Abstract
Barrett’s metaplasia is one of the commonest premalignant lesions in the western world following colorectal adenomas. One in 50 of the adult population develops Barrett’s as a consequence of chronic gastro-oesophageal reflux. The mucosal inflammation seen within patients with gastro-oesophageal reflux seems likely to drive the growth of the metaplastic mucosa and also help direct further oncological change, yet the molecular events that characterize the pathway from inflammation to metaplasia to dysplasia and adenocarcinoma are poorly understood. There is hope that understanding the role of oesophageal inflammation will provide important insight into the development of Barrett's metaplasia and oesophageal cancer. This chapter will discuss the inflammation seen within context of Barrett’s oesophagus and also clinical trials which hope to address this common premalignant disease. There are several ongoing clinical trials which are aiming to provide data using anti-inflammatory therapies to tackle this important premalignant condition. There is new data presented which suggests that data from the aspirin esomeprazole chemoprevention trial (AspECT) may hold the clue to disease treatment and that the cytokine TNF-α seems to be a key signalling molecule in the metaplasia–dysplasia–carcinoma sequence. Specifically it appears that both epigenetic and inherited genetics cooperate to modulate the prognosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Barrett’s metaplasia
- Oesophageal adenocarcinoma
- Inflammation
- Non-steroid anti-inflammatories
- Proton pump inhibitors
Oesophageal Adenocarcinoma (OA) is a fatal cancer and is increasing in incidence in the western world. OA is currently fifth in the most common of fatal cancers with annual incidence and death rates increasing by 3% per year (Jankowski and Hawk 2006).
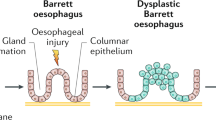
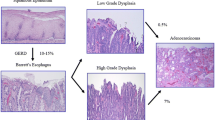
OA has a poor prognosis mainly because patients usually present at a late stage of the disease and the 5 year survival rates for the disease are about 16% (Jemal et al. 2008). Oesophageal Adenocarcinoma has been shown to be commonly associated with a premalignant condition called Barrett’s Metaplasia (BM) in which the stratified squamous epithelium of the oesophagus is replaced by a metaplastic columnar epithelium (Lagergren 2005; Nandurkar and Talley 1999). BM is thought to develop as a result of gastro-oesophageal reflux disease in which acid and bile reflux into the oesophagus (Eisen et al. 1997; Zhang et al. 2009). It follows that OA is thought to be a microcosm of evolution, developing sequentially along the metaplasia-dysplasia-adenocarcinoma sequence (Jankowski et al. 2000). Progression is attributed to a series of genetic and epigenetic events that ultimately allow for clonal selection of Barrett’s cells via subversion of intrinsic control mechanisms regulating cellular proliferation and/or apoptosis (Jankowski et al. 1999). This section will look at the implications and associations of inflammation in this progression and how we can target inflammation to help treat this fatal disease.
1 Inflammation in GI Cancer
The link between inflammation and cancer was first suggested by Rudolph Virchow in 1863 and now the epidemiological data shows a clear association between the two (Balkwill and Mantovani 2001; Coussens and Werb 2002). In the GI tract, many inflammatory tumours are seen and the cause of the inflammation can be infective; such as a virus or caused by a non-infective irritant; such as a chemical. A list of the associated GI cancers can be seen in Table 1 (Balkwill and Mantovani 2001).
Inflammation as a response to tissue damage is seen commonly in GI cancers (Fig. 1). Inflammation is part of the body's immune system; the immune system is comprised of the innate and adaptive systems. The innate system is the body’s first line of defense against pathogens and injury; it is a non-specific line of attack, comprised of inflammation, complement system and leukocytes and cells of the immune system. A more sustained and specific line of defense for the body is the adaptive immune system which comprises specific leukocytes called lymphocytes and antibody production. The adaptive immune system allows the immune system to recognise and remember specific pathogens and to mount a stronger attack next time the body comes into connect with it.
Inflammation is the very first response to damage or pathogens and is stimulated by chemical factors released by injured cells and serves to establish a physical barrier against the spread of infection. Also it promotes healing of any damaged tissue. Inflammation is normally self limiting; however, dysregulation of any of the converging factors can lead to abnormality and pathogenesis. During tissue injury associated with wounding, cell proliferation is enhanced while the tissue regenerates; proliferation and inflammation subside after the assaulting agent is removed or the repaired completed. In contrast, proliferating cells that sustain DNA damage and/or mutagenic assault continue to proliferate in microenvironments rich in inflammatory cells and growth/survival factors that support their growth. In a sense, tumours act as wounds that fail to heal (Dvorak 1986).
