Abstract
Staphylococcus aureus (SA) secretes enterotoxins, small proteins that act as superantigens because of their potent effect on the immune system. The main mode of action of superantigens is the coupling of the major histocompatibility complex molecule with the T-cell receptor. The effect is a powerful stimulation of the adaptive immune system in a polyclonal (non-antigen-specific) way, resulting in a T-helper-2-biased inflammation. This superantigen mechanism is involved in the pathogenesis of nasal polyps (NP) in about 50% of the cases. The superantigenic effect is hallmarked by immunoglobulin changes in biopsies: high total IgE, polyclonal IgE to multiple allergens, and IgE specific to SA enterotoxins. Serum immunoglobulins coincide only partially with biopsy findings. Patients with this IgE pattern have an increased risk of asthma and aspirin-exacerbated respiratory disease (AERD). Future treatments with topical or systemic antibiotics and monoclonal antibodies to IgE and interleukin-5 (IL-5) are being studied.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nasal Polyp
- Nasal Polyposis
- Eosinophil Cationic Protein
- Staphylococcal Enterotoxin
- Staphylococcus Aureus
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Core Messages
-
Staphylococcus aureus (SA) secretes enterotoxins, small proteins that act as superantigens because of their potent effect on the immune system.
-
The main mode of action of superantigens is the coupling of the major histocompatibility complex molecule with the T-cell receptor.
-
The effect is a powerful stimulation of the adaptive immune system in a polyclonal (non-antigen-specific) way, resulting in a T-helper-2-biased inflammation.
-
This superantigen mechanism is involved in the pathogenesis of nasal polyps (NP) in about 50% of the cases.
-
The superantigenic effect is hallmarked by immunoglobulin changes in biopsies: high total IgE, polyclonal IgE to multiple allergens, and IgE specific to SA enterotoxins. Serum immunoglobulins coincide only partially with biopsy findings.
-
Patients with this IgE pattern have an increased risk of asthma and aspirin-exacerbated respiratory disease (AERD).
-
Future treatments with topical or systemic antibiotics and monoclonal antibodies to IgE and interleukin-5 (IL-5) are being studied.
10.1 Introduction
The discovery of IgE antibodies to Staphylococcus aureus enterotoxins (SAE) A and B in nasal polyp tissue homogenates [4] indicated for the first time that these bacterial products could be involved in the pathogenesis of nasal polyposis. Nasal polyposis, also referred to as chronic rhinosinusitis (CRS) with nasal polyps (NP) [19], is mostly characterized by an eosinophilic, T helper 2 type of inflammation, driven by interleukin-5 (IL-5) and eotaxin which orchestrate chemotaxis, activation, and increased survival of eosinophils [2–4, 53]. This disease can be differentiated from chronic rhinosinusitis without nasal polyps (CRSsNP), which has a T helper 1 (Th1) type of inflammation with increased levels of interferon-gamma and transforming growth factor beta 1 [60]. A subgroup of NP shows high nasal colonization rates with Staphylococcus aureus (SA), an increased local polyclonal IgE synthesis, correlating with the degree of eosinophilic inflammation, and has an increased prevalence of asthma and aspirin hypersensitivity [4].
There is a wealth of data to support the hypothesis of the role of SA enterotoxins in nasal polyposis. In this chapter, we summarize the current evidence of an active role of SAE in nasal polyposis and contemplate on the possible clinical implications. After introducing the superantigenic properties of the staphylococcal enterotoxins, we present evidence for an increased nasal colonization with SA in NP and a specific humoral immune response to SAE. We provide an insight into the possible mechanisms that can elicit a polyclonal, Th2 skewed, eosinophilic milieu characteristic of NP and discuss current and future therapeutic approaches directed toward these key events in the pathophysiology of NP (Fig 10.1).
10.2 Superantigenic Properties of Staphylococcus aureus Enterotoxins
Since its discovery in the 1880s [40], SA has been recognized as an important pathogen in human disease, including minor skin infections, food poisoning, life-threatening infections, septicemia, and toxic shock syndrome [35]. Despite its powerful pathogenic capabilities, approximately 20% of the population are persistent nasal carriers of SA, and up to 60% of individuals are colonized with SA intermittently [65]. In contrast with intermittent carriers, persistent carriers tend to be colonized with the same bacterial strain over time. The versatile virulence is determined largely by its ability to regulate the production of surface and secreted proteins by a set of more than 50 genes known as the virulon [42]. Secreted proteins include extracellular enzymes, such as catalase and coagulase, and a group of host-damaging proteins known as exotoxins. Of the latter, the enterotoxins have potent gastrointestinal effects and are the cause of staphylococcal food poisoning [55]. An increasing number of staphylococcal toxins are described. The classical members, Staphylococcal enterotoxin A to E, are designated SEA-SEE, and newer toxins have been assigned a letter in the order of discovery (SEG-SEJ). However, some toxins lack proof of emetic properties, and they are considered as enterotoxin-like toxins (SElK-SElR, SElU), together with toxic shock syndrome toxin-1 (TSST-1) [34].
The staphylococcal enterotoxin-related toxins (further referred to as SAE) share the ability to mount a massive inflammatory reaction resulting from a polyclonal activation of T and B lymphocytes that is independent of a specific adaptive immune response, a unique interaction for which they are known as superantigens, as first described by Kappler and Marrack in 1989 [38]. It has been suggested that pathogens evolved to produce superantigens, thereby evading an efficient adaptive immune response from the host, thus aiding in colonization and spread of the pathogen [52]. Superantigens from other bacteria have been described, including Streptococcus pyogenes, Streptococcus dysgalactiae, Mycoplasma arthritidis, Yersynia pseudotuberculosis, and Peptostreptococcus magnus [20].
