Abstract
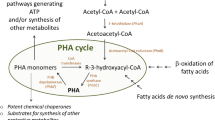
Polyhydroxyalkanoates (PHAs) are energy- and intracellular carbon-storage compounds that can be mobilized and used when carbon is a limiting resource. Intracellular accumulation of PHA enhances the survival of several bacterial species under environmental stress conditions imposed in water and soil, such as UV irradiation, salinity, thermal and oxidative stress, desiccation, and osmotic shock. The ability to endure these stresses is linked to a cascade of events concomitant with PHA degradation and the expression of genes involved in protection against damaging agents. PHA synthesis involves enzymatic and transcriptional regulation, where the RpoS central stationary phase regulator sigma factor has been shown to be implicated. The energy generated during PHA degradation can also be used to drive various important energy-consuming pathways. In addition to its relevance for the plastic industry, PHA has important applications for agriculture, as those related to the production of reliable commercial inoculants, and in controlled release of insecticides when incorporated into degradable PHA granules.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
A wide variety of taxonomically different groups of microorganisms (Bacteria and Archaea domains) produce intracellular homopolymers or copolymers containing different alkyl groups at the β position, described as polyhydroxyalkanoates (PHAs). These polymers are used as energy- and carbon-storage compounds (Anderson and Dawes 1990). PHAs are structurally simple macromolecules that accumulate inside discrete granules to levels that can be as high as 90% of the cell dry weight. They are generally believed to play a role as a sink for carbon and reducing equivalents when other nutrient supplies are limiting resources, and when the bacterial population is not growing exponentially in batch culture (Senior and Dawes 1973; Williams and Peoples 1996; Madison and Huisman 1999). These molecules exhibit material features that are similar to those of some common plastics such as polypropylene (Williams and Peoples 1996; Madison and Huisman 1999). In this chapter we will mainly focus on the ecological significance of PHAs.
Since the identification and characterization of the enzymes involved in PHA synthesis, hundreds of genes from a wide range of prokaryotes have been cloned or identified as putative PHA biosynthesis genes. Microorganisms use different pathways for synthesis of PHA. One of the best studied PHAs is poly[(R)-3-hydroxybutyrate] (PHB). Among the enzymes involved in PHB synthesis, β-ketothiolase (PhaA) and acetoacetyl-CoA reductase (PhaB) are involved in general lipid metabolism. In contrast, a third enzyme, PHA synthase (PhaC), is exclusively involved in the biosynthesis of this polymer, being responsible for the polymerization of β-hydroxyalkanoyl-CoA monomers into poly(β-hydroxyalkanoate) (Pötter and Steinbüchel 2005; Philip et al. 2007). In addition to these enzymes, a noncatalytic group of proteins called phasins (PhaPs) are important for granule organization. PhaPs have been found associated with the surface of the granules, being involved in their stabilization and coalescence, and their absence has a significant effect on polymer synthesis (Pötter and Steinbüchel 2005).
The PHA degradation pathway as described in most bacteria studied begins with the depolymerization of PHA to d-3-hydroxybutyrate monomers by PHA depolymerase (encoded by phaZ). Extracellular and intracellular PHA degradation have been described (Jendrossek and Handrick 2002) for utilization of PHAs present in the environment or accumulated in PHA granules, respectively (Tanio et al. 1982; Saegusa et al. 2001; Jendrossek and Handrick 2002; Pötter and Steinbüchel 2005).
2 The Role of PHA in Cell Survival Under Stress
PHAs have attracted attention as environmentally friendly polymers owing to their biodegradability, thermoplastic properties, and biocompatibility (Philip et al. 2007). Consequently, many resources have been invested in the isolation of microorganisms capable of synthesizing PHAs with different desirable industrial properties and from different sources. The function of PHAs as intracellular carbon-storage compounds has been the subject of most of the research in assigning a role for these polymers (Macrae and Wilkinson 1958; Sierra and Gibbons 1962; Hippe 1967; Hippe and Schlegel 1967). However, with the identification of new PHA-synthesizing microorganisms and the investigation of the role that PHAs play in bacterial fitness, it became evident that this polymer is more than just an intracellular carbon-storage compound that can be mobilized and used when carbon becomes a limiting resource. It is actually known that intracellular accumulation of PHAs enhances the survival of several bacteria under environmental stress conditions imposed in water and in the soil (Kadouri et al. 2005; Zhao et al. 2007). In other words, PHAs endow bacteria that are able to synthesize them with an ecological advantage. The roles played by PHAs in bacterial environmental fitness are summarized in Table 1.
Under certain circumstances, free-living bacterial cells with a high content of PHAs may survive longer than those that lack PHA or have a low PHA content, either because they are protected from adverse factors, or because they can utilize their reserve material longer and more efficiently than those bacteria that produce low contents of PHA or lack this ability at all (Dawes and Senior 1973; Matin et al. 1979; Kadouri et al. 2002). For example, the PHB-producing bacterium Azospirillum brasilense showed increased survival upon starvation in phosphate buffer as compared with its non-PHB-producing mutant (phaC minus mutant) (Fig. 1). As in A. brasilense, in Sinorhizobium meliloti, other plant-associated bacteria, and in the PHB-accumulating Pseudomonas sp. isolated from an Antarctic environment, PHA content was also shown to correlate positively with increased survival rates after exposure to adverse conditions such as salinity, thermal and oxidative stress, UV irradiation, desiccation, and osmotic pressure (Tal and Okon 1985; Ayub et al. 2004; Arora et al. 2006).
The effect of poly[(R)-3-hydroxybutyrate] (PHB) on survival capability of starved bacteria. Cells of Azospirillum brasilense Sp7 (filled triangles) and phaC mutant (filled circles) were grown on a medium with a high carbon to nitrogen ratio for 24 h and transferred to phosphate buffer, where they were incubated for 12 days. Bacterial density was determined using dilution plating (Reproduced from Kadouri et al. 2002, with kind permission from the American Society for Microbiology)
Proteomics-based research coupled with chemical determination of PHA content revealed that the denitrifying proteobacterium Aromatoleum aromaticum accumulates PHB during growth in the presence of the pollutant solvents toluene and ethylbenzene (Trautwein et al. 2008). The authors suggested that PHB formation in this bacterium is not induced by an imbalanced nutrient supply but rather by stress due to impaired coupling of alkylbenzene catabolism and denitrification. It was proposed that PHB could serve as a sink for reducing equivalents, ensuring continuous alkylbenzene degradation, and/or a type of hydrophobic trap for aromatic compounds (Trautwein et al. 2008).
Legionella pneumophila also accumulates PHA. The persistence of this bacterium in the environment is aided by its ability to adapt to a variety of different ecological niches, as intracellular parasites of amebae, as free-living members of complex biofilm communities, or as planktonic cells. In this bacterium, PHA accumulation in granules supports long-term survival in the culturable state under starvation, and accumulated PHA serves as an energy-reserve material to promote persistence of legionellae in stressful low-nutrient environments outside the amebic host (James et al. 1999).
Evidence has been provided suggesting that spore formation and germination, as well as cyst production, may be related to PHA biosynthesis and utilization. In Bacillus cereus and in Clostridium botulinum, PHA is accumulated maximally just prior to the formation of spores and is degraded during the process of sporulation (Valappil et al. 2007). In these cases, PHA may serve as a carbon and energy source for sporulation (Kominek and Halvorson 1965; Nakata 1965; Emeruwa and Hawirko 1973). López et al. (1995) observed that in a PHA negative mutant of B. megaterium, in contrast to the wild type, sporulation occurred immediately after exposure to river water, and survival of vegetative cells was clearly compromised, suggesting that in an oligotrophic environment cells depleted of an intracellular carbon source may be committed to earlier sporulation than normal cells. A heat shock was required for germination of PHA negative mutant spores, suggesting that PHA or its degradation products are involved in this process (López et al. 1995).
In Azotobacter vinelandii, PHA is utilized as a carbon and energy source during encystment (Lin and Sadoff 1968; Segura et al. 2003). Mutations in the phaB and phaC genes in A. vinelandii had no impact on encystment or on cyst viability under laboratory conditions; however, the possibility that under natural conditions PHA metabolism does have such effects cannot be ruled out (Segura et al. 2003).
