Abstract
Ocular fungal infections, or ophthalmic mycoses, are being increasingly recognized as an important cause of morbidity and blindness; certain types of ophthalmic mycoses may even be life-threatening in particular endogenous and exogenous fungal endophthalmitis. In this chapter, a review of literature is given on the types of common ocular fungal infections, with recent trends in corneal fungal keratitis and factors influencing such infections. Occurrence of both pathogenic and opportunistic fungal pathogens is described for both normal and diseased human eyes. Diagnoses of fungal infection are most critical. Occurrence of such fungi in other habitats, especially in soil and the hospital environment, suggests that soil is the main reservoir of ocular filamentous fungi which may be transmitted to human eyes by different routes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 An Introduction to Ocular Fungal Infection
In the last few decades, the association of fungi with eye infections has again received increased attention in ophthalmology. Our means of combating bacterial infections are good but defenses against fungal pathogens are relatively weak. Fungal infection is generally classified as endogenous or exogenous oculomycosis and extension oculomycosis. Various types of defenses are imparted naturally for the eye to be protected from pathogen attacks and may be classified as (1) anatomical defenses, (2) mechanical defenses (by eye lashes), (3) immunological defenses and (4) defenses provided by tear film, conjunctiva etc. For example, the nonkeratinized squamous epithelium of the conjunctiva and cornea serves as a protective anatomic barrier against pathogens. The tear film comprises three layers, namely oil, aqueous, and mucous, and is produced by the meibomian glands, the lacrimal glands, and the goblet cells of the conjunctiva respectively. The aqueous layer comprises the majority of the 7 mm thick tear film. The tear pH, 7.14–7.82, probably contributes to the neutralization of toxic substances. Tear flow mechanically bathes the anterior surface of the eye, preventing the adherence of micro-organisms, and flushes allergens and foreign particles into the lacrimal excretory system. In addition, the mucous layer of the tear film entraps foreign material, which facilitates its removal (Klotz et al. 2000)
A first report of keratitis in humans was published by Prof. Theodor Laber in 1879. With the help of Prof. Reincke, a botanist, he identified the organism as Aspergillus glaucus. Since then several workers have reported occurrence of fungi from diseased eyes. In the occurrence and distribution pattern of fungi in the eye reported up to 1972, there were more than 83 species belonging to 49 genera. It has been found that various fungi isolated from an infected eye could also be recovered from a normal eye (Seegal and Khorazo 1972; Ahmad 1988). Common fungi isolated from adult normal eyes belong to various genera such as Aspergillus, Candida, Cephalosporium, Cladosporium, Curvularia, Fusarium, Helimenthosporium, Mucor, Rhizopus, Penicillium, Trichophyton, etc. (Tables 4.1 and 4.2). The great majority of these fungi are considered to have their normal habitat in soil and vegetable matter. Due to exposure of the eye to the atmosphere and its close continuity with skin, it is not surprising to recover various types of fungi from otherwise normal conjunctiva. At least 95 species out of 44 genera have been isolated from corneal ulcers. A few of them may be potential pathogens of humans, but the great majority of these fungi had normal habitat either in the soil or vegetable matter as saprophytes.
Wilson et al. (1991) listed about 105 species from 35 genera of fungi as causative agents of keratitis and other ophthalmic mycoses; however, the criteria by which these fungi were considered to be genuine ophthalmic pathogens, and not simply contaminants inadvertently introduced into specimens during or after collection, were not clearly delineated. An evaluation made by McGinnis (1980) of more than 300 reports pertaining to human fungal infections published in the literature from the late 1940s to the beginning of 1979 encountered similar difficulties. This assessment included reports on 30 genera (60 species) of fungi isolated from ophthalmic infections, principally keratitis; some reports pertaining to 32 species in 19 genera of fungi satisfied strict criteria of acceptability. Criteria recommended include an adequate clinical history that suggests a mycotic infection, the association of fungus in the clinical specimens, and also that the morphology of the fungus in the clinical specimens should be consistent with the reported etiologic agent.
In the case of fungal infections of the eye, it is erroneous to make a distinction between pathogenic and saprophytic fungi. A decreased host resistance, local lesion, incidental to the primary diseases and disturbances brought about by modern therapy, including broad-spectrum antibiotics and corticosteroids, permits the growth of usually saprophytic fungi (Ahmad 1988).
Fungal infection of the eye may be endogenous endophthalmitis or exogenous endophthalmitis. Other infections are to cornea and adjacent structures (eyelids, conjunctiva, and lacrimal system). The term endogenous endophthalmitis implies that blood-borne spread of micro-organisms to the eye has occurred. Therefore, infection in the eye is the result of metastatic spread of infection from a distant site, for example, infected heart valves or the urinary tract. In this manner the eye becomes the site of numerous micro-abscesses. The epidemiology of endogenous endophthalmitis reflects both the natural habitats of the involved fungi and the habits and health status of the patients.
Endogenous endophthalmitis may be caused by Candida, Aspergillus as a direct result of the success of modern medical practice that sustains patients’ lives with broad-spectrum antibiotics, indwelling central venous lines, parenteral nutrition, abdominal surgery, and cytotoxic chemotherapy. Virtually any intravascular prosthesis or device may become contaminated by blood-borne opportunistic fungi, and fungemia arising from such infection may lead to endogenous endophthalmitis. Exogenous endophthalmitis occurs through introduction of micro-organisms into the eye from trauma or surgery. It can also be the end result of pre-existing scleritis or keratitis (Borderie et al. 1997; Klotz et al. 2000).
The number of fungi implicated in various exogenous and endogenous ophthalmic infections has been increasingly reported in recent years, such as yeasts, principally Candida sp., including Candida glabrata (Cameron et al. 1998), Candida famata (Rao et al. 1991), and Fusarium sp. (Pflugfelder et al. 1988). Furthermore, patients with AIDS may contact many different fungal infections of the eye and adjacent structures. Various common fungi in eye infections associated with AIDS patients have been reported, such as Cryptococcus sp., Pneumocystis sp., Candida sp., Histoplasma sp., Aspergillus sp., Fusarium sp., and Bipolaris sp. (Table 4.3).
Paecilomyces lilacinus is a ubiquitous soil saprophyte implicated in cases of keratitis and endophthalmitis after trauma (Westenfeld et al. 1996; Okhravi et al. 1997). However, a large outbreak of P. lilacinus exogenous endophthalmitis followed intraocular lens implantation; the lenses had been contaminated by a bicarbonate solution used to neutralize the sodium hydroxide sterilant added to the lenses. Acremonium kiliense (Fridkin et al. 1996) has caused infections following lens surgery. Fungal pathogens in posttraumatic endophthalmitis are legion and similar to those causing fungal keratitis. Some reports have identified Fusarium moniliforme (Srdic et al. 1993), Exophiala jeanselmei (Hammer et al. 1983), Pseudo-Allescheria boydii (Carney et al. 1996), Aspergillus niger (Jager et al. 1994), Scytalidium dimidiatum (Al-Rajhi et al. 1993), Helminthosporium spp. (Das et al. 1994), S. schenckii (Witherspoon et al. 1990), Penicillium chrysogenum (Eschete et al. 1981), and L. theobromae (Borderie et al. 1997).
Thomas (2003a) has extensively reviewed various types of ocular pathogenic fungi and grouped them as (1) hyaline filamentous fungi, (2) dematiaceous (phaeoid) fungi, (3) yeast and zygomycetious fungi, and (4) thermally dimorphic fungi, implicated in ophthalmic infections and other specific pathogenic fungi.
Ophthalmologists and optometrists in particular, and clinicians in general, need to be aware of the pathogenesis of fungal eye infections. More than 70 species representing 40 genera of fungi have been reported to cause fungal keratitis. Filamentous fungi form the major etiologic agents of fungal keratitis. Fusarium sp. (37%–62%) and Aspergillus sp. (24–30%) have been implicated as main pathogens. Dematiaceous fungi are the cause of 8–16.7% of cases of fungal keratitis (Bharathi et al. 2003). Most filamentous fungi associated with corneal ulceration in the tropics are found widely within the environment. Yeast can also cause keratitis. The most common causes of fungal keratitis are Fusarium solani and other Fusarium sp., Aspergillus sp., and Curvularia sp. (Thomas 1994). There may be a hierarchy of fungi capable of producing keratitis, e.g., from most to least capable, Fusarium, Acremonium, and Phialophora spp. This hierarchy is predicated upon their individual ability to invade and destroy the cornea (Liesegang 1998).
4.1.1 Predisposing Risk Factors for Fungal Keratitis
Ocular fungal infections, or ophthalmic mycoses, are being increasingly recognized as an important cause of morbidity and blindness, and even certain types of ophthalmic mycoses may be life-threatening (Yohai et al. 1994; Levin et al. 1996). Keratitis (corneal infection) occurs most frequently (Srinivasan et al. 1991), but the orbit, lids, lacrimal apparatus, conjunctiva, sclera, and intraocular structures may also be involved (Fig. 4.1). However, here we will discuss infections related to corneal keratitis. A comprehensive review of fungal diseases of the eye, in particular endogenous and exogenous fungal endophthalmitis, has been published by different workers (Klotz et al. 2000; Thomas 2003a, b; Nayak 2008). A number of factors are known to promote mycotic ocular infections, as elaborated in Fig. 4.2.