Unlike conventional T-cell activation via specific recognition by the T-cell receptor (TCR) of processed antigen peptides in the major histocompatibility complex (MHC) molecule, SAE directly activate T-cells via bridging the MHC class II molecule with the TCR directly, without being processed by an antigen presenting cell (APC). Superantigens bind to one of the domains of the MHC class II molecule on APCs in a region distant from the peptide-binding cleft, and to the Vβ-domain in the β chain of the TCR. This bypasses specific antigen recognition [20]. To date, there are only 52 Vβ gene segments described that code for the Vβ domain, and consequently, superantigen binding can result in polyclonal activation of lymphocytes. It is estimated that SAE are able to stimulate up to 20–30% of the T-cell population, compared to <0.01% by conventional antigen recognition. Staphylococcal enterotoxin-related superantigens are specific for one or more Vβ domains, linking them to specific T-cell populations and creating a superantigen-specific Vβ signature [23] (Fig 10.2).
Stimulation of a T lymphocyte by an antigen presenting cell (APC). Left: conventional antigens are processed by the APC and presented in the peptide binding cleft of the major histocompatibility complex (MHC) class II molecule. Upon recognition by the T cell receptor (TCR), signal is transduced to the T cell. Right: superantigens are not processed by an APC and activate the TCR directly by crosslinking the TCR to the MHC class II molecule, distant from the complementarity-determining regions. (Illustration courtesy of Dr. T. Van Zele, 2006)
There are several ways by which staphylococcal superantigens exert their function on immune effector cells. T-cell superantigens stimulate CD4+ and CD8+ T cells and can induce either a Th1-type or Th2-type CD4+ T-cell activation, with subsequent release of IFN-γ, TNF-α or IL-4, IL-5, and IL-13. The latter may occur due to direct T-cell activation but also indirectly via stimulation of APCs. The type of the T-helper response (Th1 or Th2) can be influenced by the concentration of superantigens, the nature of the APC, and costimulatory molecules. Mandron et al. [36] showed that SEB activates monocyte-derived dendritic cells (DCs) to secrete IL-2 and that these activated DCs polarize naïve T cells to a Th2 type.
Despite the polyclonal T-cell expansion in acute diseases such as toxic shock syndrome, chronic stimulation by superantigens may lead to an oligoclonal T-cell pattern, presumably resulting from the concerted action with the conventional T-cell activation mechanism, where clones recognizing antigens are selected after chronic exposure [33]. Moreover, after polyclonal expansion, superantigen stimulation induces clonal deletion and anergy of remaining T-cell populations [28]. Loss of immunosuppressive effects of naturally occurring regulatory T cells (CD4+ CD25+) has been described in different inflammatory conditions; in atopic dermatitis, SEB has been shown to suppress their activity [43].
A polyclonal humoral immune response is evoked by SAE in a T-cell dependent way by cross-linking MHC class II molecules on B-lymphocytes and the TCR. In addition, SAE can enhance Th2 response by inducing isotype switching to IgE and augmenting the synthesis of IgE [23]. Furthermore, SEA and SED, together with Staphylococcal protein A (SpA), may act as a B-cell superantigen by directly binding to VH3 or VH4 domains of the B-cell receptor, resulting in enhanced survival of these subsets of B-cells.
The findings of both T-lymphocytes and IgE specific to SAE (SAE-IgE) indicate that SAE may also be involved as conventional antigens, in which the SAE are processed into oligopeptides and presented in the antigen-binding groove of the MHC molecule. It is hypothesized that the superantigen and the conventional response may act in concert, where polyclonal stimulation of both T- and B-lymphocytes allows for an increased specific humoral or cellular response to SAE [23]. Finally, staphylococcal superantigens may have a direct effect on proinflammatory and other cells, such as eosinophils, macrophages, epithelial cells, and fibroblasts. SpA is also able to induce degranulation of mast cells by crosslinking FcεRI molecules via binding to VH3 IgE domains, and is therefore called a superallergen [37] (Fig 10.3).
10.3 Invasion of Nasal Tissue by Staphylococcus aureus
SA frequently colonizes the nasal cavity, with an average persistent colonization rate in 20–30% of individuals [65]. Although SA can be isolated in acute and chronic rhinosinusitis, a disease-modifying role of SA in CRS without NP has never been proven. Microbiology studies of the middle nasal meatus in CRS present conflicting results; however, in controlled studies, SA has been isolated in comparable rates in controls and CRS patients [1, 18].
We reported for the first time an increased colonization rate in middle meatus nasal swabs from CRS with NP (63.7%) compared with CRS without NP (27.3%) [59]. Even higher rates were detected in NP patients with asthma (66.7%) and aspirin hypersensitivity (87.5%), whereas there was no significant difference in the colonization rate between CRS without NP and controls. Furthermore, repeated cultures over time in patients with NP indicated long-term colonization. The colonization rates in these patients were paralleled by IgE antibodies to SAE, total IgE, and eosinophil cationic protein (ECP) in nasal tissue homogenates. These findings were corroborated in a second study by our group, showing a colonization rate of 71% in CRS with NP vs. 25% in controls [21]. On the other hand, conflicting results with our above studies have been reported [41], with detection of staphylococci in nasal lavage samples and in minced biopsies, in comparable levels between CRS with NP, CRS without NP, and controls, using conventional culture methods, PCR and FISH.
