the production of dissolved cations and silicic acid, and the formation of secondary minerals (Puura and Neretnieks 2000). For example, the incongruent destruction of the sodium-rich plagioclase feldspar albite (NaAlSi3O8) may produce montmorillonite (simplified as Al2Si4O10(OH)2) or kaolinite (Al2Si2O5(OH)4), depending on the amount of leaching:
The incongruent destruction of other feldspars such as the calcium-rich plagioclase anorthite (CaAl2Si2O8), and that of orthoclase, sanidine, adularia or microcline (KAlSi3O8) can be written as follows:
In most natural environments, the surface water contains dissolved carbon dioxide. The following reaction represents the incongruent weathering of K-feldspar under such conditions more accurately (Ollier and Pain 1997):
In the above chemical reactions (Reactions 2.10, 2.11, 2.12, 2.13, and 2.14), plagioclase and K-feldspar consume hydrogen ions in solution or generate bicarbonate ions. In addition, the by-products of feldspar and chlorite weathering are Na+, K+, Ca2+, silicic acid (H4SiO4) and the clay minerals kaolinite (Al2Si2O5(OH)4), illite (K2Al4(Si6Al2O20)(OH)4), or montmorillonite (simplified as Al2Si4O10(OH)2). The silicic acid or silica may precipitate as opaline silica or cryptocrystalline chalcedony (SiO2). New quartz is only rarely formed, and then it usually overgrows on pre-existing quartz grains. Clay minerals such as smectite, illite and kaolinite may weather, and their dissolution consumes hydrogen ions as the minerals dissolve (Rozalén et al. 2008; Shaw and Hendry 2009). For example, the dissolution of kaolinite can be represented by the following reaction:
If the dissolved Al3+ is allowed to precipitate as gibbsite (Al(OH)3), this neutralizing mechanism is lost because an equal amount of hydrogen will be released into solution (Deutsch 1997):
On the other hand, if gibbsite already exists as a solid phase in the waste rocks , it provides additional neutralizing ability because it can consume dissolved hydrogen ions. Similarly, ferric hydroxide solids (Reaction 2.17) previously precipitated during pyrite oxidation can be redissolved in acidic waters, thereby consuming hydrogen ions:
Quartz (SiO2), chalcedony (SiO2), opal (SiO2 · nH2O), and other silica minerals do not consume hydrogen when they weather to form silicic acid (Reaction 2.18). Silicic acid is a very weak acid and does not contribute significant hydrogen ions to solution. The acid is unable to donate protons to a solution unless the pH is greater than 9 (Deutsch 1997).
2.6.2 Carbonates
Carbonate minerals play an extremely important role in acid buffering reactions. Minerals such as calcite (CaCO3), dolomite (CaMg(CO3)2), ankerite (Ca(Fe,Mg)(CO3)2), or magnesite (MgCO3) neutralize acid generated from sulfide oxidation. Calcite is the most important neutralizing agent, because of its common occurrence in a wide range of geological environments and its rapid rate of reaction compared to dolomite. Similarly to pyrite weathering, grain size , texture and the presence of trace elements in the crystal lattice of carbonates may increase or decrease their resistance to weathering (Plumlee 1999; Strömberg and Banwart 1999). Calcite neutralizes acid by dissolving and complexing with hydrogen ion to form bicarbonate (HCO3 –) and carbonic acid (H2CO3) (Al et al. 2000; Blowes and Ptacek 1994; Strömberg and Banwart 1999; Stumm and Morgan 1995). Depending on the pH of the weathering solution, acidity is consumed either by the production of bicarbonate in weakly acidic to alkaline environments (Reaction 2.19) or by the production of carbonic acid in strongly acidic environments (Reaction 2.20).
Overall, the dissolution of calcite neutralizes acidity and increases pH and alkalinity in waters. A reversal of the Reactions 2.19 and 2.20 is possible when there is a change in temperature, loss of water or loss of carbon dioxide. Reprecipitation of carbonates will occur, which in turn releases hydrogen ions, causing the pH to fall.
The presence or absence of carbon dioxide strongly influences the solubility of calcite (Sherlock et al. 1995; Stumm and Morgan 1995). Calcite dissolution can occur in an open or closed system, depending on whether carbon dioxide is available for gas exchange. If water is in contact with a gas phase, then carbon dioxide can enter the solution and calcite dissolution occurs in a so-called open system (Reaction 2.21). In the open system, there is an increased solubility of calcite (Stumm and Morgan 1995). The unsaturated zones of sulfidic waste rock piles represent such open systems. In contrast, in the water saturated zone of sulfidic waste rock piles or tailings, there is no carbon dioxide gas phase. Here, calcite dissolves in a closed system (Reaction 2.22):
Therefore, in an open mine waste environment there is increased calcite dissolution because the calcite is exposed to a carbon dioxide gas phase. More bicarbonate is generated and more hydrogen ions are consumed than it would be the case in a closed mine waste environment (Sherlock et al. 1995).
Dissolution of other carbonates such as dolomite , ankerite or magnesite will similarly result in the consumption of hydrogen ions and in the release of bicarbonate, calcium and magnesium ions and carbonic acid. However, calcite is more easily dissolved than dolomite or ankerite. Siderite (FeCO3) is a common gangue mineral in coal deposits and various metal ores. The neutralizing effect of siderite depends on the redox conditions of the weathering environment. Under reducing conditions, siderite dissolves to form bicarbonate and Fe2+ ions. In contrast, in an open system with abundant oxygen, the dissolution of siderite has no neutralizing effect. While the generation of bicarbonate consumes hydrogen ions, any Fe2+ generated will undergo hydrolysis and precipitation (Reactions 2.5 and 2.6). This in turn generates as much hydrogen ions as are consumed by the generation of bicarbonate (Blowes and Ptacek 1994; Ptacek and Blowes 1994; Rose and Cravotta 1998). Hence, under well oxidized conditions, the net neutralizing effect of siderite dissolution is zero (Skousen et al. 1997).
2.6.3 Exchangeable Cations
A final neutralizing source in the subsurface are the cations (Ca2+, Mg2+, Na+, K+) present on the exchange sites of micas, clays and organic matter (Deutsch 1997; Strömberg and Banwart 1999). These exchangeable cations can be replaced by cations dissolved in weathering solutions. During sulfide oxidation, dissolved hydrogen and Fe2+ ions are produced which will compete for the cation exchange sites. The newly generated hydrogen and Fe2+ ions are removed from solution and temporarily adsorbed onto the exchange sites of the solid phases. Such reactions of clays with dissolved Fe2+ and hydrogen ions, respectively, can be represented as (Deutsch 1997; Rose and Cravotta 1998):
Clays may also undergo solid transformations during acid leaching whereby a potassium-bearing illite consumes hydrogen and is thereby transformed to a potassium-free smectite clay mineral (Puuru et al. 1999):
2.6.4 Reaction Rates
The weathering rate (i.e. weathering kinetics ) of individual minerals in sulfidic wastes is influenced by: (a) the mineral’s composition, crystal size, crystal shape, surface area , and crystal perfection; (b) the pH and dissolved carbon dioxide content of the weathering solution; (c) temperature; (d) redox conditions; and (e) access of weathering agent and removal of weathering products (Hodson 2006; Salmon and Malmström 2006; Sherlock et al. 1995). For example, there is a large difference in weathering rates between fine-grained waste and larger waste rock particles (diameters >0.25 mm). Smaller particles (diameters <0.25 mm) with their larger surface areas contribute to the great majority of sulfide oxidation as well as silicate and carbonate dissolution (Strömberg and Banwart 1999).
Different minerals reacting with acidic solutions have a variable resistance to weathering (Table 2.3). Minerals such as olivine and anorthite are more reactive and less stable in the surficial environment than K-feldspar, biotite, muscovite and albite (Fig. 2.4). The rates of the different acid buffering reactions are highly variable, and the major rock-forming minerals have been classified according to their relative pH-dependent reactivity (Table 2.4). Compared with the weathering rates of even the most reactive silicate minerals, the reaction rate s of carbonates are relatively rapid, particularly that of calcite (Strömberg and Banwart 1999). Carbonates can rapidly neutralize acid. In an extreme case, calcite may even be dissolved at a faster rate than pyrite. As a consequence, drainage from a calcite-bearing waste may have a neutral pH, yet the quality of the mine drainage can eventually deteriorate and turn acid as the calcite dissolves faster than the pyrite.
The stability of minerals during weathering (Sherlock et al. 1995)
Silicate minerals are abundant in sulfidic wastes, and their abundance may suggest that a waste rich in silicates has a significant buffering capacity . However, silicates do not necessarily dissolve completely, and the chemical weathering rate of silicates is very slow relative to the production rate of acid by pyrite oxidation. Therefore, rock-forming silicates do not buffer acid to a significant degree, and they only contribute token amounts of additional long-term buffering capacity to sulfidic wastes (Jambor et al. 2000c). Nonetheless, silicate mineral dissolution can maintain neutral conditions if the rate of acid production is quite slow and if abundant fine-grained, fast weathering silicates are present.
2.7 Coal Mine Wastes
Coal mining and processing generate the largest quantity of mine wastes (Fig. 2.5). The environmental issues related to coal wastes are attributable to the exposure of reduced earth materials (coal, sulfides, and Fe2+-bearing carbonates) to oxygen (Younger 2004). The consequences of oxidation of coal and associated strata range from the release of acid waters due to pyrite oxidation to the spontaneous combustion of the wastes.
Coals were initially deposited in reduced environments such as swamps and peat bogs. This depositional environment also resulted in the presence of fine-grained sedimentary rocks enclosing the coal seams (i.e. mudstones, sandstones). Hence, coals and their associated sediments commonly contain iron sulfides including major pyrite and possible traces of marcasite, galena, chalcopyrite and sphalerite. Oxidation of these sulfides may lead to AMD and metal and metalloid release (Kolker and Huggins 2007).
Coals are readily combustible sedimentary rocks, possessing significant carbon, hydrogen and sulfur contents. The total sulfur content of coals vary, ranging from a few 0.1 wt.% to extreme examples reaching 10 wt.%. Sulfur in coal occurs in three sulfur forms: (a) pyritic sulfur, (b) sulfate sulfur, and (c) organic sulfur. Much of the sulfur is organically bound within solid carbonaceous materials (i.e. the coal macerals), and this form of sulfur does not contribute to the acid generation of coal wastes. Sulfate sulfur is generally the result of oxidation of pyrite in the coal and is an indicator of weathering of the coal before or after mining. Thus, it is important to determine what percentage of the total sulfur is incorporated into acid-generating pyrite. Such knowledge allows an evaluation of the acid production of coal seams and associated rock types. At coal mines, AMD is commonly brought about by the oxidation of pyrite which is finely disseminated through the coals and associated sedimentary rocks.
Pyrite is not the only Fe2+-bearing mineral that undergoes oxidation when coal-bearing rocks are exposed to the atmosphere (Younger 2004). Carbonate minerals such as siderite (FeCO3) and ankerite (Ca(Mg,Fe)(CO3)2) are common gangue minerals of coal-bearing strata and these carbonates contain Fe2+. The weathering of siderite consumes hydrogen ions as long as the released Fe2+ does not undergo oxidation and hydrolysis because the hydrolysis of Fe3+ releases hydrogen protons. Thus, siderite dissolution in an oxidizing environment has no neutralizing effect on acid waters (Sect. 2.4.2). By contrast, the dissolution of ankerite consumes more hydrogen protons than the subsequent oxidation and hydrolysis of the released iron (Younger 2004). Consequently, ankerite possesses a net neutralization potential for acid waters.
Sulfide-bearing coal and associated sediments possess distinct geochemical compositions. Trace elements that are commonly enriched in coals and associated strata include trace metals (e.g. Cu, Pb, Zn, Ni, Co, Cd, Hg, U) and metalloids (e.g. As, Sb, Se). These elements are contained in sulfides, silicates, carbonates and organic matter (e.g. Kolker and Huggins 2007; Qi et al. 2008; Yang 2006; Zheng et al. 2007). Oxidation of sulfides and organic matter, weathering of silicate and carbonate minerals, and leaching of trace elements and other metals from coal mine wastes may impact on the receiving environment (Sect. 3.4.4).
2.7.1 Spontaneous Combustion of Pyritic Wastes
Coal and certain base metal, uranium, iron and phosphate ore deposits are hosted by sedimentary sequences, some of which contain pyritic, carbonaceous shales and mudstones. The exothermic oxidation of sulfides and organic matter in these rock types can lead to a significant increase in temperature in pyritic, carbonaceous rocks. The elevated temperatures have the potential to cause premature detonation of explosives in a charged blasthole with catastrophic consequences (Briggs and Kelso 2003). This is particularly the case for ammonium nitrate-based explosive products.
The development of even higher temperatures may lead to the spontaneous ignition of coal and carbonaceous, pyritic shales and mudstones, which has been observed naturally (Mathews and Bustin 1984). It can also occur in underground workings , open pit faces, waste rock dumps, and slag heaps (Bullock and Bell 1997; Puura et al. 1999; Sidenko et al. 2001). It is visible as smoke, comprising a variety of gases such as water vapour, sulfur dioxide, carbon dioxide, carbon monoxide, and methane. In particular, colliery spoil and carbonaceous, pyritic waste rock dumps have the tendency to burn and smoke. Vent sites are commonly coated with condensate minerals such as sulfates, halides and native sulfur (Masalehdani et al. 2009).
Coal and carbonaceous rocks often contain abundant very fine-grained, micrometer sized framboidal pyrite. Spontaneous combustion of this material is initiated through its exposure to atmospheric oxygen or oxygenated ground water. This leads to the slow exothermic oxidation of pyrite, carbon and organic matter which in turn results in a gradual rise in temperature of the rock. Any fine-grained rock materials will act as heat insulators, and the heat will not be able to escape. At some stage, enough heat is generated to ignite the carbon or organic matter . The oxidation reactions are significantly accelerated as soon as significant amounts of atmospheric oxygen or oxygen dissolved in water are supplied to the carbonaceous material, and large surface area s are exposed, for example, as a result of mining. Next, rapid oxidation of this hot pyritic, carbonaceous rock is initiated, and spontaneous combustion occurs. The organic carbon and sulfur begin to burn. Smoke and steam are released resembling volcanic fumaroles. The combustion of carbon and organic matter increases the heat of the rock which in turn increases the rate of sulfide oxidation. If there is sufficient oxygen during the combustion process, the pyrite is converted to hematite and sulfur oxides:
If there is not enough oxygen for complete oxidation, hydrogen sulfide is formed. In extreme cases of oxidation, temperatures reach 1200°C and localized melting of the rocks and wastes occurs. In such cases, the outer dump layer cracks, and surface venting of gases from sulfidic materials becomes significant. The spontaneous combustion and subsequent cooling of coal spoil and pyritic waste rock dumps produce waste materials of complex mineralogical composition, including slag -type phases, thermal metamorphic or pyrometamorphic minerals, and weathering related minerals (Dokoupilová et al. 2007; Puura et al. 1999; Sidenko et al. 2001).
If combustion has already begun in mine waste dumps, disturbing the burning heap – by excavating or reshaping it – will only provide additional atmospheric oxygen to the waste, and the rate of combustion will increase. Various methods are used to combat combustion in mine wastes, including: (a) covering the entire surface of burning spoil heaps including the batters with a thick layer of inert material; (b) compaction of the near-surface material; (c) application of a final cover layer with good water retention properties; (d) injection of water; and (e) water spraying (Fig. 2.6). However, compaction may eventually lead to cracking of the seal by pressurized gases. Also, the use of excessive amounts of water may generate steam and eventually cause steam explosions.
In order to prevent premature detonation of explosives or spontaneous combustion in carbonaceous, pyritic rocks, the rocks need to be characterized for their pyrite and organic carbon contents and their temperature. Such characterization should occur before or during mining. This will ensure that any high-risk material will undergo special handling prior to their finite disposal. Disposal options include dumping small heaps of wastes and leaving them to oxidize and cool prior to finite capping with benign wastes.
2.8 Formation and Dissolution of Secondary Minerals
The weathering of sulfides releases sulfate, metals, metalloids and other elements into solution. This water can contact more sulfide minerals and accelerate their oxidation (i.e. acid producing reactions). Alternatively, it can contact gangue mineral s , some of which react to neutralize some or all of the acid (i.e. acid buffering reactions). Above all, the reactive sulfide and gangue minerals will contribute various ionic species to the weathering solution. In fact, in many sulfidic materials the acid producing, acid buffering and non-acid generating reactions release significant amounts of dissolved cations and anions into pore water s. As a result, the waters become highly saline. Some ions will remain in solution in ionic form, where they can interact with minerals and be adsorbed. Sheet silicates such as chlorite, talc, illite and smectite are especially able to adsorb metal ions from pore solutions (Dinelli and Tateo 2001). Few ions will remain in solution indefinitely and enter ground or surface water s. Other ions will interact in the weathering solution, reach saturation levels and precipitate as secondary minerals in the waste. The formation of secondary minerals is the most common form of element fixation in pore waters of sulfidic wastes. A significant fraction of the metals released by sulfide oxidation is retained in the wastes as secondary mineral precipitates (Farkas et al. 2009; Lin 1997; Lin and Herbert 1997; Smuda et al. 2007). Such secondary mineral formation is not exclusive to the wastes themselves; numerous salts approach saturation in ground waters, streams and leachates associated with the weathering of sulfidic wastes. Therefore, a wide range of secondary minerals are known to precipitate in oxidizing sulfidic wastes and AMD environments (Table 2.5). Also, the formation of secondary minerals is not exclusive to sulfidic wastes and AMD waters. It may occur in any saline water regardless of its pH.
