Abstract
A number of plant proteins inhibit protein synthesis by irreversibly inactivating the 60S ribosomal subunit in a catalytical, that is, enzymatic, manner. For this property, they are called ribosome-inactivating proteins (RIPs). Several RIPs are utilized in the preparation of therapeutic heteroconjugates (immunotoxins), obtained either by chemical conjugation of a vehicle molecule to an RIP or by genetic fusion of a targeting molecule and an RIP. In the present review, we will focus on the properties of RIPs and of their immunotoxins. The most recent advancements in this domain will be reported in the following paragraphs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Systemic Lupus Erythematosus
- Autologous Bone Marrow Transplantation
- Posterior Capsule Opacification
- Adenine Residue
- Fusion Toxin
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
There are a number of recent reviews on ribosome-inactivating proteins (RIPs) (Van Damme et al. 2001; Girbés et al. 2004; Hartley and Lord 2004; Stirpe 2004; Stirpe and Battelli 2006), and therefore only the main features will be dealt with here.
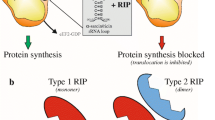
RIPs have been divided into two main groups: type 1, single chain proteins with enzymatic activity, and type 2, consisting of two unequal chains cross-linked by disulphide bond(s) – an A chain with enzymatic activity and a B chain with the properties of a lectin with specificity for sugars. A barley protein (JIP60) consisting of an A-type chain linked to a peptide with an unknown function has been identified and designated as a type-3 RIP (Peumans et al. 2001) (Fig. 1). The B chains of type 2 RIPs bind to carbohydrates (in general galactosyl residues) on the surface of virtually all cells, allowing and facilitating the entry of the whole molecule into the cells. Hence, some type 2 RIPs are potent toxins, ricin being the best known while others are not so toxic (see below). Type 1 proteins, lacking a B chain, enter into cells with difficulty and, consequently, have a relatively low toxicity. Type 1 RIPs, however, are very toxic if linked to molecules capable of delivering them inside the cell.
Schematic representation of the structure of ribosome-inactivating proteins (from Stirpe 2004)
Ricin has been known for more than a century, and pokeweed antiviral protein (PAP) was the first type 1 RIP identified by its inhibitory effect on protein synthesis (Irvin 1975). Subsequently, a number of RIPs of either type were identified, and many were purified and characterized (for a recent review see Girbés et al. 2004). More recently, some two-chain lectins were identified, which consist of A and B chains with lectin and enzymatic activity typical of the corresponding chains of type 2 RIPs; they are, however, endowed with low cytotoxicity, comparable to that of type 1 RIPs (see below) and are commonly referred to as nontoxic type 2 RIPs.
A list of known type 2 RIPs is shown in Table 1. The more numerous type 1 RIPs are listed in Girbés et al. (2004), and a list of those used to prepare immunotoxins will be provided below.
RIPs are officially denominated “rRNA N-glycosidases” (EC 3.2.2.22, Endo 1988), as it was found that they remove a specific adenine residue (A4324 in rat liver rRNA) from eukaryotic rRNAs. They also depurinate DNA and other polynucleotides (Barbieri et al. 1997), and the denomination “adenine polynucleotide glycosylases” was also proposed (Barbieri et al. 2001).
2 Distribution
RIPs are present in many plants, including some edible ones (Barbieri et al. 2006). More type 1 RIPs are known than type 2, and the notion was put forward that they could be ubiquitous. In some plants, they are present in many or even in all tissues examined (e.g., saporins in Saponaria officinalis, Ferreras et al. 1993) and in cultured cells, whereas in other plants they are confined to one or few tissues (e.g., ricin in the seeds of Ricinus communis). In many plants, the expression of RIPs was found to be enhanced in senescent tissues, or in conditions of stress or infection (reviewed in Stirpe and Battelli 2006).
RIPs were also found in mushrooms and algae, and are produced by some bacteria (Shiga and Shiga-like toxins (Reisbig et al. 1981; Obrig 1997)). In animal cells and tissues, an enzymatic activity, which removes adenine from DNA as RIPs do (see below), was found, and like RIPs in plants (Girbés et al. 1996), it is higher in virally infected and stressed cells (Barbieri et al. 2001).
2.1 Enzymatic Activity
Ricin was found to inhibit protein synthesis using whole cells (Lin et al. 1971) and cell-free systems (Olsnes and Pihl 1972), and so do the toxic type 2 RIPs, some ten of which are known (Stirpe and Battelli 2006). Type 1 RIPs and the isolated A chains of type 2 RIPs have lower effects on the protein synthesis of whole cells, in which they enter with difficulty, but are very potent in cell-free systems, where they cause an irreversible damage of ribosomes by removing a single adenine residue (A4324 in rat liver rRNA) from a GAGA sequence in a highly conserved loop at the top of a stem in 28S rRNA (Fig. 2) (reviewed by Endo 1988). This activity is common to all RIPs examined (Stirpe et al. 1988). It is noteworthy that some, but not all, RIPs require various cofactors for maximal inhibitory activity on translation (Carnicelli et al., 1992), e.g. gelonin requires a specific tRNA (Brigotti et al. 2002).
Schematic representation of the enzymatic action of ribosome-inactivating proteins on rRNA. RIPs cleave a single adenine base (A4324 in 28S rat rRNA) at a site adjacent to the site of attack by α-sarcin, which cleaves the phosphodiester bond between G4325 and A4326 in rat 28S rRNA (from Stirpe 2004)
Some RIPs, however, remove more than one adenine residue per ribosome (Barbieri et al. 1994) and all RIPs examined remove adenine residues from DNA and other polynucleotides (Barbieri et al. 1997; Nicolas et al. 1998). The latter property seems to be another characteristic specific to RIPs, to the point that it was used to detect new RIPs (Pelosi et al. 2005). Also, ricin and other RIPs remove adenine from the poly(A) tail of poly(ADP-ribosylated poly(ADP-ribose) polymerase (activated PARP, Barbieri et al. 2003). PAP depurinates capped mRNA (Hudak et al. 2002). A lyase activity of RIPs was also reported (reviewed in Van Damme et al. 2001; Aceto et al. 2005), which in some cases was due to contamination by nucleases (reviewed in Stirpe 2004).
2.2 Toxicity
It was mentioned above that some type 2 RIPs are potent toxins, and indeed for some years it was assumed that all type 2 RIPs were highly toxic. The high toxicity was explained by the combined action of the two chains: the B chain binds to galactosyl-terminated residues on the surface of most cells, allowing and facilitating the entry of the toxins into the cells, where the A chain is separated and can exert its enzymatic activity, damaging ribosomes and possibly other structures, with consequent cell damage and death.
A number of lectins (Ricinus agglutinin, lectins from Sambucus, camphor tree and iris) are type 2 RIPs, with the same enzymatic and lectin properties as those of ricin and related toxins, and still have very low cytotoxicity (reviewed in Van Damme et al. 2001; Girbés et al. 2003; He and Liu 2003). The reasons for the difference are largely unknown, and could be in the binding to, and entry into, cells, the intracellular pathway, as well as the excretion from, and resistance to destruction by cells. Inside cells, some RIPs traffic to the Golgi apparatus and from there to the cytoplasm, some others are degraded by lysosomes, and some are expelled from the cells (reviewed by Sandvig and van Deurs 2002). Differences in each of these destinations and processes could result in different cytotoxicity. Indeed, nigrin b, a nontoxic type 2 RIP from Sambucus nigra bark (Girbés et al. 1993), enters cells equally as well as ricin, but is more rapidly and extensively degraded in, and excreted from, cells (Battelli et al. 1997), whereas volkensin, which has a higher toxicity than ricin, is excreted by cells mostly nondegraded, thus being still active and capable of entering other cells (Battelli et al. 2004). Studies with ricin have clarified that in order to reach their cytosolic substrates, ricin as well as several other toxins (Lord et al. 2003) undergoes retrograde transport to the endoplasmic reticulum (ER) before translocating across the ER membrane. To achieve this export, these toxins exploit the ERAD (ER-associated protein degradation) pathway but must escape, at least in part, the normal degradative fate of ERAD substrates in order to intoxicate the cell. Toxins that translocate from the ER have an unusually low lysine content that reduces the likelihood of ubiquitination and ubiquitin-mediated proteasomal degradation. Regarding intracellular trafficking, it was also observed that despite the fact that the 3D-fold and residues responsible for the N-glycosidase activity are well conserved between saporin and ricin A-chain (RTA), it appears that these two toxins follow different intracellular routes in mammalian intoxicated cells: RTA cytotoxicity is blocked by brefedin A (BFA), a fungal agent disrupting the Golgi complex, and can be enhanced by the addition of a C-terminal KDEL ER retrieval motif. In contrast, neither treatment with BFA nor addition of a KDEL motif has any significant effect on saporin-mediated cytotoxicity (Vago et al. 2005). Although several lines of evidence suggest that saporin (and other type I RIPs) can cross cellular membranes, the site and mechanism(s) of their translocation may differ from the one used by the catalytic subunits of type II RIPs, with ERAD pathways playing little or no role in their productive intoxication paths (Vago et al. 2005; Geden et al. 2007).
3 Properties of RIPs
The properties of type 1 and type 2 RIPs are summarized in Table 2.
Once inside cells, RIPs depurinate rRNA, and this causes a “ribotoxic stress response” which is characterized by activation of several protein kinases (Iordanov et al. 1997). This in turn causes the release of TNF and other proinflammatory cytokines observed in cells and animals poisoned with ricin and other toxic type 2 RIPs. (reviewed in Stirpe and Battelli 2006).
Both type 2 (e.g., ricin (Griffiths et al. 1987), viscumin (Büssing 1996), abrin (Hughes et al. 1996; Narayanan et al. 2004) and high doses of type 1 RIPs (e.g., saporin, Bergamaschi et al. 1996) cause apoptosis and subsequently, or at higher doses, severe necrosis both in cultured cells and in the organs of poisoned animals. In animals, the liver is most often affected, with differences among both RIPs and animals, and inflammation may be present, severe in the case of ricin poisoning (review in Battelli 2004). Inflammation seems to have an important role in the pathogenesis of ricin toxicity, since its inhibition attenuates the lesions and reduces mortality of ricin-poisoned mice (Mabley et al. 2009). It was reported that apoptosis does not directly correlate with the protein synthesis inhibition (Hu et al. 2001; Suzuki et al. 2000; Brigotti et al. 2002) and saporin-6 and its mutants induce caspase-dependent apoptosis in U937 cells via the mitochondrial or intrinsic pathway, its N-glycosidase activity and protein synthesis inhibition being not required for apoptosis induction (Sikriwal et al. 2008).
Interestingly, all toxic type 2 RIPs are transported retrogradely along peripheral nerves (reviewed in Wiley and Lappi 1995), but only modeccin and volkensin (Wiley and Stirpe 1988) and stenodactylin (Monti et al. 2007) are transported retrogradely when injected in the central nervous system (CNS). The reasons for these differences are not known, and may reside in the characteristics of the B chains.
RIPs are also allergenic. The allergenicity of ricin is well known from observations in factories producing castor oil (Thorpe SC et al. 1988). Formation of IgE has been observed in mice after administration of ricin (Thorpe et al. 1989) and of several type 1 RIPs (Zheng et al. 1991), and in laboratory personnel working with RIPs (Szalai et al. 2005).
3.1 Other Biological Properties
PAP, the first known type 1 RIP, was identified as an antiviral protein (reviewed by Irvin 1975). Subsequently, PAP and other RIPs were found to have antiviral activity against both plant and animal viruses (reviewed by Battelli and Stirpe 1995). The antiviral activity was thought to be due to an easier entry of RIPs into infected cells which were killed, with consequent arrest of viral replication. More recently, however, it was suggested that RIPs could act by damaging directly viral RNA (review in He et al. 2008).
Also, RIPs have some fungicidal (Vivanco et al. 1999; Ng 2004) and insecticidal activity (reviewed by Bertholdo-Vargas et al. 2009).
3.2 Possible Uses
Some practical applications of RIPs of both types, either as such or modified, have been envisaged in medicine and agriculture.
For their activity against viruses, type 1 RIPs have been tested as antiviral agents in plants, animals, and humans. In agriculture, plants transfected with RIP genes showed increased resistance to viral and fungal infection, although they were damaged if the RIP was expressed above a certain level (reviewed in Stirpe and Battelli 2006).
Trichosanthin (McGrath et al. 1989) and PAP (Zarling et al. 1990) inhibit HIV replication in vitro, but the attempts to treat HIV-infected patients were unsuccessful (Byers et al. 1994).
In old traditional Chinese medicine, extracts of tubers of Trichosanthes kirilowii were used to induce abortion (Anonymous 1976). This is due to an RIP, trichosanthin, (Yeung et al. 1988), which is currently used in China to induce early and midterm abortion with over 95% success rate and minimal side effects. It was found that momordin and other RIPs have abortifacient activity (Ng et al. 1992). Trichosanthin is highly toxic to trophoblasts and choriocarcinoma-derived cells (Battelli et al. 1992) and causes necrosis of syncytiotrophoblastic cells and fragmentation of placental villi, with consequent large areas of necrosis in the placenta and death of the fetus (Anonymous 1976). The protein was also proposed for the therapy of hydatidiform moles and choriocarcinoma (reviewed in Ng et al. 1992; Shaw et al. 1994). Recently, a unique new intracellular delivery route has been demonstrated for trichosanthin, its well documented invasive properties being able to hijack exosome-mediated intercellular trafficking (Zhang et al. 2009).
In experimental medicine, RIPs have been conjugated to several molecules (lectins, hormones, growth factors, and especially antibodies) capable of delivering them to cells to be selectively eliminated. This matter will be dealt with extensively below.
Ricin, being a potent and easy to obtain poison, has been used for suicidal and homicidal purposes, and there are fears that it could be used as a weapon for warfare or terrorism in the form of powder or aerosol to be inhaled (Waterer and Robertson 2009). The toxin is included among the potential biological weapons by the United States Center of Disease Control and Prevention.