As the above studies used endoscopically guided swabs from the middle meatus, these results do not necessarily reflect the presence of SA within the nasal mucosal tissue. While SA has traditionally been regarded as an extracellular pathogen, there is increasing evidence that SA has the ability to invade and survive in nonphagocytic eukaryotic cells such as keratinocytes and respiratory epithelial cells [11]. An intracellular reservoir of SA in three patients with recurrent/chronic rhinosinusitis undergoing sinus surgery has been shown by confocal immunofluorescence microscopy in nasal epithelial cells, mucous gland cells, myofibroblasts, and CD45-positive phagocytes [11]. These findings were confirmed in a population of CRS patients undergoing sinus surgery, where intracellular SA could be demonstrated in the nasal epithelium of 17 of the 27 patients [49]. Long-term carriage of identical clonal strains in CRS suggests that intracellular invasion presents an escape mechanism for host defence or antibiotic therapy. This finding may point to the involvement of SA small colony variants (SCV), strains that show a decreased growth rate, decreased hemolytic activity, increased intracellular survival, and decreased antibiotic susceptibility; however, evidence of involvement in nasal pathology is lacking [63]. The role of biofilms in CRS is being studied extensively (reviewed in [25]), but studies explicitly involving NP are scarce [8, 39]. However, as biofilms have been shown to be related to protracted disease and antibiotic resistance, their role in the continuous immune stimulation by SA superantigens in NP is of particular interest.
We recently demonstrated intraepithelial presence of SA in a subgroup of NP using immunohistochemistry. Interestingly, SEB could be colocalized to the intracellular SA, indicating a potential local intracellular production of SA enterotoxins (Patou, unpublished). Investigating invasive SA in different CRS subgroups, we used peptide nucleic acid fluorescence in situ hybridization (PNA-FISH) technique to stain for SA in nasal tissue samples [17]. Intramucosal presence of SA was comparable between control and CRS without NP groups. Although we did not demonstrate a significantly higher rate of intramucosal presence in NP per se, we showed for the first time that the presence of intramucosal SA is significantly augmented in aspirin-sensitive asthmatic NP patients compared to polyp patients without such comorbidities.
10.4 Augmented Immune Response to SAE in Polyps
In 2001, we presented a role for staphylococcal superantigens in NP [4]. Investigating the relationship between atopy, local IgE concentration, and parameters of eosinophilic inflammation, we demonstrated local IgE specific to staphylococcal enterotoxins (SAE-IgE) SEA and SEB in a subgroup of polyp patients with high local IgE concentrations and a multiclonal IgE pattern. This represented up to 50% of the NP patients in the study. These polyps showed higher concentrations of sCD23, ECP, IL-5, eotaxin, and cysteinyl leukotrienes (CysLT), and a higher eosinophil count compared to control tissue and to polyps with low IgE. These patients had a higher prevalence of asthma, and the inflammatory parameters and IgE concentrations in polyps were not related to atopy.
We subsequently reported a higher colonization rate of SA in NP (63.6%) which was paralleled by an increased presence of SAE-IgE (SEA, SEC, TSST-1) (in 27.8%), total IgE, and ECP; observations that further increased in subgroups with asthma and with aspirin-exacerbated respiratory disease (AERD), detecting SAE-IgE in 53.8 and 80%, respectively [59]. The colonization rates of SA always exceeded the rate of detection of SAE-IgE, indicating that colonization may not necessarily lead to the generation of a humoral immune response. Furthermore, ECP and total IgE were increased where IgE to SAE was detected, suggesting a role for SA in eosinophilic inflammation and high IgE concentrations. These results were confirmed in a further study where SAE-IgE (SEA-SEE, TSST-1) was demonstrated in 50% of NP, compared to 0% in control tissue [21]. Total IgE, the ratio of IgE to albumin concentrations, and eosinophil count was higher in the tissue of polyps that were positive for SAE-IgE. In accordance with these findings, a study from a South-Chinese hospital showed that 10/27 NP were positive for SAE-IgE vs. 0/15 controls, although those rates may be lower in other parts of China [66]. Suh et al. found IgE to SEA and SEB in one third of aspirin-sensitive nasal polyps (ASNP) compared to one fifth in aspirin-tolerant nasal polyps (ATNP) [54]. The levels of SEA-IgE and SEB-IgE were closely correlated with total IgE, ECP, and IL-5 concentrations.
Most of the in vivo evidence of enterotoxin secretion is indirect, by demonstration of staphylococcal enterotoxins-specific IgE. A study [9] isolated enterotoxin-producing SA strains in 55% of NP patients, although it is not clear whether and to what extent these organisms secrete superantigens in vivo. Seiberling et al. [51] detected common staphylococcal toxins (SEA, SEB, SEC1-3, SED, TSST-1) using ELISA in 48% of polyp patients and in 7.7% of CRS without NP. Nine out of fifteen positive patients demonstrated more than one toxin. It is common for SA to produce more than one toxin at a time. In a Chinese study, the same superantigens were detected by ELISA in 12 of 22 NP, compared to none in CRS without NP or controls [64].
The classical superantigens (SEA through SEE and TSST-1) have been characterized and studied intensively, and most IgE responses described are directed against one or more of these proteins. Recently, the egc gene cluster, encoding SEG, SEI, SEM, SEN, and SEO was identified in SA [30]. We identified enterotoxin genes in 75% of SA strains detected in middle nasal meatus swabs, and showed an amplification of the egc gene cluster in 67.5% of strains [62]. As there are no validated tests for the measurement of specific IgE against egc cluster enterotoxins, previous data regarding specific IgE production against SAE might underestimate the impact of enterotoxins. Interestingly, there were no differences in enterotoxin genes between SA isolated from controls compared with NP patients, suggesting that the specific immunological response of the host to SAE rather than the panel of enterotoxin genes produced by the organism determines the clinical outcome.