2.8.1 Pre-mining and Post-mining Secondary Minerals
Secondary minerals are defined as those that form during weathering. Weathering of sulfides may occur before, during or after mining. Thus, a distinction has to be made between secondary minerals formed by natural processes prior to mining and those formed after the commencement of mining (Nordstrom and Alpers 1999a).
Sulfide oxidation prior to mining results in the formation of secondary minerals . For example, if a sulfide orebody has been exposed by erosion and weathered by surface water s descending through the unsaturated zone, a near-surface oxidized layer of secondary minerals forms (Courtin-Nomade et al. 2009; Gomes and Favas 2006; Williams 1990). Some of these secondary minerals are relatively insoluble in ground and surface waters. They effectively capture the metals and reduce the release of metals into the environment. Hence, leaching of completely oxidized wastes can produce non-acid mine waters. Nonetheless, an abundance of relatively soluble sulfates such as gypsum may still result in saline, sulfate-rich drainage waters.
Sulfide oxidation during and after mining results in the formation of secondary minerals . Post-mining secondary minerals form because waste and ore have been exposed to the atmosphere and subsequently weathered. Such post-mining oxidation products occur as cements and masses within the waste and as crusts at or near the waste’s surface. The surface precipitates are commonly referred to as efflorescences. They are particularly common in waste piles, underground workings, stream beds and seepage areas, and on pit faces (Figs. 2.7, 2.8 and 2.9).
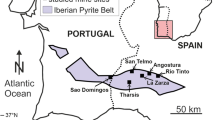
Face of the Río Tinto smelting slag dump, Spain. Mineral efflorescences commonly occur as white sulfate salt precipitates (gypsum, epsomite, hexahydrite, bloedite, copiapite, roemerite) in protected overhangs and at seepage points at the base of the slag dump. The slags generate ephemeral drainage, which runs from the dump into the Río Tinto and contributes to its acidification and metal load
The distinction of pre- from post-mining secondary minerals can be a challenging task because some minerals, particularly the soluble sulfates, may have formed during the pre- and post-mining stage. Stable isotope analyses coupled with textural analyses have proven useful in differentiating pre- and post-mining sulfates (Campbell and Lueth 2008). The precipitation of post-mining secondary minerals takes place in response to one of these following processes (Nordstrom and Alpers 1999a):
-
Oxidation and hydrolysis of the dissolved cation (Fe2+);
-
Hydrolysis of the dissolved cation (e.g. Fe3+, Al3+);
-
Reaction of acid mine waters with acid buffering minerals or alkaline waters;
-
Mixing of acid mine waters with neutral pH waters;
-
Oxidation of sulfides in humid air;
-
Concentration of the mine water due to evaporation .
Evaporation is an important mechanism in the formation of mineral salts. This process concentrates any cations and anions in mine waters until they reach mineral saturation, forming secondary minerals . Not all precipitates are crystalline, and many solids are of a poorly crystalline or even amorphous nature. The initial minerals that precipitate tend to be poorly crystalline, metastable phases that may transform to more stable phases over time (Murad et al. 1994; Nordstrom and Alpers 1999a). Consequently, the collection and identification of metastable phases using conventional laboratory techniques are troublesome, and materials should be collected and stored in airtight containers at temperatures resembling field conditions. By contrast, airborne and ground infrared spectrometry can be used to identify and map secondary iron minerals. This approach allows the discrimination and mapping of different iron minerals in exposed outcrops, waste dumps, watersheds, and streams impacted by tailings spillages (Ackman 2003; Dalton et al. 2000; Ferrier et al. 2009; Sams and Veloski 2003; Sams et al. 2003; Swayze et al. 2000; Williams et al. 2002; Velasco et al. 2005). In turn, the pH value of mine drainage waters can be inferred from the colour and spectral reflectance of the precipitates because the occurrence of different iron minerals is controlled by pH as well as other parameters.
2.8.2 Solubility of Secondary Minerals
Secondary minerals can be grouped into sulfates, oxides, hydroxides and arsenates, carbonates, silicates, and native elements (Table 2.5). The type of secondary minerals formed in mine wastes is primarily controlled by the composition of the waste. For example, coal spoils commonly possess iron, aluminium, calcium, magnesium, sodium, and potassium sulfates (e.g. Zielinski et al. 2001), whereas metalliferous waste rocks tend to contain abundant iron, aluminium and heavy metal sulfate salts.
Some of the secondary minerals are susceptible to dissolution, whereby a wide range in solubility has been noted. For example, simple hydrous metal sulfates are very soluble in water, whereas the iron and aluminium hydroxysulfates are relatively insoluble. In addition, there are a number of secondary sulfates and carbonates which are poorly soluble such as barite (BaSO4), anglesite (PbSO4), celestite (SrSO4), and cerussite (PbCO3). As a result, once these minerals are formed, they will effectively immobilize the contained elements. The minerals act as sinks for sulfate, barium, strontium, and lead in oxidizing sulfidic wastes, and their precipitation controls the amount of sulfate, barium, strontium, and lead in AMD solutions.
The water soluble hydrous metal sulfates with divalent cations (Me2+SO4 · nH2O) are the most dominant secondary mineral types (Jambor et al. 2000a, b). These hydrous sulfates may redissolve in water and release their ions back into solution:
Alternatively, the hydrous sulfates may dehydrate to less hydrous or even anhydrous compositions. For example, melanterite (FeSO4 · 7H2O) may precipitate first, which may then dehydrate to rozenite (FeSO4 · 4H2O) or szomolnokite (FeSO4 · H2O). Also, the hydrous Fe2+ sulfates may oxidize to Fe2+-Fe3+ or Fe3+ sulfate salts. For instance, the Fe2+ mineral melanterite (FeSO4 · 7H2O) may oxidize to the mixed Fe2+-Fe3+ mineral copiapite (Fe5(SO4)6(OH)2 · 20H2O) (Frau 2000; Jerz and Rimstidt 2003). The newly formed secondary minerals are more stable and resistant to redissolution compared to their precursors. Thus, secondary minerals may exhibit a paragenetic sequence whereby the minerals formed in a distinct order. The general trend for the simple hydrous sulfate salts is that the Fe2+ minerals form first, followed by the mixed Fe2+-Fe3+ minerals, and then the Fe3+ minerals (Jambor et al. 2000a, b).
Secondary minerals, be they relatively soluble or insoluble, possess large surface area s. Consequently, they adsorb or coprecipitate significant quantities of trace elements including metals and metalloids. The precipitates effectively immobilize elements in acid mine waters and hence provide an important natural attentuation and detoxification mechanism in mine waters (Berger et al. 2000; Lin 1997; Nordstrom and Alpers 1999a). However, this immobilization of metals is only temporary as many mineral efflorescences, particularly the simple hydrous metal sulfates, tend to be soluble and release their stored metals back into mine waters upon dissolution.
The presence of soluble secondary minerals in mine wastes is strongly influenced by the prevalent climatic conditions. In humid climates, soluble sulfate minerals may not accumulate because of extensive leaching. In arid and semi-arid climates, soluble minerals may persist because of high evaporation rates and a lack of rainfall.
2.8.3 Acid Consumption and Production
The precipitation of some secondary minerals may influence the mine water pH as their formation generates or consumes hydrogen ions. Generally, the formation of Fe3+ or Al3+ hydroxides generates acid, whereas the precipitation of Fe2+, Mn2, Fe3+ and Al3+ sulfate salts such as jarosite (KFe3(SO4)2(OH)6), alunite (KAl3(SO4)2(OH)6), coquimbite (Fe2(SO4)3 · 9H2O), jurbanite (Al(SO4)(OH) · 5H2O), halotrichite (FeAl2(SO4)4 · 22H2O), or melanterite (FeSO4 · 7H2O) consumes acid. However, this consumption of acidity is only temporary as these minerals, particularly the simple hydrous metal sulfates, tend to be soluble and release their stored acidity upon dissolution (Cravotta 1994) (Table 2.2). A generalized reaction for this temporary acid consumption can be written as follows:
The precipitation and redissolution of secondary minerals in sulfidic wastes may greatly influence the acidity and chemical composition of ground, surface and pore water s (Chap. 3). As a consequence, the amounts and types of secondary salts need to be determined in sulfidic mine wastes.
2.8.4 Coatings and Hardpans
The formation of secondary minerals does not only influence the mine water chemistry but it also impacts on potential water-rock reactions. For example, rapid precipitation of secondary minerals – during sulfide oxidation or carbonate dissolution – may coat or even encapsulate the acid producing or buffering mineral. Such coatings will make the mineral less susceptible to continued weathering and dissolution. Coatings developing on oxidizing sulfide grains can be exceptionally fine-grained (i.e. nm sized phases) and can consist of complex assemblages of amorphous, poorly crystalline and crystalline secondary phases (Petrunic et al. 2009).
Prolonged precipitation of secondary minerals may occur at the surface or at a particular depth of tailings dams and waste rock piles. Such continuous precipitation results in the formation of laterally extensive or discontinuous surface or subsurface layers (Alakangas and Öhlander 2006a; Boorman and Watson 1976; Blowes et al. 1991; Graupner et al. 2007; Hakkou et al. 2008a; Holmström et al. 1999; McGregor and Blowes 2002; McSweeney and Madison 1988; Moncur et al. 2005) (Fig. 2.10). Precipitated minerals include hydroxides (e.g. goethite , ferrihydrite, lepidocrocite ), sulfates (e.g. jarosite , gypsum, melanterite ), or sulfides (e.g. covellite), which fill the intergranular pores and cement the waste matrices.
In waste rock piles and tailings dams , secondary minerals typically precipitate below the zone of oxidation and at the interface between oxic and anoxic layers (Fig. 2.11). A distinct vertical colour change in the waste, from reddish-brown-yellow at the top to grey below, generally indicates the transition from an oxidized layer to reduced material. If the precipitation layer dries out and cements, it forms a so-called hardpan . This layer acts as horizontal barrier to the vertical flow of pore water s. A hardpan may also form within the zone of oxidation at a depth where the pore water reacts with acid neutralizing carbonates. The pH of the pore water rapidly rises due to carbonate dissolution, and iron precipitates as iron hydroxides which cement the waste.
Simplified diagram illustrating the formation of a hardpan layer in sulfidic wastes (after Jambor et al. 2000b). In this example, hardpan formation occurs at the water table between the saturated and unsaturated zone. A hardpan layer may also form within the unsaturated zone due to chemical reactions between an acidic leachate and a neutralizing layer
The formation of hardpans in sulfidic wastes can be induced in order to control sulfide oxidation. The addition of limestone , lime (Ca(OH)2), magnesite (MgCO3), brucite (Mg(OH)2), or other neutralizing materials, at or just below the surface of sulfidic waste, will help to generate artifical hardpans or so-called chemical covers or chemical caps of gypsum, jarosite and iron hydroxides (Chermak and Runnells 1996, 1997; Ettner and Braastad 1999; Pérez-López et al. 2007a, b; Shay and Cellan 2000). Regardless whether the hardpan is naturally formed or chemically induced using neutralizing materials, a hardpan reduces the extent of wind and water erosion at the tailings surface, limiting dust dispersion. It also protects the underlying materials from further oxidation and limits AMD generation through various processes: (a) it prevents ingress of oxygenated ground and pore water into water saturated parts of the sulfidic waste; (b) it limits the movement of atmospheric oxygen through reactive unsaturated sulfidic wastes; (c) it reduces the waste’s porosity ; and (d) it accumulates heavy metal s and metalloids through mineral precipitation, and adsorption and coprecipitation processes. However, elements not permanently fixed in insoluble minerals are susceptible to dissolution and mobilization back into pore waters. Such hardpans do not protect the sulfidic materials from further oxidation nor do they cause permanent sequestration of trace elements (Lottermoser and Ashley 2006a).
2.9 Acid Generation Prediction
AMD generation can result in surface and ground water contamination that requires expensive water treatment and involves potential liability in perpetuity. An accurate prediction of the acid producing potential of sulfidic wastes is, therefore, essential. A prediction of acid generation requires a good understanding of the physical, geological, geochemical and mineralogical characteristics of the sulfidic wastes. Data acquisition for acid generation prediction includes the completion of:
-
Geological modeling;
-
Geological, geochemical, mineralogical and petrographic descriptions;
-
Geochemical static and kinetic tests; and
-
The use of computer models for oxygen movement and geochemical processes.
2.9.1 Geological Modeling
Geological modeling is a basic technique for assessing the acid generation potential of sulfidic wastes. It involves classification of the deposit and deduction of potential acidity problems (Table 2.6). The reasoning behind this method is that ore deposits of the same type have the same ore and gangue mineral s and accordingly, the same acid producing and acid buffering materials (Kwong 1993; Plumlee 1999; Seal et al. 2000). However, the method has very limited application because it assumes that factors influencing acid generation such as pyrite surface area , abundance of sulfides or waste dump characteristics are constant for the mine sites and ore deposits being compared. The comparisons are very unreliable, yet they may provide some initial insight in the overall likelihood of acid generation. The technique may be applied to stratigraphically equivalent coal mines or ore deposits in volcano-sedimentary sequences. Thus, geological modeling and classification of an ore deposit is an initial crude step in ranking the deposit in terms of its potential to produce AMD .
2.9.2 Geological, Petrographic, Geochemical and Mineralogical Descriptions
A prediction on acid generation should begin well before sulfidic wastes are produced at mine sites. Preliminary evaluations can be performed as early as the exploration drilling and early mining of an orebody. Fundamental basic data for waste characterization and acid generation prediction include: existing lithologies; structural features; ore and gangue textures and mineralogy; particle size distribution; depth of oxidation; and whole rock geochemistry. Geological data such as pyrite content, geochemical analyses (S, C, CO3, metals), and static test data can be used to construct a three-dimensional block model of different waste rock units prior to mining (Bennett et al. 1997).
Characterization of sulfidic waste materials involves mineralogical, mineral chemical and geochemical investigations (Lin 1997; Marescotti et al. 2008). Mineralogical observations (using X-ray diffraction, optical microscopy, scanning electron microscopy, and transmission electron microscopy) should note the size, shape, surface area s, degree of crystallinity, distribution, and oxidation state of sulfides, gangue mineral s , and weathering products. As the abundance of pyrite and other sulfides in mine wastes is a crucial aspect of AMD generation, their quantity can be determined using advanced X-ray diffraction techniques (Oerter et al. 2007). Textural descriptions are also important as they can reveal protective encapsulation of sulfides in weathering resistant gangue minerals such as quartz .
Investigations using electron microprobe and synchrotron based analyses demonstrate the abundance, siting and speciation of metals, metalloids and other elements in solid waste phases (Lu et al. 2005; Schuwirth et al. 2007). The potential mobility of metals and metalloids in mine wastes and waste contaminated soils and sediments can be evaluated using partial and sequential extraction techniques (Álvarez-Valero et al. 2009; Cappuyns et al. 2007; Dold 2003; Falk et al. 2006; Heikkinen and Räisänen 2009; Hudson-Edwards and Edwards 2005; Ibrahim and Jaber 2007; Lavergren et al. 2009; Ostergren et al. 1999; Pérez-López et al. 2008; Slowey et al. 2007). Sequential leaching tests demonstrate that heavy metals may be present as cations: (a) on exchangeable sites; (b) incorporated in carbonates; (c) incorporated in easily reducible iron and manganese oxides and hydroxides; (d) incorporated in moderately reducible iron and manganese oxides and hydroxides; (e) incorporated in sulfides and organic matter ; and (f) incorporated in residual silicate and oxide minerals. The successive extractions are assumed to remove individual phases of the material selectively, while causing relatively insignificant dissolution of other phases. However, it is obvious that these differentiated wet chemistry analyses are associated with several problems. (a) the reactions involved in the leaching procedures are not selective; (b) readsorption and precipitation processes may occur; and (c) sample preparation, including drying, commonly causes transformation of labile phases to more stable components (e.g. Conesa et al. 2008).
Moreover, the bioavailability of metals and metalloids in mine wastes and their potential transfer to plants, animals and humans can be established using various extraction tests. These tests also allow an evaluation of the risk associated with the exposure of humans and animals to contaminated materials (Bruce et al. 2007; Schaider et al. 2007).
Thus, geological, petrographic, mineralogical and geochemical descriptions of sulfidic wastes provide important information on: (a) the nature and distribution of acid producing and acid buffering minerals; (b) the mineralogical siting of metals and metalloids; (c) the potential release and mobility of elements during weathering; and (d) the bioavailability of elements in mine wastes and the risk associated with human and animal exposure.
2.9.3 Sampling
The distribution of acid producing and acid consuming minerals is generally heterogeneous on micro- to macroscopic scales. Different ore lenses, coal seam s and waste materials may represent acid producing or acid buffering units. Sulfidic wastes cannot be treated as a homogeneous mass, and the accurate prediction of AMD requires an appropriate samping strategy and sampling density (Modis and Komnitsas 2007).
Waste samples can be obtained during exploration drilling and mining. However, representative sampling from drill cores is very difficult to achieve. The properties of vein deposits highlight the problems of sampling from drill cores for acid generation prediction (Dobos 2000). For example, a mesothermal gold vein deposit comprises of a rock mass, which is non-acid generating, and a series of acid generating veins with abundant pyrite (Fig. 2.12). Drilling and sampling of a composite over the entire drill section will yield a sample, largely comprising of the non-acid generating host rock. By contrast, blasting of this material will cause the rock to break along the veins, resulting in the exposure of a disproportionate amount of pyrite veins. If this mined material is dumped, it will generate more acid than the initially drilled and geochemically tested material. Therefore, geologically controlled sampling is most important in order to ensure that the analyzed samples are representative of the type and distribution of acid producing and acid buffering minerals (Dobos 2000). Otherwise, significant errors may occur when averages of static or kinetic test data are used: (a) to predict the likelihood of acid generation from a particular waste pile; or (b) to forecast the composition of seepage waters emanating from waste dumps.