Predisposing factors include trauma, contact lenses, and topical steroids. Trauma is the most important predisposing cause, followed by ocular and systemic defects, and prior application of corticosteroids. Previous history of ocular trauma (especially if organic matter is involved), agricultural occupations, age, recurrent ocular disease, exposure keratopathy, chronic keratitis, chronic use of steroids, systemic immunosuppressive disease also plays a role in fungal keratitis.
4.1.1.1 Corneal Trauma
Injury to the cornea is the leading cause of microbial keratitis, particularly fungal keratitis. The nature of injury is often vegetative in origin, which may consist of trauma with plant twigs, rice-husk, cotton plant, etc. A history of corneal trauma with vegetable matter or organic matter is reported in 55%–65% of fungal keratitis (Bharathi et al. 2003). Trauma leads to destruction of the epithelium and Bowman’s membrane, impairing the barrier to infection. The underlying stroma becomes excessively hydrated and possibly altered in such a way as to constitute a more favorable site for fungus to grow. Keratomycosis caused by filamentous fungi is an occupational hazard of farmers and agricultural workers. The seasonal variation noted in most series most probably represents occupational injuries associated with harvesting. On the other hand, mycotic infections, especially Candida spp., may develop in pre-existing lesions such as herpetic scars or neurotrophic keratitis, which alter local ocular immune defense (Sudan and Sharma 2003). Micro-organisms do not usually penetrate intact corneal epithelium. However, following trauma opportunistic fungi and bacteria may complicate the underlying disease, especially if caused by vegetative material. This is also the case with corneal diseases with epithelial disintegration, such as exposure keratitis, chronic corneal epithelial erosions, corneal degeneration, herpesvirus keratitis, and/or suppressed local immune system (corticosteroid treatment; Richter et al. 2003). A study from the northern United States reported trauma as the inciting event in only 8.3% of cases, whereas, in the southern United States, trauma was identified as a principal risk factor in 44% of children who had microbial keratitis and in 27% among 227 cases of microbial keratitis reported in a nonreferral county practice in southern California (Srinivasan 2004).
4.1.1.2 Contamination Through Contact Lenses
Several published case reports have identified contact lens wear as a risk factor for fungal keratitis in industrialized countries (29%; Hoflin-Lima and Roizenblatt 2002). Patients wearing any type of contact lens can get fungal keratitis. Most of the pathologies related to contact lens wearing occur in the anterior segment of the eyeball and clinically can be seen as serious inflammation. Nevertheless, using contact lenses without any coexisting symptoms does not guarantee their sterility, and may also be a source of infection. The factors causing infectious complications are connected with hygiene negligence, such as: improper disinfecting of contact lenses, usage of contaminated lenses, contact lens containers, and cleaning solutions, wearing contact lenses during eye infections, and contamination of contact lenses through the introduction of micro-organisms from the environment (Buczek-Kalakucka and Polz-Dacewicz 2006)
4.1.1.3 Use of Contaminated Topical Steroids
Many ophthalmologists identify topical steroids as the principal risk factor in enhancing ocular fungal growth. Steroid use as initial therapy was reported in 1%–30% of patients having microbial keratitis. Topical corticosteroid therapy has been associated with increased incidence and worsening of fungal keratitis (Bharathi et al. 2003).
The increased incidence is probably due to altered local immune response and increased rates of conjunctival colonization by fungi. Additionally, it indirectly promotes fungal replication and corneal invasion by interfering with the host’s inflammatory response. The systemic use of corticosteroids may predispose to fungal keratitis by causing immunosuppression. Candida spp. commonly colonizes conjunctive and eye lid margins of normal individuals. However, it may produce keratitis in patients with impaired immune response. The local ocular resistance may be lowered by atopic disease, eyelid malposition, dry-eye conditions, and neurotrophic or herpetic keratitis predisposing to fungal keratitis (Sudan and Sharma 2003).
4.1.1.4 Other Factors
Other disorders, including corneal surface disorders, dry eye, bullous keratopathy, and exposure keratitis, are associated with the development of suppurative keratitis (Thomas 2003b). Several case reports of fungal keratitis after photorefractive keratectomy and Lasik have been published (Periman et al. 2003).
4.2 Infections of the Cornea
Fungal infection of the cornea, e.g., mycotic keratitis or fungal keratitis, was first observed in 1879 in Germany by Leber, and is now a major public health problem in the tropical parts of many developing nations, including India (Agrawal et al. 1994; Gunawerdena et al. 1994; Hagan et al. 1995; Thomas 2003a, b). Scarring of the cornea as a result of keratitis is one of the preventable causes of blindness and usually carries an unfavorable prognosis due to its protracted course and need for specific therapy (Baradkar et al. 2008). Corneal blindness is a major public health problem worldwide and infectious keratitis is one of the predominant causes. According to the World Health Organization, corneal diseases are a major cause of vision loss and blindness, second only to cataract in overall importance. It is estimated that ocular trauma and corneal ulceration result in 1.5–2 million new cases of corneal blindness annually (Whitcher et al. 2001). The incidence of ocular fungal infections has increased in the last few years due to the improvement in microbiologic diagnostic techniques and because of the introduction of new therapeutic measures such as widespread use of broad-spectrum antibiotics, immunosuppressive drugs, and corticosteroids (Saha and Das 2006).
The fungi most often isolated from human corneal ulcers are also known to inhabit normal eyes, and also commonly occur as saprobes in the soil and vegetable matter. Due to the abundance of fungi in human surroundings, it is difficult to assign a pathogenic role to them when they are isolated from the diseased eyes. The problem is further aggravated by the presence of predisposing factors such as the use of immunosuppressive agents, antibiotics, and physical injury to the eye. It was therefore thought that a simultaneous study in infected human eyes, normal human eyes, and the surroundings would be of help in elucidating some aspects of fungal infections of the eye (Sandhu et al. 1981).
If not treated early, this condition may lead to corneal blindness. The causal agent is largely filamentous fungi, although yeasts, particularly Candida, may also be responsible in a small number of cases. Fungal infections of the cornea (fungal keratitis or keratomycosis) may constitute 6%–53% of all cases of ulcerative keratitis, depending upon the country of origin of the study (Thomas 1994). Recently, Nayak (2008) has extensively reviewed such fungal infections of the eye with special focus on keratomycosis. The major risk factors for mycotic keratitis are trauma, systemic illness, previous eye surgery, contact lenses, and diabetes (Rosa et al. 1994; Gunawerdena et al. 1994; Panda et al. 1997; Srinivasan et al. 1997; Tanure et al. 2000; Gopinathan et al. 2002; Basak et al. 2005; Chowdhary and Singh 2005; Ula et al. 2009).
Most fungal keratitis occurs after trauma to the cornea in agricultural workers, usually, but not always, with fungus-contaminated plant material (leaves, grain, branches, or wood). The disease may also occur in gardeners and, following corneal trauma, from indoor plants as well. Occasionally the object striking the cornea is metal. The trauma to the cornea may be so slight as to be forgotten by the patient. Furthermore, fungal keratitis may also occur with contact lens wear. Trauma to the cornea with vegetable matter either introduces the fungus directly into a corneal epithelial defect or, alternatively, the defect may become infected following the trauma (Klotz et al. 2000).
Keratomycosis is a suppurative, usually ulcerative, corneal disease. Infection is exogenous, with the organism entering through the corneal epithelium. The vast majority of cases of fungal keratitis are due to septate, filamentous and saprophytic fungi, mainly Aspergillus sp. and Fusarium sp.: others may include Alternaria sp., Curvularia sp., Penicillium sp., Cladosporium sp. and Acremonium sp., etc. and occasionally zygomycetes such as Absidia or Rhizopus spp. (Table 4.4; Schwartz et al. 1978; Marshall et al. 1997; Doczi et al. 2004). On the other hand, the abnormal or compromised cornea, e.g., chronic dry eye, is subject to infection with yeasts, usually Candida sp. Such uncommon Candida sp. as Candida lipolytica and Candida humicola have, however, been reported to cause posttraumatic keratitis (Nitzulescu and Niculescu 1975, 1976; Doczi et al. 2004) and Candida guilliermondii after corneal transplant (Ainbinder et al. 1998, Doczi et al. 2004). Fungal keratitis is recognizable by the presence of a coarse granular infiltration of the corneal epithelium and anterior stroma (Fig. 4.1). The corneal defect usually becomes apparent within 24–36 h after the trauma. Some of the reports with multiple numbers of cases published in the last decade are briefly presented here.