3.3 Role in Nature
The persistence throughout the evolution of these proteins suggests that they may have an important function, useful to the organisms producing them. Several hypotheses have been considered, such as that they could be storage proteins in seeds, or that they may have a defensive role against predators or parasites and fungal, bacterial, and/or viral infections. While these functions cannot be excluded, their higher expression in senescent, wounded, or stressed tissues suggested that they could play a more physiological role and they may also be involved in the mechanism of programmed cell death.
4 RIP-Based Immunotoxins
Application of native RIPs has been explored for the treatment of several diseases (see also Sect. 3.2); it soon became clear however that the potent cytotoxic effect mediated by RIPs could be made more specific and powerful if they were linked (either chemically or via gene fusion) to a targeting molecule.
The general term immunotoxins, first introduced to describe chemical conjugates of RTA and antibodies (Jansen et al. 1982), was subsequently extended to include, however inaccurately, other types of targeted RIPs making use of nonantibody vehicle molecules (e.g., cytokines, peptides, aptamers). For the sake of simplicity, we will also adopt henceforth the term immunotoxins to describe all types of vehicle–toxin combinations, irrespective of the targeting molecule.
4.1 Chemical Immunotoxins
Chemical immunotoxins are obtained by linking RIPs to a vehicle molecule by means of a cross-linking agent. Such immunotoxins are often as cytotoxic as type 2 RIPs. The synthesis is generally accomplished by modifying the carrier molecule and the RIP portion of the conjugate lacking available –SH groups with a heterobifunctional reagent that introduces and activates disulfide groups. When hemitoxins are used (e.g., RTA or abrin A-chain), a free SH group becomes available because of the reduction of the disulfide bond holding the A and the B subunits together. The linkage used to join the targeting molecule and the toxin must meet the following criteria: (1) it should not impair the binding capacity of the targeting molecule (i.e., antibody, growth factor, cytokine, or hormone); (2) it must allow the active toxin component to enter the cytosol and kill the cell; for this reason cross-linkers introducing nonreducible thioether bonds are not preferred; and (3) for in vivo use, the link must be stable enough to remain intact while the immunotoxin is transported through the tissues to its intended site of action. Among the most frequently used heterobifunctional reagents are N-succinimidyl-3-(2-pyridylthio) propionate (SPDP) and 2-iminothiolane (2-IT) (Carlsson et al. 1978; Lambert et al. 1985). Second generation cross-linkers introducing hindered disulfide linkages were later generated to improve stability of the conjugates in vivo. These include sodium S-4-succinimidyloxycarbonyl-α-methyl benzyl sulphonate (SMBT) and 4-succinimidyloxycarbonyl-α-methyl-α(2 pyridyldithio) toluene (SMPT) (Thorpe et al. 1987).
The most used conjugates were initially made by conjugating through a disulfide bond the catalytic A subunits of type 2 RIPs such as ricin or abrin, each of which had been separated from its binding B domain by reduction (Krolick et al. 1980; Blythman et al. 1981). Even without its binding domain, RTA was taken up nonspecifically by macrophages and hepatic nonparenchimal Kupffer cells (Fulton et al. 1988). This uptake was due to glycosylated side residues of RTA binding to mannose receptors on the liver cells (Bourrie et al. 1986). The most appropriate technique for reducing nonspecific uptake of RTA was through chemical deglycosylation. Deglycosylated RTA (dgA) immunotoxins had a significantly prolonged lifetime in mice, leading to an improved therapeutic index (Blakey et al. 1987; Fulton et al. 1988). Because the ricin B-chain facilitates the cytotoxicity of RTA-containing immunotoxins (Ramakrishnan et al. 1989), whole ricin has been targeted after chemically blocking its oligosaccharide binding sites to prevent nontarget cell binding. Carbohydrate binding sites of ricin were blocked with ligands prepared by chemical modification of glycopeptides containing triantennary N-linked oligosaccharides (Lambert et al. 1991). The resulting “blocked ricin” (bR) was then chemically conjugated to antibodies to make immunotoxins (Table 3).
A further strategy of cross-linking often used with type 1 RIPs utilizes the bifunctional cross-linker 2-iminothiolane; the RIP is first reacted with 2-iminothiolane and the free sulfydryl group protected with Ellman’s reagents. The derivatized RIP is then reduced with mercaptoethanol and added to an unreduced derivatized antibody.
4.2 Recombinant Immunotoxins
Recombinant DNA technology has led to the cloning of several RIPs and to the development of RIP-containing constructs of immunotoxins. Most of the RIPs used to obtain recombinant immunotoxins have been cloned following the initial cloning of the RTA gene in Escherichia coli by O'Hare et al. (1987). Indeed, in the following years, saporin (Benatti et al. 1989), abrin (Wood et al. 1991), PAP (Kataoka et al. 1993), dianthin (Legname et al. 1993), gelonin (Nolan et al. 1993), and bryodin (Gawlak et al. 1997) were expressed in heterologous systems and obtained in recombinant form.
The availability of recombinant RIPs in turn paved the way to the subsequent development of numerous recombinant immunotoxins. Many examples of such immunotoxins can be found in other sections of this review in relation to other aspects of the work conducted with both chemically synthesized and recombinant RIPs and RIP-immunotoxins function and applications. In the present section, only some representative recombinant RIP-immunotoxins will be mentioned.
One of the aspects that had to be considered when designing fusion toxins with type 1 RIPs or type 2 hemitoxins is the requirement for intracellular release of the catalytic domain to the cytosol. In an attempt to construct a fusion toxin containing RTA from which free A-chain could be generated, interleukin-2 (IL-2) was fused to recombinant RTA through a linker that contained a proteolytic cleavage site for diphtheria toxin or for clotting factor Xa (Cook et al. 1993). Although the recombinant toxin could be cleaved extracellularly, it could not selectively target cells because the ligand and the toxin were no longer connected. Later, IL-2 was fused to a mutant form of PAP, but the fusion toxin was not purified and was not highly cytotoxic (Dore et al. 1997). Ligands fused to RIPs have produced recombinant immunotoxins with significant cytotoxic activity, including one containing an antiCD40 antibody and bryodin (Francisco et al. 1997), one containing urokinase binding domain and saporin (Fabbrini et al. 1997), and one containing human fibroblast growth factor and saporin (Tetzke et al. 1997). For these molecules, however, it is not known whether the recombinant molecule entered the target cells intact, or the ligand was unstable after internalization, allowing the catalytic domain alone to translocate to the cytosol. The ability of stable ligands to predictably separate from the catalytic domain is a crucial feature of recombinant immunotoxins (Kreitman 1997) and a unique feature found only in native bacterial toxins.
4.3 In Vitro Cytotoxicity
The cytotoxicity of type 1 RIP-based immunotoxins appears to be generally more variable than that of corresponding immunotoxins made with type 2 RIPs or with bacterial toxins (Kreitman 1997). This has been attributed to the lack of a B chain which appears to facilitate the translocation of the enzymatic subunit across cell membranes in addition to its function in cell surface binding. This has prompted a number of investigations aimed at exploring potentiating substances that could be coadministered with the RIP-based immunotoxins to enhance their cytotoxic potential and strategies designed to augment their cytotoxic effect against target cells.
4.4 Enhancement of Cytotoxicity
4.4.1 Lysosomotropic Amines and Carboxylic Ionophores
Lysosomotropic amines and carboxylic ionophores are able to increase dramatically the cytotoxic potency of weakly cytotoxic immunotoxins. In some instances, even noncytotoxic immunotoxins may acquire considerable cytotoxic potency. These compounds accelerate the cell intoxication process and greatly reduce the number of immunotoxin molecules required for cytotoxicity. They may act by several mechanisms, including inhibition of lysosomal hydrolases, traffic alteration along the endosome-Golgi route, and inhibition of the extracellular recycling of internalized material.
4.4.2 Ammonium Chloride (NH4Cl)
NH4Cl is one of the most extensively studied reagents used for enhancing RIP-immunotoxin activity. Raising the pH within acidic organelles (e.g., lysosomes and endosomes) to which the immunotoxins are routed is considered to be one of the mechanisms involved in increasing immunotoxin cytotoxicity (Poole and Ohkuma 1981). It is likely that the lipophylic NH3 can diffuse across the plasma and lysosomal membranes and become protonated to NH +4 within the intracellular organelles, where its entrapment causes a pH increase, thus inhibiting the function of acidic proteolytic enzymes. Using RTA-based immunotoxins, it was found that NH4Cl could only increase the cytotoxicity of the immunotoxin when the pH was raised to above 7 and that NH4Cl acts on internalized molecules for a very short time, suggesting that this enhancer affects an early intracellular step (Casellas et al. 1984; Ravel and Casellas 1990). It is however intriguing that the cytotoxic effect of immunotoxins made with other RIPs (i.e., saporin, gelonin) could not be potentiated upon treatment with NH4Cl (Siena et al. 1988; Goldmacher et al. 1989; Battelli et al. 1998). Differences in trafficking may partly explain these contradictory observations.
4.4.3 Chloroquine
Chloroquine is a well known drug used for the therapy of malaria and, being a clinical drug, might be more suitable for use in combination with immunotoxins in patients. Chloroquine can enhance the cytotoxicity of RTA-based immunotoxins up to 2,500-fold (Casellas et al. 1984). However, as observed for NH4Cl, immunotoxins made with type 1 RIPs are in general much less sensitive to the potentiating effect of chloroquine (Ramakrishnan and Houston 1984; Goldmacher et al. 1989; Lizzi et al. 2005).
4.4.4 Other Lysosomotropic Amines (Methylamine, Amantadine)
Poole and Ohkuma (1981) have shown that weakly basic substances also can increase the intralysosomal pH in a concentration-dependent manner. Methylamine is a weak base which affects the intralysosomal pH. A concentration of 10 mM enhanced the activity of an anti-CD5 immunotoxin on CEM cells by over 13,000-fold (Casellas et al. 1984). The drug 1-adamantanamine hydrochloride (amantadine) also is a potent enhancer of the cytotoxic activity of anti-CD5 RTA-based immunotoxins against peripheral blood T cells (Siena et al. 1987) and can restore the activity of an anti-IgM–saporin conjugate whose efficacy is impaired by the presence of human bone marrow (Bregni et al. 1988). Amantadine may be more advantageous than NH4Cl because it is a licensed drug used for the prophylaxis of the influenza; although the in vitro concentration used in the study cited above (1 mmol/L) may be difficult to achieve in the blood of patients, it may nevertheless be used to purge the bone marrow of patients from malignant cells or from allogeneic mature T cells which are often responsible for graft-versus-host-disease (GVHD) reactions.
4.4.5 Carboxylic Ionophores
Carboxylic ionophores such as monensin are well studied reagents able to enhance immunotoxin efficacy. Monensin, grisorixin, lasalocid, and nigericin are all able to enhance the effect of RIPs-based immunotoxins (especially those made with RTA); however, other ionophores such as nonactin, valinomycin, and calicimycin have no effect on immunotoxin toxicity. The present section will focus essentially on the effects brought about both in vitro and in vivo by monensin, which is the most widely used and described carboxylic ionophore for immunotoxin potentiation.
Monensin is a molecule capable of ion complexation through a cyclic form stabilized by hydrogen bonding between the carboxyl and hydroxyl groups (Mollenhauer et al. 1990). Monensin is able to collapse Na+ and H+ gradients across cell membranes and may increase the pH of acidic vesicles like lysosomes through the exchange of Na+ for H+. As an immunotoxin potentiator, monensin was first described in 1984 (Raso and Lawrence 1984; Casellas et al. 1984) and was found to function at very low concentrations (nanomolar range) in vitro yielding significant increase in the cytotoxicity of RTA-based immunotoxins, with IC50s in the range 10−12–10−14 M. When assayed to evaluate the potentiation of antibody–gelonin conjugates, however, its effects were close to nil (Goldmacher et al. 1989). As shown by Raso and Lawrence (1984), monensin accelerates the kinetics of target cell intoxication. Lysosomotropic amines and carboxylic ionophores raise the pH and so it has been suggested that they may act by reducing the rate of degradation of the immunotoxin (Casellas et al. 1982, 1984). However, Raso and Lawrence (1984) and Jansen et al. (1992) have shown that monensin potentiates RTA immunotoxins at concentrations that do not affect lysosomal pH, suggesting that an alternative mechanism may be operating. In fact, at micromolar concentrations monensin increases the pH in the lysosomes; however, vacuolization of the Golgi and enhancement of immunotoxins can be obtained at 100-fold lower concentrations of 50 nM (Jansen et al. 1992). Indeed, studies have shown that lysosomotropic amines can delay the delivery of immunotoxins to lysosomes Carrière et al. 1985), keeping them longer inside peripheral endosomes and possibly diverting them to other subcellular compartments which facilitate their escape to the cytosol. Along these lines, Ravel et al. (1992) demonstrated that the presence of NH4Cl or monensin, both dramatically enhancing the effects of RTA immunotoxins, did not affect the rate of internalization or the intracellular localization of the immunotoxin, suggesting that these activators could act at a postendocytotic level on a limited number of immunotoxin molecules.
To evaluate the possible in vivo potentiation of RTA immunotoxins by monensin, and our group (Colombatti et al. 1990) have used monensin chemically cross-linked to the carrier protein human serum albumin (HSA). This compound can enhance the immunotoxin effect to the same extent as it does in in vitro experiments. However, since disulfide cross-linked HSA–monensin is rapidly inactivated by human serum, we (Candiani et al. 1992) synthesized thioether cross-linked HSA–monensin conjugates, which are resistant to treatments with reducing agents (e.g., glutathione, dithiothreitol) and show potentiating activity identical to that of free monensin and disulfide HSA-linked monensin achieving a potentiation of 45–35,000-fold. Immunotoxin potentiation by both disulfide- and thioether-linked types of HSA–monensin conjugates is inhibited by whole human serum, the serum blocking factor(s) residing mostly in a Mr 40,000–90,000 protein fraction (Candiani et al. 1992). In support of this finding, Jansen et al. (1992) found that a serum glycoprotein of an approximate molecular mass of 45 kDa (sGP3.5) was responsible for plasma inhibition of monensin potentiating effect on immunotoxins. The protein sGP3.5 could be involved in the physiological regulation of intracellular trafficking.