Schematic diagram showing a macroscopic ore texture of a mesothermal gold deposit (after Dobos 2000). Acid producing pyrite veins are hosted by non-acid producing country rocks. The traces of drill holes into the veined rock are also indicated
Waste rock piles and coal spoil heaps of historic mining operations commonly require characterization and acid prediction . It has been suggested that the most economic sampling strategy to adequately characterize existing waste rock piles is a homogeneous composite of 15–30 samples (Munroe et al. 1999; Smith et al. 2000). However, sulfidic waste piles, particularly those dumped some time ago, may have developed a vertical mineralogical and chemical zonation. Sampling restricted to dump surfaces will disregard sulfidic, partly oxidized or secondary mineral enriched wastes at depth. Hence, drilling and profile sampling may be required to obtain sample materials representative of the entire waste dump (Farkas et al. 2009).
2.9.4 Geochemical Tests
Geochemical tests should not be conducted without detailed mineralogical and geochemical investigations of the material. Particularly, the acquisition of pure static and kinetic test data without a detailed knowledge of the mineralogical composition of the waste represents a waste by itself. Detailed procedures for various static and kinetic tests, and instructions on how to interpret them, are found in Morin and Hutt (1997). Laboratory methods for the geochemical analysis of environmental samples, including sulfidic wastes, are given by Crock et al. (1999).
2.9.4.1 Static Tests
Static tests are geochemical analyses of sulfidic waste which are used to predict the potential of a waste sample to produce acid. Details of these tests are documented in the literature (Jambor 2003; Jambor et al. 2007; Mitchell 2000; Morin and Hutt 1997; Sobek et al. 1978; Smith et al. 1992; White et al. 1999). Static tests are empirical procedures, and there is a confusing array of tests to measure and to document acid production and acid neutralization . In addition, static tests and reporting conventions vary (North America: AP, NP, NNP, NPR; Australia and the Asia Pacific region: MPA, ANC, NAPP). Fortunately, static tests can be assigned to three major categories:
-
Saturated paste pH and electrical conductivity. A representative fine-grained or crushed waste sample is saturated with distilled water to form a paste. The pH and electrical conductivity (EC) of the paste can be determined in the field or after a period of equilibration (12 –24 hours) (Morin and Hutt 1997). Paste pH measurements conducted in the field allow a rapid distinction of acid generating rocks from acid neutralising rocks. Hughes et al. (2007) recommend the use of paste pH and a simple portable carbonate dissolution test in the field for distinguishing rocks that are potentially acid forming from those that are acid neutralising. A pH value of less than 4 generally indicates that the sample is acid generating, and an EC value of greater than 20 μScm–1 indicates a high level of total dissolved solids in the waste’s leachate. Paste pH and EC values of wastes and soils forming on waste rock dumps may change over time because sulfide minerals within the materials weather and release ions into solution and the materials are flushed by infiltration and runoff waters (Borden 2001).
-
Acid Base Accounting (ABA ). Acid Base Accounting refers to the numerical data used to predict acid generation. The three components of the ABA are: (1) determination of acid production; (2) determination of acid consumption; and (3) calculation of net acid production or consumption using the data from (1) and (2).
-
1.
Determination of acid production. The Acid Potential (AP), Acid Production Potential (APP), or Maximum Potential Acidity (MPA) tests establish the maximum amount of sulfuric acid produced from sulfidic wastes. This is measured by analyzing the sample for its sulfur content. For the MPA and APP, the weight per cent sulfur is then converted to kilograms of sulfuric acid per tonne of waste (MPA value in kg H2SO4t–1=wt.% S×30.625). For the AP, the weight per cent sulfur is converted to kilograms of calcium carbonate per tonne of waste that would be required to neutralize the acidity (AP value in kg CaCO3t–1=wt.% S×31.25).
-
2.
Determination of acid consumption. The Neutralization Potential (NP), Acid Neutralizing Capacity (ANC) or Acid Consumption (AC) tests measure the amount of acid the sample can neutralize. This is determined by analyzing the acidity consumption of a sample in acid (HCl or H2SO4). Consequently, the tests establish the buffering capacity of a sample due to dissolution and weathering of gangue mineral s , or in other words, the ability of a sample to neutralize acid generated from sulfide oxidation. The NP and ANC are determined by adding acid to a sample, and then back titrating with hydroxide to determine the amount of acid the sample has consumed. The ANC value is reported in the form of kilograms of sulfuric acid consumption per tonne of waste (kg H2SO4t–1), whereas the NP value is given in the form of kilograms of calcium carbonate consumption per tonne of waste (kg CaCO3t–1).
-
3.
Calculation of net acid production or consumption. The Net Acid Producing Potential (NAPP) represents the theoretical balance of a sample’s capacity to generate acid. By contrast, the Net Neutralization Potential (NNP) gives the waste’s capacity to neutralize any acid generated.
-
3.1
NAPP calculations are based on the net acidity of samples (i.e. kilograms of H2SO4 per tonne of waste) (Environment Australia 1997). The NAPP is defined as being the difference between the Maximum Potential Acidity (MPA) and the Acid Neutralizing Capacity (ANC), whereby the MPA value is subtracted from the ANC value. A positive NAPP value indicates the sample should generate acid, whereas a negative value indicates the potential for acid neutralization (Fig. 2.13).
$${\rm{NAPP}} = {\rm{MPA}}-{\rm{ANC}}$$or
$${\rm{NAPP }}\;({\rm{kg}}{\rm{H}}_{\rm{2}} {\rm{SO}}_{\rm{4}} {\rm{t}}^{-{\rm{1}}} ) = {\rm{S}}\;({\rm{wt.}}\% )\;{\rm{3}}0.{\rm{625}}-{\rm{ANC }}\;({\rm{kg}}{\rm{H}}_{\rm{2}} {\rm{SO}}_{\rm{4}} {\rm{t}}^{-{\rm{1}}} ) $$((2.29)) -
3.2
NNP calculations are based on the net neutralizing potential available in the samples (i.e. kilograms of CaCO3 per tonne of waste) (Mitchell 2000; Skousen et al. 2002; White et al. 1999). The NNP or ABA is defined as being the difference between the Acid Potential (AP) and the Neutralization Potential (NP). In theory, the NNP value is the net amount of limestone required to exactly neutralize the potential acid-forming rock.
-
3.1
$${\rm{NNP\ or\ ABA = NP - AP}}$$or
$${\rm{NNP}}\,{\rm{or}}\,{\rm{ABA}}\,\left( {{\rm{kgCaCO}}_{\rm{3}} {\rm{t}}^{{\rm{ - 1}}} } \right) = {\rm{NP}}\left( {{\rm{kgCaCO}}_{\rm{3}} {\rm{t}}^{{\rm{ - 1}}} } \right) - {\rm{AP}}\left( {{\rm{kgCaCO}}_{\rm{3}} {\rm{t}}^{{\rm{ - 1}}} } \right)$$((2.30)) -
1.
Geochemical classification plot for mine wastes based on their wt.% sulfur and Acid Neutralizing Capacity (ANC) values. Wastes plot into the field of positive or negative Net Acid Producing Potential (NAPP) (after Smart et al. 2002). A higher ANC/MPA ratio may be used as a safety factor (2, 3 or 4 rather than 1) to classify waste samples as acid or non-acid generating
Theoretically, rocks with positive NNP values have no potential for acidification whereas rocks with negative NNP values do. In practice, a safety factor is applied and rocks with a significant positive NNP value are generally regarded as having no acidification potential (>+20 or +30 kg CaCO3t–1). Rocks with a significant negative NNP value (<–20 or –30 kg CaCO3t–1) are potentially acid generating. Materials with intermediate NNP values have uncertain acid generation potentials (–20 or –30 kg CaCO3t–1<NNP<+20 or +30 kg CaCO3t–1).
Alternatively, the ratio NP/AP, known as the Neutralization Potential Ratio (NPR), or the ratio NP/MPA can be used as the criterion to evaluate the capacity of the material to generate AMD (Heikkinen and Räisänen 2008; Price et al. 1997; Skousen et al. 2002) (Fig. 2.14). Theoretically, a NP/AP ratio less than 1 generally implies that the sample will eventually lead to acidic conditions (Sherlock et al. 1995). A ratio greater than 1 is indicative that the sample will not produce acid upon weathering. In practice, a safety factor is applied and rocks with a NP/AP ratio greater than 2, 3 or 4 are non-acid generating, whereas samples with a NP/AP ratio less than 1 have a likely acidification potential (Price et al. 1997).
Geochemical classification plot for mine wastes based on their wt.% sulfidic sulfur content and NP/AP ratio, showing the probable fields of AMD generation (after Price et al. 1997)
-
Net Acid Generation (NAG ) or Net Acid Production (NAP). The NAG test directly evaluates the generation of sulfuric acid in sulfidic wastes. It is based on the principle that a strong oxidizing agent accelerates the oxidation of sulfides. The test simply involves the addition of hydrogen peroxide to a pulverized sample and the measurement of the solution pH after 24 hours, when the oxidation reaction is thought to be complete (final NAG pH). If the NAG pH is below a critical value, then the sample has the potential to generate acid in the field (Liao et al. 2007; Schafer 2000). Variations of the NAG test procedure include the static, sequential and kinetic NAG test (Miller 1996, 1998a). A final NAG pH greater than or equal to 4.5 classifies the sample as non-acid forming. A final NAG pH result of less than 4.5 confirms that sulfide oxidation generates an excess of acidity and classifies the material as higher risk. The NAP test is similar to the NAG test and involves the addition of hydrogen peroxide and titration of the peroxide-sample slurry to a neutral pH using hydroxide . The amount of acidity consumed is reported in kilograms of calcium carbonate per tonne of waste (kg CaCO3t–1).
-
Results of NAG and NAPP tests will place a waste material into one of several categories including acid consuming (ACM), non-acid forming low sulfur (NAF-LS), non-acid forming high sulfur (NAF-HS), potentially acid forming low capacity (PAF-LC), and potentially acid forming high capacity (PAF-HC) (Table 2.7, Fig. 2.15) (Miller 1996, 1998a). If a site contains PAF-HC or PAF-LC material, then kinetic test data need to be acquired, and AMD management practices have to be established (Miller 1996, 1998a). However, attention must also be given to NAF-HS and ACM material if they host soluble secondary minerals such as gypsum. Drainage from such materials may be neutral to alkaline but exceptionally saline, thereby exceeding water quality guidelines for sulfate. In addition, neutral to alkaline drainage waters may carry exceptionally high contents of metals such as zinc, molybdenum or cadmium and metalloids such as arsenic , antimony , or selenium (Sect. 3.4.3).
Geochemical classification plot for mine wastes based on their Net Acid Producing Potential (NAPP) and NAG pH values. Wastes plot as NAF (non-acid forming), PAF (potentially acid forming) and UC (uncertain) wastes (after Smart et al. 2002)
The main advantage of these static tests is their simplicity, and most static tests can be perfomed at mine sites. However, the determination of the acid generating potential is not standardized. Also, the static tests are based on several assumptions and are, therefore, associated with many problems (Jambor 2000, 2003; Jambor et al. 2003; Miller 1996; Morin and Hutt 1997; Paktunc 1999; Weber et al. 2004; White et al. 1999):
-
The tests use powdered or crushed samples for analysis which artificially increase the grain size and expose more mineral grains to reactions.
-
Total sulfur analyses are not representative of the AP, APP or MPA because sulfur may also be present in non-acid producing sulfides or non-reactive or non-acid producing sulfates such as gypsum, anhydrite, barite or even organic material. Sulfur present in organic matter does not participate in acid generation (Casagrande et al. 1989). Organic matter is particularly common in coal spoils and washery wastes. Therefore, an acid generation prediction of such wastes using total sulfur analyses will be unreliable. Also, many sulfidic wastes contain non-acid generating sulfate minerals. It is possible to analyze for sulfidic sulfur contained in sulfides and for sulfate sulfur contained in secondary sulfate minerals (e.g. Yin and Catalan 2003). However, current bulk geochemical analytical techniques are not capable of distinguishing pyritic sulfur from sulfur present in acid producing sulfates or in other sulfides that may or may not generate acid.
-
Framboidal pyrite is more reactive than euhedral forms due to the greater specific surface area (Weber et al. 2004). As a result, NAPP testing is biased by the rapid acid generating oxidation of framboidal pyrite prior to and during the ANC test.
-
The possible coating of acid producing sulfides by secondary minerals is not taken into account, and it is assumed that the acid producing and acid consuming minerals will react completely.
-
Organic carbon is oxidized by hydrogen peroxide during NAG testing which interferes with the acidity of the solution.
-
The static tests do not allow the much slower acid buffering reactions of silicates to take place which, however, contribute only very minor amounts to the neutralization potential of sulfidic wastes (Heikkinen and Räisänen 2008; Jambor et al. 2000c).
Overall, static tests may under- or overestimate the acid production of a particular sample. As a result, numerous authors have proposed improvements and alternatives to existing static tests (e.g. Lawrence and Scheske 1997; Li et al. 2007a; Morin and Hutt 1997). These proposed methods seek to provide better estimates of sulfur speciation and acid producing minerals in the waste. Regardless of these modifications to existing laboratory protocols and the introduction of new field and laboratory tests, static tests only predict the acid potential of individual samples and not of entire waste dumps. The tests are adequate tools for preliminary evaluation of AMD and are best used as rapid screening tools to assess the likelihood of acid generation from particular sulfidic wastes (Miller 1996, 1998a).
2.9.4.2 Kinetic Tests
Kinetic tests simulate the weathering and oxidation of sulfidic waste samples. They are generally used to follow up the findings of static testing. Kinetic tests expose the sulfidic waste over time, from several months to several years, to moisture and air (e.g. Acero et al. 2007c; Alakangas and Öhlander 2006b; Falk et al. 2006; Hakkou et al. 2008b; Malmström et al. 2006; Mitchell 2000; Morin and Hutt 1997; Munk et al. 2006; Smith et al. 1992; Trois et al. 2007; Younger et al. 2002). The experiments can be accelerated to simulate long-term weathering of waste materials in a shorter time frame. Water is thereby added to the waste more frequently than it would occur under normal field conditions.
Generally, kinetic tests involve the addition of water to a known quantity of waste. Leach columns and humidity cells are the most frequently used laboratory test techniques, whereby water is dripped or trickled onto one kilogram to one tonne of sample. The acid producing and acid buffering reactions are allowed to proceed, and the leachate is periodically collected and analyzed for its composition including pH and EC as well as sulfate, metal and metalloid concentrations. Mineralogical and geochemical characterization of the sulfidic waste has to be carried out prior to and after experimentation. In laboratory kinetic tests, relatively small samples are monitored under controlled conditions (Fig. 2.16), whereas field kinetic tests monitor relatively large samples under less controlled conditions in large bins or drums (Fig. 2.17).
The main advantage of these simulated weathering techniques is that they consider the weathering rates of sulfides and gangue mineral s . The tests can provide an indication of the oxidation rate, lag period for the onset of acid generation, and effectiveness of blending or layering of different wastes. The tests also provide data on the load (i.e. mgs–1) or fluxes (ta–1) of metals, metalloids and other elements in leachates and seepage waters from waste disposal facilities. They thereby indicate the water quality in the short and long term.
Kinetic tests are not standardized, and a great number of kinetic test designs have been developed. Any interpretation of kinetic analytical results has to scrutinize experimental design, analytical techniques and local environmental conditions. Furthermore, the interpretation of kinetic data has to consider that the data can be in great contrast to the actual field data (Ardau et al. 2009). The reason for such discrepancies may be due to the experimental designs which hardly resemble actual waste profiles where numerous variables such as oxygen diffusion , water infiltration, microbial populations, secondary mineral formation, changes in mineralogical composition, evolution of the surface state of sulfides, and other environmental conditions control AMD generation. Hence, several authors have evaluated laboratory kinetic tests for measuring rates of weathering and have proposed improvements and alternatives to existing kinetic tests (Benzaazoua et al. 2004; Cruz et al. 2001b; Frostad et al. 2002; Hollings et al. 2001; Kargbo and He 2004).
Kinetic field trials at the mine site have distinct advantages over laboratory tests. More prolonged water-rock interaction allows the completion of chemical reactions over time and more complete chemical equilibration. Most importantly, the tests permit accurate replication of the local climate and selection of appropriate sample material and volume (Bethune et al. 1997 ; Morin and Hutt 1997; Smith et al. 1992). In particular, field-based trial dumps allow the determination of acid generation parameters under actual field conditions (Fig. 2.18). Small waste piles are constructed with an appropriate liner , and piezometers and lysimeters may be installed. Different dry covers may be installed above sulfidic wastes, allowing an evaluation of their long-term performance (Alakangas and Öhlander 2006b). Leachate, run-off and pore water compositions and volumes can then be investigated. The trials reduce the inaccuracies resulting from small test samples and allow a more realistic assessment of the AMD processes and potential.