2 Gastro-Oesophageal Reflux Disease
Gastro-oesophageal reflux disease (GORD) is a common problem in western countries; it is thought that it is the most common complaint made to general physicians (Cameron et al. 1990). It is considered that around 50% of the population experience symptoms of heartburn or substantial burning sensation in the chest at least monthly and about 20% experience symptoms weekly (Dent et al. 2005). We know that obesity (El-Serag 2008), smoking and drinking alcohol are all considered risk factors associated with GORD (Friedenberg et al. 2008) and that any individual can make lifestyle choices such as healthy eating to help reduce their risk and improve their quality of life.
The causes of reflux disease can include one or more of the following;
-
Transient relaxation of the lower oesophageal sphincter
-
Decreased resting tone of the lower oesophageal sphincter
-
Impaired oesophageal clearance
-
Decreased salivation
-
Bile/acid regurgitation from the duodenum into the stomach
-
Delayed gastric emptying
More often than not patients also present with a hiatus or hiatal hernia, which prevents the efficient closing of the lower oesophageal sphincter, and thus leads to an increase in reflux of acid and bile salts into the oesophagus (Koek et al. 2008).
3 Inflammation and Gastro-Oesophageal Reflux Disease
As previously described reflux of caustic gastric contents into the oesophagus can cause symptoms such as heartburn and nausea, but these factors can also result in local tissue injury leading to erosive oesophagitis and stricture formation (Savarino and Dulbecco 2004). Upon injury, the damaged oesophageal cells start to release inflammatory mediators such as cytokines and chemokines, a list of the implicated substances are shown in Table 2. This leads to migration of inflammatory cells to the site of damaged tissue. Cells are recruited including T-lymphocytes, neutrophils and also there is the activation of inflammatory mediator nuclear factor kappa β (NF-κβ) (Balkwill and Mantovani 2001). The intended result of the inflammatory response is clearance of the causative agent which is subsequently followed by stem cell proliferation, mucosal remodelling and eventually healing (Jankowski et al. 2000). But if the causative agent is not removed and there is deregulation of these processes, it can lead to chronic inflammation and in around 10% of cases initiation of metaplasia (Jankowski et al. 2000). But why all patients experiencing GORD do not go onto develop metaplasia is still unclear, it is possible that the duration and severity of symptoms are important risk factors. The components of the inflammatory infiltrate have implications in deregulation of cellular proliferation as well as impacting on cellular adhesion and angiogenesis. The cytokine profile of Barrett’s oesophagus differs from oesophagitis, with an anti-inflammatory response characterised by increased levels of T Helper 2 cytokines and a reduction in signalling through the transforming growth factor b (TGF-b) pathway (Fitzgerald et al. 2002; Fitzgerald et al. 2002). In addition, it has been shown that Barrett’s oesophagus has a higher proportion of Th2 effector cells than Th1 effector cells when compared with reflux oesophagitis, demonstrating a shift to a humoral inflammatory response rather than the pro-inflammatory state that characterises oesophagitis (Moons et al. 2005). The importance of these changes is not fully understood. Additionally the inflammatory infiltrate may induce increased expression of FAS ligands on cells, which may protect them from immune surveillance and render them resistant to apoptosis (Younes et al. 2000).
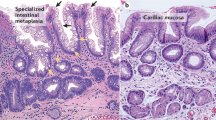
4 Inflammation and Barrett’s Metaplasia
In Barrett’s oesophagus, the native non-stratified squamous epithelium is replaced by a mucin-secreting columnar-lined intestinal-type epithelium, which is thought to be more resistant to continued duodeno-gastro-oesophageal reflux (Eksteen et al. 2001; Shaheen and Richter 2009). This change in cell type is hypothesised to be in response to a phenotypic change at the level of the pluripotent stem cells in the glands, native squamous oesophagus and oesophageal glands (Jankowski et al. 2000; Jankowski et al. 1999) and in response to stimulation by inflammatory cytokines and growth factors, some of which are detailed below. The stromal compartment of Barrett's oesophagus is increasingly being recognised to play a role in oesophageal carcinogenesis. A recent study by Saadi et al. (2009) showed that increased protein levels of inflammatory-related genes were significantly up-regulated in oesophageal adenocarcinoma.
4.1 TNF-α
Of the mediators of the NF-kB pathway TNF-α, in particular, has been shown to increase along the metaplasia-dysplasia-carcinoma sequence leading to an increase in the proto-oncogene c-myc via the β-catenin mediated pathway (Tselepis et al. 2002). TNF-α has been shown also to be expressed in the base of the Barrett’s glands, the proposed location of the stem cells (Tselepis et al. 2002). As well as having implications with increasing proliferation TNF-α and other pro-inflammatory cytokines are able to modulate the levels of both pro and anti-apoptotic proteins Bcl-2 and Bax. A study by the author (unpublished) has shown that TNF-α can cause migration in Barrett’s cells (Fig. 2).