Other formulations of monensin were subsequently explored to allow easier application in vivo. Incorporation of monensin in unilamellar vesicles (liposomes) (Griffin et al. 1993), and monoclonal antibody-targeted liposomes (Singh et al. 1994) were 100-fold more efficacious in immunotoxin potentiation as compared to nontargeted monensin–liposomes.
4.4.6 Antagonists of Ca++ Channels and Other Compounds
Ca++ channel blockers and their derivatives have been studied to evaluate their ability as immunotoxin enhancers. They can often provide several logs increase of immunotoxin efficacy. Their mechanism of action does not appear to be associated with the Ca++ channel function but might be related to the prevention of lysosomal degradation of the immunotoxin.
4.4.7 Verapamil and Its Derivatives
Verapamil was shown to enhance the cytotoxicity of anti-EGFR RTA-immunotoxins up to 40-fold (Akiyama et al. 1984). On the other hand, no influence was observed on an anti-CD22–RTA conjugate (van Horssen et al. 1999). It is likely that the potentiation afforded is in relation with the target antigen or with the histotype of the target cell.
4.4.8 Perhexiline and Indolizines
Perhexiline maleate is another Ca++ channel antagonist and is able to enhance immunotoxin cytotoxicity. An anti-CD5 RTA-immunotoxin could be enhanced 30–20,000-fold on human T leukemia cells (Jaffrezou et al. 1990). It is thought that perhexiline may act by altering the membrane lipid composition through its inhibitory action on acid sphingomyelinases leading to modifications in the intracellular trafficking of the immunotoxins.
A novel class of Ca++ blockers (indolizines SR33557 and SR33287) demonstrated potentiating effects greater than those observed with verapamil using an anti-CD5 RTA-immunotoxin (Jaffrezou et al. 1992).
All-trans retinoic acid was also found to specifically increase receptor-mediated intoxication of RTA-immunotoxins by more than 10,000-fold. Direct microscopical examination demonstrates that the Golgi apparatus undergoes morphological changes upon treatment with retinoic acid, suggesting that it may alter the intracellular trafficking of internalized immunotoxins.
4.4.9 Ricin B-Chain
Many immunotoxins obtained by linking the enzymatic effector subunit of the type 2 RIP ricin (i.e., RTA) to targeting molecules have been described (Colombatti 2002). However, the cytotoxicity of RTA-based immunotoxins is generally more variable than that of the corresponding immunotoxins made with the whole toxin (Colombatti 2002). This has been attributed to the lack of the B-chain which may facilitate RTA translocation across the cell membrane in addition to its cell surface binding.
Specific cytotoxic agents were therefore prepared by linking intact ricin to antibodies in a manner that produces obstruction of the galactose-binding sites on the B chain of the toxin and thereby diminishes the capacity of the conjugate to bind nonspecifically to cells (Thorpe et al. 1984; Cattel et al. 1988). The “blocked” ricin conjugates combine the advantages of high potency, which is often lacking in antibody–RTA conjugates, with high specificity, which previously was lacking in intact ricin conjugates. Moreover, several strategies were also explored to supply isolated B-chain to RTA-containing preparations. Addition of free B-chain in vitro (Youle and Neville 1982; McIntosh et al. 1983), or ricin-B-chain coupled to antitarget antigen antibody or to antimouse antibodies recognizing the cell surface (Vitetta et al. 1983, 1984) enhanced activity. To overcome the possible limitations inherent in using ricin B-chain-containing samples in vivo, ricin B-chain was chemically modified to reduce its ability to bind terminal galactose residues on the cell surface (Vitetta 1986). More recently Frankel et al. (1997) applied molecular biology techniques to fuse IL-2 and a ricin B-chain variant, expressed in insect cells, with modifications of aminoacid residues in each of the three galactose-binding subdomains.
4.4.10 Viruses
Viruses utilize specialized envelope structures to enter the cytosol of the infected cells. Following binding to the cell surface receptors, viruses traffic to acidic intracellular compartments (e.g., endosomes) where domains of the viral coat are activated, thereby triggering the interaction of viral proteins with the organelles’ membranes and the disruption of the endosomal membrane.
In a simplistic approach, adenoviruses were first used to enhance the cytotoxic effects of RTA coupled to antitransferrin receptor antibodies (FitzGerald et al. 1983) or to anticolon carcinoma cell antibodies (Griffin et al. 1987). Two fusogenic peptides of the influenza virus (HA23 and HA24) were employed to enhance the efficacy of anti-HIV immunotoxin by mixing the immunotoxins with peptide preparations (Tolstikov et al. 1997). Peptide HA23 enhanced the activity of the immunotoxin by four- to fivefold. Greater potentiation was achieved by fusing a vesicular stomatitis virus (VSV)-derived peptide to the cDNA coding for RTA (Chignola et al. 1995). Three chimeric proteins were successively obtained by fusing together the dianthin gene and DNA fragments encoding for the following membrane-acting peptides: the N-terminus of protein G of the vesicular stomatitis virus (KFT25), the N terminus of the HA2 hemagglutinin of influenza virus (pHA2), and a membrane-acting peptide (pJVE) (Lorenzetti et al. 2000). Genetic fusion of these membrane-acting peptides to enzymatic cytotoxins resulted in the acquisition of new physico-chemical properties and in the enhancement of the cytotoxic potential of immunotoxins constructed with the modified dianthin.
4.4.11 Saponins
Saponins are plant glycosides that consist of a steroid, steroid alkaloid, or triterpenoid aglycone and one or more sugar chains that are covalently linked by glycosidic binding to the aglycone. Heisler et al. (2005) and Fuchs et al. (2009) investigated whether saponins are able to enhance the efficacy of an immunotoxin consisting of epidermal growth factor (EGF) linked to saporin. Preapplied saponin enhances the target cell-specific cytotoxic effect, dependent on the cell line, between 3,560- and 385,000-fold with a maximum IC50 of 0.67 pM. Nontarget cells are not affected at the same concentration. Thus saponins not only preserve the target specificity of the chimeric toxin but also broaden the therapeutic window with simultaneous dose lowering. A drawback of saponins in tumor therapy is their nontargeted spreading throughout the whole body and their innate toxicity.
5 Animal Studies
Before evaluating the clinical safety and efficacy of an immunotoxin, preclinical investigations are carried out in animal models. An enormous literature has been produced in the past on these matters. This subject has been dealt with in a number of excellent reviews (Kreitman 1997; Wong et al. 2005) and only general aspects will be treated here.
To determine whether an immunotoxin might be effective in vivo, murine models are most commonly produced in which mice are grafted with human xenografts of tumor cell lines. In this regard, solid tumors have been found in general more difficult to treat than disseminated leukemia of the same cell line (van Horssen et al. 1996) and this is reproduced in the greater clinical response of hematological tumors as compared to that of solid tumors (see also below). Animal models are hampered however by great limitations that may lead to overestimation of the potential beneficial effects of immunotoxin treatment in humans. In fact, once antitumor activity is found to be appreciable in vitro, it is still not clear that the agent would result in responses in patients. One reason for this is that cell lines may grossly overestimate the number of antigen binding sites/cells in patients. Thus primary tumor cells freshly isolated from patients often display a much lower number of target antigens/cell. It must also be considered that regulation of antigen expression is sometimes crucially dependent upon conditions and microenvironments which are found exclusively in vivo. Therefore, primary tumor cells are often tested ex vivo to determine sensitivity to the immunotoxin and to predict possible outcomes in vivo. Another problem with murine models is that much greater unwanted toxicity may occur in patients than in mice because unwanted cross-reactions appearing in humans may not be detected in mice and the murine target antigen may not even bind the immunotoxin in the same manner as the human antigen, resulting in different pharmacokinetics and pharmacodynamics of the agent. Indeed, early clinical trials with immunotoxins were sometimes discontinued because of significant toxicity stemming from unpredicted cross-reactions with essential organs (Weiner et al. 1989, Gould et al. 1989). For this reason, nonhuman primates that display the antigen on their normal cells are used for toxicity experiments. Even so, expensive experiments of this type are often not predictive of human toxicity.
One additional problem with immunotoxin treatment that emerged clearly in animal models is the development of resistant mutants after treatment of animals with only one immunotoxin, as demonstrated in an AKR mouse model, where Thy1.1-negative mutants caused fatal relapses in 20% of the animals treated with an immunotoxin to the Thy1.1 antigen of mouse lymphoma cells (Thorpe PE et al. 1988). Use of immunotoxin cocktails, that is, a combination of two or more immunotoxins against different antigens on the same target cell, reduces the likelihood of mutant cell escape. Analysis of malignant cells re-established ex vivo from tumors that had relapsed after therapy with one immunotoxin showed high sensitivity toward immunotoxins directed against a different target antigen. In addition, 90% of mice treated with a cocktail of two or three immunotoxins after tumor challenge had continuous complete remissions, as compared with only 40% of animals treated with the same dose of a single immunotoxin (Engert et al. 1995). This concept has also been confirmed recently in a severe combined immunodeficiency (SCID) mouse model (Flavell et al. 2001) where it was demonstrated that treatment with a cocktail of anti-CD7 and anti-CD38 saporin-based immunotoxins was clearly superior to the treatment with one single immunotoxin in eliminating grafted human T-ALL cells. New developments achievable through the use of recombinant DNA techniques will allow the engineering of bispecific or multispecific immunotoxins for a more thorough eradication of target tumor cells.
Many valuable results were obtained in the recent past using saporin-containing immunotoxins and other conjugates directed against cells of the nervous system. This concept of “molecular” surgery has been extensively reported in various excellent reviews (Wiley and Lappi 2003; Lappi and Wiley 2004). Among the main results reported, Alzheimer’s disease was reproduced by selective destruction of the basal forebrain structures (Wiley et al. 1995). Moreover, conjugates of saporin and substance P have been used to selectively eliminate sensory neurons (reviewed in Wiley and Lappi 2003), and their potential use for treating patients with chronic debilitating pain has been proposed (Ralston 2005; Wiley and Lappi 2005).
The remainder of this review will focus on RIP-based immunotoxins tested in patients to date.
6 Ex Vivo Bone Marrow Purging with Immunotoxins
Bone marrow transplantation (BMT) is used in the treatment of leukemias and lymphomas which have failed, or are likely to fail, first-line chemotherapy. An increasing number of patients with metastatic disease of various solid tumors receive autologous BMT as well. While the development of several GVHDs is the major complication in allogeneic BMT, relapses due to infusion of bone marrow or peripheral stem cells contaminated with residual malignant cells are one of the most challenging problems in autologous transplantation. Ex vivo treatment of the patients’ stem cells with monoclonals, immunotoxins, magnetic beads, or cytostatic drugs before reinfusion (“purging”) is expected to reduce the frequency of relapses after autologous BMT.
A large number of studies were conducted to evaluate the feasibility of purging bone marrow with RIPs-based immunotoxins in the past decades. The reader is referred to an excellent review for a more detailed description of different immunotoxins used and the various settings studied (Frankel et al. 1996). Here we will just mention the first studies made with RIP-containing immunotoxins as examples. Immunotoxins directed against the CD5 and CD7 T-lineage differentiation antigens were used to purge autografts of patients with high-risk T-lineage acute lymphoblastic leukemia (Uckun et al. 1990). In general, the combination of different methods for bone marrow purging (e.g., simultaneous treatment with immunotoxins and chemotherapeutic agents) is more effective in killing residual tumor cells. Relapses which occur after effective bone marrow purging are often caused by insufficient pretransplant conditioning (Uckun et al. 1990).
Before a patient receives an allogeneic BMT, healthy donor bone marrow is often purged of T-lymphocytes in order to prevent GVHD. Immunotoxins have been successfully used for T-cell depletion in allografts, yet extensive T-cell elimination has a negative impact on engraftment of donor marrow and disrupts the graft-versus-leukemia effect (Blazar et al. 1991).
Benefits of purging tumor cells from the bone marrow, however, have not been reliably confirmed in phase II and III trials in several hematologic diseases (Alvarnas and Forman 2004). Limitations of purging include possible loss of progenitor cells, delayed engraftment, and qualitative immune defects following transplant. Further studies will be needed to clarify these points.
7 Clinical Studies
A number of studies have evaluated the effects and potentials of RIP-based immunotoxin treatment in the clinics. However, in spite of the great number of RIPs discovered in the past, only a handful of them have been studied as a potential macromolecular therapeutic in human diseases (Table 3).
Only investigations that have used RIP-based immunotoxins will be dealt with here. The reader is referred to excellent reviews for descriptions of immunotoxins made with bacterial toxins (e.g., diphtheria toxin, Pseudomonas exotoxin A) and their effects in human diseases (Pastan et al. 2007).
Experimental studies suggest that immunotoxins have optimal efficacy when inoculated as a single short course of treatment in patients with minimal disease (Ghetie et al. 1994). However, phase I clinical trials are designed to test the safety of a drug and can be carried out in patients who generally are affected by diseases not responding to conventional therapies and with bulky tumors. Only when the side effects, immunogenicity, maximum tolerated dose (MTD), and pharmacokinetic parameters have been established, can the immunotoxin proceed to phase II trials where patients with less advanced disease can be selected. In phase II trials, therapeutic efficacy can be tested and if a response rate between 20 and 40% is achieved (partial or complete remission) the drug under study will further proceed into phase III clinical trials. To date several immunotoxins have completed phase II/III trials and their efficacy, although it was found encouraging in many occasions, is still under evaluation. In the following paragraphs we will review the major achievements obtained thus far with RIP-based immunotoxins in a clinical setting. The main results in terms of response to therapy will be reported here. Toxicity phenomena and other drawbacks of immunotoxin treatment will be cumulatively described in a separate section (see below).