2.9.5 Modeling the Oxidation of Sulfidic Waste Dumps
Oxygen is essential for the oxidation of sulfides in waste dumps. Simple calculations can demonstrate that the availability of oxygen controls the oxidation rate of sulfidic waste (Gibson and Ritchie 1991). For example, a 50 t sulfidic waste rock pile has a sulfur concentration of 2 wt.%. The waste dump, therefore, contains 1 t of sulfur which will require, based on the stoichiometric ratio, 1.75 t of oxygen for its oxidation to sulfate. A 50 t waste pile with a porosity of 0.3 contains approximately 8×10–3 t of oxygen, which is only 1/200 of the 1.75 t needed for complete oxidation (Gibson and Ritchie 1991). Consequently, in order to accomplish complete oxidation of the waste, oxygen must travel into the heap from the atmosphere.
Indeed, the transport of oxygen to the oxidation sites is considered the rate limiting process in dumps and tailings deposits (Ritchie 1994a, b, c). The gas-phase transport of oxygen in waste dumps from the surface to the oxidation sites at depth occurs by: (a) diffusion (i.e. flow of oxygen induced by a gas concentration gradient); (b) convection (i.e. flow of air induced by wind action, barometric pressure changes, or thermal convection driven by the heat generated from the exothermic pyrite reaction); and (c) advection (i.e. flow of air induced by a thermal or pressure gradient) (Ritchie 1994a, b, c; Rose and Cravotta 1998). Minor amounts of oxygen may also be transported into the dump via liquid-phase diffusion and advection (i.e. flow of oxygen via infiltrating precipitation).
The relative contribution of diffusion , convection or advection to overall gas transport is dependent on a variety of parameters including the position of the waste within the dump, the component materials and minerals, and the way in which the dump has been constructed. Diffuse transport of oxygen through the gas-filled pore spaces is thought to dominate in unsaturated, newly built waste dumps (Aachid et al. 2004; Kim and Benson 2004; Ritchie 1994b). Uniform diffusion into such waste materials will result in oxygen profiles with horizontally flat oxygen concentration contours. Gas convection is limited to the edges of waste dumps, and since dump edges are a small fraction of the total dump volume, convection is disregarded in the modeling of the oxidation rate of pyritic waste dumps (Ritchie 1994b). However, convective gas flux has been reported from newly constructed waste dumps (Cathles 1994). In addition, localized convections have been observed in aged waste dumps, as indicated by high oxygen concentrations at depth and complex oxygen concentration profiles. The advective and convective modes of oxygen transport appear to predominate in porous waste dumps containing abundant coarse-grained rock fragments (Rose and Cravotta 1998). The diffuse mode of oxygen transport predominates in less permeable waste materials composed of small fragments.
The reactivity of a sulfidic waste pile and its oxidation behaviour in the long term can be described using laboratory experiments and pyrite oxidation rates (Sracek et al. 2006). The intrinsic oxidation rate is calculated through a series of mathematical equations (Ritchie 1994a, b, c). These equations quantify the physical mechanisms which control the oxidation of a pyritic waste heap. For instance, the oxygen consumption rate represents the rate at which oxygen is consumed by the dump material (in units of kilograms of oxygen per cubic metre of waste per second; kg–1m–3s–1 or molkg–1s–1). The term quantifies the loss of oxygen from the pore space by oxidation reactions in the waste. A typical oxygen consumption rate value calculated for waste rock dumps is in the order of 10–8 to 10–11 kg–1m–3s–1 (Bennett et al. 1994; Hollings et al. 2001; Ritchie 1995). In this model, it is assumed that oxygen is only consumed by pyrite. However, oxygen may also be consumed by the oxidation of other sulfides, native elements and organic matter . Furthermore, the sulfide oxidation rate is dependent on a large number of variables including temperature, pH, Fe3+ concentration, particle size distribution, mineral surface area , bacterial population, trace element substitution , degree of pyrite crystallinity and so forth. Finally, sulfide oxidation rates within a single dump appear to be variable; a dump may contain pockets of more highly oxidizing materials, particularly toward the dump edges (Linklater et al. 2005). Thus, these weathering models will need further refinement.
2.10 Monitoring Sulfidic Wastes
The recognition of sulfide oxidation does not necessarily require sophisticated equipment and measurements. In fact, some of the common indicators of sulfide oxidation can be recognized in the field:
-
Abundant yellow to red staining on rocks and flocculants in seepage points, streams and ponds due to the formation of secondary iron minerals and colloids ;
-
Sulfurous odours;
-
Unsuccessful colonization of waste materials by vegetation ;
-
Abundant mineral efflorescences within and on exposed waste materials;
-
Increasing magnetic susceptibility due to the abundance of magnetic secondary iron oxides and carbonates;
-
Increasing waste temperature due to exothermic pyrite oxidation;
-
Decreasing oxygen concentration in pore gas es due to oxygen consumption; and most importantly;
-
Decreasing pH, increasing EC, and increasing sulfate, metal (Cu, Zn etc) and major cation (Na, K, Ca, Mg) concentrations in drainage waters with time (Miller 1995).
The latter three indicators of sulfide oxidation are used to monitor sulfidic wastes. Sulfidic waste rock dumps, tailings dams and heap leach piles need monitoring in order to detect at the earliest point in time whether the waste material will “turn acid” and generate AMD . Also, rehabilitated waste repositories need monitoring to establish the effectiveness of the control technique used to curtail sulfide oxidation. The monitoring techniques are designed to identify the early presence of, or the changes to, any products of the acid producing reactions in sulfidic wastes. The products of the acid producing reactions are usually quantified by one or more of the following parameters (Hutchison and Ellison 1992):
-
Water analyses of dissolved contaminant concentrations and loads (Chap. 3.8).
-
Temperature profiles. Pyrite oxidation is an exothermic reaction, and the effects of heat generation can be assessed by remote or in situ sensing. Remote sensing using thermal infrared spectrometry is best suited for identifying zones of high acid generation of exposed sulfidic materials such as open pits (Hutchison and Ellison 1992). However, remote sensing or airborne geophysical techniques are not appropriate for detecting the onset of acid generating conditions in covered or piled sulfidic wastes. In situ temperature sensing is used to detect temperatures and temperature gradients in sulfidic waste rock piles or tailings. Temperature sensitive electrical probes (thermistors) or thermocouples are placed into a series and lowered down installed PVC pipes. Rapid increases in temperature profiles are symptomatic of the exothermic oxidation reactions of pyrite.
-
Oxygen concentration within gas pores. Sulfide oxidation reactions in the unsaturated zone of sulfidic waste piles and tailings are oxygen consuming. Hence, the depletion of oxygen within the gas phase can be indicative of sulfide oxidation, and a knowledge of pore gas compositions will allow an evaluation of sulfide oxidation reactions within the waste pile. Firstly, pore gas sampling using appropriately constructed probe holes is performed (Lundgren 2001). Next, oxygen analyzers determine the oxygen concentration and finally, oxygen concentration profiles are acquired. Oxygen is generally supplied to the interior of a fine-grained waste rock pile by diffusion , which is induced by concentration differential from the atmosphere. The concentration profile within such a pile will show decreasing oxygen with increasing depth below the surface. Under these conditions, active oxidation zones associated with acid generation can be detected as they result in sharp oxygen partial pressure gradients.
2.11 Environmental Impacts
Modern mine site rehabilitation of sulfidic waste dumps commonly involves addition of a soil cover onto mine wastes and planting of vegetation (Sect. 2.10.2). A suitable plant substrate with adequate moisture retention and nutrient availability is usually required to allow the establishment of plants over otherwise bare rock. The establishment of native vegetation directly on sulfidic wastes is difficult to achieve. If the wastes are not capped, the visible environmental impacts of sulfidic waste dumps and spoil heaps include waste erosion and a depauperate or even absent flora. For example, the surface of coal spoil heaps with their inherent salt content, sodicity of the waste and pronounced salt crust commonly does not support any vegetation (Bullock and Bell 1997; Schaaf and Hüttl 2006). A sparse or non-existent vegetation cover can also caused by a lack of soil nutrients (N, P) and organic matter , as well as the potentially high salinity and acidity and high metal content in the surface layers of wastes and contaminated soils (Figs. 2.19 and 2.20). The lack of vegetation on abandoned waste rock dump can also be due to the physical properties of the rock types making up the waste (Craw et al. 2007a). The lack of vegetation on wastes increases erosion rates. The erosion processes exacerbate the moonscape appearance of wastes and increase the areas affected by waste particles. Investigations of the environmental impacts of mine wastes require an assessment of the concentration of elements in soils, sediments, plants and biota in background and contaminated sample populations, which will allow the distinction of natural geogenic from induced anthropogenic factors (e.g. Martínez López et al. 2008).
2.11.1 Soil and Sediment Contamination
Wind and water erosion may disperse wastes from their disposal sites and unconstrained chemical leaching of wastes may release contaminants to the surrounds. Reactive and unreactive waste particles are transported into soils and streams and may affect areas many kilometers downstream of the mine site (Cidu and Fanfani 2002; Hudson-Edwards et al. 1999; Loredo et al. 1999; Pirrie et al. 1997) (Fig. 2.21, Case Study 2.1, Scientific Issue 2.3). Consequently, soils surrounding mine waste piles and mine sites are well known for their elevated metal and metalloid concentrations (Abreu et al. 2008a; Batista et al. 2007; Chopin and Alloway 2007; Díez et al. 2009; Fernández-Caliani et al. 2009; Hojdová et al. 2009; Kelepertsis et al. 2006; Loredo et al. 2008; Lottermoser et al. 1999; Molina et al. 2006; Navarro et al. 2008b; Peng et al. 2007; Rufo et al. 2007; Teršič et al. 2009). The dispersion of mine wastes may lead to the contamination of stream systems, floodplains and coastal environments as well as drinking water supplies (Ashley et al. 2003a; Jiménez-Cárceles et al. 2008; Koski et al. 2008; Miller et al. 2007; Mlayah et al. 2009; Velleux et al. 2006). Element concentrations in soils, sediments and waters may exceed environmental quality guidelines and impact on ecosystem health. Metals and metalloids originally contained in waste particles may be released to ground, surface and pore waters and become bioaccessible to plants and animals (Furman et al. 2006; Reglero et al. 2008).
2.14 Control of Sulfide Oxidation
Uncontrolled sulfide oxidation can lead to the generation of AMD . In order to prevent sulfide oxidation and the generation of AMD, appropriate control strategies are needed. Strategies for the control of sulfide oxidation require the exclusion of one or more of the factors that cause and accompany oxidation, that is, sulfide minerals, bacteria , water, iron and oxygen. The aim of these methods is to reduce the interaction between the waste and the other reactants. Established control strategies include barriers (i.e. wet and dry covers), selective handling and isolation, co-disposal and blending with other materials, addition of organic waste s, and bacterial inhibition (Brown et al. 2002; Evangelou 1998; Environment Australia 1997; Miller 1998b; Parker 1999; SMME 1998). More technologically advanced and innovative strategies involve induced hardpan formation, grouting or mineral surface treatments (Scientific Issue 2.4, Fig. 2.22). Both established and innovative sulfide oxidation control strategies are generally designed to induce one or more of the following:
-
Exclusion of water;
-
Exclusion of oxygen;
-
pH control;
-
Control of Fe3+ generation;
-
Control of bacterial action; and
-
Removal and/or isolation of sulfides.
Scanning electron microphotograph of phosphate coatings on pyrite and chalcopyrite . In a laboratory experiment, soluble phosphate ions were added to a polysulfidic waste. Formation of phosphate phases occurred, coating the surfaces of pyrite and chalcopyrite. These phosphates protect the sulfides from further oxidation (Photo courtesyof D. Harris)
2.16 Summary
Sulfidic mine wastes , especially those which contain high concentrations of pyrite, are the major sources of AMD . Pyrite oxidation may occur via biotic or abiotic and direct or indirect oxidation processes. Biotic indirect oxidation of pyrite is an important acid generating process whereby pyrite is oxidized by oxygen and Fe3+ in the presence of microorganisms . Pyrite oxidation is a complex process because it not only involves chemical, electrochemical and biochemical reactions, but it also varies with environmental conditions. The following factors all work to enhance the speed of pyrite oxidation: large surface area ; small particle size ; high porosity ; poor crystallinity; significant trace element substitutions of and solid inclusions within the pyrite; acidic pH values of the solution in contact with pyrite; no direct physical contact with other sulfides; high oxygen and high Fe3+ concentrations in the oxidizing medium; high temperature; abundant microbial activity; and alternate wetting and drying of the sulfide grain.
Sulfides other than pyrite have different acid production potentials, stabilities and rates of reaction. The metal/sulfur ratio in sulfides influences how much sulfuric acid is liberated by oxidation. Also, the amount of iron sulfides present strongly influences whether and how much acid is generated during weathering. Iron sulfides (e.g. pyrite, marcasite , pyrrhotite ) or sulfides having iron as a major constituent (e.g. chalcopyrite , iron-rich sphalerite ) generate the most acidity . In contrast, sulfide minerals which do not contain iron in their crystal lattice (e.g. covellite, galena or iron-poor sphalerite) do not have the capacity to generate significant amounts of acid. This is because Fe3+ is not available as the important oxidant , and iron hydrolysis cannot occur which would generate additional hydrogen ions. The production of acid also occurs through the dissolution of secondary soluble Fe2+, Mn2+, Fe3+ and Al3+ sulfate salts, and the precipitation of secondary Fe3+ and Al3+ hydroxides.
Any acid generated can be consumed through the reaction of the hydrogen ions with gangue mineral s . The weathering of silicates results in the consumption of hydrogen ions, formation of secondary minerals , and release of dissolved cations and silicic acid. The dissolution of hydroxides and carbonates as well as cation exchange processes on clay minerals also consume acid. The gangue minerals reacting with the acidic solutions have a variable resistance to weathering and therefore, exhibit different reaction rate s. Carbonate minerals show the highest reactivity and highest acid consumption, whereas silicates have significantly slower reaction rates and provide only token amounts of additional long-term buffering capacity to sulfidic wastes.
Weathering of sulfidic wastes produces mine waters laden with dissolved salts. The dissolved salts may approach saturation in pore water s, ground waters, streams and leachates associated with the oxidation and leaching of sulfidic wastes. In fact, numerous secondary minerals are known to precipitate in AMD environments. Some secondary minerals may redissolve in AMD waters thereby influencing the mine water chemistry; others may precipitate and coat acid buffering or acid producing minerals. Massive precipitation of secondary minerals in wastes results in the formation of laterally extensive or discontinuous subsurface or surface layers which act as horizontal barriers to vertical water movements. Thus, the secondary mineralogy of sulfidic wastes plays an important role in controlling how readily and how much acid, metals and sulfate are liberated to drainage waters.
The prediction of AMD generation from sulfidic wastes is possible using geological and petrographic descriptions, geological modeling, static and kinetic tests, and mathematical models. These tools may be used to estimate potential sulfide oxidation and dissolved metal mobility. Sulfidic waste dumps are major sources of AMD. Oxygen advection, convection and diffusion occur in such wastes, which can result in the production of acid. Fine-grained wastes have much greater surface area s and hence, a greater acid generation potential than coarse-grained wastes, yet the fine grain size limits oxygen diffusion, water ingress and acid generation.
Monitoring techniques of sulfidic wastes are designed to identify the early presence of or the changes to any products of the acid producing reactions. The products can be identified by obtaining waste temperature measurements, oxygen pore gas concentration profiles, and leachate analyses for dissolved contaminant concentrations and loads. Rapid increases in temperature profiles of waste dumps indicate the exothermic oxidation of sulfides, whereas the depletion of oxygen concentration within gas pores is also indicative of sulfide oxidation.
Control techniques of sulfide oxidation are based on the exclusion of one or more of the factors that cause and accompany oxidation; that is, sulfide minerals, bacteria, water, iron and oxygen. Controlling sulfide oxidation may reduce or even eliminate the possibility of AMD generation. The destructive sulfide oxidation processes are driven by the ready exchange of oxygen with the atmosphere. Hence, reducing oxygen availability is the most effective control on the oxidation rate. This is generally achieved using dry or wet covers. The advantage of wet covers is that oxygen diffuses very slowly and has limited solubility in water. In contrast, a dry cover with a low oxygen permeability restricts water and oxygen movement into and through the waste. The dry cover reduces both the oxidation rate of sulfides and the transport of leachates from the waste. Other established and experimental methods for the prevention of sulfide oxidation and AMD development include selective handling and isolation, co-disposal and blending , mineral surface treatments, addition of organic waste s, and bacterial inhibition.
Further information on sulfidic wastes can be obtained from web sites shown in Table 2.8.