Most investigations on prokaryotic PHA have been performed on proteobacteria, but cyanobacteria (Hein et al. 1998; Asada et al. 1999; Hai et al. 2001) and various members of the Archaea domain (Hezayen et al. 2000, 2002; Han et al. 2007; Lu et al. 2008) are PHA-producing organisms. Cyanobacteria can accumulate PHA (mainly PHB) under photoautotrophic or mixotrophic growth conditions in the presence of acetate; however, the relative PHA content is significantly lower than in other prokaryotes (Asada et al. 1999). Under conditions of nitrogen and sulfur starvation, and during light irradiation and recovery of vegetative growth by addition of nitrate, cyanobacteria accumulate PHA as storage products of fixed carbon (Asada et al. 1999; Hai et al. 2001).
The data gathered in these various studies suggest a complex role for PHA in stress alleviation. The PHA granules may offer protection against UV irradiation, by protecting DNA from damage, and increase bacterial resistance to oxidative, thermal, and osmotic shock, among others.
3 Molecular Evidence Supporting a Role for PHA Synthesis in Stress Endurance
The role of PHA in bacterial cell protection using molecular approaches has been assessed in several studies. For instance, with the aim of evaluating how bacterial inoculants of A. brasilense can be improved in important parameters (e.g., quality, longevity, reliability, efficacy), wild-type and mutant strains were challenged for their resistance to physical and chemical stresses. The ability of phaC and phaZ mutants of A. brasilense to survive, tolerate, or alleviate various stresses, such as UV irradiation, heat and osmotic shock, desiccation, and oxidative stress, was significantly impaired as compared with that of the wild type (Kadouri et al. 2002, 2003a, b; Figs. 2, 3). In addition, PHA accumulation supported cell multiplication in the absence of an exogenous carbon source in A. brasilense (Kadouri et al. 2002), in a similar manner as for Cupriavidus necator (formerly Ralstonia eutropha; Handrick et al. 2000). Interestingly, PHA was shown to maintain nitrogenase activity and aerotaxis, two physiological features that are extremely energy consuming (Tal and Okon 1985).
Effect of heat (a) and UV irradiation (b) on the survival rate of A. brasilense 7030 (filled triangles) and of the phaZ mutant (filled circles). The initial number of cells for each experiment was 3 × 108. Each value represents the mean and the standard error of three replicates from one representative experiment. Each experiment was done three times and yielded similar results (Reproduced from Kadouri et al. 2003a, with permission of Springer-Verlag)
Electron micrographs of thin sections of A. brasilense 7030 (a) and the phaZ mutant (b), grown for 48 h in high carbon to nitrogen medium, and of A. brasilense 7030 (c) and the phaZ mutant (d) following a 72-h starvation period in phosphate buffer. Arrows indicates PHB granules, bars, 1 μm. Thirty sections of each strain were examined and showed identical findings (Reproduced from Kadouri et al. (2003a), with permission of Springer-Verlag)
Aeromonas hydrophila is a heterotrophic bacterium found in warm climates, and in fresh, salty, marine, estuarine, chlorinated, and unchlorinated water. In addition, it is resistant to refrigeration and cold temperatures. This bacterium produces a PHA copolyester consisting of (R)-3-hydroxybutyrate and (R)-3-hydroxyhexanoate (PHBHHx; Chen et al. 2001). To understand the relationship between enhanced survival ability and PHA accumulation of A. hydrophila, the physiological behaviors of a wild type and a phaC mutant were compared. The ability of the phaC mutant to survive UV irradiation, heat and cold treatment, ethanol, osmotic pressure, and oxidative stress was significantly impaired as compared with that of the wild type. Thus, PHBHHx synthesis and accumulation in A. hydrophila is another example of positive correlation between resistance to environmental stresses and PHA accumulation (Zhao et al. 2007).
Studies done by Ruiz et al. (2004) demonstrated the association between PHA depolymerization and stress tolerance in Pseudomonas oleovorans. Experiments carried out during early stationary phase cultures (a carbon starvation condition that provokes a rapid PHA degradation) of P. oleovorans and its phaZ minus mutant showed that the mutant strain was more sensitive to heat and oxidative shocks than the wild type. In P. putida, impaired survival and resistance to oxidative stress of an rpoS mutant was shown under conditions inducing PHA accumulation (Raiger-Iustman and Ruiz 2008).
Altogether, the above-mentioned studies with different bacteria showed that PHA mutants affected in both anabolic and catabolic PHA pathways are affected in their tolerance to diverse stress conditions, suggesting that stress endurance can be traced to a normal functioning of the PHA cycle, and not exclusively to the presence of the polymer.
4 Regulation of PHA Synthesis
The mechanisms by which PHA favors stress alleviation are not yet fully understood. However, it is known that the PHA metabolism is regulated at both enzymatic and transcriptional levels, by cofactor inhibition and availability of metabolites, and by specific and global transcriptional regulatory factors, respectively (Kessler and Witholt 2001).
At the enzymatic level, it has been shown that, in PHA-producing bacteria, the intracellular levels of acetyl-CoA and free CoA, and both high intracellular level of NAD(P)H and high ratio of NAD(P)H/NAD(P) play a central role in the regulation of PHA synthesis (Haywood et al. 1988; Lee et al. 1995; Kessler and Witholt 2001).
An early work on C. necator suggested an association between PHA utilization and both respiration and oxidative phosphorylation (Hippe and Schlegel 1967). The effector guanosine tetraphosphate (ppGpp) was shown to increase messenger RNA translation of the sigma factor σs encoded by rpoS (Gentry et al. 1993; Brown et al. 2002), which is involved in PHA synthesis (see below). In P. oleovorans, it was found that the rise in ATP and ppGpp levels was concomitant with PHA degradation (Ruiz et al. 2001). This phenomenon was only observed in the wild-type strain but not in a PHA depolymerase-deficient mutant unable to degrade the polymer (Ruiz et al. 2001).
As stated above, evidence from recent years indicates that the central stationary phase regulator RpoS is involved in PHA metabolism. In Escherichia coli, RpoS controls the general stress response, inducing the expression of genes involved in protection against viability loss in nutrient-poor environments, such as those inducing PHA synthesis in several microorganisms. The half-life of RpoS is related to the cell nutrient status. The proteolysis of RpoS is mediated by the ClpXP protease. During starvation, aberrant misfolded proteins compete for ClpXP, reducing RpoS degradation (Ferenci 2007).
The synthesis of PHA and its regulation in A. vinelandi have been recently reviewed by Galindo et al. (2007). An extracellular signal is detected by the two-component global regulatory system formed by the histidine sensor kinase GacS and the response regulator GacA, activating rpoS transcription. At the transcriptional level, rpoS expression appears to be modulated by the GacSA system and by the intracellular levels of ppGpp. During stationary phase, RpoS stimulates the transcription of the phaBAC operon, through the pB2 promoter, and the transcriptional activator phaR, though its pR2 promoter. Consequently, PhaR activates the transcription of the phaBAC operon through the pB1 promoter. In contrast, during exponential phase there is no PHA production because PhaA activity is inhibited by the allosteric control produced by the acetyl-CoA to CoA ratio, and by low levels of transcription of phaBAC due to the lack of RpoS.
An enhanced expression of rpoS in A. hydrophila has been linked to the enhanced resistance to environmental stress conferred by PHBHHx (Zhao et al. 2007). In P. oleovorans, the increase of the intracellular concentration of RpoS during PHA depolymerization was related to an enhanced cross-tolerance to different stress agents (Ruiz et al. 2004). Interestingly, under PHA accumulation and nonaccumulation conditions, an rpoS mutant of P. putida had similar and lower survival under oxidative stress, respectively, as compared with the wild-type strain (Raiger-Iustman and Ruiz 2008).
The relevance of additional sigma factors in regulation of the PHA metabolism has to be considered since in P. aeruginosa PAO1, PHA accumulation from gluconate was found to require a functional RpoN sigma factor, whereas PHA accumulation in cells growing on fatty acids was only reduced in the absence of RpoN (Timm and Steinbüchel 1992). In addition, RpoS has not been documented in Azospirillum species.
5 PHA in Soil and in Plant–Microbe Interactions
Soil is a heterogeneous, discontinuous, and structured environment with a high diversity of microhabitats in which conditions can change rapidly (Postma et al. 1989). Thus, bacteria in soil have to cope with fluctuating – in time and space – biotic and abiotic stresses (van Elsas and van Overbeek 1993). One strategy by which bacteria can improve their establishment, proliferation, and survival in competitive niches such as soil and the rhizosphere is the accumulation and degradation of PHA (Okon and Itzigsohn 1992; Kadouri et al. 2005). In general, conditions of suboptimal growth are conducive to the production of PHAs (Madison and Huisman 1999).