10.5 Mechanisms Leading to Polyps
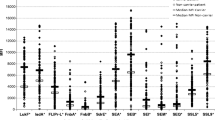
Evidence for the involvement of a response of T lymphocytes to staphylococcal superantigens has been shown in a series of studies showing proliferation of T lymphocytes bearing specific Vβ domains. Bernstein et al. [9] demonstrated significant clonal expansion of T cells with specific Vβ domains (Vβ skewing) in three NP patients. In a further study with 12 NP patients, Vβ skewing was demonstrated using flow cytometry in polyp lymphocytes of seven patients, whereas this expansion was not detectable in peripheral blood lymphocytes [57]. Subsequently, this group reported expansion of polyp lymphocytes expressing TCRs with specific Vβ domains in all of 18 polyp patients[16]. The average number of Vβ clones per CRS with NP patient was seven in polyp lymphocytes but only two in peripheral blood lymphocytes. In another study, 7 of 20 subjects exhibited skewing in Vβ domains with strong association to SAE [15]. In Chinese patients, an increased percentage of Vβ-expressing T cells was observed in toxin-positive polyps [64]. Many of the clonally expanded Vβ domains found in these studies are known to be associated with specific SAE. Moreover, the ratio of skewing of polyp lymphocytes compared with peripheral blood lymphocytes points to a local expansion of these lymphocytes (Fig 10.4).
In a recent study, we elucidated the modulatory effects of SEB and SpA exposure on NP cytokine secretion in an ex vivo setting [44]. Nasal polyp and inferior turbinate fragments were suspended in culture medium and stimulated with SEB and SpA for 30 min and 24 h. Spontaneous release of IL-5, IL-13, TNF-α, and IL-10 was greater in NP than in control tissue. Twenty-four-hour stimulation with SEB caused a significant increase of Th1 and Th2 cytokines (IFN-γ, IL-2, IL-4, IL-5, IL-10, IL-13) in inferior turbinates and to a greater extent in polyp tissue. By calculation of the ratio of increase in polyps to that in control tissue, it became apparent that the cytokine production was increased predominantly in Th2 cytokines (IL-4, IL-5) but an increase in T-regulatory cytokine production (IL-10 and TGF-β) was disfavored by SEB stimulation. This study clearly confirmed that SEB can polarize mucosal inflammation to a Th2 pattern. SEB may contribute to persistent inflammation by the suppression of T-regulatory lymphocytes, in line with our previous findings, where we showed a decreased FOXP3 and TGF-β1 expression in NP compared with CRS without NP and controls [58].
By detailed analysis of the pattern of increased IgE in NP and in serum, three groups of NP can be discerned [4, 21]: (1) no detectable specific IgE and low total IgE, (2) An “allergic” type of IgE expression characterized by increased concentrations of total IgE and selected specific IgE antibodies to aeroallergens corresponding to those found in serum and to skin prick test positivity and (3) polyclonal pattern of IgE expression with specific IgE to a majority of allergens and increased total IgE, reflecting only partially the serum IgE response and independent of skin prick test positivity. The “allergic” type can overlap with the “polyclonal” type. The polyclonal pattern was detected in 10 of 20 NP in our first study and in 16 of 24 NP in our second study, and there were SAE-IgE in, respectively, 10 and 12 of these NP, indicating that SAE are most often involved in the polyclonal IgE response. Toxins other than the classical staphylococcal enterotoxins, or bacterial products from other organisms might have acted as superantigens in some cases.
Although extravasation of serum proteins has been shown in NP [3], there is indirect evidence for a local production of IgE rather than a local reflection of a systemic production. Total IgE and SAE-IgE concentrations were in all cases higher in NP tissue compared to serum [21]; SAE-IgE may be detected in the serum of NP patients, unrelated to atopic status, especially when asthma coexists [14, 56]. Moreover, the IgE/albumin ratios in polyp tissue and serum were dissociated, and specific IgE antibodies in polyp tissue showed only a partial relation to serum IgE antibodies, indicating that tissue IgE is rather the result of a local IgE production than of extravasation [21].
When NP were analyzed for T and B lymphocytes and for IgE by immunohistochemistry, there were lymphoid accumulations seen in all samples, and lymphoid follicular structures were seen in 25% of polyps, whereas no secondary lymphoid tissue could be shown in control samples [21]. Follicular structures stained positive for B cells (CD20) and T cells (CD3), and for IgE and CD23, whereas FcεRI was found only outside the follicles. Lymphoid accumulations stained positive for plasma cells (CD38), CD3, IgE, and FcεRI but not for CD23. We demonstrated the binding of biotinylated SEA to both follicular structures and lymphoid aggregations. These data support the hypothesis of a local organization of secondary lymphoid tissue with polyclonal activation of B cells due to the stimulation by staphylococcal enterotoxins.
Acting as B cell superantigens, there is evidence that SAE can directly alter the B cell repertoire, apart from cross-linking TCR with MHC on APC. By crosslinking MHC class II molecules on B lymphocytes with TCR on T-lymphocytes, SAE can stimulate B cells in a T cell-dependent way. SpA, a surface protein of SA, can directly induce the proliferation of B cells. Moreover, TSST-1 induces isotype switching and synthesis of IgE, depending on CD40L expression on B cells [29]. A more recent study showed a direct effect by demonstrating TSST-1-induced expression of B7.2 on B cells, enhancing the Th2 response and regulating IgE production [27]. In the mucosal tissue of atopic patients, mRNA for the ε chain of IgE was found in a significant proportion of B cells using in situ hybridization, supporting the hypothesis of a local IgE synthesis in upper airway mucosa. Coker et al. [12] showed that local clonal expansion of B cells, somatic hypermutation, and class switching occur in the nasal mucosa. A significantly biased expression of the VH5 regions of the IgE molecule [13] suggests that superantigens may modulate IgE production.
