Abstract
Refractory hypoxemia is a characteristic feature of severe ARDS. Over the last 30 years, prone positioning is one intervention clinicians have used to improve oxygenation in these patients. Prone positioning improves oxygenation via better ventilation-to-perfusion matching and improved lung mechanics while reducing the potential for ventilator-associated lung injury. In both pediatric and adult studies, prone positioning has been found to be a safe and relatively noninvasive maneuver for patients with ARDS. Though prone positioning clearly improves oxygenation, clinical trials have not demonstrated improvements in survival or morbidity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
FormalPara Educational Goals-
To describe the physiologic effects of prone positioning on normal and diseased lungs
-
To review the outcomes of clinical studies evaluating prone positioning as a treatment for acute lung injury and acute respiratory distress syndrome in adults and children
-
To discuss the indications and contraindications for prone positioning in pediatric patients
-
To highlight the steps clinicians can take to use prone positioning safely in critically ill children
1 Introduction
Refractory hypoxemia is a characteristic feature of severe ARDS. Over the last 30 years, prone positioning is one intervention clinicians have used to improve oxygenation in these patients. Prone positioning improves oxygenation via better ventilation-to-perfusion matching and improved lung mechanics while reducing the potential for ventilator-associated lung injury. In both pediatric and adult studies, prone positioning has been found to be a safe and relatively noninvasive maneuver for patients with ARDS. Though prone positioning clearly improves oxygenation, clinical trials have not demonstrated improvements in survival or morbidity.
2 Basic Principles
It has long been known that changes in body position alter lung volumes, gas exchange, and perfusion. In 1933, Beams and Christie first noted that lung vital capacity was lower when in the supine position than in the upright position (Christie and Beams 1933). That same year, Hurtado and Frey found that the functional residual capacity (FRC) was also lower in the supine position (Hurtado and Frey 1933). Later, in 1955, by studying subjects using helium washout, Blair and Hickam showed that these positional changes were also associated with changes in gas mixing (Blair and Hickam 1955). The first description of the potential for prone positioning to reverse these effects came in 1961, when Moreno and Lyons studied lung volumes in patients transitioning from sitting to supine and prone positions. They demonstrated that FRC was higher in the prone position than in the supine position (Moreno and Lyons 1961). Notably, a subsequent study comparing supine and prone positioning in pediatric patients has revealed no difference in FRC (Numa et al. 1997).
Prone positioning was first proposed as a therapeutic modality for improvement of lung mechanics in 1974, when Bryan described improved oxygenation in patients under general anesthesia and neuromuscular blockade when placed prone (Bryan 1974). Several investigators subsequently demonstrated that prone positioning improved oxygenation in patients with ARDS (Douglas et al. 1977; Piehl and Brown 1976). Over the last 30 years, there has been increasing interest in prone positioning, which has been used in all age groups to improve oxygenation and respiratory mechanics.
3 Physiologic Effects
3.1 Normal Lungs
In normal lungs, changes in body posture affect oxygenation by (1) changes in distribution of alveolar inflation and ventilation, (2) by differences in regional perfusion, and (3) by changes in lung and chest wall mechanics. When supine, alveolar inflation and ventilation are preferentially distributed to nondependent ventral regions. The difference in the transpulmonary gradient between dependent and nondependent regions explains the difference in alveolar expansion between the regions. The transpulmonary gradient, the difference between the alveolar and pleural pressures, is higher in the ventral regions compared to dorsal regions of the lung in the supine position, which preferentially favors ventilation of ventral (nondependent) lung.
The difference in the transpulmonary gradient is due to several factors, including lung weight, heart mass, diaphragm displacement by intra-abdominal contents, and chest shape. The force of gravity and the mass of dependent lung regions both act to favor alveolar distention and increased transpulmonary gradient in nondependent lung areas. Under physiologic conditions, the mass of the heart also contributes by compressing underlying lung tissue (Hyatt et al. 1985). When patients are supine, the weight of the abdominal contents may compress dependent regions of the diaphragm and therefore increase pleural pressure, decreasing the transpulmonary gradient. This is particularly true in patients under general anesthesia and neuromuscular blockade (Froese and Bryan 1974).
When a patient is moved from the supine to the prone position, alveolar ventilation redistributes from the ventral regions to the dorsal regions. In addition, prone positioning results in a more homogeneous transpulmonary pressure gradient when compared to the supine position (Mutoh et al. 1992). This change in the distribution of transpulmonary pressure is due to (1) alteration in the lung weight gradient, (2) reductions in the mass of the heart transmitted to underlying lung, (3) decreased intra-abdominal pressure in the prone position which decreases cephalic displacement of the diaphragm, and (4) changes in regional chest wall mechanics and shape in the prone position (Pelosi et al. 2002).