Supporting data for PHA production in telluric environments were provided by Wang and Bakken (1998), who screened 63 soil bacteria for PHA production. They concluded that strains capable of producing PHA were not necessarily superior to those that lack this ability. Instead, survival ability was strain-specific and depended on the growth conditions prior to starvation. In this study, most PHA-producing bacteria were found to belong to the pseudomonad, coryneform, and bacillus groups. In addition to Pseudomonas and Bacillus, Arshad et al. (2007) reported the isolation of soil PHA-producing bacteria belonging to the genera Citrobacter, Enterobacter, Klebsiella, and Escherichia, all of them enterobacteria. Among symbiotic bacteria and plant growth-promoting rhizobacteria (PGPR), PHA production has been reported in members of the genera Rhizobium, Azospirillum, Herbaspirillum, and Azotobacter (Itzigsohn et al. 1995; Catalán et al. 2005; Trainer and Charles 2006).
Many PHA-producing Bacillus strains have been isolated from soil (Wang and Bakken 1998; Yilmaz et al. 2005; Arshad et al. 2007). In a recent proteomic analysis, Luo et al. (2007) reported that the soil bacterium B. cereus increased its fatty acid metabolism when grown in a medium prepared from oak forest soil. This increased fatty acid catabolism was reflected in changes in membrane structure and accumulation of PHA. In agreement with these findings, PhaR, which is required for PhaC activity, was one of the most upregulated proteins (Luo et al. 2007). In another study, it was shown that survival rate and the total cell number (including vegetative cells and spores) of the soil PHA-accumulating bacterium B. megaterium were higher than those of PHA negative mutants (López et al. 1998).
The nature of the carbon compounds found in the soil affects the growth rates of microorganisms and their root colonization ability (Chen et al. 1996; Simons et al. 1996; Jjemba and Alexander 1999). The relationship between PHA metabolism and plant root colonization is not obvious. Among PGPR, the free-living, Gram-negative, nitrogen-fixing bacteria belonging to the genus Azospirillum are well-established models for deciphering traits important for survival, colonization, and effectiveness (Okon and Vanderleyden 1997). One such trait appears to be the secretion of plant-growth-promoting substances (e.g., auxins, gibberellins, and cytokinins) that lead to an increase of the root surface area, promoting water and mineral uptake (Dobbelaere and Okon 2007; Steenhoudt and Vanderleyden 2000). PHA accumulation in A. brasilense is likely an important trait for root colonization of this bacterium (Tal and Okon 1985; Tal et al. 1990a). In support of this assumption, it was demonstrated that under certain conditions, including high carbon-to-nitrogen ratio or low-oxygen partial pressure, A. brasilense cells accumulate above 75% of their dry weight exclusively as PHB (Nur et al. 1981; Tal and Okon 1985; Paul et al. 1990; Tal et al. 1990a, b; Itzigsohn et al. 1995).
Studies carried out with wild-type and phaC mutant strains of A. brasilense, under sterile and nonsterile conditions in soil showed that both root colonization and plant growth promotion were not affected in the mutant (Kadouri et al. 2002). The lack of influence of this mutation on these parameters may stem from the optimal plant growth conditions as well as from the relatively high inoculum level used in that study. It is still to be assessed whether the impaired stress resistance and physiological changes observed in cells with a disrupted PHA metabolism have negative implications in root colonization and plant growth promotion in the field.
Rhizobia are characterized by a free-living stage in the soil and by a symbiotic stage in the interaction with leguminous roots. The establishment of the symbiotic relationship involves a bidirectional signal exchange between the bacteria and the host plant, which leads to the formation of nitrogen-fixing root nodules. Results from studies performed to evaluate the relationship between PHA metabolism and the efficiency of the rhizobia–legume interaction have been diverse, and it seems that they vary not only because of differences between the various bacteria–host systems, but also because of differences in the experimental conditions among the studies. For instance, single strain inoculation experiments with phaC mutants of S. meliloti and Rhizobium leguminosarum bv. viciae on alfalfa and pea plants, respectively, suggested that both symbiotic systems are not affected by PHA formation (Povolo et al. 1994; Lodwig et al. 2005). On the other hand, the S. meliloti–Medicago truncatula system was severely impaired by the lack of PHA formation ability by the bacterium, as plants inoculated with the S. meliloti phaC mutant showed lower rates of nitrogen fixation, lower numbers of nodules, and reduced shoot dry weight as compared with plants inoculated with the wild-type strain (Wang et al. 2007).
In a study done by Willis and Walker (1998), coinoculation experiments of alfalfa with wild-type and phaC mutant strains of S. meliloti indicated that the wild-type strain outnumbered the PHA mutant by more than 200 times. This result indicates that the phaC mutant was less competitive, and that PHA production may provide an advantage to the bacterium during root invasion or nodule initiation. Wang et al. (2007) assessed the symbiotic efficiency of an S. meliloti double mutant impaired in phaP1 and phaP2, which encode the PHA granule-associated phasins that regulate PHA synthesis and granule formation. Plants inoculated with this mutant exhibited a reduced shoot dry weight compared with those inoculated with the wild type, but there was no corresponding reduction in nitrogen-fixation activity. Thus, it appears that in the alfalfa–S. meliloti system, PHA production by the bacterium does not play a significant role after the establishment of nodules. Moreover, bacterial phasins seem to be involved in a metabolic regulatory response and/or to influence assimilation of fixed nitrogen rather than nitrogen-fixation activity (Wang et al. 2007).
Interestingly, it has been reported that common bean plants inoculated with a phaC mutant of R. etli show an increased nitrogen-fixation capacity and enhanced growth in comparison with plants inoculated with the wild-type bacterium (Cevallos et al. 1996). An important difference between S. meliloti and R. etli is that in the former, bacteroids occupy indeterminate nodules, whereas in the latter, bacteroids occupy determinate nodules. Both bacteria produce granules of PHA (in the form of PHB) during the initial stage of invasion. However, in S. meliloti the PHB granules disappear during differentiation into the bacteroid state, and bacteroids occupying the alfalfa indeterminate nodules do not accumulate PHA after the establishment of the symbiosis (Lodwig et al. 2005). It is possible that in the case of S. meliloti, the intracellular PHA supports cell division and growth during root infection and invasion (Trainer and Charles 2006). In contrast, bacteroids of determinate nodules, such as those induced by R. etli on common bean, accumulate high levels of PHA during symbiosis. In this case, PHA could support nitrogen fixation under conditions of reduced carbon availability, and PHA accumulation and nitrogen fixation would compete for energy and reductant sources as well as for photosynthates (Cevallos et al. 1996; Trainer and Charles 2006). The relationship between carbon storage and nitrogen fixation is complex. For example, in free-living Bradyrhizobium japonicum, R. leguminosarum, and S. meliloti, at the same time as PHA is accumulated, there is production of glycogen as an additional storage compound (Lodwig et al. 2005).
Azorhizobium caulinodans, as R. etli, accumulates PHA in both free-living and symbiotic stages, but an A. caulinodans phaC mutant was totally devoid of nitrogenase activity ex plant, and induced nodules devoid of bacteria (Mandon et al. 1998). Interestingly, nitrogenase activity of the mutant was partially restored by constitutive expression of the nifA gene. Mandon et al. (1998) suggested that PHA is required for maintaining the reducing power of the cell, and that nifA expression mediates adaptation of nitrogen fixation to the levels of carbon and reducing equivalents available in the nodule. Vassileva and Ignatov (2002) studied the relationship between PHA formation and nitrogenase activity in the Galega orientalis–R. galegae system. They reported high nitrogen-fixation activity in parallel to PHA degradation when low concentrations of plant growth promoters and polyamine modulators were applied.
In summary, the fact that PHA production is a widespread trait supports the assumption that PHA accumulation plays a central role in survival, especially when bacteria are faced with starvation. In PHA-producing bacteria, PHA is a major determinant for overcoming periods of carbon and energy starvation, and may represent a basic feature for so-called environmental bacteria. However, the ability to produce PHA is apparently not absolute for improved survival ability during stress, as PHA was shown to enhance the survival of some, but not all bacteria tested, which likely rely on alternative strategies (Wang and Bakken 1998).