References
Aachid M, Mbonimpa M, Aubertin M (2004) Measurement and prediction of the oxygen diffusion coefficient in unsaturated media, with applications to soil covers. Water Air Soil Poll 156:163–193
Abraitis PK, Pattrick RAD, Kelsall GH, Vaughan DJ (2004) Acid leaching and dissolution of major sulphide ore minerals: processes and galvanic effects in complex systems. Min Mag 68:343–351
Abreu MM, Matias MJ, Magalhães MCF, Basto MJ (2008a) Impacts on water, soil and plants from the abandoned Miguel Vacas copper mine, Portugual. J Geochem Explor 96:161–170
Abreu MM, Tavares MT, Batista MJ (2008b) Potential use of Erica andevalensis and Erica australis in phytoremediation of sulphide mine environments: São Domingos, Portugual. J Geochem Explor 96:210–222
Acero P, Cama J, Ayora C (2007a) Rate law for galena dissolution in acidic environment. Chem Geol 245:219–229
Acero P, Ayora C, Carrera J (2007c) Coupled thermal, hydraulic and geochemical evolution of pyritic tailings in unsaturated column experiments. Geochim Cosmochim Acta 71:5325–5338
Ackman TE (2003) An introduction to the use of airborne technologies for watershed characterization in mined areas. Mine Water Environ 22:62–68
Agricola G (1546) De natura fossilium (trans: Bandy MC, Bandy JA (2004)). Dover Publications, New York
Agricola G (1556) De re metallica (trans: Hoover HC, Hoover LH (1950)). Dover Publications, New York
Al TA, Martin CJ, Blowes DW (2000) Carbonate-mineral/water interactions in sulfide-rich mine tailings. Geochim Cosmochim Acta 64:3933–3948
Alakangas L, Öhlander B (2006a) Formation and composition of cemented layers in low-sulphide mine tailings, Laver, northern Sweden. Environ Geol 50:809–819
Alakangas L, Öhlander B (2006b) Pilot-scale studies of different covers on unoxidised sulphide-rich tailings in northern Sweden: the geochemistry of leachate waters. Mine Water Environ 25:171–183
Alpers CN, Blowes DW, Nordstrom DK, Jambor JL (1994) Secondary minerals and acid mine-water chemistry. In: Jambor JL, Blowes DW (eds) Environmental geochemistry of sulfide mine-wastes, vol 22. Mineralogical Association of Canada, Nepean, pp 247–270 (Short course handbook)
Anawar HM, Garcia-Sanchez A, Murciego A, Buyolo T (2006) Exposure and bioavailability of arsenic in contaminated soils from the La Parrilla mine, Spain. Environ Geol 50:170–179
Anderson CWN, Brooks RR, Chiarucci A, LaCoste CJ, Leblanc M, Robinson BH, Simcock R, Stewart RB (1999) Phytomining for nickel, thallium and gold. J Geochem Explor 67:407–415
Ardau C, Blowes DW, Ptacek CJ (2009) Comparison of laboratory testing protocols to field observations of the weathering of sulfide-bearing mine tailings. J Geochem Explor 100:182–191
Ashley PM, Lottermoser BG (1999a) Geochemical, mineralogical and biogeochemical characterisation of abandoned metalliferous mine sites, southern New England Orogen. In: Proceedings of the NEO’99 Conference. Armidale, Division of Earth Sciences, University of New England, pp 409–418
Ashley PM, Lottermoser BG (1999b) Arsenic contamination at the Mole River mine, northeastern New South Wales, Australia. Austral J Earth Sci 46:861–874
Ashley PM, Lottermoser BG, Chubb AJ (2003a) Environmental geochemistry of the Mt Perry copper mines area, southeast Queensland, Australia. Geochem Explor Environ Anal 3:345–357
Ashley PM, Lottermoser BG, Collins A, Grant CD (2004) Environmental geochemistry of the derelict Webbs Consols mine, New South Wales, Australia. Environ Geol 46:596–609
Avery ER, Benning LG (2008) Anaerobic pyrite oxidation rates determined via direct volume-loss measurements: a Vertical Scanning Interferometric approach. Mineral Mag 72:15–18
Baker AJM (1981) Accumulators and excluders – strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate chemical elements – a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Banks D, Younger PL, Arnesen R-T, Iversen ER, Banks SB (1997) Mine-water chemistry; the good, the bad and the ugly. Environ Geol 32:157–174
Basta NT, McGowen SL (2004) Evaluation of chemical immobilization treatments for reducing heavy metal transport in a smelter-contaminated soil. Environ Poll 127, 73–82
Batista MJ, Abreu MM, Serrano Pinto M (2007) Biogeochemistry in Neves Corvo mining region, Iberian Pyrite Belt, Portugual. J Geochem Explor 92:159–176
Belzile N, Chen YW, Cai MF, Li Y (2004) A review on pyrrhotite oxidation. J Geochem Explor 84:65–76
Bennett JW, Gibson DK, Ritchie AIM, Tan Y, Broman PG, Jönsson H (1994) Oxidation rates and pollution loads in drainage; correlation of measurements in a pyritic waste rock dump. In: Proceedings of the international land reclamation and mine drainage conference and 3rd international conference on the abatement of acidic drainage, vol 1. United States Department of the Interior, Bureau of Mines Special Publication SP06A-94, pp 401–409
Bennett JW, Timms GP, Ritchie AIM (1999) The effectiveness of the covers on waste rock dumps at Rum Jungle and the impact in the long term. In: Proceedings of the 24th annual Minerals Council of Australia environmental workshop. Minerals Council of Australia, Dickson, pp 379–388
Bennett MW, Kempton JH, Maley PJ (1997) Applications of geological block models to environmental management. In: Proceedings from the 4th international conference on acid rock drainage, vol 1. Vancouver, pp 293–303
Benzaazoua M, Bussiere B, Dagenais AM, Archambault M (2004) Kinetic test comparison and interpretation for prediction of the Joutel tailings acid generation potential. Environ Geol 46:1086–1101
Berger AC, Bethke CM, Krumhansl JL (2000) A process model of natural attentuation in drainage from a historic mining district. Appl Geochem 15:655–666
Bernier L, Warren LA (2007) Geochemical diversity in S processes mediated by culture-adapted and environmental-enrichments of Acidithiobacillus spp. Geochim Cosmochim Acta 71:5684–5697
Bethune KJ, Lockington DA, Williams DJ (1997) Acid mine drainage: comparison of laboratory testing to mine site conditions. In: Proceedings from the 4th international conference on acid rock drainage, vol 1. Vancouver, pp 306–318
Bigham JM, Nordstrom DK (2000) Iron and aluminium hydroxysulfates from acid sulfate waters. In: Alpers CN, Jambor JL, Nordstrom DK (eds) Sulfate minerals: crystallography, geochemistry and environmental significance, vol 40. Mineralogical Society of America, Washington, DC, pp 351–403 (Reviews in mineralogy and geochemistry)
Blowes DW, Ptacek CJ (1994) Acid-neutralization mechanisms in inactive mine tailings. In: Jambor JL, Blowes DW (eds) Environmental geochemistry of sulfide mine-wastes, vol 22. Mineralogical Association of Canada, Nepean, pp 271–292 (Short course handbook)
Blowes DW, Reardon EJ, Jambor JL, Cherry JA (1991) The formation and potential importance of cemented layers in inactive sulfide mine tailings. Geochim Cosmochim Acta 55:965–978
Blowes DW, Ptacek CJ, Jambor JL (1994) Remediation and prevention of low-quality drainage from tailings impoundments. In: Jambor JL, Blowes DW (eds) Environmental geochemistry of sulfide mine-wastes, vol 22. Mineralogical Association of Canada, Nepean, pp 365–380 (Short course handbook)
Blowes DW, Jambor JL, Hanton-Fong CJ, Lortie L, Gould WD (1998) Geochemical, mineralogical and microbiological characterization of a sulphide-bearing carbonate-rich gold-mine tailings impoundment, Joutel, Québec. Appl Geochem 13:687–705
Bond PL, Druschel GK, Banfield JE (2000) Comparison of acid mine drainage microbial communities in physically and geochemically distinct ecosystems. Appl Environ Microbiol 66:4962–4971
Boon M, Snijder M, Hansfrod GS, Heijnen JJ (1998) The oxidation kinetics of zinc sulphide with Thiobacillus ferrooxidans. Hydrometallurgy 48:171–186
Boorman RS, Watson DM (1976) Chemical processes in abandoned sulphide tailings dumps and environmental implication for northeastern New Brunswick. CIM Bulletin 69:86–96
Borden R (2001) Geochemical evolution of sulphide-bearing waste rock soils at the Bingham Canyon mine, Utah. Geochem Explor Environ Anal 1:15–22
Bordon RK, Black R (2005) Volunteer revegetation of waste rock surfaces at the Bingham Canyon mine, Utah. J Environ Qual 34:2234–2242
Bosso ST, Enzweiler J, Angelica RS (2008) Lead bioaccessibility in soil and mine wastes after immobilization with phosphate. Water Air Soil Poll 195:257–273
Briggs TJ, Kelso IJ (2003) Ammonium nitrate-sulfide reactivity at the Century Zn-Pb-Ag mine, northwest Queensland, Australia. Expl Min Geol 10:177–190
Bril H, Zainoun K, Puziewicz J, Courtin-Nomade A, Vanaecker M, Bollinger JC (2008) Secondary phases from the alteration of a pile of zinc-smelting slag as indicators of environmental conditions: an example from Swietochlowice, Upper Silesia, Poland. Can Mineral 46:1235–1248
Brown M, Barley B, Wood H (2002) Minewater treatment: technology, application and policy. International Water Association Publishing
Bruce S, Noller B, Matanitobua V, Ng J (2007) In vitro physiologically based extraction test (PBET) and bioaccessibility of arsenic and lead from various mine waste materials. J Toxicol Environ Health 70:1700–1711
Bryan CG, Hallberg KB, Johnson DB (2006) Mobilisation of metals in mineral tailings at the abandoned São Domingos copper mine (Portugual) by indigenous acidophilic bacteria. Hydrometallurgy 83:184–194
Bullock SET, Bell FG (1997) Some problems associated with past mining at a mine in the Witbank coalfield, South Africa. Environ Geol 33:61–71
Bussière B, Benzaazoua M, Aubertin M, Mbonimpa M (2004) A laboratory study of covers made of low-sulphide tailings to prevent acid mine drainage. Environ Geol 45:609–622
Cabral A, Lefebvre G, Proulx ME, Audet C, Labbé M, Michaud C (1997) Use of deinking residues as cover material in the prevention of AMD generation at an abandoned mine site. In: Tailings and mine waste ’97. Balkema, Rotterdam, pp 257–266
Campbell AR, Lueth VW (2008) Isotopic and textural discrimination between hypogene, ancient supergene, and modern sulfates at the Questa mine, New Mexico. Appl Geochem 23:308–319
Cappuyns V, Swennen R, Niclaes M (2007) Application of the BCR sequential extraction scheme to dredged pond sediments contaminated by Pb-Zn mining: a combined geochemical and mineralogical approach. J Geochem Explor 93:78–90
Casagrande DJ, Finkelman RB, Caruccio FT (1989) The nonparticipation of organic sulfur in acid mine drainage. Environ Geochem Health 11:187–192
Cathles LM (1994) Attempts to model the industrial-scale leaching of copper-bearing mine waste. In: Alpers CN, Blowes DW (eds) Environmental geochemistry of sulfide oxidation. American Chemical Society Symposium Series 550, Washington, DC, pp 123–131
Chermak JA, Runnells DD (1996) Self-sealing hardpan barriers to minimize infiltration of water into sulfide-bearing overburden, ore, and tailings piles. In: Tailings and mine waste ’96. Balkema, Rotterdam, pp 265–273
Chopin EIB, Alloway BJ (2007) Distribution and mobility of trace elements in soils and vegetation around the mining and smelting areas of Tharsis, Ríotinto and Huelva, Iberian Pyrite Belt, SW Spain. Water Air Soil Poll 182:245–261
Chrysochoou M, Dermatas D, Grubb DG (2007) Phosphate application to firing range soils for Pb immobilization: the unclear role of phosphate. J Haz Mat 144:1–14
Cidu R, Fanfani L (2002) Overview of the environmental geochemistry of mining districts in southwestern Sardinia, Italy. Geochem Explor Environ Anal 2:243–251
Conesa HM, Schulin R, Nowack B (2007) A laboratory study on revegetation and metal uptake in native plant species from neutral mine tailings. Water Air Soil Pollut 183:201–212
Conesa HM, Robinson BH, Schulin R, Nowack B (2008) Metal extractability in acidic and neutral mine tailings from the Cartagena-La Unión Mining District (SE Spain). Appl Geochem23:1232–1240
Cook T, Skousen J, Hilton T (2008) Covering pre-existing, acid-producing fills with alkaline sandstone to control acid mine drainage. Mine Water Environ 27:259–264
Corkhill CL, Vaughan DJ (2009) Arsenopyrite oxidation. Appl Geochem 24:2342–2361
Corkhill CL, Wincott PL, Lloyd JR, Vaughan DJ (2008) The oxidative dissolution of arsenopyrite (FeAsS) and enargite (Cu3AsS4) by Leptospirillum ferrooxidans. Geochim Cosmochim Acta 72:5616–5633
Cornejo-Garrido H, Fernández-Lomelín P, Guzmán J, Cervini-Silva J (2008) Dissolution of arsenopyrite (FeAsS) and galena (PbS) in the presence of desferrioxamine-B at pH 5. Geochim Cosmochim Acta 72:2754–2766
Courtin-Nomade A, Grosbois C, Marcus MA, Fakra SC, Beny JM, Foster AL (2009) The weathering of a sulfide orebody: speciation and fate of some potential contaminants. Can Mineral 47:493–508
Cousins C, Penner GH, Liu B, Beckett P, Spiers G (2009) Organic matter degradation in paper sludge amendments over gold mine tailings. Appl Geochem 24:2293–2300
Craw D, Chappell D, Nelson M, Walrond M (1999) Consolidation and incipient oxidation of alkaline arsenopyrite-bearing mine tailings, Macreas Mine, New Zealand. Appl Geochem 14:485–498
Craw D, Rufaut CG, Hammit S, Clearwater SG, Smith CM (2007a) Geological controls on natural ecosystem recovery on mine waste in southern New Zealand. Environ Geol 51:1389–1400
Craw D, Rufaut C, Haffert L, Paterson L (2007b) Plant colonization and arsenic uptake on high arsenic mine wastes, New Zealand. Water Air Soil Poll 179:351–364
Crock JG, Arbogast BF, Lamothe PJ (1999) Laboratory methods for the analysis of environmental samples. In: Plumlee GS, Logsdon MS (eds) The environmental geochemistry of mineral deposits. Part A: processes, techniques and health issues, vol 6A. Society of Economic Geologists, Littleton, pp 265–287 (Reviews in economic geology)
Cruz R, Bertrand V, Monroy M, Ganzalez I (2001a) Effect of sulfide impurities on the reactivity of pyrite and pyritic concentrates; a multi-tool approach. Appl Geochem 16:803–819
Cruz R, Mendez BA, Monroy M, Gonzalez I (2001b) Cyclic voltammetry applied to evaluate reactivity in sulfide mining residues. Appl Geochem 16:1631–1640
Currey NA, Ritchie PJ, Durham AJP, Wilson GW (1999) Field performance and optimisation of two low flux soil cover systems for the prevention of acid mine drainage in a semi arid environment. In: Proceedings of the 24th annual Minerals Council of Australia environmental workshop. Minerals Council of Australia, Dickson, pp 458–465
Dalton JB, King TVV, Bove DJ, Kokaly RF, Clark RN, Vance JS, Swayze GA (2000) Distribution of acid-generating and acid-buffering minerals in the Animas River watershed as determined by AVIRIS spectroscopy. In: Proceedings from the 5th international conference on acid rock drainage, vol 2. Society for Mining, Metallurgy, and Exploration, Littleton, pp 1541–1550
Dermatas D, Chrysochoou M, Grubb DG, Xu X (2008) Phosphate treatment of firing range soils: lead fixation or phosphorus release? J Environ Qual 37:47–56
Deutsch WJ (1997) Groundwater geochemistry; fundamentals and applications to contamination. Lewis Publishers, Boca Raton
Díez M, Simón M, García I, Martín F (2009) Assessment of the critical load of trace elements in soils polluted by pyrite tailings. A laboratory experiment. Water Air Soil Poll 199:381–387
Dinelli E, Tateo F (2001) Sheet silicates as effective carriers of heavy metals in the ophiolitic mine area of Vigonzano (northern Italy). Mineral Mag 65:121–132
Dobos SK (2000) Potential problems with geologically uncontrolled sampling and the interpretation of chemical tests for waste characterisation and AMD prediction. 4th Australian workshop on acid mine drainage. Australian Centre for Mining Environmental Research, Brisbane
Dokoupilová P, Sracek O, Losos Z (2007) Geochemical behavior and mineralogical transformations during spontaneous combustion of a coal waste pile in Oslavany, Czech Republic. Mineral Mag 71:443–460
Dold B (2003) Speciation of the most soluble phases in a sequential extraction procedure adapted for geochemical studies of copper sulfide mine waste. J Geochem Explor 80:55–68
Domvile SJ, Li MG, Sollner DD, Nesbitt W (1994) Weathering behaviour of mine tailings and waste rock: a surface investigation. In: Proceedings of the international land reclamation and mine drainage conference and 3rd international conference on the abatement of acidic drainage, vol 1. United States Department of the Interior, Bureau of Mines Special Publication SP06A-94, pp 167–176