A high degree of infiltration by plasma cells in NP had been described earlier [60]. We showed increased CD19+ naïve B cells and CD138+ plasma cells but not CD20+ mature B cells in NP compared to controls using immunohistochemistry [61], implying a differentiation of memory B cells into plasma cells. In this study, we extended our observations of increased IgE to other immunoglobulin isotypes. NP showed increased total IgA, IgG, and IgE concentrations compared to CRS without NP and controls which was not the case in the serum of these patients. Of interest, polyps with detectable SAE-IgE had significantly higher concentrations of IgE and IgG, and a larger fraction of the IgG4 subset of the IgG isotype, than SAE-IgE negative polyps. The fraction of IgG4 correlated strongly with IgE concentrations and CD138 counts. These findings were not reflected in the serum of these patients, supporting the hypothesis of the modulation by SAE of the local immunoglobulin production by plasma cells and local isotype switching toward IgG4 and IgE.
Investigating the effect of staphylococcal products on NP cytokines and effector molecules, Patou et al. [44] reported an increased secretion of histamine, CysLT, PGD2, and IL-5 after stimulation with SpA. These results support the view that SpA may be acting not only as a B cell superantigen but may have a direct impact on mast cell and basophil activation. This activity, for which SpA is referred to as a superallergen, is mediated by the interaction of SpA with the VH3 region of IgE bound to FcεRI, the antigen-independent crosslinking of FcεRI which it causes resulting in activation of the effector cell [37].
Nasal symptoms and markers of inflammation do not increase with seasonal allergen exposure even in ragweed sensitive patients with NP, and nasal provocation is largely unsuccessful in NP patients [31]. A polyclonal IgE pattern in NP may however cause a permanent degranulation of mast cells by conventional aeroallergens and superantigens, thus maintaining polyp growth, but not acute allergic symptoms. This hypothesis needs further study, but may also explain similar mechanisms in nonatopic, but IgE-positive asthma.
10.6 Relation to Eicosanoid Metabolism and Aspirin Sensitivity
We reported increased SA colonization rates, total local IgE, specific IgE to SAE, and ECP in ASNP [59]. In Polish NP patients, we showed increased total IgE, SAE-IgE, IL-5, and ECP in ASNP compared to ATNP [45], suggesting a relation of staphylococcal superantigens to aspirin sensitivity by upregulating eosinophilic inflammation. Posthoc subgroup analysis revealed increased IL-5 and ECP in SAE-IgE-positive ATNP compared to SAE-IgE-negative ATNP, but these differences could not be shown in SAE-IgE-positive compared with SAE-IgE-negative ASNP groups, suggesting that aspirin sensitivity is linked indirectly to SAE by the severity of inflammation rather than via direct mechanisms. Our findings have been confirmed by Suh et al. [54], reporting increased ECP, IgE, and SAE-IgE levels in Korean polyps.
Comparing eicosanoid production in CRS with NP and CRS without NP, concentrations of leukotriene C4 synthase, 5-lipoxygenase, and CysLT were increased in different sinus disease subgroups (CRS without NP, ATNP and ASNP) in parallel and in correlation with eosinophilic inflammation severity whereas COX-2 and PGE2 were inversely correlated [46]. These data confirmed the notion that changes of eicosanoid metabolism do occur in CRS even in the absence of clinical aspirin sensitivity and appear to be related to the severity of eosinophilic inflammation. We extended our observations by demonstrating that the production of CysLT, LTB4, and LXA4 is upregulated in SAE-IgE-positive NP compared to SAE-IgE-negative NP, and correlates to SAE-IgE, IL-5, and ECP levels [47]. Taken together with these results, staphylococcal enterotoxins have an amplifying role in upper airway disease with aspirin sensitivity, without evidence for a direct causal relationship of SAE with aspirin sensitivity. However, we recently isolated inferior turbinate fibroblasts and cultured the cells in the presence of different concentrations of SEB [48]. After preincubation with IFN-γ, SEB significantly downregulated PGE2, COX-2, and EP2-receptor mRNA expression, pointing to a direct effect of staphylococcal superantigens on eicosanoid metabolism in upper airway tissue.
10.7 Clinical Implications
There is accumulating evidence that staphylococcal superantigens may have a major impact on lower airway disease such as asthma, chronic obstructive pulmonary disease, and early wheezing [6]. In a NP patient, the clinician could speculate about the activity of SAE, especially if comorbidities such as severe nonallergic asthma, aspirin sensitivity, or corticosteroid-resistant disease are present. Detection of SA by culture of swabs from the nasal middle meatus is a readily available diagnostic tool, but gives only a limited idea about an active immune response to the enterotoxins. Indeed, the colonization rates exceeded the levels of SAE-IgE, and it is the latter that correlated with the severity of inflammation [60]. Furthermore, the in vivo ability of SA to produce a superantigenic effect in the nasal tissue may vary according to the number and type of strains of the colonizing bacterium, and also depends on individual host factors, such as the genetic makeup and the inflammatory background, affecting the virulence and the interaction of enterotoxins with MHC molecules, TCR, and Igs.