6 Relevance of PHA in Microbial Communities
Most microorganisms on Earth are organized into microbial biofilms and microbial mat communities. PHA production is very relevant in these kinds of microbial organization as these are niches where microbes have to cope with moderate physical and chemical stresses, and frequently have to adapt to changing conditions.
Biofilms are sessile microbial communities embedded within a matrix and attached to a solid surface. On one hand, surface-associated multicellular communities are generally advantageous over individual planktonic cells, especially in regard to protection against unfavorable environmental conditions. On the other hand, planktonic populations can quickly reach and colonize new niches. The shift between sessile and planktonic lifestyles depends upon the integration of many environmental cues. Several biofilm-producing bacteria have also been reported to produce PHA. For instance, it was recently reported that PHA accumulation in P. aeruginosa biofilms occurs in a spatially/temporally regulated way, and that it is in competition with alginate biosynthesis, playing an important role in stress tolerance and biofilm formation (Pham et al. 2004; Campisano et al. 2008).
Mats have been described as large microbial communities composed by a multilayered sheet of bacteria, archaea, and diatoms, which are characterized by both seasonal and diel fluctuations (e.g., flooding and desiccation, diel fluctuations of temperature, light, pH, oxygen, sulfide, and nutrients, among others). A culture-independent strategy for the detection of PHA-producing bacteria from a polluted marine microbial mat was adopted by López-Cortés et al. (2008). The authors showed a higher PHA-producing microbial diversity in a marine microbial mat exposed to environmental stress by organic pollution from a cannery of marine fish (nutrient imbalance) as compared with a pristine site. Because PHA synthesis is linked to lipid metabolism, PHA producers are more competitive in environments with high concentrations of fatty acids such as active sludge and microbial mats. Also, Villanueva et al. (2007) suggested that during diel fluctuations, heterotrophic microorganisms from phototrophic mats accumulate PHA, using as a precursor the excess of carbon that is generated and excreted by photosynthetic microorganisms, reflecting that changes in PHA levels depend on the time of day. Interestingly, the isolation of PHA-producing strains from mats with potential industrial applications has been successful, positioning mats as an excellent source for such microbes (Berlanga et al. 2006; Simon-Colin et al. 2008).
7 Utilization of the Energy Obtained from PHA for Environmental Cues
In addition to being a source of storage compounds and contributing to stress endurance, PHAs can serve as sources of NADH and ultimately ATP. Under diverse environmental conditions, the ability to generate energy from PHAs can be used to drive various important energy-consuming pathways, as discussed in the folowing.
7.1 Chemotaxis
Chemotaxis is the ability bacteria have to sense gradients of compounds and to drive motility toward the most appropriate niche, and is an important trait in plant–microbe interaction. A. brasilense exhibits strong chemotaxis toward different attractants such as fructose, malate, and sweet corn seed exudates, and it was shown that this chemotaxis is significantly stronger in the wild type than in a phaC mutant strain (Kadouri et al. 2003b). It is possible that the reducing power produced during PHA degradation energizes the chemotactic process in the environment, where sources of reducing power are low. In A. brasilense, PHA oxidation involves a specific NADH-dependent dehydrogenase, which competes for tricarboxylic acid (TCA) cycle intermediates in the electron transport system (Tal et al. 1990a, b). When PHA accumulation is disrupted, more resources are available for the TCA cycle, resulting in an increased motility in the phaC mutant as compared with the wild type. In contrast, an A. brasilense phaZ mutant was shown to have motility similar to that of the wild type (Kadouri et al. 2003a). phaZ encodes a poly(β-hydroxybutyrate) depolymerase; thus, it is likely that, in contrast to the phaC mutant, the phaZ mutant is unable to generate excess reducing power (Kadouri et al. 2003a).
The redox state of the rhizosphere is one of the most important parameters for maintaining this ecological system. Thus, the energy taxis, driven by PHA catabolism, toward metabolizable substrates in plant root exudates may play a major role in plant–microbe interactions. On the other hand, rhizobia are positively chemotactic toward a variety of amino acids, dicarboxylic acids, sugars, and nodulation-gene-inducing flavonoids secreted by the roots of their hosts. Rhizobial mutants defective in motility or chemotaxis are impaired in their ability to compete for sites of nodule initiation in the host root (Caetano-Anollés et al. 1988). If as suggested for A. brasilense, PHA catabolism in S. meliloti is also involved in energy supply for chemotaxis, it could at least partially explain why the S. meliloti phaC mutant strain is less competitive than the wild type (Willis and Walker 1998).
7.2 Exopolysaccharide Production
The roles of exopolysaccharide (EPS) in bacteria are dependent on their natural environment. Most of the functions assigned to EPS are related to a protective role: the highly hydrated EPS layer with which bacteria are capable of surrounding themselves provides a shield against desiccation and predation (Kumar et al. 2007). Many bacteria produce and live within a matrix of EPS in their natural environment, for example, in soil. EPS contributes anchoring cells to different substrates, protecting them against phagocytosis, masking antibody recognition, and preventing lysis by other bacteria (Deaker et al. 2004). EPS also plays an important role in plant–bacteria interactions. In rhizobia, EPS is required for success in the different stages of the establishment of the nitrogen-fixing symbiosis, including root colonization, host recognition, infection thread formation, and nodule invasion. In protective roles, EPS is important for evasion of plant immune responses and protection from reactive oxygen species (Gonzalez et al. 1996; Cooper 2007). In azospirilla, EPS is known to be involved in cell aggregation and in root adhesion (Burdman et al. 2000a). Burdman et al. (2000b) and Bahat-Samet et al. (2004) showed that the arabinose content of A. brasilense EPS plays a role in cell aggregation and Mora et al. (2008) identified an outer-membrane protein with lectin activity that specifically binds to the EPS produced by A. brasilense during aggregation conditions. In A. brasilense, several studies support EPS and PHA production as well as cell aggregation being interdependent phenomena (Burdman et al. 1998; Kadouri et al. 2002, 2003a, b; Aneja et al. 2004; Wang et al. 2007).
In the phaC mutant of A. brasilense, a considerable increase in excreted EPS was detected over the wild-type strain when grown under a medium characterized by a high carbon-to-nitrogen ratio. In such a mutant, EPS production may act as a sink for carbon and reducing equivalents which are diverted from the blocked PHA synthesis pathway. The phaC mutant was more aggregative, and exhibited a significantly increased ability to adhere to roots relative to the wild type (Kadouri et al. 2002, 2003b). In contrast, EPS production and cell aggregation capability in the wild-type strain were higher than in the phaZ mutant under the same growth conditions (Kadouri et al. 2003a). Burdman et al. (2000a) suggested that, in addition to PHB accumulation, cell aggregation could increase survival of Azospirillum cells under diverse stress conditions. Cell aggregation as well as a functional PHA metabolism may also be important during root colonization where cell aggregation is commonly observed (Kadouri et al. 2005).
In contrast to the findings with A. brasilense, in S. meliloti it has been shown that the inability to synthesize PHA is strongly associated with reduced production of EPS (Aneja et al. 2004; Wang et al. 2007). Interestingly, the phaP1/phaP2 double mutant of S. meliloti, which as stated above is impaired in PHA production, produces more EPS and glycogen than does the wild-type strain (Wang et al. 2007). In R. etli, an aniA mutant strain exhibited a significant decrease in PHA accumulation, and a significant increase in EPS formation (Encarnación et al. 2002). The aniA gene encodes a transcriptional factor involved in the expression of genes that are important in partitioning the carbon flow in the bacterial cell, such as the ones stimulated under low-oxygen conditions and channeling of excess carbon into PHA and glycogen biosynthesis (Povolo and Casella 2000).
7.3 PHA as a Carbon and Energy Source for “Environmental Bacteria”
PHAs have attracted significant industrial interest because they are natural biodegradable thermoplastics, and they do not require special environmental conditions to be degraded. Beyond this, their biodegradability means that PHAs can be used as carbon and energy sources to support bacterial growth in different environments. When PHA-accumulating microorganisms cease dividing and undergo lysis, the polymer is released to the environment and it turns out to be readily metabolized by other microorganisms. PHAs can be degraded by the action of either intracellular or extracellular depolymerases (i-PhaZ and e-PhaZ, respectively) produced by PHA-degrading bacteria, algae, and fungi.