Druschel GK, Emerson D, Sutka R, Suchecki P, Luther GW III (2008) Low-oxygen and chemical kinetic constraints on the geochemical niche of neutrophilic iron (II) oxidizing microorganisms. Geochim Cosmochim Acta 72:3358–3370
Durães N, Bobos I, Ferreira da Silva E (2008) Chemistry and FT-IR spectroscopic studies of plants from contaminated mining sites in the Iberian Pyrite Belt, Portugual. Mineral Mag 72:405–409
Ebenå G, Hagberg J, Carlsson E (2007) Origin and distribution of low molecular weight organic acids and bacteria in a depth profile of a soil covered tailings impoundment in northern Sweden. J Geochem Explor 92:186–195
Elberling B, Schippers A, Sand W (2000) Bacterial and chemical oxidation of pyritic mine tailings at low temperatures. J Contam Hydrol 41:225–238
Elberling BO, Søndergaard J, Jensen LA, Schmidt LB, Hansen BU, Asmund G, Balić-Zunić T, Hollesen J, Hanson S, Jansson PE, Friborg T (2007) Arctic vegetation damage by winter-generated coal mining pollution released upon thawing. Environ Sci Technol 41:2407–2413
Elliott LCM, Liu L, Stogran SW (1997) Evaluation of single layer organic and inorganic cover materials for oxidized tailings. In: Tailings and mine waste ’97. Balkema, Rotterdam, pp 247–256
Ettler V, Legendre O, Bodénan F, Touray JC (2001) Primary phases and natural weathering of old lead-zinc pyrometallurgical slag from Pribram, Czech Republic. Can Mineral 39:873–888
Ettler V, Piantone P, Touray JC (2003) Mineralogical control on inorganic contaminant mobility in leachate from lead-zinc metallurgical slag: experimental approach and long-term assessment. Mineral Mag 67:1269–1283
Ettler V, Johan Z, Kříbek B, Šebek O, Mihaljevič (2009) Mineralogy and environmental stability of slags from the Tsumeb smelter, Namibia. Appl Geochem 24:1–15
Ettner DC, Braastad G (1999) Induced hardpan formation in a historic tailings impoundment, Røros, Norway. In: Tailings and mine waste ’99. Balkema, Rotterdam, pp 457–464
Eusden JD, Gallagher L, Eighmy TT, Crannell BS, Krzanowski JE, Butler LG, Cartledge FK, Emery EF, Shaw EL, Francis CA (2002) Petrographic and spectroscopic characterization of phosphate-stabilized mine tailings from Leadville, Colorado. Waste Manag 22:117–135
Evangelou VP (1995) Pyrite oxidation and its control. CRC Press, Boca Raton
Evangelou VP (1996) Pyrite oxidation inhibition in coal waste by PO4 and H2O2 pH buffered pre-treatment. Int J Surf Min Reclam Environ 10:135–142
Evangelou VP (1998) Pyrite chemistry: the key for abatement of acid mine drainage. In: Geller W, Klapper H, Salomons W (eds) Acidic mining lakes: acid mine drainage, limnology and reclamation. Springer, Heidelberg, pp 197–222
Evangelou VP (2001) Pyrite microencapsulation technologies: principles and potential field applications. Ecol Eng 17:165–178
Evangelou VP, Zhang YL (1995) A review: pyrite oxidation mechanisms and acid mine drainage prevention. Crit Rev Environ Sci Technol 25:141–199
Falk H, Lavergren U, Bergbäck B (2006) Metal mobility in alum shale from Öland, Sweden. J Geochem Explor 90:157–165
Farkas IM, Weiszburg TG, Pekker P, Kuzmann E (2009) A half-century of environmental mineral formation on a pyrite-bearing waste dump in the Mátra Mountains, Hungary. Can Mineral 47:509–524
Fernández-Caliani JC, Barba-Brioso C, González I, Galán E (2009) Heavy metal pollution in soils around the abandoned mine sites of the Iberian Pyrite Belt (Southwest Spain). Water Air Soil Poll 200:211–226
Ferrier G, Hudson-Edwards KA, Pope RJ (2009) Characterisation of the environmental impact of the Rodalquilar mine, Spain by ground-based reflectance spectroscopy. J Geochem Explor 100:11–19
Ficklin WH, Mosier EL (1999) Field methods for sampling and analysis of environmental samples for unstable and selected stable constituents. In: Plumlee GS, Logsdon MS (eds) The environmental geochemistry of mineral deposits. Part A: processes, techniques and health issues, vol 6A. Society of Economic Geologists, Littleton , pp 249–264 (Reviews in economic geology)
Forsberg LS, Ledin S (2003) Effects of iron precipitation and organic amendments on porosity and penetrability in sulphide mine tailings. Water Air Soil Poll 142:395–408
Forsberg LS, Gustafsson JP, Kleja DB, Ledin S (2008) Leaching of metals from oxidising sulphide mine tailings with and without sewage sludge application. Water Air Soil Pollut 194:331–341
Fowler TA, Holmes PR, Crundwell FK (1999) Mechanism of pyrite dissolution in the presence of Thiobacillus ferrooxidans. Appl Environ Microbiol 65:2987–2993
Frau F (2000) The formation-dissolution-precipitation cycle of melanterite at the abandoned pyrite mine of Genna Luas in Sardinia, Italy; environmental implications. Mineral Mag 64:995–1006
Frostad S, Klein B, Lawrence RW (2002) Evaluation of laboratory kinetic test methods for measuring rates of weathering. Mine Water Environ 21:183–192
Furman O, Strawn DG, Heinz GH, Williams B (2006) Risk assessment test for lead bioaccessibility to waterfowl in mine-impacted soils. J Environ Qual 35:450–458
Ganne P, Cappuyns V, Vervoort A, Buvé, Swennen R (2006) Leachability of heavy metals and arsenic from slags of metal extraction industry at Angleur (eastern Belgium). Sci Total Environ 356:69–85
Georgopoulou ZJ, Fytas K, Soto H, Evangelou B (1996) Feasibility and cost of creating an iron-phosphate coating on pyrrhotite to prevent oxidation. Environ Geol 28:61–69
Gibson DK, Ritchie AIM (1991) Options to control acid generation in existing pyritic mine waste dumps. In: Randol Gold Forum Cairns ’91, pp 109–111
Gleisner M, Herbert RB Jr, Frogner Kockum PC (2006) Pyrite oxidation by Acidithiobacillus ferrooxidans at various concentrations of dissolved oxygen. Chem Geol 225:16–29
Goh SW, Buckley AN, Lamb RN, Rosenberg RA, Moran D (2006) The oxidation states of copper and iron in mineral sulfides, and the oxides formed on initial exposure of chalcopyrite and bornite to air. Geochim Cosmochim Acta 70:2210–2228
Gomes MEP, Favas PJC (2006) Mineralogical controls on mine drainage of the abandoned Ervedosa tin mine in north-eastern Portugal. Appl Geochem 21:1322–1334
Gould WD, Bechard G, Lortie L (1994) The nature and role of microorganisms in the tailings environment. In: Jambor JL, Blowes DW (eds) Environmental geochemistry of sulfide mine-wastes, vol 22. Mineralogical Association of Canada, Nepean, pp 185–199 (Short course handbook)
Gould WD, Kapoor A (2003) The microbiology of acid mine drainage. In: Jambor JL, Blowes DW, Ritchie AIM (eds) Environmental aspects of mine wastes, vol 31. Mineralogical Association of Canada, Nepean, pp 203–226 (Short course handbook)
Graupner T, Kassahun A, Rammlmair D, Meima JA, Kock D, Furche M, Fiege A, Schippers A, Melcher F (2007) Formation of sequences of cemented layers and hardpans within sulfide-bearing mine tailings (mine district Freiberg, Germany). Appl Geochem 22:2486–2508
Hakkou R, Benzaazoua M, Bussière B (2008a) Acid mine drainage at the abandoned Kettara mine (Morocco): 1. Environmental characterization. Mine Water Environ 27:145–159
Hakkou R, Benzaazoua M, Bussière B (2008b) Acid mine drainage at the abandoned Kettara mine (Morocco): 2. Mine waste geochemical behavior. Mine Water Environ 27:160–170
Hallberg KB, Johnson DB (2005) Mine water microbiology. Mine Water Environ 24:28–37
Harmer SL, Thomas JE, Fornasiero D, Gerson AR (2006) The evolution of surface layers formed during chalcopyrite leaching. Geochim Cosmochim Acta 70:4392–4402
Harries JR, Ritchie AIM (1987) The effect of rehabilitation on the rate of oxidation of pyrite in a mine waste rock dump. Environ Geochem Health 9:27–36
Harris DL, Lottermoser BG (2006a) Phosphate stabilization of polyminerallic mine wastes. Mineral Mag 70:1–13
Heikkinen PM, Räisänen ML (2008) Mineralogical and geochemical alteration of Hitura sulphide mine tailings with emphasis on nickel mobility and retention. J Geochem Explor 97:1–20
Heikkinen PM, Räisänen ML (2009) Trace metal and As solid-phase speciation in sulphide mine tailings – indicators of spatial distribution of sulphide oxidation in active tailings impoundments. Appl Geochem 24:1224–1237
Hita R, Torrent J, Bigham JM (2006) Experimental oxidative dissolution of sphalerite in the Aznalcóllar sludge and other pyrite matrices. J Environ Qual 35:1032–1039
Hodson ME (2006) Does reactive surface area depend on grain size? Results from pH 3, 25˚C far-from-equilibrium flow-through dissolution experiments on anorthite and biotite. Geochim Cosmochim Acta 70:1655–1667
Hojdová M, Navrátil T, Rohovec J, Penížek V, Grygar T (2009) Mercury distribution and speciation in soils affected by historic mercury mining. Water Air Soil Pollut 200:89–99
Hollings P, Hendry MJ, Nicholson RV, Kirkland RA (2001) Quantification of oxygen consumption and sulphate release rates for waste rock piles using kinetic cells; Cluff lake uranium mine, northern Saskatchewan, Canada. Appl Geochem 16:1215–1230
Holmström H, Ljungberg J, Ekström M, Öhlander B (1999) Secondary copper enrichment in tailings at the Laver mine, northern Sweden. Environ Geol 38:327–342
Huang X, Evangelou VP (1994) Suppression of pyrite oxidation rate by phosphate addition: In: Alpers CN, Blowes DW (eds) Environmental geochemistry of sulfide oxidation. American Chemical Society Symposium Series 550, Washington, DC, pp 562–573
Hudson-Edwards KA, Schell C, Macklin MG (1999) Mineralogy and geochemistry of alluvium contaminated by metal mining in the Rio Tinto area, southwest Spain. Appl Geochem 14:1015–1030
Hudson-Edwards KA, Edwards SJ (2005) Mineralogical controls on storage of As, Cu, Pb and Zn at the abandoned Mathiatis massive sulphide mine, Cyprus. Mineral Mag 69:695–706
Hughes J, Craw D, Peake B, Lindsay P, Weber P (2007) Environmental characterization of coal mine waste rock in the field: an example from New Zealand. Environ Geol 52:1501–1509
Hulshof AHM, Blowes DW, Gould WD (2006) Evaluation of in situ layers for treatment of acid mine drainage: a field comparison. Water Res 40:1816–1826
Hutchison I, Ellison R (1992) Mine waste management. Lewis Publishers, Boca Raton
Ibrahim KM, Jaber JO (2007) Geochemistry and environmental impacts of retorted oil shale from Jordan. Environ Geol 52:979–984
Jambor JL (1994) Mineralogy of sulfide-rich tailings and their oxidation products. In: Jambor JL, Blowes DW (eds) Environmental geochemistry of sulfide mine-wastes, vol 22. Mineralogical Association of Canada, Nepean, pp 59–102 (Short course handbook)
Jambor JL (2000) The relationship of mineralogy to acid- and neutralization-potential values in ARD. In: Cotter-Howells JD, Campbell LS, Valsami-Jones E, Batchelder M (eds) Environmental mineralogy; microbial interactions, anthropogenic influences, contaminated land and waste management. Mineralogical Society Series no 9. Mineralogical Society, London, pp 141–159
Jambor JL (2003) Mine-waste mineralogy and mineralogical perspectives of acid-base accounting. In: Jambor JL, Blowes DW, Ritchie AIM (eds) Environmental aspects of mine wastes, vol 31. Mineralogical Association of Canada, Nepean, pp 117–145 (Short course handbook)
Jambor JL, Nordstrom DK, Alpers CN (2000a) Metal sulfate salts from sulfide mineral oxidation. In: Alpers CN, Jambor JL, Nordstrom (eds) Sulfate minerals; crystallography, geochemistry and environmental significance, vol 40. Mineralogical Society of America, Washington, DC, pp 303–350 (Reviews in mineralogy and geochemistry)
Jambor JL, Dutrizac JE, Chen TT (2000c) Contribution of specific minerals to the neutralization potential in static tests. In: Proceedings from the 5th international conference on acid rock drainage, vol 1. Society for Mining, Metallurgy, and Exploration, Littleton, pp 551–565
Jambor JL, Dutrizac JE, Raudsepp M, Groat LA (2003) Effect of peroxide on neutralization-potential values of siderite and other carbonate minerals. J Environ Qual 32:2373–2378
Jambor JL, Dutrizac JE, Raudsepp M (2007) Measured and computed neutralization potentials from static tests of diverse rock types. Environ Geol 52:1019–1031
Janzen MP, Nicholson RV, Scharper JM (2000) Pyrrhotite reaction kinetics; reaction rates for oxidation by oxygen, ferric iron, and for nonoxidative dissolution. Geochim Cosmochim Acta 64:1511–1522
Jennings SR, Dollhopf DJ, Inskeep WP (2000) Acid production from sulfide minerals using hydrogen peroxide weathering. Appl Geochem 15:235–243
Jerz JK, Rimstidt JD (2003) Efflorescent iron sulfate minerals: paragenesis, relative stability, and environmental impact. Am Mineral 88:1919–1932
Jiménez-Cárceles FJ, Álvarez-Rogel J, Alcaraz HM (2008) Trace element concentrations in saltmarsh soils strongly affected by wastes from metal sulphide mining areas. Water Air Soil Poll 188:283–295
Jin S, Fallgren PH, Morris JM, Gossard RB (2008a) Biological source treatment of acid mine drainage using microbial and substrate amendments: microcosm studies. Mine Water Environ 27:20–30
Jones RA, Koval SF, Nesbitt HW (2003) Surface alteration of arsenopyrite (FeAsS) by Thiobacillus ferrooxidans. Geochim Cosmochim Acta 67:955–965
Johnson DB (1998a) Biological abatement of acid mine drainage: the role of acidophilic protozoa and other indigenous microflora. In: Geller W, Klapper H, Salomons W (eds) Acidic mining lakes. Springer, Heidelberg, pp 285–301
Kalin M, Harris B (2005) Chemical precipitates within pyritic waste rock. Hydrometallurgy 78:209–225
Kargbo DM, He J (2004) A simple accelerated rock weathering method to predict acid generation kinetics. Environ Geol 46:775–783
Karpenko V, Norris JA (2002) Vitriol in the history of chemistry. Chem Listy 96:997–1005
Keith CN, Vaughan DJ (2000) Mechanisms and rates of sulphide oxidation in relation to the problems of acid rock (mine) drainage. In: Cotter-Howells JD, Campbell LS, Valsami-Jones E, Batchelder M (eds) Environmental mineralogy; microbial interactions, anthropogenic influences, contaminated land and waste management. Mineralogical Society Series no 9. Mineralogical Society, London, pp 117–139
Kelepertsis A, Argyraki A, Alexakis D (2006) Multivariate statistics and spatial interpretation of geochemical data for assessing soil contamination by potentially toxic elements in the mining area of Stratoni, north Greece. Geochem Explor Environ Anal 6:349–355
Kelly DP, Wood AP (2000) Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov. and Thermithiobacillus gen. nov. Int J Syst Evol Microbiol 50:511–516
Kierczak J, Néel C, Puziewicz J, Bril H (2009) The mineralogy and weathering of slag produced by the smelting of lateritic Ni ores, Szklary, southwestern Poland. Can Mineral 47:557–572
Kilgour DW, Moseley RB, Barnett MO, Savage KS, Jardine PM (2008) Potential negative consequences of adding phosphorus-based fertilizers to immobilize lead in soil. J Environ Qual 35:1733–1740
Kim H, Benson CH (2004) Contributions of advective and diffusive oxygen transport through multilayer composite caps over mine waste. J Contam Hydrol 71:193–218
Kleinmann RLP (1997) Mine drainage systems. In: Marcus JJ (ed) Mining environmental handbook: effects of mining on the environment and American environmental controls on mining. Imperial College Press, London, pp 237–244
Kleinmann RLP (1998) Bactericidal control of acidic drainage. In: Coal mine drainage prediction and pollution prevention in Pennsylvania. The Pennsylvania Department of Environmental Protection, Chapter 15, pp 15–1 to 15–6
Kock D, Schippers A (2006) Geomicrobiological investigation of two different mine waste tailings generating acid mine drainage. Hydrometallurgy 83:167–175
Kolker A, Huggins FE (2007) Progressive oxidation of pyrite in five bituminous coal samples: an As XANES and Fe Mössbauer spectroscopic study. Appl Geochem 22:778–787
Koski RA, Munk L, Foster AL, Shanks WC III, Stillings LL (2008) Sulfide oxidation and distribution of metals near abandoned copper mines in coastal environments, Prince William Sound, Alaska, USA. Appl Geochem 23:227–254
Kucha H , Martens A, Ottenburgs R, De Vos W, Viaene W (1996) Primary minerals of Zn-Pb mining and metallurgical dumps and their environmental behavior at Plombieres, Belgium. Environ Geol 27:1–15