7.1 Hematologic Tumors
Hematologic malignancies are most suitable for treating with immunotoxins, because malignant cells are often intravascular and directly accessible to intravenously administered drugs, or concentrated in lymphoid organs where access to macromolecules is less problematic than in other tissues. Additionally, patients affected by hematologic malignancies often lack sufficient immunity to reject the heterologous macromolecules administered. It is surprising, however, that in spite of this, only a handful of antigens have been targeted with RIP-based immunotoxins in hematologic patients. These are summarized in Table 3.
7.1.1 Hodgkin’s Lymphoma
In Hodgkin’s disease, Hodgkin and Reed–Sternberg cells consistently express the antigen CD30. An anti-CD30 monoclonal antibody (Ber-H2) was chemically cross-linked to saporin (S06) and the immunotoxin was given to four patients with advanced refractory Hodgkin’s disease (Falini et al. 1992). In three, there was rapid and substantial reduction in tumor mass (50–75%). Clinical responses were transient (6–10 weeks).
An anti-CD30 immunotoxin (Ki-4-dgA, constructed by chemical linkage of the antibody Ki-4 to (dgA)) was evaluated by Schnell et al. (2002) in patients with relapsed CD30+ lymphoma. Clinical response in the 15 evaluable patients included one partial remission, one minor response, and two stable diseases. The immunotoxin, however, was less well tolerated than other immunotoxins of this type, possibly because of the low number of CD30+ peripheral blood mononuclear cells, and in part because of binding of the immunotoxin to soluble CD30 antigen and the resulting circulation of immunotoxin/CD30 complexes.
The anti-CD25 immunotoxin RFT5-dgA was administered to patients with relapsed Hodgkin’s lymphoma with previous heavy treatment in two clinical trials (Engert et al. 1995; Schnell et al. 2000); in spite of the suboptimal conditions of the patients to ascertain the efficacy of treatment with an immunotoxin (relapse, high tumor cell burden, unsuccessful previous therapies), RFT5-dgA was found to be of moderate clinical efficacy (in a total of 32 patients, four partial responses, two minor response, eight stable disease, and nine progressive disease were observed).
7.1.2 Non-Hodgkin’s Lymphoma
Anti-CD22 immunotoxins were evaluated in non Hodgkin lymphomas (NHL). Fab’-RFB4.dgA was the first immunotoxin to be tested in advanced refractory B-cell NHL of low, intermediate, or high grade type (Vitetta et al. 1991). Although patients presented with large tumor masses and were heavily pretreated, 38% achieved a partial remission. The partial remission rate was even higher in patients with >50% CD22-positive tumor cells in lymphnode or bone marrow biopsies. IgG-RFB4.dgA made with whole IgG antibodies was used in similar phase I studies (Amlot et al. 1993; Sausville et al. 1995) in refractory B-NHL patients. The cumulative results of these studies were that in 42 patients in total one complete response, nine partial responses, three minor responses, and six stable diseases were observed.
Because of its smaller size, Fab’ immunotoxins were expected to penetrate into solid tumors more easily than immunotoxins made with whole IgG. However, antitumor activity of Fab’ immunotoxins was generally inferior because of reduced target cytotoxicity and shorter half-life.
Anti-CD19 immunotoxins were also studied in similar trials; the immunotoxin IgG-HD37.dgA, however, was less efficacious than IgG-RFB4.dgA (Stone et al. 1996), in accordance with a lower IC50 and a less impressive performance in mice (Ghetie et al. 1992). Of 23 evaluable patients one complete response and one partial response were observed. In a subsequent study with the same immunotoxin (Messmann et al. 2000), unpredictable clinical courses including deaths were observed. A tendency of the immunotoxin to aggregate and inaccuracies in shipping, storage, and handling may explain such results leading to the conclusion that nonaggregate-forming formulations should be carefully pursued prior to clinical trials.
A phase II trial was undertaken to determine the safety, toxicity, and potential efficacy of the B-cell restricted anti-CD19 immunotoxin anti-B4-bR (Anti-B4-bR) administered as adjuvant therapy to patients in complete remission after autologous BMT for B-cell NHL (Grossbard et al. 1999). The 4-year disease-free survival and overall survival were estimated at 56 and 72%, respectively. This study demonstrated that anti-b4-bR can be administered safely to patients as adjuvant therapy early after autologous BMT for B-cell NHL. The toxicities were tolerable and reversible.
The anti-T-NHL immunotoxin to CD7 (DA7) consisting of deglycosylated ricin A chain coupled to a mouse monoclonal antihuman CD7 antibody (Frankel et al. 1997) was also studied in 11 patients with T-cell lymphoma (>30% CD7+ malignant cells). Two partial responses and one minimal response were seen. Patients with minimal lymphoma burden or T-cell large granular lymphocyte (LGL) leukemia showed the best responses.
7.1.3 Leukemia
An immunotoxin b43(anti-CD19)PAP made by linking the PAP toxin to an anti-CD19 monoclonal (Uckun 1993) was evaluated in patients affected by therapy refractory B-lineage ALL. Four complete remissions and one partial response were observed, as well as a rapid reduction in the numbers of leukemic cells in circulation in five additional patients. Interestingly, no significant organ toxicity was noticed and the patients did not develop an immune response to either the PAP toxin or the murine monoclonal antibody.
In two studies where the CD5 antigen was taken as the target, patients affected by B-CLL refractory to alkylating agents were treated with the immunotoxin T101-RTA (Hertler et al. 1988, 1989). All patients had a rapid fall in the white blood cell count of less than 2–4 h duration after each immunotoxin infusion, most likely secondary to the antibody portion of the immunotoxin. No sustained benefit could be demonstrated in any patient, possibly because in the absence of enhancing agents, the leukemic cells of all four treated patients were resistant to T101-RTA at concentrations up to 2 μg/ml in vitro.
7.1.4 Multiple Myeloma
B-cell restricted immunotoxin anti-B4-bR was administered to five patients with previously treated multiple myeloma (Grossbard et al. 1998). No patient demonstrated a significant decline in the disease during therapy.
7.1.5 Cutaneous Lymphoma
The CD5 antigen is heterogeneously expressed on cutaneous T-cell lymphoma tumor cells, but is not expressed on normal cells except lymphocytes. A phase I trial was therefore conducted in which 14 patients with cutaneous T-cell lymphoma progressive on other therapies were treated with H65-RTA (an anti-CD5 monoclonal coupled to RTA) (LeMaistre et al. 1991). Partial responses lasting from 3 to 8 months were documented in four patients. Interestingly, the immunotoxin could be repeatedly administered safely, even in the presence of anti-immunotoxin antibodies, with significant response.
7.2 Cerebrospinal Fluid Spread of Tumors
Leptomeningeal neoplasia occurs in 5–20% of all cancer patients and results in a very poor prognosis, with a median survival of only a few months. Studies indicate that 35–40% of all breast cancer patients experience CNS involvement at some point in their disease and that 5% experience leptomeningeal involvement. In addition, primary CNS tumors and leukemias can spread diffusely to the leptomeninges, thwarting efforts at treatment. Therefore, a pilot study of intraventricular therapy with the immunotoxin 454A12-rRA in eight patients with leptomeningeal spread of systemic neoplasia (six breast carcinomas, one melanoma, and one leukemia) was conducted (Laske et al. 1997). The immunotoxin 454A12-rRA is a conjugate of a monoclonal antibody against the human transferrin receptor and recombinant RTA. In four of the eight patients, a greater than 50% reduction of tumor cell counts in the lumbar cerebrospinal fluid occurred within 5–7 days after the intraventricular dose of 454A12-rRA; however, no patient had the CSF cleared of tumor, and evidence of tumor progression was demonstrated in seven of the eight patients after treatment.
7.3 Solid Tumors
7.3.1 Small-Cell Lung Cancer (SCLC)
In patients affected by SCLC, relapse and resistance to established chemotherapy regimens often cause death. N901-blocked ricin (N901-bR), a murine monoclonal antibody-bR immunotoxin, was studied as a potential therapeutic for SCLC (Epstein et al. 1994) in 21 patients. N901-bR targets CD56, present on SCLC and cells of neuro-ectodermal origin. In this study, one patient had a documented partial response and six patients demonstrated stable disease. A further phase II trial was designed to evaluate the efficacy and toxicity of the immunotoxin N901-bR in patients with SCLC who achieved a complete or near-complete response following chemotherapy and/or radiation (Fidias et al. 2002). Nine patients enrolled in the study before it closed following a treatment-related death. Seven patients had extensive-stage disease and entered the study with a more than 90% reduction of their original tumor. Two patients with limited-stage SCLC had no evidence of disease at study entry. Toxicity and a massive immune response to the administered immunotoxin reduced the effects of the treatment in one patient with limited stage disease.
7.3.2 Bladder Cancer
Bladder tumors are relatively more accessible to passive administration of therapeutic macromolecules. Indeed, Yu et al. (1998) describe an ingenious application of an antibladder tumor monoclonal (BD1-I) chemically cross-linked to momordin for the therapy of bladder carcinomas. The immunotoxin was introduced via a catheter into the bladder and 18 patients were treated. The authors concluded that intravesical administration is very safe and effective.
7.3.3 Breast Tumors
Four women with metastatic breast cancer were treated by Weiner et al. (1989) with the immunotoxin 269F9-rRTA. The trial however was quickly suspended because patients treated with a continuous infusion schedule developed significant neurological toxicities. Also in a study by Gould et al. (1989) five patients treated with antibreast cancer immunotoxin with continuous infusion experienced severe toxic effects, including marked fluid overload and debilitating sensorimotor neuropathies. In this case, immunohistochemistry suggested that the 260F9 monoclonal targeting of the Schwann cells may have induced demyelination and subsequent neuropathy.
7.3.4 Colon Carcinoma
In other clinical trials, the application of anticolon carcinoma immunotoxins (Byers et al. 1989; LoRusso et al. 1995) was studied. The mAb 791T/36, recognizing a Mr 72,000 antigen on the surface of colon carcinoma cells was conjugated to RTA. Seventeen patients with metastatic colorectal cancer were treated in a phase I trial. Biological activity, manifest as mixed tumor regression, was seen in five patients (Byers et al. 1989). The immunoconjugate XMMCO-791/RTA (RTA linked to a murine mAb 791T which binds a glycoprotein of 72 kDa, expressed on human colorectal carcinoma, ovarian carcinoma, and osteogenic sarcoma) was used by LoRusso et al. (1995) in a phase I trial. Twelve patients with metastatic colorectal carcinoma were treated. No antitumor activity was seen.
7.3.5 Melanoma
Spitler et al. (1987) conducted a trial of a murine monoclonal antimelanoma antibody–RTA immunotoxin (XOMAZYME-MEL) in 22 patients with metastatic malignant melanoma. Encouraging clinical results were observed (one complete response and nine mixed response/stabilization of the disease), even after a single course of a low dose of immunotoxin.
In a study by Gonzalez et al. (1991), 20 patients with metastatic melanoma were treated with XOMAZYME-MEL. There was one durable complete response of 12+ month duration and one brief mixed response lasting 3 months. In a subsequent study by Selvaggi et al. (1993) of four patients treated with XOMAZYME-MEL, one experienced partial lymphnode remission for 5 months and a second patient had stable mediastinal disease for 20 months.
8 Autoimmune Diseases
The targeting of T cells can be envisaged in attempts to treat autoimmune diseases because autoreactive T cells are thought to be involved in their pathogenesis. In the following paragraphs, results obtained in rheumatoid arthritis (RA) and in systemic lupus erythematosus (SLE) are reported.
8.1 RA
The safety and activity of an immunoconjugate of RTA and anti-CD5 monoclonal antibody (anti-CD5 IC), with and without concomitant methotrexate and/or azathioprine, was evaluated for the treatment of RA in 79 patients with active disease (Strand et al. 1993). Response rates were 50–68% at 1 month and 22–25% at 6 months. Transient depletion of CD3/CD5 T cells was observed on days 2 and 5 of treatment, with reconstitution on day 15 or day 29. This initially suggested activity of anti-CD5 IC in active RA but when the efficacy of the treatment was evaluated in a total of 104 evaluable patients in a multicenter, double-blind, multiple-dose, placebo-controlled study, CD5-IC failed to produce marked or prolonged T cell depletion and was not more effective than placebo in ameliorating disease manifestations (Olsen et al. 1996).
8.2 SLE
CD5 Plus (an anti-CD5 monoclonal linked to RTA) was used in patients with SLE to determine the safety and clinical and biological effects (Stafford et al. 1994). Six patients (four with glomerulonephritis and two with thrombocytopenia) were studied. Improvement was documented in two patients with nephritis; no effect on thrombocytopenia was observed. The conclusion was that anti-CD5 ricin A chain immunoconjugate is well tolerated in patients with SLE, causes modest T cell depletion which may persist for months, and may have some clinical efficacy in lupus nephritis.
9 Other Applications
9.1 Corneal Opacification
The safety and effectiveness of an immunotoxin (MDX-RA) designed to inhibit posterior capsule opacification (PCO) was evaluated in 63 eyes of 63 patients having extracapsular cataract extraction by phacoemulsification (Clark et al. 1998); these patients were enrolled in a Phase I/II clinical investigation. The immunotoxin consists of a murine monoclonal antibody (4197X) that binds specifically to human lens epithelial cells conjugated to RTA. It was found that the immunotoxin was well tolerated and was effective in reducing PCO for up to 24 months after cataract surgery.
10 Problems and Opportunities in the Future Development of Immunotoxins
10.1 Selection of Patients
It is generally believed that immunotoxins could be most useful in the elimination of small aggregates of target cells, as is the case in the “minimal residual disease.” So far, however, immunotoxins have been administered only to patients at an advanced stage of disease, that is, with large tumor masses and with limited possibilities of benefiting from a biological therapy. Considering the low penetration of immunotoxins into solid tumors, even minor responses in these patients are impressive. If, however, immunotoxins would be used in an adjuvant setting, after surgical debulking or following established conventional type of regimens (e.g., radiotherapy and chemotherapy) small clumps of cells or even isolated tumor cells remaining might be completely removed by the administration of immunotoxins.