TNF-α causes migration in Barretts cell lines (Qh-TERT), the effect is abrogated by the TNF- α inhibitor Infliximab. (a) Nitrocellulose membranes showing migrated cells. Cells cultured with low serum do not migrate through the membrane. Cells cultured in low serum with added TNF-α do migrate, an effect which is removed by the addition of the TNF-α inhibitor Infliximab, as a control cells cultured in 10% serum also migrate through the membrane. (b) Graphical representation of 4 experiments testing the migration of Barrett’s cells cultured with TNF-α. TNF-α causes a significant increase in migration in Barretts cells (p < 0.001) and this effect is removed by Infliximab (p < 0.001)
4.2 COX-2
Cyclooxygenase-2 (COX-2) is another inducible agent with carcinogenic properties. Activation of NF-kβ possibly via TNF-α signalling can also lead to an increase in COX-2. Expression of COX-2 has been shown to be significantly increased in Barrett’s oesophagus before dyplasia development, suggesting a role early on in the Barrett’s malignancy (Wilson et al. 1998). COX-2 and derived prostaglandins including prostaglandin E2 (PGE2) can contribute to malignancy by inhibiting apoptosis, increasing proliferation and angiogenesis and inducing the production of matrix metalloproteinases (MMPs). Also COX-2 can be induced by other cytokines including IL-1α and IL-1β.
4.3 NF-κB
The transcription factor NF-κB is largely implicated in oesophageal carcinogenesis. Prior to activation most NF-κB molecules are retained in the cytoplasm, bound to one of the IκB (inhibitor of NF-κB) proteins. Upon stimulation, the IKK (IκB kinase) complex is activated which phosphorylates NF-κB-bound IκB and targets them for polyubiquitination and ultimately degradation. This allows NF-κB to enter the nucleus and bind to κB-regulatory elements and co-ordinate the transcriptional activation of many immune response genes (Baron et al. 2008; Mathers et al. 2003; Logan et al. 2008). This is known as the classical NF-κB signalling pathway and is triggered in response to pro-inflammatory cytokines and micro-organisms. Once activated, NF-κB regulates expression of over 200 genes, including genes encoding cell adhesion molecules and immune response genes including cytokines, and cell proliferation (Fitzgerald et al. 2002; O’Riordan et al. 2005). Barrett’s cells have shown to activate the NF-κB pathway as a way of avoiding apoptosis when they sustain damage, whereas normal squamous cells of the oesophagus undergo apoptosis when damaged by mutagens (Hormi-Carver et al. 2009). Most recently a study by Duggan et al. highlighted pseudokinase tribbles homolog 3 (TRB3) as a novel gene involved in the regulation of inflammation through modulation of NF-kB function (Duggan et al. 2010).
4.4 Interleukins
This group of cytokines which are produced by a variety of the body cells, function to promote the development and differentiation of T, B, and haematopoietic cells. Within the development of Barrett’s, interleukins 1b, 4, 8, and 10 have all been implicated in the disease progression. IL-1β is a pro-inflammatory cytokine involved in immune defense against infection. Studies of the inflammatory gradient in Barrett’s oesophagus show the levels of IL-1β are significantly increased at the proximal end of the segment (closest to the new squamocolumner junction) and were actually higher in the inflamed squamous than the distal Barrett’s (Fitzgerald et al. 2002). IL-8, a chemokine produced by macrophages, was also up-regulated in the proximal Barrett’s and has also been seen to be up-regulated in dysplasia and adenocarcinoma, however anti-reflux surgery can modulate its expression (Oh et al. 2007). IL-10, an anti-inflammatory cytokine, has been shown to be increased distally to the leading edge of the Barrett’s, and its expression up-regulated in association with adenocarcinoma (Fitzgerald et al. 2002). Finally alongside IL-10, IL-4 has been shown to be expressed specifically in Barrett’s and characterised by a distinct Th2 predominant cytokine profile not seen in oesophagitis. In a study by Nguyen et al., several interleukins were each associated with poor prognosis in oesophageal adenocarcinoma and profiling these genes in tissues may have clinical utility as predictors of prognosis (Nguyen et al. 2010).
It is thought that to become cancerous a cell must undergo approximately four to seven genetic alterations in either up-regulation of oncogenes or down regulation of tumour suppressor genes. Although acid and bile have a role to play in remodelling the mucosa, such as inducing DNA damage or affecting tissue differentiation, evidence showing that once reflux disease is corrected by acid suppression drugs, Barrett’s never totally regresses points to maintainance of the metaplasia and promotion to adenocarcinoma by the mild chronic inflammatory infiltrate (Fukata and Abreu 2008).