The local Ig pattern may give a more specific idea about the effect of superantigens; this pattern is only partially reflected in serum. Presence of SAE-IgE indicates a former or present stimulation of the local immune system by the respective enterotoxin. A locally high total IgE and a polyclonal IgE response, directed to multiple conventional aeroallergens, which may be unrelated to serum IgE specificities, is indicative of a superantigenic effect. In asthmatic patients, the SAE-IgE level in serum is related to disease severity [5].
In contrast to the polyvalent mechanisms of action of superantigens, currently, the therapeutic armamentarium mainly consists of topical or systemic glucocorticosteroids and surgery [19]. Therapeutic failure and recurrence account for a large part of patients treated with glucocorticorsteroids, and cellular resistance to glucocorticosteroids is considered a main cause of treatment failure [50]. Staphylococcal enterotoxins may impair corticosteroid treatment possibilities, as it has been shown that superantigens may alter steroid sensitivity and expression of glucocorticosteroid receptor beta [26].
Having an established role in NP pathophysiology, eradication of SA with antibiotics seems a logical treatment option. This has not yet been studied extensively in NP, but the benefit of antibiotic and antiseptic treatment has been shown in atopic dermatitis, a disease sharing the modifying effects of staphylococcal superantigens. An eradication scheme, consisting of oral antibiotics, topical antiseptics, and nasal mupirocin ointment resulted in a significant but temporary improvement in atopic dermatitis patients who were colonized with SA [10]. Nasal mupirocin lavage might be particularly useful in eradicating nasal SA because of its potent effect on SA in biofilms [24]. Studies investigating the therapeutic benefit of antibiotic treatment in nasal polyp disease are currently underway. Further studies are needed to suggest other treatment options including long-term treatment with intracellular active antibiotics, SA vaccination, and specific enterotoxin antagonists. Based on the hypothesis of a continuous mast cell degranulation by an overwhelming polyclonal local IgE, treatment with monoclonal antibodies to IgE could be of relevance in suppressing IgE-mediated effects in analogy to the effect in allergic disorders. A randomized double-blind placebo-controlled trial is currently performed.
In the light of the association of SAE antibodies with eosinophilic inflammation, treatment strategies antagonizing IL-5 provide an opportunity to prove the hypothesis. We recently reported a double-blind placebo-controlled randomized trial evaluating the safety and pharmacokinetics of intravenous injection of humanized anti-IL-5 antibody in NP patients [22]. We demonstrated that a single injection of anti-IL-5 is safe and well tolerated, and reduced the levels of blood eosinophilia and ECP, and IL-5Rα concentrations in both blood and nasal secretions. In half of the patients, polyp scores improved after single injection, and responders could be differentiated by increased levels of IL-5 in nasal secretions.
10.8 Summary and Perspectives
We presented evidence for a role of SA superantigens in the pathogenesis of CRS with NP by (1) showing an increased colonization rate of SAE-secreting SA strains in NP, (2) presence of superantigens in NP, (3) evidence for an immune response to SA characterized by SAE specific IgE antibodies, (4) in vitro modulation of NP cytokine pattern to a Th2 response by SEB and (5) specific T lymphocyte Vβ-skewing, characteristic of SAE. However, data supporting the superantigen hypothesis by these modalities have been shown in only 50% of NP [4, 21, 59]. Approximately 50% of NP patients do not show evidence for a superantigen effect, but share a similar eosinophilic inflammation. Currently, it remains unclear as to why only a subset of NP is showing superantigen response and why only some individuals exposed to superantigens develop NP. A genetic predisposition (expression of alleles specific to the superantigen interaction with MHC and TCR molecules) could explain part of this observation. Measurement of IgE antibodies to only the classical enterotoxins (SEA-SEE, TSST-1) could mask the possible effects of other staphylococcal superantigens or superantigens produced by different organisms. Furthermore, the observation of the variable possibility of SA to invade tissue and cells could point to the defects in mechanical or innate immunity. Genetic, epigenetic, or environmental factors are involved in epithelial antigen passage and processing, and could explain the highly variable immune response to a given staphylococcal load [32].
Of interest, we demonstrated that NP from South Chinese patients do not share the Th2-biased inflammatory pattern of polyps in European patients, as they were characterized by a neutrophilic inflammatory pattern and lacked increased IL-5, ECP, or IgE concentrations within polyp tissue [66]. Further studies revealed that Chinese polyps were characterized by a Th1/Th17 type of inflammation [67]. Those polyps may be less susceptible or may respond differently to the same exposure of SAE than European NP. Furthermore, in exploring the therapeutic role of anti-IL-5 antibodies for NP, only a subgroup of patients responded to treatment. Studying the effect of anti-IgE-antibodies on NP, it is expected to find again a subgroup of responders.
The above evidence indicates that SAEs with superantigenic activity do play an amplifying role in a subgroup of NP patients that may eventually lead to asthma comorbidity and persistent unified airway disease. The clinical identification of those patients is currently indirect, but the analysis of total and specific IgE antibodies in serum, or better in tissue biopsies, may support such diagnosis. First steps in the development of appropriate new therapeutic targets have been made, and will in the near future impact our daily clinical management [7].
Take Home Pearls
-
S. aureus enterotoxins are involved in the pathogenesis of a subgroup of nasal polyps via the superantigen mechanism.
-
These polyps have a more severe eosinophilic inflammation and a local polyclonal pattern of increased IgE.
-
This subgroup is associated with asthma and aspirin exacerbated respiratory disease.
-
Diagnostic and therapeutic tools for this specific group need to be studied further.