Furthermore, various applications can be envisaged which could be more practicable than the use of systemically administered immunotoxins. Encouraging results have been obtained in the local administration of antibrain tumor immunotoxins (Laske et al. 1997). Further, immunotoxins can be administered intravesically for the therapy of bladder cancer. In this way, immunotoxins may not enter into contact with the systemic circulation (or only negligibly) reducing the risk of an immune response and minimizing the risk of toxicity and side effects, thus allowing the use of higher dosages against cancer cells. RIP-containing immunotoxins (Thiesen et al. 1987; Battelli et al. 1996) and a fibroblast growth factor–saporin conjugate (Tetzke et al. 1997) specific for bladder tumors have been assayed, and clinical trials with immunotoxins have been conducted with promising results (Yu et al. 1998; Zang et al. 2000).
10.2 Immunogenicity
Taking into account the results obtained in a wide range of clinical trials, the incidence of immunogenicity after a single course of immunotoxin treatment ranges from 50 to 100% for solid tumors, and from 0 to 40% for hematologic malignancies. Antibodies that neutralize the cytotoxic effects can be detected by determining whether serum containing them can block the cytotoxicity of the immunotoxin against cultured cells. The presence of neutralizing antibodies in the patients’ serum lowers the levels of biologically active immunotoxin and compromises efficacy. The method most useful for other biologic agents, such as interferon (Reddy 2004) and l-asparaginase (Graham 2003), is PEGylation, which not only reduces immunogenicity but also prolongs half-life. This approach has been evaluated also for ricin, demonstrating a lower binding of antiricin antibodies after PEGylation without altering its enzymatic properties (Hu et al. 2002), and in the case of isolated RTA, the method may even increase its therapeutic potential (Youn et al. 2005). PEGylating a toxin, however, appears more challenging than PEGylating simpler molecules, because the disturbance of sites on an enzymatic toxin may reduce activity. Moreover, PEGylation does not completely prevent an immune response.
Immunologic studies have identified a large number of T-cell and B-cell epitopes on RTA, suggesting that “humanization” of the molecule or elimination of immunodominant epitopes would be extremely difficult. Indeed, use of human T-cell lines and T-cell clones allowed the identification of residue I175, which is part of the active site of the molecule, as a crucial residue for the epitope(s) recognized by two HLA-DRB1 alleles. Failure of T-cell clones to recognize RIPs showing sequences similar but not identical to that of RTA further confirmed the role of I175 as a key residue in immune recognition of RTA (Tommasi et al. 2001) but also encourages sequential use of immunotoxins with different RIPs in treatment courses. Use of a peptide scan approach and the sera of patients treated with antibody–RTA conjugates allowed the identification of a continuous B-cell epitope recognized by all patients studied, located within the stretch L161-I175 of the RTA primary sequence, close to a the T-cell epitope (Castelletti et al. 2004). The ability of anti-L161-I175 antibodies to recognize folded RTA and to affect its biological activity by inhibiting RTA-immunotoxin cytotoxicity in vitro revealed that they may exert an important role in immunotoxin neutralization in vivo. No similar studies were conducted for other RIPs, however.
Recently, bouganin, a type I ribosome inactivating protein isolated from the leaf of Bougainvillea spectabilis, was mutated to remove the T-cell epitopes while preserving the biological activity of the wild-type molecule (Cizeau et al. 2009). A genetic fusion with an anti-EpCAM Fab moiety was then produced and in vivo efficacy demonstrated using a human tumor xenograft model in SCID mice. However, effects of T-epitopes removal on immune recognition in vivo was not evaluated.
10.3 Side Effects
A variety of unwanted toxicities have been observed with immunotoxins that have limited the dose and hence the efficacy. The most common toxicity is vascular leak syndrome (VLS), which is characterized by symptoms related to extravasation of fluid into the interstitial space, such as hypoalbuminemia, edema, weight gain, hypotension, tachycardia, and weakness. These side effects are not surprising, given that a cytotoxic protein must interact with endothelial cells to exit the blood vessels. Studies have shown that RTA binds the fibronectin receptor on the endothelial cells, acting as a “disintegrin” and increasing intercellular permeability (Soler-Rodriguez et al. 1993). Other studies have suggested that specific residues on RTA and IL-2 can bind to endothelial cells through a (x)D(y) motif and elicit VLS by a mechanism independent of the normal toxin-induced cell death (Baluna et al. 1999). Such studies led to a mutant form of RTA showing less VLS in animals (Smallshaw et al. 2003).
Hepatotoxicity, a side effect frequently observed also with recombinant immunotoxins, is attributed to the binding of basic residues on the antibody to negatively charged hepatic cells (Schnell et al. 1998). Hepatotoxicity appears to be related to cytokine release, possibly by the Kupffer cells of the liver (Schnell et al. 2003).
Renal toxicity due to immunotoxins is less well defined and could be nonspecific at least in part because the kidneys are among the main routes of excretion of immunotoxins.
Toxicities due to unpredicted cross-reactions of the immunotoxin with crucial tissues were observed in the first trials with immunotoxins and were reported above. More accurate studies of specificity prevented these side effects in subsequent studies.
11 Conclusions
Several years of study in the basic science domain as well as in the clinics have confirmed that RIPs represent an invaluable tool for the improvement of human health and the setting up of new treatment regimens in many diseases.
A few general conclusions can be drawn for RIPs and their applications in vivo at the present time: (1) RIP-based immunotoxins have a wide range of potential applications in many human diseases; (2) clinical trials have established the minimum tolerated dosages and the pharmacology of many types of synthetic and recombinant immunotoxins, suggesting that continuous infusions might be preferable over the bolus administration. This, however, may increase the risk of side effects; (3) for many types of diseases, immunotoxins are not likely to work by themselves but may be a useful, sometime crucial, adjuvant of more established forms of treatment. Selection of patients that may best benefit from a combined treatment with immunotoxins may yield greater efficacy. Particularly in cancer, poorly vascularized, bulky, solid tumors may not be suitable for immunotoxin-based therapies. Circulating cells and well-vascularized lymphomas appear to be better targets; (4) in most of the trials (except those conducted in immunosuppressed lymphoma patients) anti-immunotoxin antibodies were generated. In some cases these antibodies were neutralizing, but in all cases they decreased the t 1/2 of the immunotoxin in the blood, thus reducing its therapeutic potential. Nevertheless, there were meaningful responses even in the presence of such antibodies. So far, attempts to decrease the antibody responses by the use of immunosuppressive drugs in humans have been unsuccessful, in spite of the results obtained in animal models. The many RIPs that have been described so far, the use of human or humanized targeting molecules, and the development of molecular biology techniques may allow to by-pass the immune response against the toxin moiety by swapping the toxin and selecting the one most appropriate for the applications under investigation; (5) in spite of the limitations described in the present review, the number of patients achieving partial or complete remission from immunotoxin treatment of cancer has reached impressive ratios. This can be considered as a substantial achievement, particularly considering that trials were mostly conducted in terminal patients failing all other established therapies and that many reported trials were Phase I trials, aimed at evaluating essentially safety of in vivo administration. If compared with the performance of more conventional anticancer therapies, it must be noticed that more than 90% of chemotherapeutic drugs used today produced less than 5% partial or complete remissions in Phase I clinical trials. Good progress has been made in the therapeutic use of RIP-based immunotoxins and the advent of recombinant immunotoxins will certainly represent a further advancement.
References
Aceto S, Maro D, Conforto B, Siniscalco GG, Parente A, Delli Bovi P, Gaudio L (2005) Nicking activity on pBR322 DNA of ribosome inactivating proteins from Phytolacca dioica L. leaves. Biol Chem 386:307–317
Akiyama S, Gottesman MM, Hanover JA, Fitzgerald DJ, Willingham MC, Pastan I (1984) Verapamil enhances the toxicity of conjugates of epidermal growth factor with Pseudomonas exotoxin and antitransferrin receptor with Pseudomonas exotoxin. J Cell Physiol 120:271–279
Alvarnas JC, Forman SJ (2004) Graft purging in autologous bone marrow transplantation: a promise not quite fulfilled. Oncology (Williston Park) 18:867–876
Amlot PL, Stone MJ, Cunningham D, Fay J, Newman J, Collins R, May R, McCarthy M, Richardson J, Ghetie V, Ramilo O, Thorpe PE, Uhr JW, Vitetta ES (1993) A phase I study of an anti-CD22-deglycosylated ricin A chain immunotoxin in the treatment of B-cell lymphomas resistant to conventional therapy. Blood 82:2624–2633
Anonymous (1976) The Second Laboratory SIEB. Studies on the mechanism of abortion induction by trichosanthin. Sci Sin 19(6):811–830
Baluna R, Rizo J, Gordon BE, Ghetie V, Vitetta ES (1999) Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome. Proc Natl Acad Sci USA 96:3957–3962
Barbieri L, Gorini P, Valbonesi P, Castiglioni P, Stirpe F (1994) Unexpected activity of saporins. Nature 372:624
Barbieri L, Valbonesi P, Bonora E, Gorini P, Bolognesi A, Stirpe F (1997) Polynucleotide:adenosine glycosidase activity of ribosome-inactivating proteins: effect on DNA, RNA and poly(A). Nucleic Acids Res 25:518–522
Barbieri L, Valbonesi P, Bondioli M, Alvarez ML, Dal MP, Landini MP, Stirpe F (2001) Adenine glycosylase activity in mammalian tissues: an equivalent of ribosome-inactivating proteins. FEBS Lett 505:196–197
Barbieri L, Brigotti M, Perocco P, Carnicelli D, Ciani M, Mercatali L, Stirpe F (2003) Ribosome-inactivating proteins depurinate poly(ADP-ribosyl)ated poly(ADP-ribose) polymerase and have transforming activity for 3T3 fibroblasts. FEBS Lett 538:178–182
Barbieri L, Polito L, Bolognesi A, Ciani, M, Pelosi E, Farini V, Jha A, Sharma N, Vivanc, JM, Chambery A, Parente A, Stirpe F (2006) Ribosome-inactivating proteins in edible plants and purification and characterization of a new ribosome-inactivating protein from Cucurbita moschata. Biochim Biophys Acta 1760:783–792
Battelli MG (2004) Cytotoxicity and toxicity to animals and humans of ribosome-inactivating proteins. Mini Rev Med Chem 4:513–521
Battelli MG, Montacuti V, Stirpe F (1992) High sensitivity of cultured human trophoblasts to ribosome-inactivating proteins. Exp Cell Res 201:109–112
Battelli MG, Polito L, Bolognesi A, Lafleur L, Fradet Y, Stirpe F (1996) Toxicity of ribosome-inactivating proteins-containing immunotoxins to a human bladder carcinoma cell line. Int J Cancer 65:485–490
Battelli MG, Stirpe F (1995) Ribosome-inactivating proteins from plants. In: Chessin M, DeBorde D, Zipf A (eds) Antiviral Proteins in Higher Plants (eds.) CRC Press, Boca Raton, pp. 39–64
Battelli MG, Citores L, Buonamici L, Ferreras JM, de Benito FM, Stirpe F, Girbes T (1997) Toxicity and cytotoxicity of nigrin b, a two-chain ribosome-inactivating protein from Sambucus nigra: comparison with ricin. Arch Toxicol 71:360–364
Battelli MG, Bolognesi A, Olivieri F, Polito L, Stirpe F (1998) Different sensitivity of CD30+ cell lines to Ber-H2/saporin-S6 immunotoxin. J Drug Target 5:181–191