4.5 p53
The inflammatory response and reactive oxygen species have been shown to cause mutations in genes which play an important role in carcinogenesis, one very important tumour suppressor gene involved in Barrett’s carcinogenesis is p53. p53 mutations have been shown in Barrett’s oesophagus and in low grade dysplasia, although these mutations are thought not to be the ones seen to clonally expand to progress to carcinoma. Although in High-grade dysplasia and cancer the frequency of mutations increases dramatically, with an even high rate of allelic loss of the p53 locus. A few prospective studies of p53 immunohistochemistry have shown that patients with p53 over-expression in low-grade dysplasia have an increased risk of progressing to high-grade dysplasia and cancer (Kim et al. 1997; Weston et al. 2001; Younes et al. 1997).
Although p53 is mutated early in Barrett’s there is an increase in expression as tissue becomes dysplastic. p53 is a tumour suppressor protein which has shown to be inactivated by a two-hit mechanism involving loss of heterozygosity of one allele and mutation or methylation of the second (Chao et al. 2008; Wong et al. 1997). It is also thought that it is the mutational changes occurring late in the dysplastic sequence which clonally expand throughout the region and drive the dysplasia to carcinoma.
4.6 CDX1 and CDX2
The embryonic gut is glandular throughout and CDX1 and CDX2 are homeobox proteins which play major roles in the development of the intestine, CDX2 expression arises in the proximal intestine and declines distally, whereas CDX1 expression arises in the distal intestine with overlap of both in the mid-gut. It is possible that in humans, injurious agents present in GORD activate ectopic expression of CDX1 through NF-κ signalling which in turn initiates the development of the intestinal phenotype seen with Barrett’s oesophagus. Wong et al. (2005) have found CDX1 mRNA and protein expression in all samples of Barrett’s metaplasia tested but not in normal oesophageal squamous or gastric body epithelia. Conjugated bile salts and inflammatory cytokines tumour necrosis factor alpha(TNF-alpha) and interleukin 1b (IL-1b) were found to increase CDX2 mRNA expression in vitro through NF-κB signalling. CDX2 may cooperate with CDX1 in inducing and maintaining a complete intestinal phenotype.
4.7 p16
p16 (CDKN2a/INK4a) is a cyclin dependant kinase inhibitor that regulates the cell cycle at G1/S control. Germ line mutations in p16 have been shown in melanomas but also somatic mutations in the gene have been implicated in many cancers including oesophageal adenocarcinoma. Alterations in the gene can occur as mutations, loss of heterozygosity and promoter hypermethylation (Barrett et al. 1999). p16 alterations occur early in the MDA sequence and mutated lesions have the ability to undergo clonal expansion, creating a field in which other abnormalities can arise that can lead to adenocarcinoma (Wong et al. 2001). In detail, growth advantages result in preferential expansion of a mutated clone and a mutation is said to have “gone to fixation” when it expands throughout an entire field, extinguishing all competing clones. A “selective sweep” is the process of natural selection driving a mutation to fixation. It has been suggested that loss of each of the two p16 alleles predisposes to a selective sweep, and that p16 mutation fixation occurs early in the progression of Barrett’s oesophagus (Maley et al. 2004).
4.8 Reactive Oxygen Species
The inflammatory cells including neutrophils produce reactive oxygen species (ROS) whose primary role is to remove the damaged cells but they can also induce genetic mutations, which can contribute to DNA damage (Clemons et al. 2007; Jaiswal et al. 2001). While most of these changes will lead to cell death, others may confer a survival advantage and lead to a clonal expansion of the premalignant Barrett’s cell type (Atherfold and Jankowski 2006). High levels of ROS have been identified in ulcerated gastro-oesophageal mucosa. The production of ROS can also lead to the further increase in NF-kB activity, thus enhancing the overall inflammatory response (Fig. 3).
4.9 Polymorphisms in Inflammatory Agents
There has been little work into finding polymorphisms implicated in the oesophagus but some have been identified to affect cancer risk in other GI diseases. In H. pylori induced gastric cancer individuals with the IL-1B-31*C or -511*T and IL-1RN*2/*2 genotypes are at increased risk of developing hypochlorhydria and gastric atrophy in response to H. pylori infection. In addition to IL-1 gene cluster polymorphisms, pro-inflammatory genotypes of TNF-α and IL-10 have also been identified as risk factors for gastric cancer (El-Omar et al. 2000; Figueiredo et al. 2002).