Tanure et al. (2000) reported the spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia from 24 eyes (24 patients) from 1991 to 1994. Predisposing factors included chronic ocular surface disease (OSD; 41.7%), contact lens wear (29.2%), atopic disease (16.7%), topical steroid use (16.7%), and ocular trauma (8.3%). Early identification of fungal elements was achieved by staining of corneal scrapings in 18 cases (75%). Half of the cases (12 eyes) had corneal infections caused by yeast, and the other half by filamentous fungi. Candida albicans was the most commonly isolated organism (45.8%), followed by Fusarium sp. (25%). They found Fusarium sp. the most commonly isolated filamentous fungus and C. albicans was the most frequent cause of fungal keratitis, and a past history of ocular trauma was uncommon.
Gopinathan et al. (2002) reported the retrospective laboratory results of 1,352 cases of fungal keratitis diagnosed at the L.V. Prasad Eye Institute (LVPEI) in south India from January 1991 to December 2000. Ocular trauma predisposed to infection in 736 (54.4%) of 1,354 eyes. A fungal cause was established by smears of corneal scrapings in 1,277 (95.4%) eyes. Fusarium (506, 37.2%) and Aspergillus sp. (417, 30.7%) have predominated the hyaline fungal spectrum (1,133) and Curvularia sp. (39, 2.8%) were the highest among the dematiaceous isolates (218).
Fungal keratitis is an uncommon complication in contact lens wearers: however, a study highlights use of contact lens cleaning solution as being responsible for causing a Fusarium outbreak from 2005 to 2006 in Singapore (Khor et al. 2006). During the study period, 66 patients (68 affected eyes) were diagnosed with Fusarium keratitis associated with contact lens wear; the estimated annual national incidence is 2.35 cases per 10,000 contact lens wearers. Patients ranged in age from 13 to 44 years (27.1 ± 8.4 years), of which 32 (48.5%) were men. The vast majority (65 patients; 98.5%) wore using soft, disposable contact lenses; 62 patients (93.9%) reported using one brand of contact lens cleaning solution (ReNu, Bausch & Lomb, Rochester, NY, USA), including 42 patients (63.6%) who recalled using ReNu with MoistureLoc. Most patients (81.8%) reported poor contact lens hygiene practices, including overnight use of daily wear contact lenses (19.7%), and use of contact lenses past the replacement date (43.9%). Because no predisposing factors, such as ocular trauma, pre-existing ocular diseases, topical steroid usage, or an exposure to Bausch & Lomb ReNu with the MoistureLoc cleaning solution, were involved in this case, poor contact lens hygiene was very probably the reason for this rare fungal infection. This study suggests that physicians and eye care practitioners worldwide need to be aware of the likelihood of similar outbreaks emerging among contact lens wearers.
Srinivas et al. (2007) presented a study over a period of 1 year in which they treated 12 patients with unilateral Fusarium keratitis. All patients were contact lens users who used ReNu MostureLoc contact lens solution and had no other specific predisposing conditions. Microbiological examination yielded growth of Fusarium spp. in seven patients from corneal scrapings at presentation and from three patients in subsequent corneal specimens. For two other patients, fungi were not detected from corneal scrapings, but Fusarium spp. was isolated from their contact lenses. The infections were treated with topical natamycin and amphotericin B eye drops, and with systemic itraconazole in eight patients. The infection resolved with medical treatment in eight eyes, a conjunctival flap in one eye, and a therapeutic corneal graft in one eye. Two eyes required tectonic corneal grafts for perforation. Two of the three corneal grafts failed because of graft rejection. Final visual acuities ranged from counting fingers to 1.0. This cluster of Fusarium keratitis seems to be related to the use of the ReNu MoistureLoc contact lens solution. The cure rate with medical therapy was 66%. However, corneal scarring limited visual recovery. This episode highlights the need for clinical vigilance when dealing with corneal infiltrates in contact lens users.
Chowdhary and Singh (2005) reported the epidemiologic features and laboratory results of 191 consecutive cases of fungal keratitis presenting to a tertiary-level super-specialty teaching hospital (Department of Guru Nanak Eye Center, Maulana Azad Medical College, New Delhi) from January 1999 to June 2001. Diagnosis of mycotic keratitis was established in 191 (39%) out of the total study group of 485 cases. Direct microscopic examination of KOH mounts and Gram-stained smears revealed the presence of fungal elements in the corneal scrapings in 119 (62.3%) and 114 (60%) of the subsequently fungal culture-positive cases respectively. Predisposing risk factors were noted in 79%, with corneal trauma 42%, contact lens wear 25%, and topical corticosteroids in 21% patients. The spectrum of fungi isolated included Aspergillus sp. in 78 cases (41%) followed by Curvularia sp. in 55 cases (29%).
A prospective study of corneal ulcer was conducted by Shokohi et al. (2006) between May 2004 to March 2005 in Sari, North Iran. Patients who presented with clinically suspected corneal ulcer to the Ophthalmology Department of Bou-Ali Sina University Hospital in Sari were included in this study. A total of 22 patients met the inclusion criteria of this study, among whom ten (45.5%) were females and 12 (54.5%) were males. The investigation highlighted that infections of the cornea due to filamentous fungi are frequent causes of corneal damage. In direct microscopy, branching, and septate hyphae were identified in seven patients (31.8%). Two fungi (Aspergillus fumigatus and Fusarium spp.) were isolated (28.6%). Five patients (31.8%) with fungal keratitis were males and two (28.6%) were females. Three (42.85%) patients with fungal keratitis were farmers. Trauma with plant debris and straws were noted in two patients (28.6%) with fungal keratitis. Five patients (71.4%) received topical antibiotics.
A study was carried out by Saha and Das (2006) on patients attending tertiary care hospital, East Delhi. In this study, of the 346 patients with corneal ulcers investigated, in 77 of cases (22.25%) fungal etiology was identified. Males were more commonly affected, and were mostly in the age group of 31–40 years. It was seen that trauma was the most common predisposing risk factor especially in the agriculturists and the farmers. Aspergillus flavus was the most common fungus isolated in 31.16% of cases, followed by A. fumigatus (16.88%) and Fusarium spp. (7.79%). Yeasts were also isolated in 21.62% of cases. Both yeasts and mycelial fungi were isolated in 6.5% of cases.
David et al. (2007) reported an extensive study fungal keratitis seen at Moorfields Eye Hospital over a 13-year period to January 2007. There were 66 isolates from 65 patients. Forty (60.6%) of the isolates were subspecies of Candida. Prior OSD or a penetrating keratoplasty (PK) was present in 38 patients (97.4%) with Candida infection, and 29 patients (74.4%) with Candida infection were using topical steroid at the time of diagnosis. The principal risk factors for filamentary fungal infection were trauma (eight cases, 30.8%) or cosmetic contact lens wear (eight cases, 30.8%), with OSD or a prior PK each present in five cases (19.2%). Candida was the principal isolate, usually from eyes with OSD or a prior PK treated with topical steroids.
A study conducted by Chander et al. (2008), carried out jointly by the Departments of Microbiology and Ophthalmology, Government Medical College Hospital, Chandigarh, over a period of 5 years from January 1999 to December 2003, revealed incidence of keratomycosis in and around Chandigarh. Out of 154 suspected patients, 64 cases were positive for agents. Most common fungal isolates found were Aspergillus sp. (41.18%) followed by Fusarium sp. (23.53%), Candida sp. (8.82%), Curvularia sp. (5.88%), and Bipolaris sp. (5.88%). This study highlighted hyaline filamentous fungi as most common etiological agents and mechanical trauma with vegetative matter as the most common predisposing factor (37.75%); other predisposing factors were chronic antibiotic usage (25%) and use of topical corticosteroids (7.81%).
Furthermore, a study by Baradkar et al. (2008) to identify aetiological agents and predisposing risk factors in mycotic keratitis, was conducted at Department of Microbiology, Lokmanya Tilak Municipal Medical College and General Hospital, Sion, Mumbai. Fungi were isolated in 13.07% of cases. Correlation between microscopy and culture was present in 58% of cases. The male:female ratio observed was 3:1. Filamentous fungi were isolated in 79.41% of cases and yeasts were isolated in 20.58% of cases. Predominant fungal isolates were Aspergillus sp. 17.64% (16/34), Fusarium sp. 14.7% (5/34). Curvularia sp., Penicillium sp., and C. albicans were isolated in three cases each (8.82% each); Cladosporium sp., Dreschleria sp., Acremonium sp., Aureobasidium sp. were isolated in two cases each (5.88% each), and Alternaria sp. and Bipolaris sp. in one each.