Battelli MG, Musiani S, Buonamici L, Santi S, Riccio M, Maraldi NM, Girbés T, Stirpe F (2004) Interaction of volkensin with HeLa cells: binding, uptake, intracellular localization, degradation and exocytosis. Cell Mol Life Sci 61:1975–1984
Benatti L, Saccardo MB, Dani M, Nitti G, Sassano M, Lorenzetti R, Lappi DA, Soria M (1989) Nucleotide sequence of cDNA coding for saporin-6, a type-1 ribosome-inactivating protein from Saponaria officinalis. Eur J Biochem 183:465–470
Bergamaschi G, Perfetti V, Tonon L, Novella A, Lucotti C, Glennie DM, MJ MG, Cazzola M (1996) Saporin, a ribosome-inactivating protein used to prepare immunotoxins, induces cell death via apoptosis. Br J Haematol 93:789–794
Bertholdo-Vargas LR, Martins JN, Bordin D, Salvador M, Schafer AE, Barros NM, Barbieri L, Stirpe F, Carlini CR (2009) Type 1 ribosome-inactivating proteins - entomotoxic, oxidative and genotoxic action on Anticarsia gemmatalis (Hubner) and Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). J Insect Physiol 55:51–58
Blakey DC, Watson GJ, Knowles PP, Thorpe PE (1987) Effect of chemical deglycosylation of ricin A chain on the in vivo fate and cytotoxic activity of an immunotoxin composed of ricin A chain and anti-Thy 1.1 antibody. Cancer Res 47:947–952
Blazar BR, Carroll SF, Vallera DA (1991) Prevention of murine graft-versus-host disease and bone marrow alloengraftment across the major histocompatibility barrier after donor graft preincubation with anti-LFA1 immunotoxin. Blood 78(11):3093–3102
Blythman HE, Casellas P, Gros O, Gros P, Jansen FK, Paolucci F, Pau B, Vidal H (1981) Immunotoxins: hybrid molecules of monoclonal antibodies and a toxin subunit specifically kill tumour cells. Nature 290:145–146
Bourrie BJ, Casellas P, Blythman HE, Jansen FK (1986) Study of the plasma clearance of antibody-ricin-A-chain immunotoxins. Evidence for specific recognition sites on the A chain that mediate rapid clearance of the immunotoxin. Eur J Biochem 155:1–10
Bregni M, Lappi DA, Siena S, Formosa A, Villa S, Soria M, Bonadonna G, Gianni AM (1988) Activity of a monoclonal antibody–saporin-6 conjugate against B-lymphoma cells. J Natl Cancer Inst 80:511–517
Brigotti M, Alfieri R, Sestili P, Bonelli M, Petronini PG, Guidarelli A, Barbieri L, Stirpe F, Sperti S (2002) Damage to nuclear DNA induced by Shiga toxin 1 and ricin in human endothelial cells. FASEB J 16(3):365–372
Büssing A (1996) Induction of apoptosis by the mistletoe lectins: a review on the mechanisms of cytotoxicity mediated by Viscum album L. Apoptosis 1:25–32
Byers VS, Rodvien R, Grant K, Durrant LG, Hudson KH, Baldwin RW, Scannon PJ (1989) Phase I study of monoclonal antibody-ricin A chain immunotoxin XomaZyme-791 in patients with metastatic colon cancer. Cancer Res 49:6153–6160
Byers VS, Levin AS, Malvino A, Waites L, Robins RA, Baldwin RW (1994) A phase II study of effect of addition of trichosanthin to zidovudine in patients with HIV disease and failing antiretroviral agents. AIDS Res Hum Retroviruses 10:413–420
Candiani C, Franceschi A, Chignola R, Pasti M, Anselmi C, Benoni G, Tridente G, Colombatti M (1992) Blocking effect of human serum but not of cerebrospinal fluid on ricin A chain immunotoxin potentiation by monensin or carrier protein–monensin conjugates. Cancer Res 52:623–630
Carlsson J, Drevin H, Axen R (1978) Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J 173:723–737
Carnicelli D, Brigotti M, Montanaro L, Sperti S (1992) Differential requirement of ATP and extra-ribosomal proteins for ribosome inactivation by eight RNA N-glycosidases. Biochem Biophys Res Commun 182:579–582
Carrière D, Casellas P, Richer G, Gros P, Jansen FK (1985) Endocytosis of an antibody ricin A-chain conjugate (immuno-A-toxin) adsorbed on colloidal gold. Effects of ammonium chloride and monensin. Exp Cell Res 156:327–340
Casellas P, Brown JP, Gros O, Gros P, Hellstrom I, Jansen FK, Poncelet P, Roncucci R, Vidal H, Hellstrom KE (1982) Human melanoma cells can be killed in vitro by an immunotoxin specific for melanoma-associated antigen p97. Int J Cancer 30:437–443
Casellas P, Bourrie BJ, Gros P, Jansen FK (1984) Kinetics of cytotoxicity induced by immunotoxins. Enhancement by lysosomotropic amines and carboxylic ionophores. J Biol Chem 259:9359–9364
Castelletti D, Fracasso G, Righetti S, Tridente G, Schnell R, Engert A, Colombatti M (2004) A dominant linear B-cell epitope of ricin A-chain is the target of a neutralizing antibody response in Hodgkin’s lymphoma patients treated with an anti-CD25 immunotoxin. Clin Exp Immunol 136:365–372
Cattel L, Delprino L, Brusa P, Dosio F, Comoglio PM, Prat M (1988) Comparison of blocked and non-blocked ricin-antibody immunotoxins against human gastric carcinoma and colorectal adenocarcinoma cell lines. Cancer Immunol Immunother 27:233–240
Chignola R, Anselmi C, Dalla SM, Franceschi A, Fracasso G, Pasti M, Chiesa E, Lord JM, Tridente G, Colombatti M (1995) Self-potentiation of ligand–toxin conjugates containing ricin A chain fused with viral structures. J Biol Chem 270:23345–23351
Cizeau J, Grenkow DM, Brown JG, Entwistle J, MacDonald GC (2009) Engineering and biological characterization of VB6-845, an anti-EpCAM immunotoxin containing a T-cell epitope-depleted variant of the plant toxin bouganin. J Immunother 32:574–584
Clark DS, Emery JM, Munsell MF (1998) Inhibition of posterior capsule opacification with an immunotoxin specific for lens epithelial cells: 24 month clinical results. J Cataract Refract Surg 24:1614–1620
Colombatti M (2002) Ricin A: structure, function and its clinical applications. Chimeric toxins: mechanisms of action and therapeutic applications. Harwood Academic Publishers GmbH, Switzerland, pp 37–85
Colombatti M, Dell'Arciprete L, Chignola R, Tridente G (1990) Carrier protein–monensin conjugates: enhancement of immunotoxin cytotoxicity and potential in tumor treatment. Cancer Res 50:1385–1391
Cook JP, Savage PM, Lord JM, Roberts LM (1993) Biologically active interleukin 2-ricin A chain fusion proteins may require intracellular proteolytic cleavage to exhibit a cytotoxic effect. Bioconjug Chem 4:440–447
Dore JM, Gras E, Wijdenes J (1997) Expression and activity of a recombinant chimeric protein composed of pokeweed antiviral protein and of human interleukin-2. FEBS Lett 402:50–52
Endo Y (1988) Mechanism of action of ricin and related toxins on the inactivation of eukaryotic ribosomes. Cancer Treat Res 37:75–89
Engert A, Gottstein C, Bohlen H, Winkler U, Schön G, Manske O, Schnell R, Diehl V, Thorpe P (1995) Cocktails of ricin A-chain immunotoxins against different antigens on Hodgkin and Sternberg-Reed cells have superior anti-tumor effects against H-RS cells in vitro and solid Hodgkin tumors in mice. Int J Cancer 63(2):304–309
Epstein C, Lynch T, Shefner J, Wen P, Maxted D, Braman V, Ariniello P, Coral F, Ritz J (1994) Use of the immunotoxin N901-blocked ricin in patients with small-cell lung cancer. Int J Cancer Suppl 8:57–59
Fabbrini MS, Carpani D, Bello-Rivero I, Soria MR (1997) The amino-terminal fragment of human urokinase directs a recombinant chimeric toxin to target cells: internalization is toxin mediated. FASEB J 11:1169–1176
Falini B, Bolognesi A, Flenghi L, Tazzari PL, Broe MK, Stein H, Dürkop H, Aversa F, Corneli P, Pizzolo G, Barbabietola G, Sabattini E, Pileri S, Martelli MF, Stirpe F (1992) Response of refractory Hodgkin’s disease to monoclonal anti-CD30 immunotoxin. Lancet 339:1195–1196
Ferreras JM, Barbieri L, Girbés T, Battelli MG, Rojo MA, Arias FJ, Rocher MA, Soriano F, Mendéz E, Stirpe F (1993) Distribution and properties of major ribosome-inactivating proteins (28S rRNA N-glycosidases) of the plant Saponaria officinalis L. (Caryophyllaceae). Biochim Biophys Acta 1216:31–42
Fidias P, Grossbard M, Lynch TJ Jr (2002) A phase II study of the immunotoxin N901-blocked ricin in small-cell lung cancer. Clin Lung Cancer 3:219–222
Fitzgerald DJ, Trowbridge IS, Pastan I, Willingham MC (1983) Enhancement of toxicity of antitransferrin receptor antibody–Pseudomonas exotoxin conjugates by adenovirus. Proc Natl Acad Sci USA 80:4134–4138
Flavell DJ, Boehm DA, Noss A, Warnes SL, Flavell SU (2001) Therapy of human T-cell acute lymphoblastic leukaemia with a combination of anti-CD7 and anti-CD38-SAPORIN immunotoxins is significantly better than therapy with each individual immunotoxin. Br J Cancer 84:571–578
Francisco JA, Gawlak SL, Siegall CB (1997) Construction, expression, and characterization of BD1-G28-5 sFv, a single-chain anti-CD40 immunotoxin containing the ribosome-inactivating protein bryodin 1. J Biol Chem 272:24165–24169
Frankel AE, FitzGerald D, Siegall C, Press OW (1996) Advances in immunotoxin biology and therapy: a summary of the fourth international symposium on immunotoxins. Cancer Res 56:926–932
Frankel AE, Laver JH, Willingham MC, Burns LJ, Kersey JH, Vallera DA (1997) Therapy of patients with T-cell lymphomas and leukemias using an anti-CD7 monoclonal antibody-ricin A chain immunotoxin. Leuk Lymphoma 26(3-4):287–298
Frankel AE, Fu T, Burbage C, Chandler J, Willingham MC, Tagge EP (1997) IL2 fused to lectin-deficient ricin is toxic to human leukemia cells expressing the IL2 receptor. Leukemia 11:22–30
Fuchs H, Bachran D, Panjideh H, Schellmann N, Weng A, Melzig MF, Sutherland M, Bachran C (2009) Saponins as tool for improved targeted tumor therapies. Curr Drug Targets 10:140–151
Fulton RJ, Uhr JW, Vitetta ES (1988) In vivo therapy of the BCL1 tumor: effect of immunotoxin valency and deglycosylation of the ricin A chain. Cancer Res 48:2626–2631
Gawlak SL, Neubauer M, Klei HE, Chang CY, Einspahr HM, Siegall CB (1997) Molecular, biological, and preliminary structural analysis of recombinant bryodin 1, a ribosome-inactivating protein from the plant Bryonia dioica. Biochemistry 36:3095–3103
Geden SE, Gardner RA, Fabbrini MS, Ohashi M, Phanstiel IO, Teter K (2007) Lipopolyamine treatment increases the efficacy of intoxication with saporin and an anticancer saporin conjugate. FEBS J 274:4825–4836
Ghetie V, Vitetta E (1994) Immunotoxins in the therapy of cancer: from bench to clinic. Pharmacol Ther 63:209–234
Ghetie MA, Tucker K, Richardson J, Uhr JW, Vitetta ES (1992) The antitumor activity of an anti-CD22 immunotoxin in SCID mice with disseminated Daudi lymphoma is enhanced by either an anti-CD19 antibody or an anti-CD19 immunotoxin. Blood 80:2315–2320
Ghetie MA, Tucker K, Richardson J, Uhr JW, Vitetta ES (1994) Eradication of minimal disease in severe combined immunodeficient mice with disseminated Daudi lymphoma using chemotherapy and an immunotoxin cocktail. Blood 84(3):702–707
Girbés T, Citores L, Ferreras JM, Rojo MA, Iglesias R, Muñoz R, Arias FJ, Calonge M, Garcia JR, Méndez E (1993) Isolation and partial characterization of nigrin b, a non-toxic novel type 2 ribosome-inactivating protein from the bark of Sambucus nigra L. Plant Mol Biol 22:1181–1186
Girbés T, Ferreras JM, Iglesias R, Citores L, De TC, Carbajales ML, Jiménez P, de Benito FM, Muñoz R (1996) Recent advances in the uses and applications of ribosome-inactivating proteins from plants. Cell Mol Biol (Noisy-le-grand) 42:461–471
Girbés T, Ferreras JM, Arias FJ, Muñoz R, Iglesias R, Jiménez P, Rojo MA, Arias Y, Perez Y, Benitez J, Sanchez D, Gayoso MJ (2003) Non-toxic type 2 ribosome-inactivating proteins (RIPs) from Sambucus: occurrence, cellular and molecular activities and potential uses. Cell Mol Biol (Noisy-le-grand) 49:537–545
Girbés T, Ferreras JM, Arias FJ, Stirpe F (2004) Description, distribution, activity and phylogenetic relationship of ribosome-inactivating proteins in plants, fungi and bacteria. Mini Rev Med Chem 4:461–476
Goldmacher VS, Blattler WA, Lambert JM, McIntyre G, Stewart J (1989) Cytotoxicity of gelonin conjugated to targeting molecules: effects of weak amines, monensin, adenovirus, and adenoviral capsid proteins penton, hexon, and fiber. Mol Pharmacol 36:818–822
Gonzalez R, Salem P, Bunn PA Jr, Zukiwski AA, Lamb R, Benjamin RS, Spitler L, Wedel N, Robinson WA (1991) Single-dose murine monoclonal antibody ricin A chain immunotoxin in the treatment of metastatic melanoma: a phase I trial. Mol Biother 3:192–196
Gould BJ, Borowitz MJ, Groves ES, Carter PW, Anthony D, Weiner LM, Frankel AE (1989) Phase I study of an anti-breast cancer immunotoxin by continuous infusion: report of a targeted toxic effect not predicted by animal studies. J Natl Cancer Inst 81:775–781