With these genetic changes and new microenvironment produced by the infiltrating inflammatory cells, the pluripotent stem cells in Barrett’s glands/native oesophagus/oesophageal glands are able to acquire the mutations they need to result in the observed metaplastic change while avoiding cell apoptosis or atrophy. Although all these changes occur when the normal squamous oesophagus changes into Barrett’s oesophagus, only a small percentage (0.5–2%) of patients with Barrett’s will progress to adenocarcinoma there is a need to identify biomarkers to identify these patients at risk (Tischoff and Tannapfel 2008; Fitzgerald 2005). There are a few biomarkers which have been investigated but none have yet to be taken into the clinic (Kerkhof et al. 2007). Most recently a large scale genome wide association study is being undertaken in patients with Barrett's oesophagus, this work is being carried out by the Eagle consortium as part of the WTCCC2. It is hoped that this study will identify common variants in alleles which predispose people to developing Barrett's oesophagus.
5 Anti-Inflammatory Therapies and Clinical Management of Oesophageal Adenocarcinoma
5.1 Proton Pump Inhibitors
Reflux associated diseases including Barrett’s are treated with proton pump inhibitors (PPI’s) to help reduce the reflux and prevent neoplastic change associated with the infiltrate, although it has been shown that there are patients who do not respond to PPI’s (Fass and Sifrim 2009). PPI’s have numerous beneficial effects including symptoms control, reduction of inflammation and promotion of the development of squamous islands (Shaheen 2005). However even with these benefits PPI therapy has been shown to cause hypergastrinemia and has not prevented recent increase in the incidence of oesophageal cancer.
5.2 Non-Steroidal Anti-Inflammatories
Aspirin and other non-steroidal anti-inflammatory drugs are increasingly used in the treatment of the disease and are thought to be implicated early in the disease progression. The use of non-steroidal anti-inflammatory drugs (NSAIDs) has been shown to reduce the incidence of several cancers associated with chronic inflammation. The principle target of NSAIDs is cyclo-oxygenase (COX).
5.2.1 COX
COX converts arachidonic acid to prostaglandins and exists as two isoforms, COX-1 is expressed on many tissues and maintains physiological funcions, whereas COX-2 is expressed at low levels in normal tissues and is up-regulated by pro-inflammatory cytokines, growth factors and tumour promoters. COX-2 has been shown to be over-expressed in patients with Barrett’s metaplasia and oesophageal adenocarcinoma (Fullard et al. 2006) and also tumours with high expression have been shown to have a more aggressive phenotype (Buskens et al. 2002). Recent advances in the understanding of the cellular and molecular mechanisms of the anti-cancer effects of NSAIDs and COX-2 inhibitors have demonstrated that these drugs target both tumour cells and the tumour vasculature.
5.2.2 Studies into Using NSAID’s
Aspirin is commonly used as an NSAID in chemoprevention (Bosetti et al. 2009), it has multiple effects on the inflammatory process, and several studies have tried to assess whether users of aspirin or other NSAIDs have an associated reduced risk of oesophageal cancers. Most importantly, a recent meta-analysis performed by Rothwell and colleagues showed that daily aspirin can reduce the risk of death in several common cancers (Rothwell et al. 2011).
A large study by Thun et al. (Jemal et al. 2008) showed a reduced risk reduction by 40% for oesophageal cancer in recurrent users of aspirin. From a meta-analysis undertaken by Corley et al. (Corley et al. 2003) it was observed that the overall protective effect of aspirin is 50% compared to 25% for other NSAIDs, but the use of these drugs has to be frequent to achieve this association. There are thoughts that the patients who take aspirin frequently will also be undertaking other lifestyle choices such as vitamins and that this may be having additional effects, also there are concerns over the toxicity of NSAIDs, which are associated with GI bleeds. This shows that evidence from prospective studies such as the one by Thun and Rothwell can give limited data and what is really required is a large scale clinical trial (Moayyedi et al. 2010).
6 Clinical Trials and Future Treatment of Acid Reflux and Oesophageal Cancer
6.1 Current Treatment for Oesophageal Adenocarcinoma
The main treatment options for patients presenting with high-grade dysplasia or Oesophageal adenocarcinoma is currently surgery but this has a low 5 year survival rate.
Observational studies have shown that NSAID use reduces the risk of disease progression in Barrett’s Oesophagus (Vaughan et al. 2005). There are still concerns over the use of anti-inflammatories after the Victor trial was ended after a significant increase in the cardiovascular adverse events (Kerr et al. 2007). Although aspirin has been shown to be effective in colorectal adenomas in patients with a history of lesions (Cole et al. 2009).
6.2 Anti-TNF Therapy
Previously in this report TNF-α has been shown to be implicated in Oesophageal adenocarcinoma by upregulating oncogene transcription, TNF-α is shown to be a possible therapeutic target for cancer and in 2004 the first clinical trial using TNF-anatagonists in cancer treatment were undertaken in Breast cancer (Madhusudan et al. 2004), the trial showed safety and biological activity of the treatment and further trials in other advanced cancers have confirmed this (Harrison et al. 2007; Brown et al. 2008). There is now a potential to investigate the use of anti-TNF therapy in the treatment of Barrett’s metaplasia/barrett’s adenocarcinoma.