Ula et al. (2009) presented a report involving patient demographics, clinical and laboratory findings, and treatment and outcomes of 46 cases of culture-proven fungal keratitis diagnosed from January 2004 to November 2007, being compared with 23 cases of fungal keratitis previously collected over a similar period from January 1999 to November 2002 at the Massachusetts Eye and Ear Infirmary. They showed that during 2004–2007, the rate of fungal keratitis was 1.0 cases per month, an increase from the baseline rate of 0.5 cases per month during 1999–2002. The proportion of cases caused by filamentous fungi increased from 30% (1999–2002) to 65% (2004–2007; p = 0.01). Soft contact lens wear accounted for 41% of fungal keratitis cases in 2004–2007, as compared with 17% in 1999–2002. Patients with a history of corneal transplant had the highest rate of therapeutic keratoplasties (67%) and had the poorest visual outcome (40% counting fingers or less). In the contact lens group, 94% of patients maintained vision of at least 20/40 and only 12% required surgery to control the infection. The study concluded that there has been an increase in fungal keratitis in the Boston area and a change in the causative pathogens and risk factors for infection. Filamentous fungi now account for the majority of fungal keratitis cases, whereas yeasts were the predominant pathogen in the past. Soft contact lens wear is currently the most common risk factor for development of fungal keratitis.
Elmer (2009) reviewed the cases of Alternaria keratitis diagnosed and treated at the University of Illinois Eye and Ear Infirmary from 1999 to 2007 for clinical presentation, antifungal therapy, and final visual acuity. They found that Alternaria keratitis has a varied clinical presentation and may present without macroscopic pigmentation.
A study carried out by Saha et al. (2009) at Priyamvada Birla Arvind Eye Hospital, West Bengal, from January to December 2008 highlighted Aspergillus sp. as the predominant group (55.40%) of fungi causing keratitis among 110 patients, followed by C. albicans (18.91%) and Fusarium sp. (10.81%). Agricultural activity-related ocular trauma was the principal cause of such keratitis.
4.3 Diagnosis of Fungal Infections of the Eye
Fungal infections of the cornea are frequently caused by species of Fusarium, Aspergillus, Curvularia, and Candida. Fungal keratitis usually manifests as a rapidly developing process, with fungi frequently found deep in the equine cornea. Fungi and bacteria are capable of promoting enzymatic degradation of the cornea. This process is aggravated by attracted neutrophils, which degranulate in the corneal stroma and release additional lytic enzymes. The diagnosis of keratomycosis is made by the finding of fungal elements during cytologic examination of corneal scrapings or mycologic culture, or during histopathologic examination of a keratectomy specimen (Richter et al. 2003).
Culture remains the cornerstone of diagnosis; direct microscopic detection of fungal structures in corneal scrapes or biopsies permits a rapid presumptive diagnosis. The most critical pieces of information regarding infections of the eye are the clinical history, clinical examination, and accurate identification of the causative micro-organisms. A good history and eye examination may provide sufficient information to suggest the pathogenesis of the disease and likely micro-organisms. For example, diminishing vision and pain in the eye of a patient wearing contact lenses in the presence of a corneal ulcer strongly suggest an infectious keratitis caused by bacteria, saprophytic fungi, or amoebae. The diagnosis of fungal infections requires the clinician to (1) establish the presence of ophthalmic pathology (which may require special instruments, such as a scanning slit confocal microscope; Florakis et al. 1997), (2) obtain tissue in which the fungus is visualized, and (3) isolate the responsible fungus. Fungal isolation by culture is particularly important since tissue strains frequently do not allow one to determine the identity of filamentous fungi or yeasts with any degree of certainty. Isolation allows one to perform both authoritative identification and antifungal testing when necessary. In most other circumstances the clinician will be obliged to establish the diagnosis by isolating the causative micro-organism directly from the eye or adnexal tissue.
Aspergillus, Fusarium spp. (Ando and Takatori 1982; Sehgal et al. 1981), and occasionally C. albicans (Schwab and Dawson 1995) are cultured from the conjunctiva of healthy and diseased eyes; therefore, it is difficult to establish saprophytic fungal isolates as pathogens of the conjunctiva unless a biopsy is performed, and this is rarely necessary. Pneumocystis carinii may cause conjunctivitis, but this organism cannot be cultured (Ruggli et al. 1997). Material may be expressed from an infected lacrimal duct or, if required, an incision can be made and tissue can be obtained for culture and appropriate stains. Not infrequently, invasive orbital disease arises from the paranasal sinuses, usually the ethmoid and sphenoid sinuses. A computerized tomography (CT) scan of the orbit and paranasal sinuses will establish the extent of disease, and biopsy, curettage, and drainage of the infected sinus can obtain adequate material. Aspergillus spp., zygomycetes, and other filamentous fungi are the usual pathogens. Rhinocerebral zygomycosis involving the orbit can often be diagnosed by biopsy of necrotic tissue from the hard palate or nose. The use of an exceedingly thin, round-ended platinum spatula or, alternatively, a scalpel blade or small needle allows for scrapings to be obtained from the corneal surface for stains and cultures (Wilhelmus et al. 1994). Ample tissue is needed, so multiple corneal scrapings are usually performed. Biopsy of the cornea or keratoplasty may be required to provide sufficient diagnostic material. Saprophytic filamentous fungi more often than not cause post-traumatic keratomycosis. Rarely are yeasts involved in post-trauma keratitis (Thomas 1994).
4.3.1 The Detection of Fungal Elements
In tissue or smears detection of fungal elements is enhanced and detected with significantly greater sensitivity using acridine orange (Kanungo et al. 1991), Calcofluor white (Chander et al. 1993), or lactophenol cotton blue (LCB; Thomas et al. 1991; Byrne et al. 1995) stains. The first two stains have the added advantage of demonstrating other pathogens, such as bacteria, amoebic exocysts, and micro-sporidial spores. This may be important, because traumatic injuries to the cornea may involve more than one pathogen. Similarly, the use of a battery of fluorescein-conjugated lectins has been shown to be useful in detection of ocular mycoses (Robin et al. 1986). The use of such tests as a chitin assay (Lamps et al. 1995) or polymerase chain reaction (PCR; Okhravi et al. 1998) may prove useful, but these assays currently suffer from a cumbersome technique in the former and lack of detection across fungal genera in the later.
4.3.2 Culture and Identification
Apart from the conventional culture techniques on SDA slants, and LCB preparation of the growth for distinguishing between yeasts and mycelia, and for the identification of mycelia fungi, one can also opt for a slide culture technique (Rippon 1982) which visualizes aerial hyphae of molds, making the microscopic identification easier. Yeasts can be speciated by looking for chlamydospore formation on cornmeal agar and germ tube production, as well as by using various sugar fermentation and assimilation tests, urease test, and other biochemical tests (Rippon 1988).
4.3.3 Molecular Methods for the Diagnosis of Mycotic Keratitis
PCR assay, PCR-SSCP (single strand conformation polymorphism) and PCR-RFLP (restriction fragment length polymorphism) techniques have also been standardized for fungal identification (Chen et al. 2002). Of these, the PCR is universally accepted as the most popular technique as it can yield quick results, confirming the diagnosis of mycotic keratitis within a few hours, whereas culture takes at least 5–6 days for a positive detection (Ferrer et al 2002). Gaudio et al. (2002) developed a PCR-based assay to amplify a part of the fungal 18S r-RNA gene, which was used for detection of fungal DNA in corneal scrapings. PCR and fungal culture results matched in 74% of cases. Thus at present PCR assay seems quite promising for the diagnosis of fungal keratitis, offering a definite advantage over culture methods. However, its main drawback is its occasional false positivity that can be overcome by application of stringency in laboratory procedures and proper standardization of the techniques. Despite this, PCR remains an effective method for diagnosing keratomycosis. It is also a more sensitive and rapid method than the conventional mycologic procedures. In addition, PCR is of great benefit in rapidly detecting the presence of the organism difficult to culture. The sensitivity of PCR, taking culture as the gold standard, was quite high, between 89% and 94%, whereas specificity ranged from 50% to 88% (Ferrer et al. 2002; Gaudio et al. 2002). Some of the well-cited studies on molecular identification of ocular fungi are summarized here.
Kappe et al. (1998) used molecular probe for detection of pathogenic fungi in the presence of human tissue. Four primer systems, amplifying fragments of the gene coding for the small ribosomal subunit (18S r-RNA), were characterized with pure cultures of 65 medically relevant fungal species plus two mushrooms. A primer cocktail (TR1/CA1-TR2/AF2) amplified 59 of 67 fungal species; the universal fungal primer 1 (UF1), in combination with the eukaryotic primers S3 or EU1, amplified 64 and 65 of 67 fungal species respectively. The design of an additional primer (RZY1) enabled the amplification of the missing members of the zygomycetes. The primer systems amplified all the medically relevant fungi tested. These included eight Candida spp. and seven other yeast species, 13 dermatophytes, 32 molds (including six zygomycetes and five dimorphic fungi), and two mushrooms. Eleven controls including DNA from Schistosoma mansoni, Escherichia coli, Mycobacterium tuberculosis and human tissue were not amplified. The oligonucleotide CA hybridized with C. albicans, Candida tropicalis, and Candida parapsilosis; the oligonucleotide TR hybridized with the 13 dermatophytes; the oligonucleotide AF hybridized with A. fumigatus, A. flavus, Aspergillus terreus, Aspergillus nidulans, Aspergillus versicolor, Aspergillus tamarii, Aspergillus clavatus, and Aspergillus fischeri, but not with A. niger or A. versicolor; and the oligonucleotide HC hybridized with three varieties of Histoplasma capsulatum. These oligonucleotides did not hybridize with the other fungi or the controls. The specificity of the designed primer systems was confirmed by selective amplification of fungal DNA from human lung tissue spiked with fungal biomass and from vitrectomy fluid of a patient with Candida endophthalmitis.