Graham ML (2003) Pegaspargase: a review of clinical studies. Adv Drug Deliv Rev 55:1293–1302
Griffin TW, Childs LR, FitzGerald DJ, Levine LV (1987) Enhancement of the cytotoxic effect of anti-carcinoembrionic antigen immunotoxins by adenovirus and carboxylic ionophores. J Natl Cancer Inst 79:679–685
Griffin T, Rybak ME, Recht L, Singh M, Salimi A, Raso V (1993) Potentiation of antitumor immunotoxins by liposomal monensin. J Natl Cancer Inst 85:292–298
Griffiths GD, Leek MD, Gee DJ (1987) The toxic plant proteins ricin and abrin induce apoptotic changes in mammalian lymphoid tissues and intestine. J Pathol 151:221–229
Grossbard ML, Fidias P, Kinsella J, O'Toole J, Lambert JM, Blättler WA, Esseltine D, Braman G, Nadler LM, Anderson KC (1998) Anti-B4-blocked ricin: a phase II trial of 7 day continuous infusion in patients with multiple myeloma. Br J Haematol 102:509–515
Grossbard ML, Multani PS, Freedman AS, O'Day S, Gribben JG, Rhuda C, Neuberg D, Nadler LM (1999) A phase II study of adjuvant therapy with anti-B4-blocked ricin after autologous bone marrow transplantation for patients with relapsed B-cell non-Hodgkin’s lymphoma. Clin Cancer Res 5:2392–2398
Hartley MR, Lord JM (2004) Cytotoxic ribosome-inactivating lectins from plants. Biochim Biophys Acta 1701:1–14
He WJ, Liu WY (2003) Cinnamomum: a multifunctional type II ribosome-inactivating protein. Int J Biochem Cell Biol 35:1021–1027
He YW, Guo CX, Pan YF, Peng C, Weng ZH (2008) Inhibition of hepatitis B virus replication by pokeweed antiviral protein in vitro. World J Gastroenterol 14:1592–1597
Heisler I, Sutherland M, Bachran C, Hebestreit P, Schnitger A, Melzig MF, Fuchs H (2005) Combined application of saponin and chimeric toxins drastically enhances the targeted cytotoxicity on tumor cells. J Control Release 106:123–137
Hertler AA, Schlossman DM, Borowitz MJ, Laurent G, Jansen FK, Schmidt C, Frankel AE (1988) A phase I study of T101-ricin A chain immunotoxin in refractory chronic lymphocytic leukemia. J Biol Response Mod 7:97–113
Hertler AA, Schlossman DM, Borowitz MJ, Blythman HE, Casellas P, Frankel AE (1989) An anti-CD5 immunotoxin for chronic lymphocytic leukemia: enhancement of cytotoxicity with human serum albumin–monensin. Int J Cancer 43:215–219
Hu R, Zhai Q, Liu W, Liu X (2001) An insight into the mechanism of cytotoxicity of ricin to hepatoma cell: roles of Bcl-2 family proteins, caspases, Ca(2+)-dependent proteases and protein kinase C. J Cell Biochem 81:583–593
Hu RG, Zhai QW, He WJ, Mei L, Liu WY (2002) Bioactivities of ricin retained and its immunoreactivity to anti-ricin polyclonal antibodies alleviated through pegylation. Int J Biochem Cell Biol 34:396–402
Hudak KA, Bauman JD, Tumer NE (2002) Pokeweed antiviral protein binds to the cap structure of eukaryotic mRNA and depurinates the mRNA downstream of the cap. RNA 8:1148–1159
Hughes JN, Lindsay CD, Griffiths GD (1996) Morphology of ricin and abrin exposed endothelial cells is consistent with apoptotic cell death. Human Exp Toxicol 15:443–451
Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SL, Magun BE (1997) Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol Cell Biol 17:3373–3381
Irvin JD (1975) Purification and partial characterization of the antiviral protein from Phytolacca americana which inhibits eukaryotic protein synthesis. Arch Biochem Biophys 169:522–528
Jaffrezou JP, Levade T, Kuhlein E, Thurneyssen O, Chiron M, Grandjean H, Carrière D, Laurent G (1990) Enhancement of ricin A chain immunotoxin activity by perhexiline on established and fresh leukemic cells. Cancer Res 50:5558–5566
Jaffrezou JP, Levade T, Thurneyssen O, Chiron M, Bordier C, Attal M, Chatelain P, Laurent G (1992) In vitro and in vivo enhancement of ricin-A chain immunotoxin activity by novel indolizine calcium channel blockers: delayed intracellular degradation linked to lipidosis induction. Cancer Res 52:1352–1359
Jansen FK, Blythman HE, Carrière D, Casellas P, Gros O, Gros P, Laurent JC, Paolucci F, Pau B, Poncelet P, Richer G, Vidal H, Voisin GA (1982) Immunotoxins: hybrid molecules combining high specificity and potent cytotoxicity. Immunol Rev 62:185–216
Jansen FK, Jansen A, Derocq JM, Carrière D, Carayon P, Veas F, Jaffrezou JP (1992) Golgi vacuolization and immunotoxin enhancement by monensin and perhexiline depend on a serum protein. Implications for intracellular trafficking. J Biol Chem 267:12577–12582
Kataoka J, Ago H, Habuka N, Furuno M, Masuta C, Miyano M, Koiwai A (1993) Expression of a pokeweed antiviral protein in Escherichia coli and its characterization. FEBS Lett 320:31–34
Kreitman RJ (1997) Getting plant toxins to fuse. Leuk Res 21:997–999
Krolick KA, Villemez C, Isakson P, Uhr JW, Vitetta ES (1980) Selective killing of normal or neoplastic B cells by antibodies coupled to the A chain of ricin. Proc Natl Acad Sci USA 77:5419–5423
Lambert JM, Senter PD, Yau-Young A, Blättler WA, Goldmacher VS (1985) Purified immunotoxins that are reactive with human lymphoid cells. Monoclonal antibodies conjugated to the ribosome-inactivating proteins gelonin and the pokeweed antiviral proteins. J Biol Chem 260:12035–12041
Lambert JM, McIntyre G, Gauthier MN, Zullo D, Rao V, Steeves RM, Goldmacher VS, Blättler WA (1991) The galactose-binding sites of the cytotoxic lectin ricin can be chemically blocked in high yield with reactive ligands prepared by chemical modification of glycopeptides containing triantennary N-linked oligosaccharides. Biochemistry 30:3234–3247
Lappi DA, Wiley RG (2004) Immunotoxins and neuropeptide–toxin conjugates experimental applications. Mini Rev Med Chem 4:585–595
Laske DW, Muraszko KM, Oldfield EH, DeVroom HL, Sung C, Dedrick RL, Simon TR, Colandrea J, Copeland C, Katz D, Greenfield L, Groves ES, Houston LL, Youle RJ (1997) Intraventricular immunotoxin therapy for leptomeningeal neoplasia. Neurosurgery 41:1039–1049
Legname G, Gromo G, Lord JM, Monzini N, Modena D (1993) Expression and activity of pre-dianthin 30 and dianthin 30. Biochem Biophys Res Commun 192:1230–1237
LeMaistre CF, Rosen S, Frankel A, Kornfeld S, Saria E, Meneghetti C, Drajesk J, Fishwild D, Scannon P, Byers V (1991) Phase I trial of H65-RTA immunoconjugate in patients with cutaneous T-cell lymphoma. Blood 78:1173–1182
Lin JY, Liu K, Chen CC, Tung TC (1971) Effect of crystalline ricin on the biosynthesis of protein, RNA, and DNA in experimental tumor cells. Cancer Res 31:921–924
Lizzi AR, D'Alessandro AM, Zeolla N, Brisdelli F, D'Andrea G, Pitari G, Oratore A, Bozzi A, Ippoliti R (2005) The effect of AZT and chloroquine on the activities of ricin and a saporin-transferrin chimeric toxin. Biochem Pharmacol 70:560–569
Lord JM, Deeks E, Marsden CJ, Moore K, Pateman C, Smith C, Spooner RA, Watson P, Roberts LM (2003) Retrograde transport of toxins across the endoplasmic reticulum membrane. Biochem Soc Trans 31:1260–1262
Lorenzetti I, Meneguzzi A, Fracasso G, Potrich C, Costantini L, Chiesa E, Legname G, Menestrina G, Tridente G, Colombatti M (2000) Genetic grafting of membrane-acting peptides to the cytotoxin dianthin augments its ability to de-stabilize lipid bilayers and enhances its cytotoxic potential as the component of transferrin–toxin conjugates. Int J Cancer 86:582–589
LoRusso PM, Lomen PL, Redman BG, Poplin E, Bander JJ, Valdivieso M (1995) Phase I study of monoclonal antibody-ricin A chain immunoconjugate Xomazyme-791 in patients with metastatic colon cancer. Am J Clin Oncol 18:307–312
Mabley JG, Pacher P, Szabo C (2009) Activation of the cholinergic anti-inflammatory pathway reduces ricin-induced mortality and organ failure in mice. Mol Med 15:166–172
McGrath MS, Hwang KM, Caldwell SE, Gaston I, Luk KC, Wu P, Ng VL, Crowe S, Daniels J, Marsh J (1989) GLQ223: an inhibitor of human immunodeficiency virus replication in acutely and chronically infected cells of lymphocyte and mononuclear phagocyte lineage. Proc Natl Acad Sci USA 86:2844–2848
McIntosh DP, Edwards DC, Cumber AJ, Parnell GD, Dean CJ, Ross WC, Forrester JA (1983) Ricin B chain converts a non-cytotoxic antibody–ricin A chain conjugate into a potent and specific cytotoxic agent. FEBS Lett 164:17–20
Messmann RA, Vitetta ES, Headlee D, Senderowicz AM, Figg WD, Schindler J, Michiel DF, Creekmore S, Steinberg SM, Kohler D, Jaffe ES, Stetler-Stevenson M, Chen H, Ghetie V, Sausville EA (2000) A phase I study of combination therapy with immunotoxins IgG-HD37-deglycosylated ricin A chain (dgA) and IgG-RFB4-dgA (Combotox) in patients with refractory CD19(+), CD22(+) B cell lymphoma. Clin Cancer Res 6:1302–1313
Mollenhauer HH, Morre DJ, Rowe LD (1990) Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim Biophys Acta 1031:225–246
Monti B, D'Alessandro C, Farini V, Bolognesi A, Polazzi E, Contestabile A, Stirpe F, Battelli MG (2007) In vitro and in vivo toxicity of type 2 ribosome-inactivating proteins lanceolin and stenodactylin on glial and neuronal cells. Neurotoxicology 28:637–644
Narayanan S, Surolia A, Karande AA (2004) Ribosome inactivating protein and apoptosis: abrin causes cell death via mitochondrial pathway in Jurkat cells. Biochem J 377:233–240
Ng TB (2004) Antifungal proteins and peptides of leguminous and non-leguminous origins. Peptides 25:1215–1222
Ng TB, Chan WY, Yeung HW (1992) Proteins with abortifacient, ribosome inactivating, immunomodulatory, antitumor and anti-AIDS activities from Cucurbitaceae plants. Gen Pharmacol 23:579–590
Nicolas E, Beggs JM, Haltiwanger BM, Taraschi TF (1998) A new class of DNA glycosylase/apurinic/apyrimidinic lyases that act on specific adenines in single-stranded DNA. J Biol Chem 273:17216–17220
Nolan PA, Garrison DA, Better M (1993) Cloning and expression of a gene encoding gelonin, a ribosome-inactivating protein from Gelonium multiflorum. Gene 134:223–227
Obrig TG (1997) Shiga toxin mode of action in E. coli O157:H7 disease. Front Biosci 2:d635–d642
O'Hare M, Roberts LM, Thorpe PE, Watson GJ, Prior B, Lord JM (1987) Expression of ricin A chain in Escherichia coli. FEBS Lett 216:73–78
Olsen NJ, Brooks RH, Cush JJ, Lipsky PE, St Clair EW, Matteson EL, Gold KN, Cannon GW, Jackson CG, McCune WJ, Fox DA, Nelson B, Lorenz T, Strand V (1996) A double-blind, placebo-controlled study of anti-CD5 immunoconjugate in patients with rheumatoid arthritis. The Xoma RA Investigator Group. Arthritis Rheum 39:1102–1108
Olsnes S, Pihl A (1972) Ricin – a potent inhibitor of protein synthesis. FEBS Lett 20:327–329
Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ (2007) Immunotoxin treatment of cancer. Annu Rev Med 58:221–237
Pelosi E, Lubelli C, Polito L, Barbieri L, Bolognesi A, Stirpe F (2005) Ribosome-inactivating proteins and other lectins from Adenia (Passifloraceae). Toxicon 46:658–663
Peumans WJ, Hao Q, Van Damme EJ (2001) Ribosome-inactivating proteins from plants: more than RNA N-glycosidases? FASEB J 15:1493–1506
Poole B, Ohkuma S (1981) Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol 90:665–669
Ralston HJ III (2005) Pain and the primate thalamus. Prog Brain Res 149:1–10
Ramakrishnan S, Houston LL (1984) Inhibition of human acute lymphoblastic leukemia cells by immunotoxins: potentiation by chloroquine. Science 223:58–61
Ramakrishnan S, Bjorn MJ, Houston LL (1989) Recombinant ricin A chain conjugated to monoclonal antibodies: improved tumor cell inhibition in the presence of lysosomotropic compounds. Cancer Res 49:613–617
Raso V, Lawrence J (1984) Carboxylic ionophores enhance the cytotoxic potency of ligand- and antibody-delivered ricin A chain. J Exp Med 160:1234–1240
Ravel S, Casellas P (1990) Internalization of the cytotoxic molecules of T101 F(ab′)2-(ricin-A-chain) immunotoxin into human T-leukemic cells. Eur J Biochem 192:469–473
Ravel S, Colombatti M, Casellas P (1992) Internalization and intracellular fate of anti-CD5 monoclonal antibody and anti-CD5 ricin A-chain immunotoxin in human leukemic T cells. Blood 79:1511–1517
Reddy KR (2004) Development and pharmacokinetics and pharmacodynamics of pegylated interferon alfa-2a (40 kD). Semin Liver Dis 24(Suppl 2):33–38
Reisbig R, Olsnes S, Eiklid K (1981) The cytotoxic activity of Shigella toxin. Evidence for catalytic inactivation of the 60 S ribosomal subunit. J Biol Chem 256:8739–8744
Sandvig K, van Deurs B (2002) Transport of protein toxins into cells: pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett 529:49–53