Abbreviations
- AERD:
-
Aspirin-exacerbated respiratory disease
- ASNP:
-
Aspirin-sensitive nasal polyps
- ATNP:
-
Aspirin-tolerant nasal polyps
- CysLT:
-
Cysteinyl leukotrienes
- ECP:
-
Eosinophil cationic protein
- IFN-γ:
-
Interferon-gamma
- IL:
-
Interleukin
- MHC:
-
Major histocompatibility complex
- NP:
-
Nasal polyps
- SA:
-
Staphylococcus aureus
- SAE:
-
Staphylococcus aureus enterotoxin-like toxins
- SAE-IgE:
-
IgE antibodies to SAE
- SEA–SEU:
-
Staphylococcal enterotoxin A–U
- TCR:
-
T cell receptor
- TGF-β:
-
Transforming growth factor-beta
- Th:
-
T helper
- TNF-α:
-
Tumor necrosis factor-alpha
- TSST-1:
-
Toxic shock syndrome toxin-1
References
Araujo E, Palombini BC et al (2003) Microbiology of middle meatus in chronic rhinosinusitis. Am J Rhinol 17(1):9–15
Bachert C, Wagenmann M et al (1997) IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol 99(6 Pt 1):837–842
Bachert C, Gevaert P et al (2000) Nasal polyposis: from cytokines to growth. Am J Rhinol 14(5):279–290
Bachert C, Gevaert P et al (2001) Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol 107(4):607–614
Bachert C, Gevaert P et al (2003) IgE to Staphylococcus aureus enterotoxins in serum is related to severity of asthma. J Allergy Clin Immunol 111(5):1131–1132
Bachert C, Gevaert P et al (2007) Role of staphylococcal superantigens in airway disease. Chem Immunol Allergy 93:214–236
Bachert C, Van Bruaene N et al (2009) Important research questions in allergy and related diseases: 3-chronic rhinosinusitis and nasal polyposis – a GALEN study. Allergy 64(4):520–533
Bendouah Z, Barbeau J et al (2006) Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol Head Neck Surg 134(6):991–996
Bernstein JM, Ballow M et al (2003) A superantigen hypothesis for the pathogenesis of chronic hyperplastic sinusitis with massive nasal polyposis. Am J Rhinol 17(6):321–326
Breuer K, Haussler S et al (2002) Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol 147(1):55–61
Clement S, Vaudaux P et al (2005) Evidence of an intracellular reservoir in the nasal mucosa of patients with recurrent Staphylococcus aureus rhinosinusitis. J Infect Dis 192(6):1023–1028
Coker HA, Durham SR et al (2003) Local somatic hypermutation and class switch recombination in the nasal mucosa of allergic rhinitis patients. J Immunol 171(10):5602–5610
Coker HA, Harries HE et al (2005) Biased use of VH5 IgE-positive B cells in the nasal mucosa in allergic rhinitis. J Allergy Clin Immunol 116(2):445–452
Conley DB, Tripathi A et al (2004) Chronic sinusitis with nasal polyps: staphylococcal exotoxin immunoglobulin E and cellular inflammation. Am J Rhinol 18(5):273–278
Conley DB, Tripathi A et al (2006) Superantigens and chronic rhinosinusitis: skewing of T-cell receptor V beta-distributions in polyp-derived CD4+ and CD8+ T cells. Am J Rhinol 20(5):534–539
Conley DB, Tripathi A et al (2006) Superantigens and chronic rhinosinusitis II: analysis of T-cell receptor V beta domains in nasal polyps. Am J Rhinol 20(4):451–455
Corriveau M, Zhang N et al (2009) Detection of Staphylococcus aureus in nasal tissue with peptide nucleic acid – fluorescence in situ hybridisation. Am J Rhinol 23(5):461–465
Damm M, Quante G et al (2004) Nasal colonization with Staphylococcus aureus is not associated with the severity of symptoms or the extent of the disease in chronic rhinosinusitis. Otolaryngol Head Neck Surg 131(3):200–206
Fokkens W, Lund V et al (2007) European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl (20):1–136
Fraser JD, Proft T (2008) The bacterial superantigen and superantigen-like proteins. Immunol Rev 225:226–243
Gevaert P, Holtappels G et al (2005) Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy 60(1):71–79
Gevaert P, Lang-Loidolt D et al (2006) Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol 118(5):1133–1141
Gould HJ, Takhar P et al (2007) The allergic march from Staphylococcus aureus superantigens to immunoglobulin E. Chem Immunol Allergy 93:106–136
Ha KR, Psaltis AJ et al (2008) In vitro activity of mupirocin on clinical isolates of Staphylococcus aureus and its potential implications in chronic rhinosinusitis. Laryngoscope 118(3):535–540
Harvey RJ, Lund VJ (2007) Biofilms and chronic rhinosinusitis: systematic review of evidence, current concepts and directions for research. Rhinology 45(1):3–13
Hauk PJ, Hamid QA et al (2000) Induction of corticosteroid insensitivity in human PBMCs by microbial superantigens. J Allergy Clin Immunol 105(4):782–787
Hofer MF, Harbeck RJ et al (1999) Staphylococcal toxins augment specific IgE responses by atopic patients exposed to allergen. J Invest Dermatol 112(2):171–176
Ivars F (2007) Superantigen-induced regulatory T cells in vivo. Chem Immunol Allergy 93:137–160
Jabara HH, Geha RS (1996) The superantigen toxic shock syndrome toxin-1 induces CD40 ligand expression and modulates IgE isotype switching. Int Immunol 8(10):1503–1510
Jarraud S, Peyrat MA et al (2001) egc, A highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J Immunol 166(1):669–677
Keith PK, Conway M et al (1994) Nasal polyps: effects of seasonal allergen exposure. J Allergy Clin Immunol 93(3):567–574
Kern RC, Conley DB et al (2008) Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol 22(6):549–559