Two types of PHA polymers have been described: (1) native PHA granules containing lipids and proteins that are rapidly hydrolyzed by i-PhaZs and (2) denatured PHA granules, which are crystalline and hardly hydrolyzed by i-PhaZs but are efficiently degraded by ubiquitous e-PhaZs into water-soluble products (Tokiwa and Calabia 2004). Several environmental bacteria, algae, and fungi are able to “attack” PHA granules and to solubilize the PHA polymer. The polymer is then degraded by e-PhaZs, producing oligomers, which in some cases can be further degraded by hydrolases into monomers. The breakdown products can be utilized as a carbon and energy source by these organisms (Philip et al. 2007).
On the basis of the size of the PHA polymer that can be degraded, e-PhaZs are divided into two groups: short-chain-length PHA (SCL-PHA) and medium-chain-length PHA (MCL-PHA) depolymerases. The majority of PHA-degrading microbes produce only one type of e-PhaZ. Only a few bacterial species have been reported to produce both kinds of depolymerases, thus being able to degrade both SCL-PHA and MCL-PHA (Kim et al. 2007). The rate of PHA degradation is dependent on environmental conditions including temperature, pH, moisture, and nutrient supply, as well as on properties related the PHA substrate, such as monomer composition, crystallinity, additives, and surface area (Philip et al. 2007). Pseudomonas and Stenotrophomonas are the predominant MCL-PHA degraders in soil and marine environments (Kim et al. 2007). However, also microorganisms from the families Pseudonocardiaceae, Micromonosporaceae, Thermomonosporaceae, Streptosporangiaceae, and Streptomycetacease have been reported to degrade PHA in the environment (Philip et al. 2007).
8 Phylogenetic Aspects of PHA Metabolism and Their Relationship with the Environment
Systematic phylogenetic analyses of genes involved in PHA biosynthesis and degradation were recently carried out by Kadouri et al. (2005) and Kalia et al. (2007). From 253 sequenced genomes, Kalia et al. (2007) identified 71 and 111 complete phaA and phaB sequences, respectively. The PhaA and PhaB phylogenetic trees showed 12 and 16 cases of discrepancy, respectively, as compared with 16S ribosomal DNA (rDNA) phylogenies. These inconsistencies might be explained by horizontal gene transfer (HGT). The presence of the phaC gene was detected in 72 genomes belonging to 40 genera from different taxonomical groups such as Actinobacteria, Cyanobacteria, Firmicutes, and Proteobacteria (Kalia et al. 2007). Analysis of the PhaC phylogenetic tree revealed quite a few significant deviations as compared with the 16S rDNA. Similarly, a PhaC phylogenetic tree built with 67 homologous proteins from Proteobacteria by Kadouri et al. (2005) showed that the tree was congruent with the 16S rDNA data and clustered according to the phylogenetic taxa, suggesting the existence of genotypic clusters that correspond to traditional species designations. In addition, this PhaC tree topology was in agreement with previous analyses reported by Steinbüchel and Hein (2001) and Rehm (2003).
The mechanism and regulation of PHA mobilization is poorly understood, but as mentioned, a clear distinction exists between intracellular and extracellular PHA degradation by means of PhaZ enzymes (Jendrossek and Handrick 2002). Kadouri et al. (2005) analyzed the phylogeny of the i-PhaZ. Most clusters of the i-PhaZ phylogenetic tree were highly incongruent with those of the 16S rDNA tree. Interestingly, sequencing of the genome of C. necator (formerly R. eutropha) H16 revealed this bacterium possesses six different PhaZs (Pohlmann et al. 2006), and two copies of PhaZ were detected in the genomes of R. metallidurans and Burkholderia fungorum (Kadouri et al. 2005). The multiplication of genes encoding PHA depolymerases in a genome may reflect the diversity of the PHAs that a given microorganism is able to produce and utilize. The incongruence observed in PhaZ phylogenetic tress suggests that these genes have likely been subjected to HGT. In addition, the multiplication of these genes in some bacterial genomes possibly reflects duplication events that lead to parallel evolution of different genes to increase the versatility of the organism for PHA utilization. Thus, although the process of PHA synthesis seems to be highly conserved, it appears that a variety of options for PHA utilization have been laterally acquired and/or developed in parallel by several microorganisms.
Kalia et al. (2007) also revealed for the first time the presence of all three PHA biosynthesis genes (phaA, phaB, and phaC) in some cyanobacteria and Firmicutes. Prior to this, different combinations of phaA, phaB, and/or phaC were partially detected among a few members of Archaea, actinobacteria, and cyanobacteria (Kalia et al. 2007).
In conclusion, on the basis of the highly frequent appearance of phaCAB clusters and the relatively high congruence between PhaA, PhaB, and PhaC phylogenetic trees with 16S rDNA trees, it appears that the acquisition of PHA biosynthesis genes is an ancient event, at least in Proteobacteria. However, it is becoming evident that these genes have been spread among microorganisms by HGT, thus leading to the acquisition of the PHA accumulation trait by other groups of bacteria. Interestingly, it was recently reported that the pha gene cluster of a Pseudomonas isolate from the Antarctic, which produces high amounts of PHB, is located in a genomic island within a large genetic element of approximately 32.3 kb (Ayub et al. 2007). GC content, phylogeny inference, and codon usage analyses showed that in this bacterium the phaBCA operon itself has a complex mosaic structure and indicated that the phaB and phaC genes were acquired by HGT, probably derived from Burkholderiales (Ayub et al. 2007).
Some natural and anthropogenic activities are dramatically affecting the environment. As a result, we are witnesses to an increase in the intensity of extreme weather events, desertification, reduction of the ozone layer, and acidification of the oceans among other phenomena of concern. Supraorganism systems (namely, populations of micro- and macroorganisms, organized into trophic chains and capable of biotic cycling) have to adapt to the new situations. Considering that PHA accumulation is involved in bacterial cell survival and stress endurance, and that PHA genes have been subjected to HGT, it is reasonable to hypothesize that PHA accumulation and degradation is presently an evolutionarily valuable trait that microcommunities can exploit to deal with such environmental changes.
9 PHA Applications in Agriculture
Bacterial inoculants are commercial formulations for agricultural uses containing PGPR that can be applied to the seeds or to the soil during planting. During production, transportation, and storage of inoculants, bacteria should respond to (and survive) several stress factors, such as acidity, desiccation, chemical pesticides, and nonoptimal temperatures (Rebah et al. 2007). In other words, inoculants that have the capability to support survival rates of bacterial cells are highly desirable. Thus, appropriate materials for carrying microbes must offer special properties, such as chemical and physical uniformity, high water holding capacity, and lack of toxicity, and they must be environmentally friendly. Commercial inoculants are available as solid – in powder from peat or in granular form – or as liquid formulations (Stephens and Rask 2000; Rebah et al. 2007).
Many microbial inoculants are based on solid peat formulations owing the protective properties of this material. Recently, Albareda et al. (2008) evaluated six carriers (bagasse, cork compost, attapulgite, sepiolite, perlite, and amorphous silica) as alternatives to peat. Cork compost and perlite gave as good results as peat in terms of support of B. japonicum and S. meliloti growth, maintaining a long survival of inoculated strains, as well as survival on soybean seeds. Most of the research done in this field aims at developing new carriers or improving carrier properties by adding elements such as nutrients or other synthetic products that can prolong survival (López et al. 1998).
The vast amount of information gathered especially on azospirilla and rhizobia throughout the years suggests that for an inoculant to be successful, i.e., to provide efficient root colonization and/or invasion, not only the type of carrier material is important for bacterial survival within the carrier itself, but also the metabolic state of the cells, including their capability to use intercellular storage materials such as PHB. This knowledge originating from studies showing that although the carriers may vary, plant growth promotion effects are more consistent with A. brasilense inoculants containing cells with high amount of PHB (Fallik and Okon 1996). In support of these studies, field experiments carried out in Mexico with maize and wheat revealed that increasing crop yields were obtained using peat inoculants prepared with PHB-rich Azospirillum cells (Dobbelaere et al. 2001). Additionally, experiments done with an A. brasilense phaC mutant showed that among different inoculant carriers (peat, sianic sand, and perlite), peat sustained the highest survival rates, whereas the lowest survival rates were observed in perlite. Importantly, although variations between carriers were very large, in all carriers the wild-type strain survived better than the mutant. It was thus concluded that the production of PHA is of critical importance for improving the shelf life, efficiency, and reliability of commercial inoculants (Kadouri et al. 2003b).