Sausville EA, Headlee D, Stetler-Stevenson M, Jaffe ES, Solomon D, Figg WD, Herdt J, Kopp WC, Rager H, Steinberg SM, Ghetie V, Schindler J, Uhr J, Vitetta ES (1995) Continuous infusion of the anti-CD22 immunotoxin IgG-RFB4-SMPT-dgA in patients with B-cell lymphoma: a phase I study. Blood 85:3457–3465
Schnell R, Vitetta E, Schindler J, Barth S, Winkler U, Borchmann P, Hansmann ML, Diehl V, Ghetie V, Engert A (1998) Clinical trials with an anti-CD25 ricin A-chain experimental and immunotoxin (RFT5-SMPT-dgA) in Hodgkin’s lymphoma. Leuk Lymphoma 30:525–537
Schnell R, Vitetta E, Schindler J, Borchmann P, Barth S, Ghetie V, Hell K, Drillich S, Diehl V, Engert A (2000) Treatment of refractory Hodgkin’s lymphoma patients with an anti-CD25 ricin A-chain immunotoxin. Leukemia 14:129–135
Schnell R, Staak O, Borchmann P, Schwartz C, Matthey B, Hansen H, Schindler J, Ghetie V, Vitetta ES, Diehl V, Engert A (2002) Phase I study with an anti-CD30 ricin A-chain immunotoxin (Ki-4.dgA) in patients with refractory CD30+ Hodgkin’s and non-Hodgkin’s lymphoma. Clin Cancer Res 8:1779–1786
Schnell R, Borchmann P, Staak JO, Schindler J, Ghetie V, Vitetta ES, Engert A (2003) Clinical evaluation of ricin A-chain immunotoxins in patients with Hodgkin’s lymphoma. Ann Oncol 14:729–736
Selvaggi K, Saria EA, Schwartz R, Vlock DR, Ackerman S, Wedel N, Kirkwood JM, Jones H, Ernstoff MS (1993) Pase I/II study of murine monoclonal antibody-ricin A chain (XOMAZYME-Mel) immunoconjugate plus cyclosporine A in patients with metastatic melanoma. J Immunother Emphasis Tumor Immunol 13:201–207
Shaw PC, Chan WL, Yeung HW, Ng TB (1994) Minireview: trichosanthin – a protein with multiple pharmacological properties. Life Sci 55:253–262
Siena S, Villa S, Bregni M, Bonnadonna G, Gianni AM (1987) Amantadine potentiates T lymphocyte killing by an anti-pan-T cell (CD5) ricin A-chain immunotoxin. Blood 69:345–348
Siena S, Bregni M, Formosa A, Martineau D, Lappi DA, Bonadonna G, Gianni AM (1988) Evaluation of antihuman T lymphocyte saporin immunotoxins potentially useful in human transplantation. Transplantation 46:747–753
Sikriwal D, Ghosh P, Batra JK (2008) Ribosome inactivating protein saporin induces apoptosis through mitochondrial cascade, independent of translation inhibition. Int J Biochem Cell Biol 40:2880–2888
Singh M, Griffin T, Salimi A, Micetich RG, Atwal H (1994) Potentiation of ricin A immunotoxin by monoclonal antibody targeted monensin containing small unilamellar vesicles. Cancer Lett 84:15–21
Smallshaw JE, Ghetie V, Rizo J, Fulmer JR, Trahan LL, Ghetie MA, Vitetta ES (2003) Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat Biotechnol 21:387–391
Soler-Rodriguez AM, Ghetie MA, Oppenheimer-Marks N, Uhr JW, Vitetta ES (1993) Ricin A-chain and ricin A-chain immunotoxins rapidly damage human endothelial cells: implications for vascular leak syndrome. Exp Cell Res 206:227–234
Spitler LE, del Rio M, Khentigan A, Wedel NI, Brophy NA, Miller LL, Harkonen WS, Rosendorf LL, Lee HM, Mischak RP (1987) Therapy of patients with malignant melanoma using a monoclonal antimelanoma antibody-ricin A chain immunotoxin. Cancer Res 47:1717–1723
Stafford FJ, Fleisher TA, Lee G, Brown M, Strand V, Austin HA III, Balow JE, Klippel JH (1994) A pilot study of anti-CD5 ricin A chain immunoconjugate in systemic lupus erythematosus. J Rheumatol 21:2068–2070
Stirpe F (2004) Ribosome-inactivating proteins. Toxicon 44:371–383
Stirpe F, Battelli MG (2006) Ribosome-inactivating proteins: progress and problems. Cell Mol Life Sci 63:1850–1866
Stirpe F, Bailey S, Miller SP, Bodley JW (1988) Modification of ribosomal RNA by ribosome-inactivating proteins from plants. Nucleic Acids Res 16:1349–1357
Stone MJ, Sausville EA, Fay JW, Headlee D, Collins RH, Figg WD, Stetler-Stevenson M, Jain V, Jaffe ES, Solomon D, Lush RM, Senderowicz A, Ghetie V, Schindler J, Uhr JW, Vitetta ES (1996) A phase I study of bolus versus continuous infusion of the anti-CD19 immunotoxin, IgG-HD37-dgA, in patients with B-cell lymphoma. Blood 88:1188–1197
Strand V, Lipsky PE, Cannon GW, Calabrese LH, Wiesenhutter C, Cohen SB, Olsen NJ, Lee ML, Lorenz TJ, Nelson B (1993) Effects of administration of an anti-CD5 plus immunoconjugate in rheumatoid arthritis. Results of two phase II studies. The CD5 Plus Rheumatoid Arthritis Investigators Group. Arthritis Rheum 36:620–630
Suzuki A, Doi H, Matsuzawa F, Aikawa S, Takiguchi K, Kawano H, Hayashida M, Ohno S (2000) Bcl-2 antiapoptotic protein mediates verotoxin II-induced cell death: possible association between bcl-2 and tissue failure by E. coli O157:H7. Genes Dev 14:1734–1740
Szalai K, Scholl I, Forster-Waldl E, Polito L, Bolognesi A, Untersmayr E, Riemer AB, Boltz-Nitulescu G, Stirpe F, Jensen-Jarolim E (2005) Occupational sensitization to ribosome-inactivating proteins in researchers. Clin Exp Allergy 35:1354–1360
Tetzke TA, Caton MC, Maher PA, Parandoosh Z (1997) Effect of fibroblast growth factor saporin mitotoxins on human bladder cell lines. Clin Exp Metastasis 15:620–629
Thiesen HJ, Juhl H, Arndt R (1987) Selective killing of human bladder cancer cells by combined treatment with A and B chain ricin antibody conjugates. Cancer Res 47:419–423
Thorpe PE, Ross WC, Brown AN, Myers CD, Cumber AJ, Foxwell BM, Forrester JT (1984) Blockade of the galactose-binding sites of ricin by its linkage to antibody. Specific cytotoxic effects of the conjugates. Eur J Biochem 140:63–71
Thorpe PE, Wallace PM, Knowles PP, Relf MG, Brown AN, Watson GJ, Knyba RE, Wawrzynczak EJ, Blakey DC (1987) New coupling agents for the synthesis of immunotoxins containing a hindered disulfide bond with improved stability in vivo. Cancer Res 47:5924–5931
Thorpe PE, Wallace PM, Knowles PP, Relf MG, Brown AN, Watson GJ, Blakey DC, Newell DR (1988) Improved antitumor effects of immunotoxins prepared with deglycosylated ricin A-chain and hindered disulfide linkages. Cancer Res 48:6396–6403
Thorpe SC, Kemeny DM, Panzani R, Lessof MH (1988) Allergy to castor bean. I. Its relationship to sensitization to common inhalant allergens (atopy). J Allergy Clin Immunol 82:62–66
Thorpe SC, Murdoch RD, Kemeny DM (1989) The effect of the castor bean toxin, ricin, on rat IgE and IgG responses. Immunology 68:307–311
Tolstikov VV, Cole R, Fang H, Pincus SH (1997) Influence of endosome-destabilizing peptides on efficacy of anti-HIV immunotoxins. Bioconjug Chem 8:38–43
Tommasi M, Castelletti D, Pasti M, Fracasso G, Lorenzetti I, Sartoris S, Pera C, Ferrara GB, Tridente G, Colombatti M (2001) Identification of ricin A-chain HLA class II-restricted epitopes by human T-cell clones. Clin Exp Immunol 125:391–400
Uckun FM (1993) Immunotoxins for the treatment of leukaemia. Br J Haematol 85:435–438
Uckun FM, Kersey JH, Vallera DA, Ledbetter JA, Weisdorf D, Myers DE, Haake R, Ramsay NK (1990) Autologous bone marrow transplantation in high-risk remission T-lineage acute lymphoblastic leukemia using immunotoxins plus 4-hydroperoxycyclophosphamide for marrow purging. Blood 76:1723–1733
Vago R, Marsden CJ, Lord JM, Ippoliti R, Flavell DJ, Flavell SU, Ceriotti A, Fabbrini MS (2005) Saporin and ricin A chain follow different intracellular routes to enter the cytosol of intoxicated cells. FEBS J 272:4983–4995
Van Damme EJ, Hao Q, Barre A, Vandenbussche F, Desmyter S, Rougè P, Peumans WJ (2001) Ribosome-inactivating proteins: a family of proteins that do more than inactivate ribosomes. Crit Rev Plant Sci 20:395–465
van Horssen PJ, Preijers FW, van Oosterhout YV, de Witte T (1996) Highly potent CD22-recombinant ricin A results in complete cure of disseminated malignant B-cell xenografts in SCID mice but fails to cure solid xenografts in nude mice. Int J Cancer 68:378–383
van Horssen PJ, van Oosterhout YV, Evers S, Backus HH, van Oijen MG, Bongaerts R, de Witte T, Preijers FW (1999) Influence of cytotoxicity enhancers in combination with human serum on the activity of CD22-recombinant ricin A against B cell lines, chronic and acute lymphocytic leukemia cells. Leukemia 13:241–249
Vitetta ES (1986) Synergy between immunotoxins prepared with native ricin A chains and chemically-modified ricin B chains. J Immunol 136:1880–1887
Vitetta ES, Cushley W, Uhr JW (1983) Synergy of ricin A chain-containing immunotoxins and ricin B chain-containing immunotoxins in in vitro killing of neoplastic human B cells. Proc Natl Acad Sci USA 80:6332–6335
Vitetta ES, Fulton RJ, Uhr JW (1984) Cytotoxicity of a cell-reactive immunotoxin containing ricin A chain is potentiated by an anti-immunotoxin containing ricin B chain. J Exp Med 160:341–346
Vitetta ES, Stone M, Amlot P, Fay J, May R, Till M, Newman J, Clark P, Collins R, Cunningham D, Ghetie V, Uhr JW, Thorpe PE (1991) Phase I immunotoxin trial in patients with B-cell lymphoma. Cancer Res 51:4052–4058
Vivanco JM, Savary BJ, Flores HE (1999) Characterization of two novel type I ribosome-inactivating proteins from the storage roots of the Andean crop Mirabilis expansa. Plant Physiol 119:1447–1456
Waterer GW, Robertson H (2009) Bioterrorism for the respiratory physician. Respirology 14:5–11
Weiner LM, O’Dwyer J, Kitson J, Comis RL, Frankel AE, Bauer RJ, Konrad MS, Groves ES (1989) Phase I evaluation of an anti-breast carcinoma monoclonal antibody 260F9-recombinant ricin A chain immunoconjugate. Cancer Res 49(14):4062–4067
Wiley RG, Lappi DA (2003) Targeted toxins in pain. Adv Drug Deliv Rev 55:1043–1054
Wiley RG, Lappi DA (2005) Molecular neurosurgery with targeted toxins. Humana Press Inc, Totowa, NJ
Wiley RG, Stirpe F (1988) Modeccin and volkensin but not abrin are effective suicide transport agents in rat CNS. Brain Res 438:145–154
Wiley RG, Berbos TG, Deckwerth TL, Johnson EM Jr, Lappi DA (1995) Destruction of the cholinergic basal forebrain using immunotoxin to rat NGF receptor: modeling the cholinergic degeneration of Alzheimer’s disease. J Neurol Sci 128:157–166
Wong L, Suh DY, Frankel AE (2005) Toxin conjugate therapy of cancer. Semin Oncol 32:591–595
Wood KA, Lord JM, Wawrzynczak EJ, Piatak M (1991) Preproabrin: genomic cloning, characterisation and the expression of the A-chain in Escherichia coli. Eur J Biochem 198:723–732
Yeung HW, Li WW, Feng Z, Barbieri L, Stirpe F (1988) Trichosanthin, alpha-momorcharin and beta-momorcharin: identity of abortifacient and ribosome-inactivating proteins. Int J Pept Protein Res 31:265–268
Youle RJ, Neville DM Jr (1982) Kinetics of protein synthesis inactivation by ricin-anti Thy 1.1 monoclonal antibody hybrids. Role of the ricin B subunit demonstrated by reconstitution. J Biol Chem 257:1598–1601
Youn YS, Na DH, Yoo SD, Song SC, Lee KC (2005) Carbohydrate-specifically polyethylene glycol-modified ricin A-chain with improved therapeutic potential. Int J Biochem Cell Biol 37:1525–1533
Yu L, Gu F, Zhang C, Xie S, Guo Y (1998) Targeted diagnosis and treatment of superficial bladder cancer with monoclonal antibody BDI-1. Chin Med J (Engl) 111:404–407
Zang Z, Xu H, Yu L, Yang D, Xie S, Shi Y, Li Z, Li J, Wang J, Li M, Guo Y, Gu F (2000) Intravesical immunotoxin as adjuvant therapy to prevent the recurrence of bladder cancer. Chin Med J (Engl) 113:1002–1006
Zarling JM, Moran PA, Haffar O, Sias J, Richman DD, Spina CA, Myers DE, Kuebelbeck V, Ledbetter JA, Uckun FM (1990) Inhibition of HIV replication by pokeweed antiviral protein targeted to CD4+ cells by monoclonal antibodies. Nature 347:92–95
Zhang F, Sun S, Feng D, Zhao WL, Sui SF (2009) A novel strategy for the invasive toxin: hijacking exosome-mediated intercellular trafficking. Traffic 10:411–424
Zheng SS, Wai WL, Hin WY, Wu AR (1991) Kinetics of IgE antibody response to trichosanthin α-momorcharin and beta-momorcharin in mice. Chin Med J (Engl) 104:292–299
Acknowledgments
The help and advice of Dr. Serena Fabbrini (Istituto di Biologia e Biotecnologia Agraria, CNR, Milano, Italy) are gratefully acknowledged.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Fracasso, G., Stirpe, F., Colombatti, M. (2010). Ribosome-Inactivating Protein-Containing Conjugates for Therapeutic Use. In: Lord, J., Hartley, M. (eds) Toxic Plant Proteins. Plant Cell Monographs, vol 18. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-12176-0_12
Download citation
DOI: https://doi.org/10.1007/978-3-642-12176-0_12
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-12175-3
Online ISBN: 978-3-642-12176-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)