Okhravi et al. (1998) determined the usefulness of PCR and RFLP analysis in the identification and speciation of Candida spp. that causes ocular infection. Oligonucleotide primers based on the cytochrome P450 L1 A1 demethylase gene were used to successfully amplify by PCR a single 1.0 kb and a single 500 bp DNA fragment from C. albicans, C. tropicalis, Candida krusei, C. glabrata, C. parapsilosis, and Candida pelliculosa genomic DNA. RFLPs within the PCR product were identified after restriction enzyme digestion. The sensitivity of the amplification reaction after two rounds of PCR was 10 fg genomic C. albicans DNA or one copy of the gene. No amplification product was obtained when DNA from C. guilliermondii, A. fumigatus, F. solani, human leukocytes, or ten species of bacteria was used as a template. Experiments with spiked normal vitreous demonstrated equal sensitivity as long as the volume of vitreous did not exceed 20% of the total PCR volume. RFLP analysis of the PCR product generated from each species obtained from the first- and second-round amplification products enabled species identification after digestion with specific endonucleases. Application of the technique to four clinical samples was successful. PCR-RFLP analysis has great potential in the rapid detection and identification of Candida spp. and in the provision of a useful laboratory tool.
Ferrer et al. (2002) used molecular techniques to determine whether sequence analysis of internal transcribed spacer/5.8S ribosomal DNA (rDNA) can be used to detect fungal pathogens in patients with ocular infections (endophthalmitis and keratitis). Internal transcribed spacer 1 (ITS1) and ITS2 and 5.8S rDNA were amplified by PCR and seminested PCR to detect fungal DNA. Fifty strains of 12 fungal species (yeasts and molds) were used to test the selected primers and conditions of the PCR. PCR and seminested PCR of this region were carried out to evaluate the sensitivity and specificity of the method. It proved possible to amplify the ITS2/5.8S region of all the fungal strains by this PCR method. All negative controls (human and bacterial DNA) were PCR-negative. The sensitivity of the seminested PCR amplification reaction by DNA dilutions was one organism per PCR, and the sensitivity by cell dilutions was fewer than ten organisms per PCR. Intraocular sampling or corneal scraping was undertaken for all patients with suspected infectious endophthalmitis or keratitis (nonherpetic) respectively, between November 1999 and February 2001. PCRs were subsequently performed with 11 ocular samples. The amplified DNA was sequenced, and aligned against sequences in GenBank at the National Institutes of Health. The results were PCR-positive for fungal primers for three corneal scrapings, one aqueous sample, and one vitreous sample; one of them was negative by culture. Molecular fungal identification was successful in all cases. Bacterial detection by PCR was positive for three aqueous samples and one vitreous sample; one of these was negative by culture. Amplification of ITS2/5.8S rDNA and molecular typing shows potential as a rapid technique for identifying fungi in ocular samples.
Various other workers have used molecular techniques for identification and diagnoses of pathogenic fungi from eye infection and other sources.
4.4 Soil and Other Environments as Reservoir of Ocular Fungi
Fungi are ubiquitous members of soil microbial communities, but constitute a varying proportion of the biomass in different systems. They tend to dominate in soils containing high proportions of organic matter and low pH, and generally constitute a smaller proportion in intensively managed mineral soils. They are involved in a plethora of functional roles in soil. The fungi are an immensely diverse group of organisms, encompassing a huge range of forms from microscopic single-celled yeasts to large macrofungi, as exemplified by the well-known mushrooms and toadstools and the largest of fruitbodies, the giant puffball (Bridge and Spooner 2001).
The great majority of the >80,000 fungal species so far named and described are likely to occur in the soil environment at some stage in their life-cycle. Fungi therefore have many different functions in soils, which include both active roles, such as the degradation of dead plant material, and inactive roles where propagules are present in the soil as resting states. Survey of the soil fungal diversity, which were popular during the 1960s and 1970s, have reappeared in the literature with the advent of DNA-based, culture-independent methods of analysis. The development of molecular techniques has provided a new range of tools that can provide clear insights into specific interactions and activities in soil environments. The combination of broad-spectrum PCR detection, coupled with SSCP or denaturing gradient gel electrophoresis, can give more accurate answers to fundamental questions on ecosystem diversity. This technique does not however distinguish between active and resting stages, and in order to interpret results accurately, some a priori knowledge is necessary (Bridge and Spooner 2001). Thus it is not surprising that opportunistic and pathogenic fungi are isolated from soil. It has long been known that soil is the main reservoir of ocular fungi. However, in recent years the number of fungal infections in human eyes has increased, which triggers an interest to examine the source and reservoir of such fungi and how they cause of various fungal infections in the eye.
Fungi are ubiquitous in the natural environment, appearing in air, water, and soil. The diversity of free living micro-soil fungi has been well-established and known for a very long time (Srivastava and Mishra 1972; Alexander 1985; Ahmad 1988; Paul 2007; Imran 2009). Occurrence of such fungi from environmental sources including air is expected. In poorly ventilated buildings with damaged and poor air-conditioning systems, there may be an increase in the concentration of mycotoxicogenic molds, Penicillium and Aspergillus spp. (Garrison et al. 1993). Similarly, airborne microflora in hospital rooms were the subject of numerous studies as a potential cause of hospital infections (Herman 1980; Li and Hou; 2003; Arnow et al. 1991; Pini et al. 2004). A study from Tabbara and Al Jabarti (1998) reported an outbreak of Aspergillus endophthalmitis in five patients after cataract extraction during hospital construction in Jeddah, Saudi Arabia. Severe postoperative uveitis occurred in all five patients and failed to subside with topical steroid therapy. The causative organism was identified as A. fumigatus in each case.
In a well-designed study, air-borne fungi in the Departments of Dermatology, Venereology and Allergology of the Medical University in Wroclaw, Poland were studied by Lukaszuk et al. (2007). Thus, air is the common source of fungal spores and the fungal aerosols and can enter into the host body by direct contact or through inhalation. On the other hand, soil is considered as the major reservoir for almost all types of fungi — saprophytes, pathogenic, symbiotic, etc. These fungi are associated with plants and or soil have good opportunity to come in contact to human eye specially to agricultural workers, farmers, during sowing, plowing field, crop harvesting and processing of crops. Other reservoir of fungi which may come in contact to human is animals and organic matter including; animal wastes FYM may be potential source of ocular fungi. Fungi associated with normal eye may constitute potential ocular pathogenic or opportunistic fungi. In a separate study of soil fungi from the same geographical area, occurrence of the soil fungi isolated and identified includes species of Aspergillus, Trichoderma, Geotrichum, Alternaria, Monilia, and Mycelia sterilia (Zafar and Ahmad 2005).
A more extensive investigation of the soil fungi from agricultural soil of Aligarh showed 2.5 × 105 to 7.9 × 105 CFUg−1 of filamentous soil fungi. Common members of the genera were Aspergillus, Penicillium, Rhizopus, Curvularia, Trichoderma, Trichophyton, Mucor, Mycelia sterillia, Monotospora, Verticillium, Alternaria, Cladosporium, Hormodendrum, Trichothecium; and Fusarium (Imran 2009).
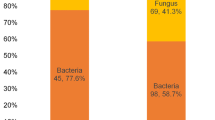
A comparative study of the occurrence of fungi from diseased human eye (patients attended the Institute of Ophthalmology, JawaharLal Nehru Medical College and hospital for eye microbiological examination) during the year 1988 was analyzed by Ahmad (1988), and is presented in Table 4.5. In this comparative study occurrence of the filamentous fungi from diseased human eye, soil, plant material, and animal body surface indicated the common occurrence of various fungi both from diseased human eye and environmental sources. The Aspergillus was the most common fungi isolated both from environment and diseased eyes. Among Aspergillus isolates, A. niger (Fig. 4.3a), A. glaucus, A. flavus and A. terreus (Fig. 4.3b) were commonly isolated. Other fungi such as Alternaria (Fig. 4.3c) and Microsporum (Fig. 4.3d) were also isolated from soil and normal eye.
a Microscopic photographs of A. niger isolated from diseased eye: (a) conidiophore, (b) radiate head, and (c) chain of conidia. b Microscopic photographs of A. terreus isolated from diseased eye: (a) conidiophore, and (b) vesicle having fertile sterigmate. c Microscopic photographs of Alternaria sp. isolated from diseased eye: (a) septate hyphae, (b) conidium showing longitudinal, and (c) transverse septations. d Microscopic photographs of Microsporum sp. isolated from diseased eye: (a) septate hyphae, (b) macroconidia showing septations, and (c) microconidium
Occurrence of such fungi from the hospital environment, including air, was also reported by Lukaszuk et al. (2007) from Poland. A. niger, Penicillium citrinum and C. albicans were isolated more frequently in the air of the Department, of Dermatology, Venereology and Allergology of the Medical University in Wroclaw.