6.3 Aspirin and PPI’s
The AspECT trial got underway in 2005 with recruitment reaching its target of 2500 patients in February 2009. It is a national, multi-centred, phase III clinical trial recruiting Barrett’s patients to one of four arms, consisting of low or high dose PPI’s and aspirin or no aspirin long term. The trial aims to follow-up patients for 10 years and will report on the incidence of end points including high-grade dysplasia or adenocarcinoma. After 3 years more than 85% of patients tolerated their initial dose of medicine and the drop out rate has been 7%, an interim analysis is expected in 2011 (Das et al. 2009).
6.4 Surveillance of Barrett’s Patients
There are questions being asked about the surveillance of Barrett’s patients and how we can better manage this condition (Armstrong 2008; Barritt and Shaheen 2008). Since only 0–2% of Barrett’s patients go onto develop adenocarcinoma there is a question over the need for constant endoscopy, better data are required to determine whether patients with mild gastro-oesophageal reflux disease would benefit from increased surveillance (Fullard et al. 2006) and linked to the AspECT trial, the BOSS study will challenge this question in Barrett’s patients (Jankowski and Barr 2006).
6.5 Other Studies
There are several other clinical trials which are investigating the effects of anti-inflammatories in GI cancer (Jankowski and Hawk 2006) (Table3). As well as Victor there were other studies which showed an increase in cardiovascular events after taking anti-inflammatories but none have been seen in the AspECT trial. Hopefully a combined therapy of aspirin and PPI’s can be shown to have a significant effect on the development of adenocarcinoma and therefore we can be able to control the increase in cases of these cancers being seen in the west.
In this regard it is possible that PPI therapy may abrogate the GI ulcer complications seen with low dose aspirin therapy (Cuzick et al. 2009; Jankowski and Hunt 2008). Recently an expert independent review has nominated aspirin as the first choice chemoprevention agent for testing in the clinic (Cuzick et al. 2009).
References
Armstrong D (2008) Should patients with Barrett’s oesophagus be kept under surveillance? The case for. Best Pract Res Clin Gastroenterol 22(4):721–739
Atherfold PA, Jankowski JA (2006) Molecular biology of Barrett’s cancer. Best Pract Res Clin Gastroenterol 20(5):813–827
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357(9255):539–545
Baron JA et al (2008) Cardiovascular events associated with rofecoxib: final analysis of the APPROVe trial. Lancet 372(9651):1756–1764
Barrett MT et al (1999) Evolution of neoplastic cell lineages in Barrett oesophagus. Nat Genet 22(1):106–109
Barritt AS, Shaheen NJ (2008) Should patients with Barrett’s oesophagus be kept under surveillance? The case against. Best Pract Res Clin Gastroenterol 22(4):741–750
Benamouzig R et al (2001) APACC, a French prospective study on aspirin efficacy in reducing colorectal adenoma recurrence: design and baseline findings. Eur J Cancer Prev 10(4):327–335
Benamouzig R et al (2003) Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology 125(2):328–336
Bosetti C, Gallus S, La Vecchia C (2009) Aspirin and cancer risk: a summary review to 2007. Recent Results Cancer Res 181:231–251
Brown ER et al (2008) A clinical study assessing the tolerability and biological effects of infliximab, a TNF-alpha inhibitor, in patients with advanced cancer. Ann Oncol 19(7):1340–1346
Buskens CJ et al (2002) Prognostic significance of elevated cyclooxygenase 2 expression in patients with adenocarcinoma of the esophagus. Gastroenterology 122(7):1800–1807
Cameron AJ et al (1990) Prevalence of columnar-lined (Barrett’s) esophagus. Comparison of population-based clinical and autopsy findings. Gastroenterology 99(4):918–922
Chao DL et al (2008) Cell proliferation, cell cycle abnormalities, and cancer outcome in patients with Barrett’s esophagus: a long-term prospective study. Clin Cancer Res 14(21):6988–6995
Clemons NJ, McColl KE, Fitzgerald RC (2007) Nitric oxide and acid induce double-strand DNA breaks in Barrett’s esophagus carcinogenesis via distinct mechanisms. Gastroenterology 133(4):1198–1209
Cole BF et al (2009) Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomised trials. J Natl Cancer Inst 101(4):256–266
Corley DA et al (2003) Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 124(1):47–56
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867