Kuechler R, Noack K (2007) Comparison of the solution behavior of a pyrite-calcite mixture in batch and unsaturated sand column. J Contam Hydrol 90:203–220
Kwong YTJ (1993) Mine site acid rock drainage assessment and prevention; a new challenge for a mining geologist. In: Proceedings of the international mining geology conference, Kalgoorlie, pp 213–217
Kwong YTJ, Swerhone GW, Lawrence JR (2003) Galvanic sulphide oxidation as a metal-leaching mechanism and its environmental implications. Geochem Explor Environ Anal 3:337–343
Langmuir D (1997) Aqueous environmental geochemistry. Prentice Hall, Upper Saddle River
Lasaga AC, Berner RA (1998) Fundamental aspects of quantitative models for geochemical cycles. Chem Geol 145:161–175
Lavergren U, Åström ME, Bergbäck B, Holmström H (2009) Mobility of trace elements in black shale assessed by leaching tests and sequential chemical extraction. Geochem Explor Environ Anal 9:71–79
Lawrence RW, Scheske M (1997) A method to calculate the neutralization potential of mining wastes. Environ Geol 32:100–106
Ledin M, Pedersen K (1996) The environmental impact of mine wastes; roles of microorganisms and their significance in treatment of mine wastes. Earth-Sci Rev 41:67–108
Lee CSL, Qi SH, Zhang G, Luo CL, Zhao LYL, Li XD (2008) Seven thousand years of records on the mining and utilization of metals from lake sediments in central China. Environ Sci Technol 42:4732–4738
Lehner S, Savage K (2008) The effect of As, Co, and Ni impurities on pyrite oxidation kinetics: batch and flow-through reactor experiments with synthetic pyrite. Geochim Cosmochim Acta 72:1788–1800
Lehner S, Savage K, Ciobanu M, Cliffel DE (2007) The effect of As, Co, and Ni impurities on pyrite oxidation kinetics: an electrochemical study of synthetic pyrite. Geochim Cosmochim Acta 71:2491–2509
Lengke MF, Tempel RN (2003) Natural realgar and amorphous AsS oxidation kinetics. Geochim Cosmochim Acta 67:859–871
Lengke MF, Sanpawanitchakit C, Tempel RN (2009) The oxidation and dissolution of arsenic-bearing sulfides. Can Mineral 47:593–613
Li J, Xie ZM, Xu JM, Sun YF (2006) Risk assessment for safety of soils and vegetables around a lead/zinc mine. Environ Geochem Health 28:37–44
Li J, Smart RSC, Schumann RC, Gerson AR, Levay G (2007a) A simplified method for estimation of jarosite and acid-forming sulfates in acid mine wastes. Sci Total Environ 373:391–403
Li MG, St-Arnoud L (1997) Hydrogeochemistry of secondary mineral dissolution: column leaching experiment using oxidized waste rock. In: Proceedings of the 4th international conference on acid rock drainage, vol 1. Vancouver, pp 465–477
Liao B, Huang LN, Ye ZH, Lan CY, Shu WS (2007) Cut-off net acid generation pH in predicting acid-forming potential in mine spoils. J Environ Qual 36:887–891
Lim HS, Lee JS, Chon HT, Sager M (2008) Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au-Ag mine in Korea. J Geochem Explor 96:223–230
Lin Z (1997) Mineralogical and chemical characterization of wastes from the sulfuric acid industry in Falun, Sweden. Environ Geol 30:152–162
Lin Z, Herbert RB Jr (1997) Heavy metal retention in secondary precipitates from a mine rock dump and underlying soil, Dalarna, Sweden. Environ Geol 33:1–12
Linklater CM, Sinclair DJ, Brown PL (2005) Coupled chemistry and transport modeling of sulphidic waste rock dumps at the Aitik mine site, Sweden. Appl Geochem 20:275–293
Liu J, Aruguete DM, Jinschek JR, Rimstidt JD, Hochella MF Jr (2008a) The non-oxidative dissolution of galena nanocrystals: insights into mineral dissolution rates as a function of grain size, shape, and aggregation state. Geochim Cosmochim Acta 72:5984–5996
Liu Q, Li H, Zhou L (2008b) Galvanic interactions between metal sulfide minerals in a flowing system: implications for mines environmental restoration. Appl Geochem 23:2316–2323
Liu R, Wolfe AL, Dzombak DA, Horwitz CP, Stewart BW, Capo RC (2008c) Electrochemical study of hydrothermal and sedimentary pyrite dissolution. Appl Geochem 23:2724–2734
Liu R, Wolfe AL, Dzombak DA, Stewart BW, Capo RC (2008d) Comparison of dissolution under oxic acid drainage conditions for eight sedimentary and hydrothermal pyrite samples. Environ Geol 56:171–182
Loredo J, Ordonez A, Gallego JR, Baldo C, Garcia-Iglesias J (1999) Geochemical characterisation of mercury mining spoil heaps in the area of Mieres (Asturias, northern Spain). J Geochem Explor 67:377–390
Loredo J, Álvarez R, Ordóñez A, Bros T (2008) Mineralogy and geochemistry of the Texeo Cu-CO mine site (NW Spain): screening tools for environmental assessment. Environ Geol 55:1299–1310
Lottermoser BG (2002) Mobilization of heavy metals from historical smelting slag dumps, north Queensland, Australia. Mineral Mag 66:475–490
Lottermoser BG (2005) Evaporative mineral precipitates from a historical smelting slag dump, Río Tinto, Spain. Neues Jb Miner Abh 181:183–190
Lottermoser BG, Ashley PM (2006a) Mobility and retention of trace elements in hardpan-cemented cassiterite tailings, north Queensland, Australia. Environ Geol 50:835–846
Lottermoser BG, Ashley PM, Lawie DC (1999) Environmental geochemistry of the Gulf Creek copper mine area, northeastern New South Wales, Australia. Environ Geol 39:61–74
Lottermoser BG, Ashley PM, Munksgaard NC (2008) Biogeochemistry of Pb-Zn gossans, northwest Queensland, Australia: implications for mineral exploration and mine site rehabilitation. Appl Geochem 23:723–742
Lu L, Wang R, Chen F, Xue J, Zhang P, Lu J (2005) Element mobility during pyrite weathering; implications for acid and heavy metal pollution at mining-impacted sites. Environ Geol 49:82–89
Lundgren T (2001) The dynamics of oxygen transport into soil covered mining waste deposits in Sweden. J Geochem Explor 74:163–173
Luther GW (1987) Pyrite oxidation and reduction; molecular orbital theory considerations. Geochim Cosmochim Acta 51:3193–3199
Malmström ME, Gleisner M, Herbert RB (2006) Element discharge from pyritic mine tailings at limited oxygen availability in column experiments. Appl Geochem 21:184–202
Marescotti P, Carbone C, De Capitani L, Grieco G, Lucchetti G, Servida D (2008) Mineralogical and geochemical characterization of open-air tailing and waste-rock dumps from the Libiola Fe-Cu sulphide mine (Eastern Liguria, Italy). Environ Geol 53:1613–1626
Martínez-Ruiz C, Fernández-Santos B, Putwain PD, Fernández-Gómez MJ (2007) Natural and man-induced revegetation on mining wastes: changes in the floristic composition during early succession. Ecol Eng 30:286–294
Martínez López J, Llamas Borrajo J, de Miguel García E, Rey Arrans J, Hidalgo Estévez MC, Sáez Castillo AJ (2008) Multivariate analysis of contamination in the mining district of Linares (Jaén, Spain). Appl Geochem 23:2324–2336
Masalehdani MNN, Mees F, Dubois M, Coquinot Y, Potdevin JL, Fialin M, Blanc-Valleron MM (2009) Condensate minerals from a burning coal-waste heap in Avion, Northern France. Can Mineral 47:573–591
Mathews WH, Bustin RM (1984) Why do the Smoking Hills smoke? Can J Earth Sci 21:737–742
Matlock MM, Howerton BS, Atwood DA (2003) Covalent coating of coal refuse to inhibit leaching. Adv Environ Res 7:495–501
McKibben MA, Tallant BA, del Angel JK (2008) Kinetics of inorganic arsenopyrite oxidation in acidic aqueous solutions. Appl Geochem 23:121–135
McSweeney K, Madison FW (1988) Formation of a cemented subsurface horizon in sulfidic minewaste. J Environ Qual 17:256–262
Miller JR, Lechler PJ, Mackin G, Germanoski D, Villarroel LF (2007) Evaluation of particle dispersal from mining and milling operations using lead isotopic fingerprinting techniques, Rio Pilcomayo Basin, Bolivia. Sci Total Environ 384:355–373
Miller SD (1995) Geochemical indicators of sulfide oxidation and acid generation in the field. In: Grundon NJ, Bell LC (eds) Proceedings of the 2nd Australian acid mine drainage workshop. Australian Centre for Minesite Rehabilitation Research, Brisbane, pp 117–120
Miller SD (1996) Advances in acid drainage: prediction and implications for risk management. In: Proceedings of 3rd international and 21st annual Minerals Council of Australia environmental workshop, vol 1. Minerals Council of Australia, Dickson, pp 149–157
Miller SD (1998b) Theory, design and operation of covers for controlling sulfide oxidation in waste rock dumps. In: McLean RW, Bell LC (eds) Proceedings of the 3rd Australian acid mine drainage workshop. Australian Centre for Minesite Rehabilitation Research, Brisbane, pp 115–126
Mitchell P (2000) Prediction, prevention, control and treatment of acid rock drainage. In: Warhurst A, Noronha L (eds) Environmental policy in mining; corporate strategy and planning for closure. Lewis Publishers, Boca Raton, pp 117–143
Mlayah A, Ferreira da Silva E, Rocha F, Ben Hamza C, Charef A, Noronha F (2009) The Oued Mellègue: mining activity, stream sediments and dispersion of base metals in natural environments, North-western Tunisia. J Geochem Explor 102:27–36
Modis K, Komnitsas K (2007) Optimum sampling density for the prediction of acid mine drainage in an underground sulphide mine. Mine Water Environ 26:237–242
Molina JA, Oyarzun R, Esbrí JM, Higueras P (2006) Mercury accumulation in soils and plants in the Almadén mining district, Spain: one of the most contaminated sites on Earth. Environ Geochem Health 28:487–498
Moncur MC, Ptacek CJ, Blowes DW, Jambor JL (2005) Release, transport and attenuation of metals from an old tailings impoundment. Appl Geochem 20:639–659
Moncur MC, Jambor JL, Ptacek CJ, Blowes DW (2009) Mine drainage from the weathering of sulfide minerals and magnetite. Appl Geochem 24:2362–2373
Morin KA, Hutt NM (1997) Environmental geochemistry of minesite drainage. MDAG Publication, Vancouver
Morrison AL, Gulson BL (2007) Preliminary findings of chemistry and bioaccessibility in base metal smelter slags. Sci Total Environ 382:30–42
Müller N, Franke K, Schreck P, Hirsch D (2008) Georadiochemical evidence to weathering of mining residues of the Mansfeld mining district, Germany. Environ Geol 54:869–877
Munk LA, Faure G, Koski R (2006) Geochemical evolution of solutions derived from experimental weathering of sulfide-bearing rocks. Appl Geochem 21:1123–1134
Munroe EA, McLemore VT, Kyle P (1999) Waste rock pile characterization, heterogeneity, and geochemical anomalies in the Hillsboro Mining District, Sierra County, New Mexico. J Geochem Explor 67:391–405
Murad E, Schwertmann U, Bigham JM, Carlson L (1994) Mineralogical characteristics of poorly crystallized precipitates formed by oxidation of Fe2+ in acid sulfate waters. In: Alpers CN, Blowes DW (eds) Environmental geochemistry of sulfide oxidation. American Chemical Society Symposium Series 550, Washington, DC, pp 190–200
Natarajan KA, Subramanian S, Braun JJ (2006) Environmental impact of metal mining – biotechnological aspects of water pollution and remediation – an Indian experience. J Geochem Explor 88:45–48
Navarro A, Cardellach E, Mendoza JL, Corbella M, Domènech LM (2008a) Metal mobilization from base-metal smelting slag dumps in Sierra Almagrera (Almería, Spain). Appl Geochem 23:895–913
Navarro MC, Pérez-Sirvent C, Martínez-Sánchez MJ, Vidal J, Tovar PJ, Bech J (2008b) Abandoned mine sites as a source of contamination by heavy metals: a case study in a semi-arid zone. J Geochem Explor 96:183–193
Ngoc KC, Nguyen NV, Dinh BN, Thanh SL, Tanaka S, Kang Y, Sakurai K, Iwasaki K (2009) Arsenic and heavy metal concentrations in agricultural soils around tin and tungsten mines in the Dai Tu district, N Vietnam. Water Air Soil Poll 197:75–89
Nicholson RV, Scharer JM (1994) Laboratory studies of pyrrhotite oxidation kinetics. In: Alpers CN, Blowes DW (eds) Environmental geochemistry of sulfide oxidation. American Chemical Society Symposium Series 550, Washington, DC, pp 14–30
Nicholson RV, Gillham RW, Reardon EJ (1990) Pyrite oxidation in carbonate-buffered solution; 2. Rate control by oxide coatings. Geochim Cosmochim Acta 54:395–402
Nordstrom DK, Alpers CN (1999a) Geochemistry of acid mine waters. In: Plumlee GS, Logsdon MS (eds) The environmental geochemistry of mineral deposits. Part A: processes, techniques and health issues, vol 6A. Society of Economic Geologists, Littleton, pp 133–160 (Reviews in economic geology)
Nugraha C, Shimada H, Sasaoka T, Ichinose M, Matsui K, Manege I (2009) Geochemistry of waste rock at dumping area. Int J Min Reclam Environ 23:132–143
Oerter EJ, Brimhall GH Jr, Redmond J, Walker B (2007) A method for quantitative pyrite abundance in mine rock piles by powder X-ray diffraction and Rietveld refinement. Appl Geochem 22:2907–2925
Ollier CD, Pain CF (1997) Regolith, soils and landforms. Wiley, New York
Ostergren JD, Brown GE, Parks GA, Tingle TN (1999) Quantitative speciation of lead in selected mine tailings from Leadville, CO. Environ Sci Technol 33:1627–1636
Oyarzun R, Cubas P, Higueras P, Lillo J, Llanos W (2009) Environmental assessment of the arsenic-rich, Rodalquilar gold–(copper-lead-zinc) mining district, SE Spain: data from soils and vegetation. Environ Geol 58:761–777
Pagnanelli F, Luigi M, Mainelli S, Toro L (2007) Use of natural materials for the inhibition of iron oxidizing bacteria involved in the generation of acid mine drainage. Hydrometallurgy 87:27–35
Paktunc AD (1999) Mineralogical constraints on the determination of neutralization potential and prediction of acid mine drainage. Environ Geol 39:103–112
Parker G (1999) A critical review of acid generation resulting from sulfide oxidation: processes, treatment and control. In: Acid Drainage. Australian Minerals & Energy Environment Foundation, Melbourne, occasional paper no 11, pp 1–182
Parsons MB, Bird DK, Einaudi MT, Alpers CN (2001) Geochemical and mineralogical controls on trace element release from the Penn Mine base-metal slag dump, California. Appl Geochem 16:1567–1593
Pedersen TF, McNee JJ, Flather DH, Mueller B, Pelletier CA (1998) Geochemical behaviour of submerged pyrite-rich tailings in Canadian lakes. In: Geller W, Klapper H, Salomons W (eds) Acidic mining lakes. Springer, Heidelberg, pp 87–125
Peng B, Piestrzynski A, Pieczonka J, Xiao M, Wang Y, Xie S, Tang X, Yu C, Song Z (2007) Mineralogical and geochemical constraints on environmental impacts from waste rock at Taojiang Mn-ore deposit, central Hunan, China. Environ Geol 52:1277–1296
Pérez-López R, Nieto JM, Álvarez-Valero AM, Ruiz de Almodóvar G (2007a) Mineralogy of the hardpan formation processes in the interface between sulfide-rich sludge and fly ash: applications for acid mine drainage mitigation. Amer Mineral 92:1966–1977
Pérez-López R, Álvarez-Valero AM, Nieto JM, Sáez R, Matos JX (2008) Use of sequential extraction procedure for assessing the environmental impact at regional scale of the Sao Domingos Mine (Iberian Pyrite Belt). Appl Geochem 23:3452–3463
Petrunic BM, Al TA, Weaver L, Hall D (2009) Identification and characterization of secondary minerals formed in tungsten mine tailings using transmission electron microscopy. Appl Geochem 24:2222–2233
Piatak NM, Seal RR, Hammarstrom JM (2004) Mineralogical and geochemical controls on the release of trace elements from slag produced by base- and precious-metal smelting at abandoned mine sites. Appl Geochem 19:1039–1064
Pirrie D, Camm GS, Sear LG, Hughes SH (1997) Mineralogical and geochemical signature of mine waste contamination, Tresillian River, Fal Estuary, Cornwall, UK. Environ Geol 29:58–65
Plumlee GS (1999) The environmental geology of mineral deposits. In: Plumlee GS, Logsdon MS (eds) The environmental geochemistry of mineral deposits. Part A: processes, techniques and health issues, vol 6A. Society of Economic Geologists, Littleton, pp 71–116 (Reviews in economic geology)
Pond AP, White SA, Milczarek M, Thompson TL (2005) Accelerated weathering of biosolid-amended copper mine tailings. J Environ Qual 34:1293–1301
Price WA, Morin K, Hutt N (1997) Guidelines for the prediction of acid rock drainage Part II. Recommended procedures for static and kinetic testing. In: Proceedings from the 4th international conference on acid rock drainage, vol 1. Vancouver, pp 15–30
Ptacek CJ, Blowes DW (1994) Influence of siderite on the pore-water chemistry of inactive mine-tailings impoundments. In: Alpers CN, Blowes DW (eds) Environmental geochemistry of sulfide oxidation. American Chemical Society Symposium Series 550, Washington, DC, pp 172–189