Furthermore, some studies have highlighted animal as reservoir for these infections. Humans coming in contact with these infected animals by one or other means may spread fungal keratitis. In India, 50% of cases of keratomycosis in human beings are reported in association with injuries by paddy straw, stalk, sheath, and thorns (Sansom et al. 2005). Among domestic animals, fungal keratitis is most common in the horse. One study of the conjunctival fungal flora of several domestic animal species, including horses and cattle, incorporated cows and horses without clinical evidence or recent history of keratitis or other ocular disease (Elligott et al. 2006). In a study investigating the fungal flora of the normal conjunctiva, fungi were isolated in 94% of the horses, in 100% of the cows, in 22% of the dogs and in 40% of the cats. Yeasts comprised 13% of the isolates of the fungal flora. The most common fungal species isolated from the normal equine eye are Aspergillus, Fusarium, Penicillium, Alternaria and Cladosporium spp. (Richter et al. 2003). Fungi were isolated from conjunctival swabs of 100% of the bovine and 95% of the equine subjects. Of a total of 95 bovine fungal isolates, 75 of which were identifiable, Penicillium sp. and Cladosporium sp. were most frequently isolated, accounting for 11 (12%) and 16 (16%) of the isolates respectively. Only three (3%) of the bovine fungal isolates were identified as Aspergillus sp. Of a total of 88 equine conjunctival fungal isolates, 81 of which were identifiable, 23 of the isolates (26%) were identified as Aspergillus sp. Penicillium sp. were the next most frequently isolated fungi among the horses, accounting for 19 (22%) of the total isolates (Elligott et al. 2006).
Thus based on the comparative occurrence of various fungal genera in various environmental sources (soil, air, plant and animals) and human eye, it can be concluded that soil is the main reservoir of the mycotic flora including ocular fungi. The possible mode of transmission of fungi from various sources to the eye is depicted in schematic diagram Fig. 4.4.
4.5 Conclusion
Fungal infections of the cornea continue to be an important cause of ocular morbidity, particularly in the agricultural communities of the developing world. A proper understanding of agent and host factors involved in these infections will improve the outcome of this condition. Fungi isolated from normal eyes, diseased eyes and various reservoirs such as soil have great morphological and even taxonomic similarities. This indicates that fungi of the eye could be easily isolated from different sources which may act as reservoir for these fungi. Future research into ophthalmic mycoses reservoirs and their contribution to the increasing number of cases of fungal infection is needed. The use of improved diagnostic techniques and to distinguish between opportunistic, pathogenic, and nonpathogenic ocular fungi present in different reservoirs and the sources of contamination of such fungi in the eye needs to be extensively examined. Rapid and accurate identification of the fungal species which have potential to cause ocular infection will permit the immediate initiation of specific preventive, prophylactic, or antifungal therapy. A possible mode of transmission and factors encouraging fungi to reside in the normal eye need to be investigated.
References
Agrawal V, Biswas J, Madhavan HN, Mangat G, Reddy MK, Saini JS, Sharma S, Srinivasan M (1994) Current perspectives in infectious keratitis. Indian J Ophthalmol 42:171–192
Ahmad I (1988) A comparative study of the pathogenic and saprophytic fungi of human eye, soil, plant and animal sources. MSc (Microbiology) dissertation, Aligarh Muslim University, Aligarh
Ainbinder D, Parmley VC, Mader TH, Nelson ML (1998) Infectious crystalline keratopathy caused by Candida guilliermondii. Am J Ophthalmol 125:723–725
Alexander M (1985) Introduction to soil microbiology, 2nd edn. John Wiley, New York
Al-Rajhi A, Awad AH, Al-Hedaithy SS, Forster RK, Caldwell KC (1993) Scytalidium dimidiatum fungal endophthalmitis. Br J Ophthalmol 77:388–390
Ando N, Takatori K (1982) Fungal flora of the conjunctival sac. Am J Ophthalmol 94:67–74
Aristimuno B, Nirankari VS, Hemady RK, Rodrigues MM (1993) Spontaneous ulcerative keratitis in immunocompromised patients. Am J Ophthalmol 115:202–208
Arnow PM, Sadigh M, Costas C, Weil D, Chudy R (1991) Endemic and epidemic aspergillosis associated with in-hospital replication of Aspergillus organism. J Infect Dis 164:998–1002
Baradkar VP, De A, Mathur M, Lanjewar M, Kumar S (2008) Mycotic keratitis from Mumbai. Bombay Hosp J 50:200–204
Bartley GB (1995) Blastomycosis of the eyelid. Ophthalmology 102:2020–2023
Basak SK, Basak S, Mohanta A, Bhowmick A (2005) Epidemiological and microbiological diagnosis of suppurative keratitis in Gangetic West Bengal, Eastern India. Indian J Ophthalmol 53:17–22
Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi R, Palaniappan R (2003) Epidemiological characteristics and laboratory diagnosis of fungal keratitis: a three-year study. Indian J Ophthalmol 51:315–321
Borderie V, Bourcier TM, Poirot JL, Baudrimont M, Prudhomme de Saint-Maur P, Laroche L (1997) Endophthalmitis after Lasiodiplodia theobromae corneal abscess. Graefes Arch Clin Exp Ophthalmol 235:259–261
Bridge P, Spooner B (2001) Soil fungi: diversity and detection. Plant Soil 232:147–154
Buczek-Kalakucka S, Polz-Dacewicz M (2006) Asymptomatic and symptomatic ocular infections related to contact lens wearing. Ann Univ Mariae Curie 61:731–734
Burnier SV, Sant’Anna AE (1997) Palpebral paracoccidioidomycosis. Mycopathologia 140:29–33
Byrne KA, Burd EM, Tabbara KF, Hyndiuk RA (1995) Diagnostic microbiology and cytology of the eye. Butterworth-Heinemann, Boston, MA
Cameron J, Badr IA, Risco JM, Abboud E, Gaonnah S (1998) Endophthalmitis cluster from contaminated donor corneas following penetrating keratoplasty. Can J Ophthalmol 33:8–13
Carney MD, Tabassian A, Guerry RK (1996) Pseudo-Allescheria boydii endophthalmitis. Retina 16:263–264
Chander J, Chakrabarti A, Sharma A, Saini JS, Panigarhi D (1993) Evaluation of Calcofluor staining in the diagnosis of fungal corneal ulcer. Mycoses 36:243–245
Chander J, Singla N, Agnihotri N, Arya SK, Deep A (2008) Keratomycosis in and around Chandigarh: a five year study from a north Indian tertiary care hospital. Indian J Pathol Microbiol 51:304–306
Chen SC, Halliday CL, Meyer W (2002) A review of nucleic acid diagnostic tests for systemic mycoses with an emphasis on polymerase chain reaction-based assays. Med Mycol 40:333–357
Chowdhary A, Singh K (2005) Spectrum of fungal keratitis in North India. Cornea 24:8–15
Coccia L, Calista D, Boschini A (1999) Eyelid nodule: a sentinel lesion of disseminated cryptococcosis in a patient with acquired immunodeficiency syndrome. Arch Ophthalmol 117:271–272
Das T, Gopinathan U, Sharma S (1994) Exogenous Helminthosporium endophthalmitis. Br J Ophthalmol 78:492–493
David GJ, Stephen TJ, Andrew R, John DKG (2007) Fungal keratitis in London: microbiological and clinical evaluation. Cornea 26:1082–1086
Doczi I, Gyetvai T, Kredics L, Nagy E (2004) Involvement of Fusarium spp. in fungal keratitis. Clin Microbiol Infect 10(9):773–776
Elligott CR, Wilkie DA, Kuonen VJ, Bras ID, Neihaus A (2006) Primary Aspergillus and Fusarium keratitis in a Holstein cow. Vet Ophthalmol 9:175–178
Elmer TY (2009) Alternaria keratitis: clinical presentation and resolution with topical fluconazole or intrastromal voriconazole and topical caspofungin. Cornea 28:116–119
Eschete M, King JW, West BC, Oberle A (1981) Penicillium chrysogenum endophthalmitis. Mycopathologia 74:125–127