Cuzick Jack, Otto Florian, Baron John A, Brown Powel H, Burn John, Greenwald Peter, Jankowski Janusz, Vecchia Carlo La, Meyskens Frank, Senn Hans Jörg, Thun Michael (2009) Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol 10:501–507
Das D, Chilton AP, Jankowski JA (2009) Chemoprevention of oesophageal cancer and the AspECT trial. Recent Results Cancer Res 181:161–169
Dent J et al (2005) Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 54(5):710–717
Duggan SP et al (2010) An integrative genomic approach in oesophageal cells identifies TRB3 as a bile acid response gene, which regulates NF-kappaB activation and cytokine levels. Carcinogenesis 31(5): 936-945
Dvorak HF (1986) tumours: wounds that do not heal Similarities between tumour stroma generation and wound healing. N Engl J Med 315(26):1650–1659
Eisen GM et al (1997) The relationship between gastroesophageal reflux disease and its complications with Barrett’s esophagus. Am J Gastroenterol 92(1):27–31
Eksteen JA et al (2001) Inflammation promotes Barrett’s metaplasia and cancer: a unique role for TNFalpha. Eur J Cancer Prev 10(2):163–166
El-Omar EM et al (2000) Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404(6776):398–402
El-Serag H (2008) Role of obesity in GORD-related disorders. Gut 57(3):281–284
Fass R, Sifrim D (2009) Management of heartburn not responding to proton pump inhibitors. Gut 58(2):295–309
Figueiredo C et al (2002) Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst 94(22):1680–1687
Fischbach LA et al (2001) Anti-inflammatory and tissue-protectant drug effects: results from a randomised placebo-controlled trial of gastritis patients at high risk for gastric cancer. Aliment Pharmacol Ther 15(6):831–841
Fitzgerald RC (2005) Genetics and prevention of oesophageal adenocarcinoma. Recent Results Cancer Res 166:35–46
Fitzgerald RC et al (2002a) Inflammatory gradient in Barrett’s oesophagus: implications for disease complications. Gut 51(3):316–322
Fitzgerald RC et al (2002b) Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut 50(4):451–459
Friedenberg FK et al (2008) The association between gastroesophageal reflux disease and obesity. Am J Gastroenterol 103(8):2111–2122
Fukata M, Abreu MT (2008) Role of toll-like receptors in gastrointestinal malignancies. Oncogene 27(2):234–243
Fullard M et al (2006) Systematic review: does gastro-oesophageal reflux disease progress? Aliment Pharmacol Ther 24(1):33–45
Harrison ML et al (2007) Tumour necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J Clin Oncol 25(29):4542–4549
Heath EI et al (2003) Chemoprevention for Barrett’s esophagus trial. Design and outcome measures. Dis Esophagus 16(3):177–186
Heath EI et al (2007) Secondary chemoprevention of Barrett’s esophagus with celecoxib: results of a randomised trial. J Natl Cancer Inst 99(7):545–557
Hormi-Carver K et al (2009) Unlike esophageal squamous cells, Barrett’s epithelial cells resist apoptosis by activating the nuclear factor-kappa B pathway. Cancer Res 69(2):672–677
Jaiswal M, LaRusso NF, Gores GJ (2001) Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol 281(3):G626–G634
Jankowski J, Barr H (2006) Improving surveillance for Barrett’s oesophagus: AspECT and BOSS trials provide an evidence base. Bmj 332(7556):1512
Jankowski JA, Hawk ET (2006) A methodologic analysis of chemoprevention and cancer prevention strategies for gastrointestinal cancer. Nat Clin Pract Gastroenterol Hepatol 3(2):101–111
Jankowski J, Hunt R (2008) Cyclooxygenase-2 inhibitors in colorectal cancer prevention; better the devil you know. Cancer Epidemiol Biomarkers Prev 17:1858–1861
Jankowski JA et al (1999) Molecular evolution of the metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Pathol 154(4):965–973
Jankowski JA et al (2000) Barrett’s metaplasia. Lancet 356(9247):2079–2085
Jemal A et al (2008) Cancer statistics, 2008. CA Cancer J Clin 58(2):71–96
Kerkhof M et al (2007) Biomarkers for risk stratification of neoplastic progression in Barrett esophagus. Cell Oncol 29(6):507–517
Kerr DJ et al (2007) Rofecoxib and cardiovascular adverse events in adjuvant treatment of colorectal cancer. N Engl J Med 357(4):360–369
Kim R et al (1997) Expression of p53, PCNA, and C-erbB-2 in Barrett’s metaplasia and adenocarcinoma. Dig Dis Sci 42(12):2453–2462
Koek GH et al (2008) Multivariate analysis of the association of acid and duodeno-gastro-oesophageal reflux exposure with the presence of oesophagitis, the severity of oesophagitis and Barrett’s oesophagus. Gut 57(8):1056–1064
Lagergren J (2005) Adenocarcinoma of oesophagus: what exactly is the size of the problem and who is at risk? Gut 54(Suppl 1):i1–i5