In other agricultural aspects, it has been shown that relevant agricultural substances, such as insecticides, can be incorporated into PHA granules. If spread in the environment, PHA-degrading bacteria can slowly degrade the PHA granule, leading to controlled release of the insecticide. This pioneer idea was proposed for the first time by Holmes (1985) and was recently supported by Philip et al. (2007). However, to date, few studies have been performed on this subject, and despite its potential, this PHA application still seems to be far from commercial utilization.
10 Conclusions
PHAs have attracted the attention of many research groups as they are environmentally friendly polymers. It is becoming evident that PHA production is a widespread trait among microorganisms, suggesting that, among other strategies, PHA production plays a central role in survival under environmental stress conditions, such as those imposed in water and soil (Table 1). Despite significant advances in recent years, research is needed to understand the molecular mechanisms of regulation of both PHA accumulation and degradation, and how these processes enhance bacterial survival and fitness. Advances in this research area in the future could, for instance, benefit the industry of bacterial inoculants used for plant protection or plant growth promotion, as in these cases the capabilities of the microorganisms to establish, survive, and proliferate in the target niche is of utmost importance.
References
Albareda M, Rodriguez-Navarro DN, Camacho M, Temprano FJ (2008) Alternatives to peat as a carrier for rhizobia inoculants: solid and liquid formulations. Soil Biol Biochem 40:2771–2779
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Aneja P, Dai M, Lacorre DA, Pillon B, Charles TC (2004) Heterologous complementation of the exopolysaccharide synthesis and carbon utilization phenotypes of Sinorhizobium meliloti Rm1021 polyhydroxyalkanoate synthesis mutants. FEMS Microbiol Lett 39:277–283
Arora NK, Singhal V, Maheshwari DK (2006) Salinity-induced accumulation of poly-β-hydroxybutyrate in rhizobia indicating its role in cell protection. World J Microbiol Biotechnol 22:603–606
Arshad MU, Jamil N, Naheed N, Hasnain S (2007) Analysis of bacterial strains from contaminated and non-contaminated sites for the production of biopolymers. Afr J Biotechnol 6:1115–1121
Asada Y, Miyake M, Miyake J, Kurane R, Tokiwa Y (1999) Photosynthetic accumulation of poly-(hydroxybutytate) by cyanobacteria-the metabolism and potential of CO2 recycling. Int J Biol Micromol 25:37–42
Ayub ND, Pettinari MJ, Ruiz JA, López NI (2004) A polyhydroxybutyrate-producing Pseudomonas sp. isolated from Antarctic environments with high stress resistance. Curr Microbiol 49:170–174
Ayub ND, Pettinari KN, Méndez BS, López NI (2007) The polyhydroxyalkanoate genes of a stress resistant Antarctic Pseudomonas are situated within a genomic island. Plasmid 58:240–248
Babel W, Ackermann JU, Breuer U (2001) Physiology, regulation, and limits of the synthesis of poly(3HB). Adv Biochem Eng Biotechnol 71:125–157
Bahat-Samet E, Castro-Sowinski S, Okon Y (2004) Arabinose content of extracellular polysaccharide plays a role in cell aggregation of Azospirillum brasilense. FEMS Microbiol Lett 237:195–203
Berlanga M, Montero MT, Hernandez-Borrell J, Guerrero R (2006) Rapid spectrofluorometric screening of poly-hydroxyalkanoate-producing bacteria from microbial mats. Int Microbiol 9:95–102
Brown L, Gentry D, Elliott T, Cashel M (2002) DksA affects ppGpp induction of RpoS at a translational level. J Bacteriol 184:4455–4465
Burdman S, Jurkevitch E, Schwartsburd B, Hampel M, Okon Y (1998) Aggregation in Azospirillum brasilense: effects of chemical and physical factors and involvement of extracellular components. Microbiology 144:1989–1999
Burdman S, Okon Y, Jurkevitch E (2000a) Surface characteristics of Azospirillum brasilense in relation to cell aggregation and attachment to plant roots. Crit Rev Microbiol 26:91–110
Burdman S, Jurkevitch E, Soria-Díaz ME, Gil Serrano AM, Okon Y (2000b) Extracellular polysaccharide composition of Azospirillum brasilense and its relation with cell aggregation. FEMS Microbiol Lett 189:259–264
Caetano-Anollés G, Wall LG, De Micheli AT, Macchi EM, Bauer WD, Favelukes G (1988) Role of motility and chemotaxis in efficiency of nodulation by Rhizobium meliloti. Plant Physiol 86:1228–1235
Campisano A, Ovehage J, Rehm BHA (2008) The polyhydroxyalkanoate biosynthesis genes are differentially regulated in planktonic- and biofilm-grown Pseudomonas aeruginosa. J Biotechnol 133:442–452
Catalán AI, Ferreira F, Gill PR, Batista S (2005) Production of polyhydroxyalkanoates by Herbaspirillum seropedicae grown with different sole carbon sources and on lactose when engineered to express the lacZlacY genes. Enzyme Microb Technol 40:1352–1357
Cevallos MA, Encarnación S, Leija A, Mora Y, Mora J (1996) Genetic and physiological characterization of a Rhizobium etli mutant strain unable to synthesize poly-β-hydroxybutyrate. J Bacteriol 178:1646–1654
Chen GQ, Zhang G, Park SJ, Lee SY (2001) Industrial production of polyhydroxybutyrate-co-hydroxyhexanoate. Appl Microbiol Biotechnol 57:50–55
Chen W, Zhang Q, Coleman DC, Carroll CR, Hoffman CA (1996) Is available carbon limiting microbial respiration in the rhizosphere? Soil Biol Biochem 28:1283–1288
Cooper JE (2007) Early interactions between legumes and rhizobia: disclosing complexity in a molecular dialogue. J Appl Microbiol 103:1355–1365
Dawes JB (1986) Microbial energy reserve compounds. In: Dawes EA (ed) Tertiary level biology microbial energetics. Blackie, London, pp 145–165
Dawes EA, Senior PJ (1973) The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol 10:135–266
Deaker R, Roughley RJ, Kennedy IR (2004) Legume seed inoculation technology – a review. Soil Biol Biochem 36:1275–1288
Dobbelaere S, Okon Y (2007) The plant growth-promoting effect and plant responses. In: Elmerich C, Newton WE (eds) Associative and endophytic nitrogen-fixing bacteria and cyanobacterial associations. Springer, Dordrecht, pp 145–170
Dobbelaere S, Croonenborghs A, Thys A, Ptacek D, Vanderleyden J, Dutto JP et al (2001) Responses of agronomically important crops to inoculation with Azospirillum. Aust J Plant Physiol 28:871–879
Emeruwa AC, Hawirko RZ (1973) Poly-beta-hydroxybutyrate metabolism during growth and sporulation of Clostridium botulinum. J Bacteriol 120:399–406
Encarnación S, Vargas MC, Dunn MF, Dávalos A, Mendoza G, Mora Y, Mora J (2002) AniA regulates reserve polymer accumulation and global protein expression in Rhizobium etli. J Bacteriol 184:2287–2295
Fallik E, Okon Y (1996) Inoculants of Azospirillum brasilense: biomass production, survival and growth promotion of Setaria italica and Zea mays. Soil Biol Biochem 28:123–126
Ferenci T (2007) Sensing nutrient levels in bacterial. Nat Chem Biol 3:607–608
Galindo E, Peña C, Núñez C, Segura D, Espín G (2007) Molecular and bioengineering strategies to improve alginate and polyhydroxyalkanoate production by Azotobacter vinelandii. Microb Cell Fact 6:7. doi:10.0086/1475-2859-6-7
Gentry DR, Hernández VJ, Nguyen LH, Jensen DB, Cashel M (1993) Synthesis of the stationary-phase sigma factor σs is positively regulated by ppGpp. J Bacteriol 175:7982–7989
Gonzalez JE, York GM, Walker GC (1996) Rhizobium meliloti exopolysaccharides: synthesis and symbiotic function. Gene 179:141–146