Ferrer C, Munoz G, Alio JL, Abad JL, Colon F (2002) Polymerase chain reaction diagnosis in fungal keratitis caused by Alternaria alternata. Am J Ophthalmol 33:398–399
Florakis G, Moazami G, Schubert H, Koester CJ, Auran JD (1997) Scanning slit confocal microscopy of fungal keratitis. Arch Ophthalmol 115:1461–1463
Font R, Parsons MA, Keener MJ, Shaver RP, Foos RY (1995) Involvement of anterior chamber angle structures in disseminated histoplasmosis: report of three cases. Ger J Ophthalmol 4:107–115
Fridkin S, Kremer FB, Bland LA, Padhye A, McNeil MM, Jarvis WR (1996) Acremonium kiliense endophthalmitis that occurred after cataract extraction in an ambulatory surgical center and was traced to an environmental reservoir. Clin Infect Dis 22:222–227
Garg P, Gopinathan U, Choudhary K, Rao GN (2000) Keratomycosis – clinical and microbiologic experience with dematiaceous fungi. Ophthalmology 107:574–580
Garrison RA, Robertson LD, Koehn RD, Wynn SR (1993) Effect of heating–ventilation–air conditioning system sanitation on air borne fungal populations in residential environments. Ann Allergy 71:548–556
Garrity JD, Herman C, Imes R, Fries P, Hughes CF, Campbell RJ (1993) Optic nerve sheath decompression for visual loss in patients with acquired immunodeficiency syndrome and cryptococcal meningitis with papilledema. Am J Ophthalmol 116:472–478
Gaudio PA, Gopinanthan U, Sangwan V, Hughes TE (2002) Polymerase chain reaction based detection of fungi in infected corneas. Br J Ophthalmol 86:755–760
Glasgow B, Engstrom RE Jr, Holland GN, Kreiger AE, Wool MG (1996) Bilateral endogenous Fusarium endophthalmitis associated with acquired immunodeficiency syndrome. Arch Ophthalmol 114:873–877
Gonzales CA, Scott IU, Chaudhry NA, Luu KM, Miller D, Murray TG, Davis JL (2000) Endogenous endophthalmitis caused by Histoplasma capsulatum var. capsulatum. A case report and literature review. Ophthalmology 107:725–729
Gopinathan U, Garg P, Fernandes M, Sharma S, Athamanathan S, Rao GN (2002) The epidemiological features and laboratory results of fungal keratitis: a 10 year review at a referral eye care center in South India. Cornea 21:555–559
Guarro J, Gams W, Pujol I, Gene J (1997) Acremonium species: new emerging fungal opportunists – in vitro antifungal susceptibilities and review. Clin Infect Dis 25:1222–1229
Guarro J, Akiti T, Almada-Horta R, Leite-Filho LAM, Gene J, Ferreira-Gomes S, Aguilar C, Ortoneda M (1999) Mycotic keratitis due to Curvularia senegalensis and in vitro antifungal susceptibilities of Curvularia spp. J Clin Microbiol 37:4170–4173
Gunawerdena SA, Ranasinghe KP, Arseceuleratne SN, Scimon CR, Ajello L (1994) Survey of mycotic and bacterial keratitis in Sri Lanka. Mycopathologia 127:77–81
Hagan M, Wright E, Newman M, Dolin P, Johnson G (1995) Causes of suppurative keratitis in Ghana. Br J Ophthalmol 79:1024–1028
Hammer M, Harding S, Wynn P (1983) Post-traumatic fungal endophthalmitis caused by Exophiala jeanselmei. Ann Ophthalmol 15:853–855
Herman LG (1980) Aspergillus in patient care areas. Ann N Y Acad Sci 353:140–146
Hoflin-Lima AL, Roizenblatt R (2002) Therapeutic contact lens-related bilateral fungal keratitis. CLAO J 28:149–150
Imran M (2009) Interaction of heavy metals with indigenous isolates of free living rhizospheric fungi and their plant growth promoting potentials. PhD thesis submitted to Aligarh Muslim University, Aligarh
Jager M, Chodosh J, Huang AJ, Alfonso EC, Culbertson WW, Forster RK (1994) Aspergillus niger as an unusual cause of scleritis and endophthalmitis. Br J Ophthalmol 78:584–586
Johnson TE, Casiano RR, Kronish JW, Tse DT, Meldrum M, Chang W (1999) Sino-orbital aspergillosis in acquired immunodeficiency syndrome. Arch Ophthalmol 117:57–64
Kanungo R, Srinivasan R, Rao RS (1991) Acridine orange staining in early diagnosis of mycotic keratitis. Acta Ophthalmol 69:750–753
Kappe R, Okeke CN, Fauser C, Maiwald M, Sonntag HG (1998) Molecular probes for the detection of pathogenic fungi in the presence of human tissue. J Med Microbiol 47:811–820
Khor WB, Aung T, Saw SM, Wong TY, Tambyah PA, Tan AL, Beuerman R, Lim L, Chan WK, Heng WJ, Lim J, Loh RSK, Lee SB, Tan DTH (2006) An outbreak of Fusarium keratitis associated with contact lens wear in Singapore. JAMA 295:2867–2873
Klotz SA, Penn CC, Negvesky GJ, Butrus SI (2000) Fungal and parasitic infections of the eye. Clin Microbiol Rev 13:662–685
Lamps C, Oeltmann TN, Collins MJ Jr, Robinson RD, Logan RA, Head WS, O’Day DM (1995) Development of a chitin assay for the quantification of fungus. Curr Eye Res 14:637–641
Lee BL, Holland GN, Glasgow BJ (1996) Chiasmal infarction and sudden blindness caused by mucormycosis in AIDS and diabetes mellitus. Am J Ophthalmol 122:895–896
Levin LA, Avery R, Shore JW, Woog JJ, Baker AS (1996) The spectrum of orbital aspergillosis: a clinicopathological review. Surv Ophthalmol 41:142–154
Li CS, Hou PA (2003) Bioaerosol characteristics in hospital clean rooms. Sci Total Environ 305:169–176
Liesegang TJ (1998) Fungal keratitis. In: Kaufman HE, Barron BA, McDonald MB (eds) The Cornea. Butterworth-Heinemann, Boston, MA, pp 219–245
Locher DH, Adesina A, Wolf TC, Imes CB, Chodosh J (1998) Post-operative Rhizopus scleritis in a diabetic man. J Cataract Refract Surg 24:562–565
Lukaszuk C, Krajewska-Kulak E, Boran E, Szepietowski J, Bialynicki-Birula R, Kulak W, Rolka H, Oksiejczuk E (2007) Analysis of the incidence of the fungal pathogens in air of the Department of Dermatology, Venereology and Allergology of Medical University in Wroclaw. Adv Med Sci 52:15–17
Marshall DH, Brownstein S, Jackson WB, Mintsioulis G, Gilberg SM, Al-Zeerah BF (1997) Post-traumatic corneal mucormycosis caused by Absidia corymbifera. Ophthalmology 104:1107–1111
McGinnis MR (1980) Laboratory Handbook of Medical Mycology. Academic, New York
Morinelli EN, Dugel PU, Riffenburgh R, Rao NA (1993) Infectious multifocal choroiditis in patients with acquired immune deficiency syndrome. Ophthalmology 100:1014–1021
Muccioli C, Belfort B Jr, Neves R, Rao N (1995) Limbal and choroidal Cryptococcus infection in the acquired immunodeficiency syndrome. Am J Ophthalmol 120:539–540
Nayak N (2008) Fungal infections of the eye — laboratory diagnosis and treatment. Nepal Med Coll J 10:48–63
Nitzulescu V, Niculescu M (1975) Ophthalmopathy determined by Candida humicola. Arch Roum Pathol Exp Microbiol 34:357–361
Nitzulescu V, Niculescu M (1976) Three cases of ocular candidiasis caused by Candida lipolytica. Arch Roum Pathol Exp Microbiol 35:269–272
Okhravi N, Dart JK, Towler HM, Lightman S (1997) Paecilomyces lilacinus endophthalmitis with secondary keratitis: a case report and literature review. Arch Ophthalmol 115:1320–1324
Okhravi N, Adamson P, Mant R, Matheson M, Midgley G, Towler H, Lightman S (1998) Polymerase chain reaction and restriction fragment length polymorphism mediated detection and speciation of Candida spp. causing intraocular infection. Invest Ophthalmol Vis Sci 39:859–866
Panda A, Sharma N, Das G, Kumar N, Satpathy G (1997) Mycotic keratitis in children: epidemiologic and microbiologic evaluation. Cornea 16:295–299
Paul EA (2007) Soil microbiology, ecology and biochemistry, 3rd edn. Academic, Canada
Pavan PR, Margo CE (1993) Endogenous endophthalmitis caused by Bipolaris hawaiiensis in a patient with acquired immunodeficiency syndrome. Am J Ophthalmol 116:644–645
Periman LM, Harrison DA, Kim J (2003) Fungal keratitis after photorefractive keratectomy: delayed diagnosis and treatment in a co-managed setting. J Refract Surg 19:364–366
Petersen M, Althaus C, Santen R, Gerhaz CD (1997) Endogenous Aspergillus endophthalmitis in AIDS. Klin Monbl Augenheilkd 211:400–402