Puura E, Neretnieks I (2000) Atmospheric oxidation of the pyritic waste rock in Maardu, Estonia. 2. An assessment of aluminosilicate buffering potential. Environ Geol 39:560–566
Puura E, Neretnieks I, Kirsimäe K (1999) Atmospheric oxidation of the pyritic waste rock in Maardu, Estonia. 1. Field study and modelling. Environ Geol 39:1–19
Puziewicz J, Zainoun K, Bril H (2007) Primary phases in pyrometallurgical slags from a zinc-smelting waste dump, Swiętochłowice, Upper Silesia, Poland. Can Mineral 45:1189–1200
Qi C, Liu G, Chou CL, Zheng L (2008) Environmental geochemistry of antimony in Chinese coals. Sci Total Environ 389:225–234
Rapant S, Dietzová Z, Cicmanová S (2006) Environmental and health risk assessment in abandoned mining area, Zlata Idka, Slovakia. Environ Geol 51:387–397
Reglero MM, Monsalve-González L, Taggart MA, Mateo R (2008) Transfer of metals to plants and red deer in an old lead mining area in Spain. Sci Total Environ 406:287–297
Rimstidt JD, Vaughan DJ (2003) Pyrite oxidation: a state-of-the-art assessment of the reaction mechanism. Geochim Cosmochim Acta 67:873–880
Rimstidt JD, Chermak JA, Gagen PM (1994) Rates of reaction of galena, sphalerite, chalcopyrite and arsenopyrite with Fe(III) in acidic solutions. In: Alpers CN, Blowes DW (eds) Environmental geochemistry of sulfide oxidation. American Chemical Society Symposium Series 550, Washington, DC, pp 2–13
Ritchie AIM (1994a) The waste-rock environment. In: Jambor JL, Blowes DW (eds) Environmental geochemistry of sulfide mine-wastes, vol 22. Mineralogical Association of Canada, Nepean, pp 133–161 (Short course handbook)
Ritchie AIM (1994b) Sulfide oxidation mechanisms; controls and rates of oxygen transport. In: Jambor JL, Blowes DW (eds) Environmental geochemistry of sulfide mine-wastes, vol 22. Mineralogical Association of Canada, Nepean, pp 201–245 (Short course handbook)
Ritchie AIM (1995) Application of oxidation rates in rehabilitation design. In: Grundon NJ, Bell LC (eds) Proceedings of the 2nd Australian acid mine drainage workshop. Australian Centre for Minesite Rehabilitation Research, Brisbane, pp 101–116
Romano P, Blazquez ML, Alguacil FJ, Munoz JA, Ballester A, Gonzalez F (2001) Comparitive study on the selective chalcopyrite bioleaching of a molybdenite concentrate with mesophilic and thermophilic bacteria. FEMS Microbiol Lett 196:71–75
Rosado, L, Morais C, Candeias AE, Pinto AP, Guimarães F, Mirão J (2008) Weathering of S. Domingos (Iberian Pyritic Belt) abandoned mine slags. Mineral Mag 72:489–494
Rozalén ML, Huertas FJ, Brady PV, Cama J, García-Palma S, Linares J (2008) Experimental study of the effect of pH on the kinetics of montmorillonite dissolution at 25˚C. Geochim Cosmochim Acta 72:4224–4253
Ruby MV, Davis A, Nicholson A (1994) In situ formation of lead phosphates in soils as a method to immobilize lead. Environ Sci Technol 28:646–654
Rufo L, Rodríguez N, Amils R, de la Fuente V, Jiménez-Ballesta R (2007) Surface geochemistry of soils associated to the Tinto River (Huelva, Spain). Sci Total Environ 378:223–227
Salkield LU (1987) A technical history of the Rio Tinto mines: some notes on exploitation from pre-Phoenician times to the 1950 s. The Institution of Mining and Metallurgy, London
Salmon SU, Malmström ME (2006) Quantification of mineral dissolution rates and applicability of rate laws: laboratory studies of mill tailings. Appl Geochem 21:269–288
Salomons W (1995) Environmental impact of metals derived from mining activities; processes, predictions, prevention. J Geochem Explor 52:5–23
Sams JI, Veloski GA (2003) Evaluation of airborne thermal infrared imagery for locating mine drainage sites in the Lower Kettle Creek and Cook Run Basins, Pennsylvania, USA. Mine Water Environ 22:85–93
Sams JI, Veloski GA, Ackman TE (2003) Evaluation of airborne thermal infrared imagery for locating mine drainage sites in the lower Youghiogheny River Basin, Pennsylvania, USA. Mine Water Environ 22:94–103
Sánchez España JS, Pamo EL, Pastor ES (2007) The oxidation of ferrous iron in acidic mine effluents from the Iberian Pyrite Belt (Odiel Basin, Huelva, Spain): field and laboratory rates. J Geochem Explor 92:120–132
Sánchez España JS, Toril EG, López Pamo E, Amils R, Ercilla MD, Pastor ES, Martin-Úriz PS (2008a) Biogeochemistry of a hyperacidic and ultraconcentrated pyrite leachate in San Telmo mine (Iberian Pyrite Belt, Spain). Water Air Soil Poll 194:243–257
Sand W, Jozsa PG, Kovacs ZM, Săsăran N, Schippers A (2007) Long-term evaluation of acid rock drainage mitigation measures in large lysimeters. J Geochem Explor 92:2005–211
Sato M (1992) Persistency-field Eh-pH diagrams for sulfides and their application to supergene oxidation and enrichment of sulfide ore bodies. Geochim Cosmochim Acta 56:3133–3156
Schaaf W, Hüttl RF (2006) Direct and indirect effects of soil pollution by lignite mining. Water Air Soil Poll: Foc 6:353–364
Schaider LA, Senn DB, Brabander DJ, McCarthy KD, Shine JP (2007) Characterization of zinc, lead, and cadmium in mine waste: implications for transport, exposure, and bioavailability. Environ Sci Technol 41:4164–4171
Schippers A, Sand W (1999) Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl Environ Microbiol 65:319–321
Schippers A, Kock D, Schwartz M, Bottcher ME, Vogel H, Hagger M (2007) Geomicrobiological and geochemical investigation of a pyrrhotite-containing mine waste tailings dam near Selebi-Phikwe in Botswana. J Geochem Explor 92:151–158
Schrenk MO, Edwards KJ, Goodman RM, Hamers RJ, Banfield JF (1998) Distribution of Thiobacillus ferrooxidans and Leptospirillum ferrooxidans: implications for generation of acid mine drainage. Science 279:1519–1522
Schubert M, Osenbrück K, Knöller K (2008) Using stable and radioactive isotopes for the investigation of contaminant metal mobilization in a metal mining district. Appl Geochem 23:2945–2954
Schuwirth N, Voegelin A, Kretzschmar R, Hofmann T (2007) Vertical distribution and speciation of trace metals in weathering flotation residues of a zinc/lead sulfide mine. J Environ Qual 36:61–99
Seignez N, Gauthier A, Bulteel D, Damidot D, Potdevin JL (2008) Leaching of lead metallurgical slags and pollutant mobility far from equilibrium conditions. Appl Geochem 23:3699–3711
Shaw SA, Hendry MJ (2009) Geochemical and mineralogical impacts of H2SO4 on clays between pH 5.0 and -3.0. Appl Geochem 24:333–345
Shay DA, Cellan RR (2000) Use of a chemical cap to remediate acid rock conditions at Homestake’s Santa Fe mine. In: Tailings and mine waste ’00. Balkema, Rotterdam, pp 203–210
Sherlock EJ, Lawrence RW, Poulin R (1995) On the neutralization of acid rock drainage by carbonate and silicate minerals. Environ Geol 25:43–54
Shevade AV, Erickson L, Pierzynski G, Jiang S (2001) Formation and stability of substituted pyromorphite: a molecular modeling study. J Hazard Subst Res 3:2–1 to 2–12
Shipitalo MJ, Bonta JV (2008) Impact of using paper mill sludge for surface-mine reclamation on runoff water quality and plant growth. J Environ Qual 37:2351–2359
Sidenko NV, Gieré R, Bortnikova SB, Cottard F, Pal’chik NA (2001) Mobility of heavy metals in self-burning waste heaps of the zinc smelting plant in Belovo (Kemerovo Region, Russia). J Geochem Explor 74:109–125
Sidenko NV, Khozhina EI, Sherriff BL (2007) The cycling of Ni, Zn, Cu in the system “mine tailings – ground water – plants”: a case study. Appl Geochem 22:30–52
Singer PC, Stumm W (1970) Acid mine drainage: rate-determining step. Science 167:1121–1123
Skousen J, Renton J, Brown H, Evans P, Leavitt B, Brady K, Cohen L, Ziemkiewicz P (1997) Neutralization potential of overburden samples containing siderite. J Environ Qual 26:673–681
Skousen J, Simmons J, McDonald LM, Ziemkiewicz P (2002) Acid-base accounting to predict post-mining drainage quality on surface mines. J Environ Qual 31:2034–2044
Slowey AJ, Johnson SB, Newville M, Brown GE Jr (2007) Speciation and colloid transport of arsenic from mine tailings. Appl Geochem 22:1884–1898
Smart R, Skinner B, Levay G, Gerson A, Thomas J, Sobieraj H, Schumann R, Weisener C, Weber P, Miller S, Stewart W (2002) ARD testbook. Project P387A prediction and kinetic control of acid mine drainage. AMIRA International, Melbourne
Smith A, Robertson A, Barton-Bridges J, Hutchison IPG (1992) Prediction of acid generation potential. In: Hutchison IPG, Ellison RD (eds) Mine waste management. Lewis Publishers, Boca Raton, pp 123–199
Smith AML, Hudson-Edwards KA, Dubbin WE, Wright K (2006) Dissolution of jarosite [KFe3(SO4)2(OH)2] at pH 2 and 8; insights from batch experiments and computational modelling. Geochim Cosmochim Acta 70:608–621
Smith KS, Ramsey CA, Hageman PL (2000) Sampling strategy for the rapid screening of mine-waste dumps on abandoned mine lands. In: Proceedings from the 5th international conference on acid rock drainage, vol 2. Society for Mining, Metallurgy, and Exploration, Littleton, pp 1453–1461
Smith MW, Brady KBC (1998) Alkaline addition. In: Coal mine drainage prediction and pollution prevention in Pennsylvania. The Pennsylvania Department of Environmental Protection, Chapter 13, 13–1 to 13–13
Smuda J, Dold B, Friese K, Morgenstern P, Glaesser W (2007) Mineralogical and geochemical study of element mobility at the sulphide-rich Excelsior waste rock dump from the polymetallic Zn-Pb-(Ag-Bi-Cu) deposit, Cerro de Pasco, Peru. J Geochem Explor 92:97–110
Sneddon IR, Orueetxebarria M, Hodson ME, Schofield PF, Valsami-Jones E (2008) Field trial using bone meal amendments to remediate mine waste derived soil contaminated with zinc, lead and cadmium. Appl Geochem 23:2414–2424
Sobek AA, Schuller WA, Freeman JR, Smith RM (1978) Field and laboratory methods applicable to overburdens and minesoils. US EPA 600/2-78-054, Washington, DC
Spuller C, Weigand H, Marb C (2007) Trace metal stabilisation in a shooting range soil: mobility and phytotoxicity. J Hazard Mat 141:378–387
Sracek O, Gélinas P, Lefebvre R, Nicholson RV (2006) Comparison of methods for the estimation of pyrite oxidation rate in a waste rock pile at Mine Doyon site, Quebec, Canada. J Geochem Explor 91:99–109
Stanton MR, Gemery-Hill PA, Shanks WC III, Taylor CD (2008) Rates of zinc and trace metal release from dissolving sphalerite at pH 2.0-4.0. Appl Geochem 23:136–147
Strömberg B, Banwart SA (1999) Experimental study of acidity-consuming processes in mine waste rock; some influences of mineralogy and particle size. Appl Geochem 14:1–16
Stumm W, Morgan JJ (1995) Aquatic chemistry, 3rd edn. Wiley, New York
Svendson A, Henry C, Brown S (2007) Revegetation of high zinc and lead tailings with municipal biosolids and lime: greenhouse study. J Environ Qual 36:1609–1617
Sverdrup HU (1990) The kinetics of base cation release due to chemical weathering. Lund University Press, Lund
Swayze GA, Smith KS, Clark RN, Sutley SJ, Pearson RM, Vance JS, Hageman PL, Briggs PH, Meier AL, Singleton MJ, Roth S (2000) Using imaging spectroscopy to map acidic mine waste. Environ Sci Technol 34:47–54
Taylor JR, Waring CL, Murphy NC, Leake MJ (1998) An overview of acid mine drainage control and treatment options, including recent advances. In: McLean RW, Bell LC (eds) Proceedings of the 3rd Australian acid mine drainage workshop. Australian Centre for Minesite Rehabilitation Research, Brisbane, pp 147–159
Teršič T, Gosar M, šajn R (2009) Impact of mining activities on soils and sediments at the historical mining area in Podljubelj, NW Slovenia. J Geochem Explor 100:1–10
Trois C, Marcello A, Pretti S, Trois P, Rossi G (2007) The environment risk posed by small dumps of complex arsenic, antimony, nickel and cobalt sulphides. J Geochem Explor 92:83–95
Vaughan DJ, Craig JR (1978) Mineral chemistry of metal sulfides. Cambridge Earth Science Series, Cambridge University Press, Cambridge
Velasco F, Alvaro A, Suarez S, Herrero JM, Yusta I (2005) Mapping Fe-bearing hydrated sulphate minerals with short wave infrared (SWIR) spectral analysis at San Miguel mine environment, Iberian Pyrite Belt (SW Spain). J Geochem Explor 87:45–72
Velleux ML, Julien PY, Rojas-Sanchez R, Clements WH, England JF Jr (2006) Simulation of metals transport and toxicity at a mine-impacted watershed: California Gulch, Colorado. Environ Sci Technol 40:6996–7004
Walker FP, Schreiber ME, Rimstidt JD (2006) Kinetics of arsenopyrite oxidative dissolution by oxygen. Geochim Cosmochim Acta 70:1668–1676
Weber PA, Stewart WA, Skinner WM, Weisener CG, Thomas JE, Smart RSC (2004) Geochemical effects of oxidation products and framboidal pyrite oxidation in acid mine drainage prediction techniques. Appl Geochem 19:1953–1974
White WW, Lapakko KA, Cox RL (1999) Static-test methods most commonly used to predict acid-mine drainage: practical guidelines and interpretation. In: Plumlee GS, Logsdon MS (eds) The environmental geochemistry of mineral deposits. Part A: processes, techniques and health issues, vol 6A. Society of Economic Geologists, Littleton, pp 325–338 (Reviews in economic geology)
Williams DJ (1997) Effectiveness of co-disposing coal washery wastes. In: Tailings and mine waste ’97. Balkema, Rotterdam, pp 335–341
Williams DJ, Wilson GW, Currey NA (1997) A cover system for a potentially acid forming waste rock dump in a dry climate. In: Tailings and mine waste ’97. Balkema, Rotterdam, pp 231–236
Williams DJ, Bigham JM, Cravotta CA, Traina SJ, Anderson JE, Lyon JG (2002) Assessing mine drainage pH from the colour and spectral reflectance of chemical precipitates. Appl Geochem 17:1273–1286
Williams PA (1990) Oxide zone geochemistry. Ellis Horwood, New York
Wilson GW, Newman LL, Ferguson KD (2000) The co-disposal of waste rock and tailings. In: Proceedings from the 5th international conference on acid rock drainage, vol 2. Society for Mining, Metallurgy, and Exploration, Littleton, pp 789–796
Xenidis A, Mylona E, Paspaliaris I (2002) Potential use of lignite fly ash for the control of acid generation from sulphidic wastes. Waste Manage 22:631–641
Xu Y, Schwartz FW (1994) Lead immobilization by hydroxyapatite in aqueous solutions. J Contam Hydrol 15:187–206
Yang J (2006) Concentrations and modes of occurrence of trace elements in the Late Permian coals from the Puan Coalfield, southwestern Guizhou, China. Environ Geochem Health 28:567–576
Yang JE, Skousen JG, Ok YS, Yoo KY, Kim HJ (2006) Reclamation of abandoned coal mine waste in Korea using lime cake by-products. Mine Water Environ 25:227–232
Yin G, Catalan LJJ (2003) Use of alkaline extraction to quantify sulfate concentration in oxidized mine tailings. J Environ Qual 32:2410–2413
You LQ, Heping L, Li Z (2007) Study of galvanic interactions between pyrite and chalcopyrite in a flowing system: implications for the environment. Environ Geol 52:11–18
Younger PL (2004) Environmental impacts of coal mining and associated wastes: a geochemical perspective. In: Gieré R, Stille P (eds) Energy, waste, and the environment: a geochemical perspective, vol 236. Geological Society, London, Special Publications, pp 169–209
Younger PL, Banwart SA, Hedin RS (2002) Mine water; hydrology, pollution, remediation. Kluwer Academic Publishers, Dordrecht
Yunmei Y, Yongxuan Z, Williams-Jones AE, Zhenmin G, Dexian L (2004) A kinetic study of the oxidation of arsenopyrite in acidic solutions: implications for the environment. Appl Geochem 19:435–444
Zheng G, Kuno A, Mahdi TA, Evans DJ, Miyahara M, Takahashi Y, Matsuo M, Shimizu H (2007) Iron speciation and mineral characterization of contaminated sediments by coal mine drainage in Neath Canal, South Wales, United Kingdom. Geochem J 41:463–474
Zielinski RA, Otton JK, Johnson CA (2001) Sources of salinity near a coal mine spoil pile, north-central Colorado. J Environ Qual 30:1237–1248
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Lottermoser, B.G. (2010). Sulfidic Mine Wastes. In: Mine Wastes. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-12419-8_2
Download citation
DOI: https://doi.org/10.1007/978-3-642-12419-8_2
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-12418-1
Online ISBN: 978-3-642-12419-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)