Hai T, Hein S, Steinbüchel A (2001) Multiple evidence for widespread and general occurrence of type-III PHA synthases in cyanobacteria and molecular characterization of the PHA synthases from two thermophilic cyanobacteria: Chlorogloeopsis fritschii PCC 6912 and Synechococcus sp strain MA19. Microbiology 147:3047–3060
Han J, Lu Q, Zhou L, Zhou J, Xiang H (2007) Molecular characterization of the phaEC Hm genes, required for biosynthesis of poly(3-hydroxybutyrate) in the extremely halophilic archaeon Haloarcula marismortui. Appl Environ Microbiol 73:6058–6065
Handrick R, Reinhard S, Jendrossek D (2000) Mobilization of poly(3-hydroxybutyrate) in Ralstonia eutropha. J Bacteriol 182:5916–5918
Haywood GW, Anderson AJ, Chu L, Dawes EA (1988) The role of NADH- and NADPH-linked acetoacetyl-CoA reductases in the poly-3-hydroxybutyrate synthesizing organism Alcaligenes eutrophus. FEMS Microbiol Lett 52:259–264
Hein S, Tran H, Steinbüchel A (1998) Synechocystis sp. PCC6803 possesses a two-component polyhydroxyalkanoic acid synthase similar to that of anoxygenic purple sulfur bacteria. Arch Microbiol 170:162–170
Hezayen FF, Rehm BH, Eberhardt R, Steinbüchel A (2000) Polymer production by two newly isolated extremely halophilic archaea: application of a novel corrosion-resistant bioreactor. Appl Microbiol Biotechnol 54:319–325
Hezayen FF, Steinbüchel A, Rehm BH (2002) Biochemical and enzymological properties of the polyhydroxybutyrate synthase from the extremely halophilic archaeon strain 56. Arch Biochem Biophys 403:284–291
Hippe H (1967) Aufbau und Wiederverwertung von Poly-β-hydroxybuttersäure durch Hydrogenomonas H16. Arch Mikrobiol 56:248–277
Hippe H, Schlegel HG (1967) Hydrolyse von PHBS durch intrazelluläre Depolymerase von Hydrogenomonas H16. Arch Mikrobiol 56:278–299
Holmes PA (1985) Application of PHB: a microbially produced biodegradable thermoplastic. Phys Technol 16:32–36
Itzigsohn R, Yarden O, Okon Y (1995) Polyhydroxyalkanoate analysis in Azospirillum brasilense. Can J Microbiol 41:73–76
James BW, Mauchline WS, Dennis PJ, Keevil CW, Wait R (1999) Poly-3-hydroxybutyrate in Legionella pneumophila, an energy source for survival in low-nutrient environments. Appl Environ Microbiol 65:822–827
Jendrossek D, Handrick R (2002) Microbial degradation of polyhydroxyalkanoates. Annu Rev Microbiol 56:403–432
Jjemba PK, Alexander M (1999) Possible determinants of rhizosphere competence of bacteria. Soil Biol Biochem 31:623–632
Kadouri D, Burdman S, Jurkevitch E, Okon Y (2002) Identification and isolation of genes involved in poly β-hydroxybutyrate (PHB) biosynthesis in Azospirillum brasilense and characterization of a phbC mutant. Appl Environ Microbiol 68:2943–2949
Kadouri D, Jurkevitch E, Okon Y (2003a) Poly beta-hydroxybutyrate depolymerase (PhaZ) in Azospirillum brasilense and characterization of a phaZ mutant. Arch Microbiol 180:309–318
Kadouri D, Jurkevitch E, Okon Y (2003b) Involvement of the reserve material poly β-hydroxybutyrate (PHB) in Azospirillum brasilense in stress endurance and root colonization. Appl Environ Microbiol 69:3244–3250
Kadouri D, Jurkevitch E, Okon Y, Castro-Sowinski S (2005) Ecological and agricultural significance of bacterial polyhydroxyalkanoates. Crit Rev Microbiol 31:55–67
Kalia VC, Lal S, Cheema S (2007) Insight into the phylogeny of polyhydroxyalkanoate biosynthesis: horizontal gene transfer. Gene 389:19–26
Kessler B, Witholt B (2001) Factors involved in the regulatory network of poly-hydroxyalkanoate metabolism. J Biotechnol 86:97–104
Kim DY, Kim HW, Chung MG, Rhee YA (2007) Biosynthesis, modification, and biodegradation of bacterial medium-chain-length polyhydroxyalkanoates. J Microbiol 45:87–97
Kominek LA, Halvorson HO (1965) Metabolism of poly-beta-hydroxybutyrate and acetoin in Bacillus cereus. J Bacteriol 90:1251–1259
Kumar AS, Mody K, Jha B (2007) Bacterial exopolysaccharides – a perception. J Basic Microbiol 47:103–117
Lee IY, Kim MK, Chang HN, Park YH (1995) Regulation of poly-beta-hydroxybutyrate biosynthesis by nicotinamide nucleotide Alcaligenes eutrophus. FEMS Microbiol Lett 131:35–39
Lin LP, Sadoff HL (1968) Encystment and polymer production by Azotobacter vinelandii in the presence of beta-hydroxybutyrate. J Bacteriol 95:2336–2343
Lodwig EM, Leonard M, Marroqui S, Wheeler TR, Kindlay K, Downie JA, Poole PS (2005) Role of polyhydroxybutyrate and glycogen as carbon storage compounds in pea and been bacteroids. Mol Plant Microbe Interact 18:67–74
López NI, Floccari ME, Garcia AF, Steinbüchel A, Mendez BS (1995) Effect of poly-3-hydroxybutyrate content on the starvation survival of bacteria in natural waters. FEMS Microbiol Ecol 16:95–102
López NI, Ruiz JA, Méndez BS (1998) Survival of poly-3-hydroxybutyrate-producing bacteria in soil microcosms. World J Microbiol Biotechnol 14:681–684
López-Cortés A, Lanz-Landázuri A, García-Maldonado JQ (2008) Screening and isolation of PHB-producing bacteria in a polluted marine microbial mat. Microb Ecol 56:112–120
Lu Q, Han J, Zhou L, Zhou J, Xiang H (2008) Genetic and biochemical characterization of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) synthase in Haloferax mediterranea. J Bacteriol 190:4173–4180
Luo Y, Vilain S, Voigt B, Albrecht D, Hecker M, Brözel VS (2007) Proteomic analysis of Bacillus cereus growing in liquid soil organic matter. FEMS Microbiol Lett 271:40–47
Macrae RM, Wilkinson JF (1958) Poly-ß-hydroxybutyrate metabolism in washed suspensions of Bacillus cereus and Bacillus megaterium. J Gen Microbiol 19:210–222
Madison LL, Huisman GW (1999) Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiology and Molecular Biology Reviews 63:21–53
Mandon K, Michel-Reydellet N, Encarnación S, Kaminski PA, Leija A, Cevallos MA, Elmerich C, Mora J (1998) Poly-β-hydroxybutyrate turnover in Azorhizobium caulinodans is required for growth and affects nifA expression. J Bacteriol 180:5070–5076
Matin A, Veldhuis C, Stegeman V, Veenhuis M (1979) Selective advantage of a Spirillum sp. in a carbon-limited environment. Accumulation of poly-beta-hydroxybutyric acid and its role in starvation. J Gen Microbiol 112:349–355
Mora P, Rosconi F, Franco-Fraguas L, Castro-Sowinski S (2008) Azospirillum brasilense Sp7 produces an outer-membrane lectine that specifically binds to surface-exposed extracellular polysaccharide produced by the bacterium. Arch Microbiol 189:519–524
Nakata HM (1965) Role of acetate in sporogenesis of Bacillus cereus. J Bacteriol 91:784–788
Nur I, Steinitz YL, Okon Y, Henis Y (1981) Carotenoid composition and function in nitrogen-fixing bacteria of the genus Azospirillum. J Gen Microbiol 123:27–32
Okon Y, Itzigsohn R (1992) Poly-β-hydroxybutyrate metabolism in Azospirillum brasilense and the ecological role of PHB in the rhizosphere. FEMS Microbiol Rev 103:131–140
Okon Y, Vanderleyden J (1997) Root-associated Azospirillum species can stimulate plants. ASM News 63:366–370
Paul E, Mulard D, Blanc P, Fages J, Goma G, Pareilleux A (1990) Effects of partial O2 pressure, and agitation on growth kinetics of Azospirillum lipoferum under fermentor conditions. Appl Environ Microbiol 56:3235–3239
Pham TH, Webb JS, Rehm BHA (2004) The role of polyhydroxyalkanoate biosynthesis by Pseudomonas aeruginosa in rhamnolipid and alginate production as well as stress tolerance and biofilm formation. Microbiology 150:3405–3413
Philip S, Kashavarz T, Roy I (2007) Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J Chem Technol Biotechnol 82:233–247
Pohlmann A, Fricke WF, Reinecke F et al (2006) Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat Biotechnol 24:1257–1262