Logan RF et al (2008) Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology 134(1):29–38
Madhusudan S et al (2004) A phase II study of etanercept (Enbrel), a tumour necrosis factor alpha inhibitor in patients with metastatic breast cancer. Clin Cancer Res 10(19):6528–6534
Maley CC et al (2004) Selectively advantageous mutations and hitchhikers in neoplasms: p16 lesions are selected in Barrett’s esophagus. Cancer Res 64(10):3414–3427
Mathers JC et al (2003) Can resistant starch and/or aspirin prevent the development of colonic neoplasia? The Concerted Action Polyp Prevention (CAPP) 1 Study. Proc Nutr Soc 62(1):51–57
Moayyedi P, Jankowski JA (2010) Does long-term aspirin prevent cancer? BMJ 341
Moons LM et al (2005) Barrett’s oesophagus is characterised by a predominantly humoral inflammatory response. J Pathol 207(3):269–276
Nandurkar S, Talley NJ (1999) Barrett’s esophagus: the long and the short of it. Am J Gastroenterol 94(1):30–40
Nguyen GH et al (2010) Inflammatory and microRNA gene expression as prognostic classifier of Barrett's-associated esophageal adenocarcinoma. Clin Cancer Res 16(23):5824–5834
O’Riordan JM et al (2005) Proinflammatory cytokine and nuclear factor kappa-B expression along the inflammation-metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Gastroenterol 100(6):1257–1264
Oh DS et al (2007) Reduction of interleukin 8 gene expression in reflux esophagitis and Barrett’s esophagus with antireflux surgery. Arch Surg 142(6):554–559 (discussion 559–560)
Pendlebury S et al (2003) A trial of adjuvant therapy in colorectal cancer: the VICTOR trial. Clin Colorectal Cancer 3(1):58–60
Rothwell PM et al (2011) Effect of daily Aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377(9759):31–41
Saadi A et al (2009) Stromal genes discriminate preinvasive from invasive disease, predict outcome, and highlight inflammatory pathways in digestive cancers. PNAS 107(5):2177–2182
Savarino V, Dulbecco P (2004) Optimizing symptom relief and preventing complications in adults with gastro-oesophageal reflux disease. Digestion 69(Suppl 1):9–16
Shaheen NJ (2005) Advances in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterology 128(6):1554–1566
Shaheen NJ, Richter JE (2009) Barrett’s oesophagus. Lancet 373(9666):850–861
Tischoff I, Tannapfel A (2008) Barrett’s esophagus: can biomarkers predict progression to malignancy? Expert Rev Gastroenterol Hepatol 2(5):653–663
Tselepis C et al (2002) Tumour necrosis factor-alpha in Barrett’s oesophagus: a potential novel mechanism of action. Oncogene 21(39):6071–6081
Vaughan TL et al (2005) Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett’s oesophagus: a prospective study. Lancet Oncol 6(12):945–952
Wang Y et al (2008) Negative feedback regulation of IFN-gamma pathway by IFN regulatory factor 2 in esophageal cancers. Cancer Res 68(4):1136–1143
Weston AP et al (2001) p53 protein overexpression in low grade dysplasia (LGD) in Barrett’s esophagus: immunohistochemical marker predictive of progression. Am J Gastroenterol 96(5):1355–1362
Wilson KT et al (1998) Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett’s esophagus and associated adenocarcinomas. Cancer Res 58(14):2929–2934
Wong DJ et al (1997) p16INK4a promoter is hypermethylated at a high frequency in esophageal adenocarcinomas. Cancer Res 57(13):2619–2622
Wong DJ et al (2001) p16(INK4a) lesions are common, early abnormalities that undergo clonal expansion in Barrett’s metaplastic epithelium. Cancer Res 61(22):8284–8289
Wong NA et al (2005) CDX1 is an important molecular mediator of Barrett’s metaplasia. Proc Natl Acad Sci USA 102(21):7565–7570
Younes M et al (1997) p53 Protein accumulation is a specific marker of malignant potential in Barrett’s metaplasia. Dig Dis Sci 42(4):697–701
Younes M et al (2000) Decreased expression of Fas (CD95/APO1) associated with goblet cell metaplasia in Barrett’s esophagus. Hum Pathol 31(4):434–438
Zhang HY, Spechler SJ, Souza RF (2009) Esophageal adenocarcinoma arising in Barrett esophagus. Cancer Lett 275(2):170–177
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Velag Berlin Heidelberg
About this chapter
Cite this chapter
Nicholson, A., Jankowski, J. (2011). Acid Reflux and Oesophageal Cancer. In: Jankowski, J. (eds) Inflammation and Gastrointestinal Cancers. Recent Results in Cancer Research, vol 185. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-03503-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-03503-6_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-03502-9
Online ISBN: 978-3-642-03503-6
eBook Packages: MedicineMedicine (R0)