Kim KS, Jacob N et al (2003) In vitro and in vivo T cell oligoclonality following chronic stimulation with staphylococcal superantigens. Clin Immunol 108(3):182–189
Lina G, Bohach GA et al (2004) Standard nomenclature for the superantigens expressed by Staphylococcus. J Infect Dis 189(12):2334–2336
Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339(8):520–532
Mandron M, Aries MF et al (2006) Human dendritic cells conditioned with Staphylococcus aureus enterotoxin B promote TH2 cell polarization. J Allergy Clin Immunol 117(5):1141–1147
Marone G, Rossi FW et al (2007) Role of superallergens in allergic disorders. Chem Immunol Allergy 93:195–213
Marrack P, Kappler J (1990) The staphylococcal enterotoxins and their relatives. Science 248(4956):705–711
Mladina R, Poje G et al (2008) Biofilm in nasal polyps. Rhinology 46(4):302–307
Newsom SW (2008) Ogston’s coccus. J Hosp Infect 70(4):369–372
Niederfuhr A, Kirsche H et al (2008) Staphylococcus aureus in nasal lavage and biopsy of patients with chronic rhinosinusitis. Allergy 63(10):1359–1367
Novick RP (2003) Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48(6):1429–1449
Ou LS, Goleva E et al (2004) T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J Allergy Clin Immunol 113(4):756–763
Patou J, Gevaert P et al (2008) Staphylococcus aureus enterotoxin B, protein A, and lipoteichoic acid stimulations in nasal polyps. J Allergy Clin Immunol 121(1):110–115
Perez-Novo CA, Kowalski ML et al (2004) Aspirin sensitivity and IgE antibodies to Staphylococcus aureus enterotoxins in nasal polyposis: studies on the relationship. Int Arch Allergy Immunol 133(3):255–260
Perez-Novo CA, Watelet JB et al (2005) Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol 115(6):1189–1196
Perez-Novo CA, Claeys C et al (2006) Eicosanoid metabolism and eosinophilic inflammation in nasal polyp patients with immune response to Staphylococcus aureus enterotoxins. Am J Rhinol 20(4):456–460
Perez-Novo CA, Waeytens A et al (2008) Staphylococcus aureus enterotoxin B regulates prostaglandin E2 synthesis, growth, and migration in nasal tissue fibroblasts. J Infect Dis 197(7):1036–1043
Plouin-Gaudon I, Clement S et al (2006) Intracellular residency is frequently associated with recurrent Staphylococcus aureus rhinosinusitis. Rhinology 44(4):249–254
Pujols L, Mullol J et al (2007) Alpha and beta glucocorticoid receptors: relevance in airway diseases. Curr Allergy Asthma Rep 7(2):93–99
Seiberling KA, Conley DB et al (2005) Superantigens and chronic rhinosinusitis: detection of staphylococcal exotoxins in nasal polyps. Laryngoscope 115(9):1580–1585
Seiberling KA, Grammer L et al (2005) Chronic rhinosinusitis and superantigens. Otolaryngol Clin North Am 38(6):1215–1236; ix
Simon HU, Yousefi S et al (1997) Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol 158(8):3902–3908
Suh YJ, Yoon SH et al (2004) Specific immunoglobulin E for staphylococcal enterotoxins in nasal polyps from patients with aspirin-intolerant asthma. Clin Exp Allergy 34(8):1270–1275
Thomas D, Chou S et al (2007) Diversity in Staphylococcus aureus enterotoxins. Chem Immunol Allergy 93:24–41
Tripathi A, Conley DB et al (2004) Immunoglobulin E to staphylococcal and streptococcal toxins in patients with chronic sinusitis/nasal polyposis. Laryngoscope 114(10):1822–1826
Tripathi A, Kern R et al (2005) Staphylococcal exotoxins and nasal polyposis: analysis of systemic and local responses. Am J Rhinol 19(4):327–333
Van Bruaene N, Perez-Novo CA et al (2008) T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol 121(6):1435–1441; 1441 e1–e3
Van Zele T, Gevaert P et al (2004) Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol 114(4):981–983
Van Zele T, Claeys S et al (2006) Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 61(11):1280–1289
Van Zele T, Gevaert P et al (2007) Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy 37(12):1840–1847
Van Zele T, Vaneechoutte M et al (2008) Detection of enterotoxin DNA in Staphylococcus aureus strains obtained from the middle meatus in controls and nasal polyp patients. Am J Rhinol 22(3):223–227
von Eiff C, Peters G et al (2006) The small colony variant (SCV) concept – the role of staphylococcal SCVs in persistent infections. Injury 37(suppl 2):S26–S33
Wang M, Shi P et al (2008) The role of superantigens in chronic rhinosinusitis with nasal polyps. ORL J Otorhinolaryngol Relat Spec 70(2):97–103
Wertheim HF, Melles DC et al (2005) The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5(12):751–762
Zhang N, Holtappels G et al (2006) Pattern of inflammation and impact of Staphylococcus aureus enterotoxins in nasal polyps from southern China. Am J Rhinol 20(4):445–450
Zhang N, Van Zele T et al (2008) Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol 122(5):961–968
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Tomassen, P. et al. (2010). Staphylococcus-aureus-derived Superantigens in Nasal Polyp Disease. In: Önerci, T., Ferguson, B. (eds) Nasal Polyposis. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-11412-0_10
Download citation
DOI: https://doi.org/10.1007/978-3-642-11412-0_10
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-11411-3
Online ISBN: 978-3-642-11412-0
eBook Packages: MedicineMedicine (R0)