Postma J, van Veen JA, Walter S (1989) Influence of different initial soil moisture contents on the distribution and population dynamics on introduced Rhizobium leguminosarum. Soil Biol Biochem 21:437–442
Pötter M, Steinbüchel A (2005) Poly(3-hydroxybutyrate) granule-associated proteins: impacts on poly(3-hydroxybutyrate) synthesis and degradation. Biomacromolecules 6:552–560
Povolo S, Casella S (2000) A critical role for aniA in energy-carbon flux and symbiotic nitrogen fixation in Sinorhizobium meliloti. Arch Microbiol 174:42–49
Povolo S, Tombolini R, Morea A, Anderson AJ, Casella S, Nuti MP (1994) Isolation and characterization of mutants of Rhizobium meliloti unable to synthesize poly-β-hydroxybutyrate. Can J Microbiol 40:823–829
Raiger-Iustman LJ, Ruiz JA (2008) The alternative sigma factor, σs, affects polyhydroxyalkanoate metabolism in Pseudomonas putida. FEMS Microbiol Lett 284:218–224
Rebah FB, Prévost D, Yezza A, Tyagi RD (2007) Agro-industrial waste materials and wastewater sludge for rhizobial inoculant production: a review. Bioresour Technol 98:3535–3546
Rehm BHA (2003) Polyester synthases: natural catalysts for plastics. Biochem J 376:15–33
Rothermich MM, Guerrero R, Lenz RW, Goodwin S (2000) Characterization, seasonal occurrence, and diel fructuation of poly(hydroxyalkanoate) in photosynthetic microbial mats. Appl Environ Microbiol 66:4279–4291
Ruiz JA, López NI, Méndez BS (1999) Polyhydroxyalkanoates degradation affects survival of Pseudomonas oleovorans in river water microcosms. Rev Argent Microbiol 31:201–204
Ruiz JA, López NI, Fernandes R, Méndez B (2001) Polyhydroxyalkanoate degradation is associated with nucleotide accumulation and enhanced stress resistance and survival of Pseudomonas oleovorans in natural water microcosms. Appl Environ Microbiol 67:225–230
Ruiz JA, López NL, Méndez BS (2004) rpoS gene expression in carbon-starved cultures of the polyhydroxyalkanoate-accumulating species Pseudomonas oleovorans. Curr Microbiol 48:396–400
Saegusa H, Shiraki M, Kanai C, Saito T (2001) Cloning of an intracellular poly[D(-)-3-hydroxybutyrate] depolymerase gene from Ralstonia eutropha H16 and characterization of the gene product. J Bacteriol 183:94–100
Segura D, Cruz T, Espín G (2003) Encystment and alkylresorcinol production by Azotobacter vinelandii strains impaired in poly-beta-hydroxybutyrate synthesis. Arch Microbiol 179:437–443
Senior PJ, Dawes EA (1973) The regulation of poly-beta-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem J 134:225–238
Sierra G, Gibbons NE (1962) Production of poly-β-hydroxybutiric acid granules in Micrococcus halodenitrificans. Can J Microbiol 8:249–253
Simon-Colin C, Raguénès G, Cozien J, Guezennec JG (2008) Halomonas profundus sp. nov., a new PHA-producing bacterium isolated from a deep-sea hydrothermal vent shrimp. J Appl Microbiol 104:1425–1432
Simons M, van der Bij AJ, Brand I, de Weger LA, Wijffelman CA, Lugtenberg BJ (1996) Gnotobiotic system for studying rhizosphere colonization by plant growth-promoting Pseudomonas bacteria. Mol Plant Microbe Interact 9:600–607
Steenhoudt O, Vanderleyden J (2000) Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev 24:487–506
Steinbüchel A, Hein S (2001) Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. Adv Biochem Eng Biotechnol 71:81–123
Stephens JHG, Rask HM (2000) Inoculant production and formulation. Field Crops Res 65:249–258
Tal S, Okon Y (1985) Production of the reserve material poly-β-hydroxybutyrate and its function in Azospirillum brasilense Cd. Can J Microbiol 31:608–613
Tal S, Smirnoff P, Okon Y (1990a) Purification and characterization of D-levo-β-hydroxybutyrate dehydrogenase from Azospirillum brasilense Cd. J Gen Microbiol 136:645–650
Tal S, Smirnoff P, Okon Y (1990b) The regulation of poly-β-hydroxybutyrate metabolism in Azospirillum brasilense during balanced growth and starvation. J Gen Microbiol 136:1191–1196
Tanio T, Fukui T, Shirakura T, Saito T, Tomita K, Kaiho T, Masamune S (1982) An extracellular poly(3-hydroxybutyrate) depolymerase from Alcaligenes faecalis. Eur J Biochem 124:71–77
Timm A, Steinbüchel A (1992) Cloning and molecular analysis of the poly(3-hydroxyalkanoic acid) gene locus of Pseudomonas aeruginosa PAO1. Eur J Biochem 209:15–30
Tokiwa Y, Calabia BP (2004) Degradation of microbial polyesters. Biotechnol Lett 26:1181–1189
Trainer MA, Charles TC (2006) The role of PHB metabolism in the symbiosis of rhizobia with legumes. Appl Microbiol Biotechnol 71:377–386
Trautwein K, Kühner S, Wöhlbrand L, Halder T, Kuchta K, Steinbüchel A, Rabus R (2008) Solvent stress response of the denitrifying bacterium “Aromatoleum aromaticum” strain EbN1. App Environ Microbiol 74:2267–2274
Valappil SP, Boccaccini AR, Bucke C, Roy I (2007) Polyhydroxyalkanoates in Gram-positive bacteria: insights from the genera Bacillus and Streptomyces. Antonie Van Leeuwenhoek 91:1–17
Van Elsas JD, van Overbeek LS (1993) Bacterial responses to soil stimuli. In: Kjelleberg S (ed) Starvation in bacteria. Plenum, New York, pp 55–77
Vassileva V, Ignatov G (2002) Relationship between bacteroid poly-β-hydroxybutyrate accumulation and nodule functioning in the Galega orientalis–Rhizobium galegae symbiosis under diamine treatment. Physiol Plant 114:27–32
Villanueva L, Navarrete A, Urmeneta J, Geyer R, White DC, Guerrero R (2007) Monitoring diel variations of physiological status and bacterial diversity in an estuarine microbial mat: an integrated biomarker analysis. Microb Ecol 54:523–531
Wang JG, Bakken LR (1998) Screening of soil bacteria for poly-beta-hydroxybutyric acid production and its role in the survival of starvation. Microb Ecol 35:94–101
Wang C, Sheng X, Equi RC, Trainer MA, Charles TC, Sobral BWS (2007) Influence of the poly-3-hydroxybutyrate (PHB) granule-associated proteins (PhaP1 and PhaP2) on PHB accumulation and symbiotic nitrogen fixation in Sinorhizobium meliloti Rm1021. J Bacteriol 189:9050–9056
Williams SF, Peoples OP (1996) Biodegradable plastics from plants. Chemtech 26:38–44
Willis LB, Walker GC (1998) The phbC (poly-beta-hydroxybutyrate synthase) gene of Rhizobium (Sinorhizobium) meliloti and characterization of a phbC mutant. Can J Microbiol 44:554–569
Yilmaz M, Soran H, Beyatli Y (2005) Determination of poly-β-hydroxybutyrate (PHB) production by some Bacillus spp. World J Microbiol Biotechnol 21:565–566
Zhao HY, Li HM, Qin LF, Wang HH, Chen G-Q (2007) Disruption of the polyhydroxyalkanoate synthase gene in Aeromonas hydrophila reduces its survival ability under stress conditions. FEMS Microbiol Lett 276:34–41
Acknowledgements
The production of this chapter was supported by The Israel Science Foundation founded by The Academy of Sciences and Humanities. The work of S.C.-S. at the Hebrew University of Jerusalem was supported by a Lady Davis Trust Fellowship and by the Comisión Sectorial de Investigación Cientifíca (CSIC), Universidad de la República, Unidad de Recursos Humanos, Uruguay.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Castro-Sowinski, S., Burdman, S., Matan, O., Okon, Y. (2010). Natural Functions of Bacterial Polyhydroxyalkanoates. In: Chen, GQ. (eds) Plastics from Bacteria. Microbiology Monographs, vol 14. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-03287-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-642-03287-5_3
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-03286-8
Online ISBN: 978-3-642-03287-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)