Pflugfelder SC, Flynn HW, Zwickey TA, Forster RK, Tsiligianni A, Culbertson WW, Mandelbaum S (1988) Endogenous fungal endophthalmitis. Ophthalmology 95:19–30
Pini G, Donato R, Faggi E, Fanci R (2004) Two years of fungal aerobiocontamination survey in a Florentine haematology ward. Eur J Epidemiol 19:693–698
Rao N, Nerenberg AV, Forster DJ (1991) Torulopsis candida (Candida famata) endophthalmitis simulating Propionibacterium syndrome. Arch Ophthalmol 109:1718–1721
Read RW, Chuck RS, Rao NA, Smith RE (2000) Traumatic Acremonium atrogriseum keratitis following laser-assisted in situ keratomileusis. Arch Ophthalmol 118:418–421
Richter M, Hauser B, Kaps S, Spiess BM (2003) Keratitis due to Histoplasma spp. in a horse. Vet Ophthalmol 6:99–103
Rippon JW (1982) Laboratory Mycology Section II Culture methods, media, stains and serologic procedures. In: Rippon JW (ed) Medical Mycology: The pathogenic fungi and the pathogenic Actinomycetes, 2nd edn. WB Saunders, Philadelphia, pp 772–795
Rippon JW (1988) Candidiasis and the pathogenic yeasts. In: Rippon JW (ed) Medical Mycology: The pathogenic fungi and the pathogenic Actinomycetes, 2nd edn. WB Saunders, Philadelphia, pp 536–581
Robin J, Arffa RC, Avni I, Rao NA (1986) Rapid visualization of three common fungi using fluorescein-conjugated lectins. Invest Ophthalmol Vis Sci 27:500–506
Rodenbiker HT, Ganley JP (1980) Ocular coccidioidomycosis. Surv Ophthalmol 24:263–290
Rosa RH Jr, Miller D, Alfonso EC (1994) The changing spectrum of fungal keratitis in South Florida. Ophthalmology 101:1005–1013
Ruggli G, Weber R, Messmer EP, Font RL, Moll C, Bernauer W (1997) Pneumocystis carinii infection of the conjunctiva in a patient with acquired immune deficiency syndrome. Ophthalmology 104:1853–1856
Saha R, Das S (2006) Mycological profile of infectious keratitis from Delhi. Indian J Med Res 123:159–164
Saha S, Banerjee D, Khetan A, Sengupta J (2009) Epidemiological profile of fungal keratitis in urban population of West Bengal, India. Oman J Ophthalmol 2:114–118
Sandhu DK, Randhawa IS, Singh D (1981) The correlation between environmental and ocular fungi. Indian J Ophthalmol 29:177–182
Sansom J, Featherstone H, Barnett KC (2005) Keratomycosis in six horses in the United Kingdom. Vet Rec 156:13–17
Saran BR, Pomilla PV (1996) Retinal vascular nonperfusion and retinal neovascularization as a consequence of cytomegalovirus retinitis and cryptococcal choroiditis. Retina 16:510–512
Schwab IR, Dawson CR (1995) Conjunctiva. In: Vaughan DG, Asbury T, Riordan-Eva P (eds) General ophthalmology. Appleton & Lange, Norwalk, Conn., pp 95–122
Schwartz L, Loignon LM, Webster RG Jr (1978) Post traumatic phycomycosis of the anterior segment. Arch Ophthalmol 96:860–863
Seegal BC, Khorazo DL (1972) Microbiology of the eye. Mosby, St Louis, p 361
Sehgal S, Dhawan S, Chhiber S, Sharma M, Talwar P (1981) Frequency and significance of fungal isolations from conjunctival sac and their role in ocular infections. Mycopathologia 73:17–19
Shmookler MP, Kolsky BM (1996) Unilateral optic neuritis caused by Histoplasma capsulatum in a patient with the acquired immunodeficiency syndrome. Am J Ophthalmol 121:324–326
Shokohi T, Nowroozpoor-Dailami K, Moaddel-Haghighi T (2006) Fungal keratitis in patients with corneal ulcer in Sari, Northern Iran. Arch Iran Med 9:222–227
Slack JW, Hyndiuk RA, Harris GJ, Simons KB (1992) Blastomycosis of the eyelid and conjunctiva. Ophthal Plast Reconstr Surg 8:143–149
Specht C, Mitchell KT, Bauman AE, Gupta M (1991) Ocular histoplasmosis with retinitis in a patient with acquired immune deficiency syndrome. Ophthalmology 98:1356–1359
Srdic N, Radulovic S, Nonkovic Z, Velimirovic S, Cvetkovic L, Vico I (1993) Two cases of exogenous endophthalmitis due to Fusarium moniliforme and Pseudomonas species as associated aetiological agents. Mycoses 36:441–444
Srinivas RK, Philip LTH, Emmy LYM, Hunter YKL, Dennis LSC (2007) A case series of contact lens-associated Fusarium keratitis in Hong Kong. Cornea 26:1205–1209
Srinivasan M (2004) Fungal keratitis. Curr Opin Ophthalmol 15:321–327
Srinivasan R, Kanungo R, Goyal JL (1991) Spectrum of oculomycosis in South India. Acta Ophthalmol 69:744–749
Srinivasan M, Ganzales CA, George C (1997) Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol 81:965–971
Srivastava SN, Mishra SR (1972) Rhizopus root rot of sugar beet — a new host record in India. Indian Phytopath 25:154–155
Stevens DA, Kan VL, Judson MA, Morrison VA, Dummer S, Denning DW, Bennett JE, Walsh TJ, Patterson TF, Pankey GA (2000) Practice guidelines for diseases caused by Aspergillus. Clin Infect Dis 30:696–709
Sudan R, Sharma YR (2003) Keratomycosis: clinical diagnosis, medical and surgical treatment. JK Sci 5:3–10
Tabbara KF, Al Jabarti A (1998) Hospital construction-associated outbreak of ocular aspergillosis after cataract surgery. Ophthalmology 105:522–526
Tanure MA, Cohen EJ, Grewal S, Rapuano CJ, Laibson PR (2000) Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea 19:307–312
Thomas PA (1994) Mycotic keratitis — an underestimated mycosis. J Med Vet Mycol 32:235–256
Thomas PA (2003a) Current perspectives on ophthalmic mycoses. Clin Microbiol Rev 16:730–797
Thomas PA (2003b) Fungal infections of the cornea. Eye 17:852–862
Thomas P, Kuriakose T, Kirupashanker MP, Maharajan VS (1991) Use of lactophenol cotton blue mounts of corneal scrapings as an aid to the diagnosis of mycotic keratitis. Diagn Microbiol Infect Dis 14:219–224
Ula J, Irmgard B, Kathryn C (2009) Fungal keratitis: changing pathogens and risk factors. Cornea 6:638–643
Westenfeld F, Alston WK, Winn WC (1996) Complicated soft tissue infection with prepatellar bursitis caused by Paecilomyces lilacinus in an immunocompetent host: case report and review. J Clin Microbiol 34:1559–1562
Whitcher JP, Srinivasan M, Upadhyay MP (2001) Corneal blindness: a global perspective. Bull World Health Organ 79:214–221
Whitcup SM, Fenton RM, Pluda JM, De Smet MD, Nussenblatt RB, Chan CC (1992) Pneumocystis carinii and Mycobacterium aviumintracellulare infection of the choroid. Retina 12:331–335
Wilhelmus KR, Liesegang TJ, Osato MS, Jones DB (1994) Laboratory diagnosis of ocular infections. American Society for Microbiology, Washington, DC
Wilson LA, Sawant AD, Ahearn DG (1991) Comparative efficacies of soft contact lens disinfectant solutions against microbial films in lens cases. Arch Ophthalmol 109:1155–1157
Witherspoon C, Kuhn F, Owens SD, White MF, Kimble JA (1990) Endophthalmitis due to Sporothrix schenckii after penetrating ocular injury. Ann Ophthalmol 22:385–388
Yau TH, Rivera-Velazquez PM, Mark AS, Cytryn AS, Levy CS, Shmookler BM, Kolsky MP (1996) Unilateral optic neuritis caused by Histoplasma capsulatum in a patient with the acquired immunodeficiency syndrome. Am J Ophthalmol 121:324–326
Yohai RA, Bullock JD, Aziz AA, Markert RJ (1994) Survival factors in rhino-orbital-cerebral mucormycosis. Surv Ophthalmol 39:3–22
Zafar S, Ahmad I (2005) Fungal diversity of metal contaminated agricultural contaminated soils and in vitro fungi-toxicity of heavy metals. Poll Res 24:793–799
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Ahmad, S., Khan, M.S.A., Hussain, F.M., Ahmad, I. (2010). Fungi Associated with Eye Infections with Special Reference to Corneal Keratitis and Their Possible Reservoir. In: Ahmad, I., Owais, M., Shahid, M., Aqil, F. (eds) Combating Fungal Infections. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-12173-9_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-12173-9_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-12172-2
Online ISBN: 978-3-642-12173-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)