In 1964, West and colleagues observed that lung perfusion increases gradually from nondependent to dependent lung regions (West et al. 1964). Classically, the “gravitational theory” was used to explain this observation, which proposed that perfusion increases steadily in more dependent lung regions solely due to the effects of gravity. The prone position could therefore reverse this gravitational gradient and redistribute perfusion to the better-ventilated ventral lung. Recent studies have challenged this notion and suggest that gravity plays only a minor role in the redistribution of perfusion in the lung. In fact, it appears that more dorsal lung regions are always preferentially perfused due to the geometry of the pulmonary vasculature (Glenny and Robertson 1991; Glenny et al. 1991; Wiener et al. 1990). This observation increases the likelihood that prone ventilation reduces shunt by changes in regional ventilation rather than to changes in pulmonary perfusion.
As a child matures, the thoracic shape, compliance, and deformability change significantly. In the newborn, the ribs extend horizontally, producing a circular chest shape. The newborn’s rib cage is highly compliant and easily deformable, predisposing the newborn to paradoxical movement of the chest wall during inspiration which interferes with the mechanical coupling of diaphragmatic and chest wall movement. The high ratio of chest wall compliance to lung compliance (6.7:1) exaggerates the magnitude of the paradoxical motion between the caudal surface of the lung (driven by the diaphragm) and the remaining lung (driven by the rib cage) (Gerhardt and Bancalari 1980). In the older child, caudal inclination of the ribs increases both the efficiency of the thoracic mechanics and intrathoracic volume. In the adult, the thorax has a smaller relative anteroposterior diameter, and increased intrathoracic volumes are produced by the “bucket handle” or the “pump handle” effect of costal elevation during inspiration. These changes in the thoracic shape, along with the increased effect of gravity in the upright position, the development of the thoracic musculature, and the mineralization of bone, all result in decreased chest wall compliance and deformability in adults. Total respiratory compliance decreases progressively from 5 to 16 years of age, and the decrease in chest wall compliance prevents paradoxical motion of the chest wall during inspiration, improving mechanical coupling of the diaphragm and chest wall (Sharp et al. 1970).
Newborns, especially preterm infants, may be more prone to fatigue of respiratory muscles because of the increased workload imposed by the characteristics of their chest wall. Indeed, the work required to expand the chest wall may be up to four times the work required to produce movement of the lung (Heldt and McIlroy 1987). Lateri and Sly demonstrated that, compared with older children, infants had disproportionately higher respiratory system compliances, as an influence of chest wall composition after adjusting for the effects of lung volume (Nicolai et al. 1993). In addition to the effect of the compliant chest wall on work of breathing, there is also a significant effect on the airways which compromises the efficiency of gas exchange. The reduced outward recoil of the chest wall during end expiration in infants limits the distending pressure in the pleural space which results in airway collapse, atelectasis, decreased functional residual capacity, and impaired gas exchange (Stocks 1999).
Prone positioning has a significant impact on respiratory mechanics in healthy children. In a study of ten healthy infants undergoing surgery for clubfoot, Cox and colleagues recorded pulmonary mechanics in the supine and prone positions. The study, which was the first to examine the physiologic effects of prone positioning in healthy infants, found that both static and dynamic respiratory system compliances were significantly lower in the prone position compared to the supine position. This was not associated with any impairment of gas exchange (Cox et al. 2001).
3.2 Diseased Lungs
There are a number of physiologic benefits when prone positioning is utilized in patients with lung diseases such as ARDS, including improvement in V/Q matching, a more homogeneous transpulmonary pressure gradient, improvement of alveolar recruitment, better respiratory mechanics, and the potential for reduced ventilator-induced lung injury (VILI).
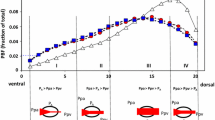
Computed tomography (CT) scans of lungs affected by ARDS have revealed that alveolar inflation follows a gravitational gradient, with nondependent regions exhibiting more expansion than dependent regions (Fig. 23.1). This suggests that more dependent regions of the lung collapse under the lung’s own weight and do not participate effectively in gas exchange. The atelectasis-prone dependent regions of the lung are therefore more likely to endure repetitive collapse and inflation (atelectrauma) during mechanical ventilation. At the same time, dependent lung regions have preferentially more perfusion than nondependent regions. The result is a mismatch in ventilation and perfusion. Prone positioning dramatically alters the distribution of alveolar ventilation and leads to enhanced ventilation-perfusion matching. Transpulmonary pressure is more evenly distributed, resulting in a more homogeneous distribution of alveolar inflation. This, in turn, allows improved ventilation of dorsal regions. Perfusion of the lung in the prone position is more homogeneous than in the supine position (Lamm et al. 1994). Overall, the result of prone positioning is an increase in the V/Q ratio. This was shown by Pappert and colleagues who administered pressure-controlled ventilation in the prone position for 2 h–12 adult patients with ARDS (Pappert et al. 1994). Using the multiple inert gas elimination technique (MIGET), the authors recorded continuous ventilation-perfusion ratios. Within 30 min of prone positioning, eight patients, the responders, showed improved oxygenation, which they attributed to reduced perfusion to shunt regions and resultant increase in the V/Q ratio. The change in perfusion from non-ventilated lung regions to those with normal V/Q ratios was thought to be due to the redistribution of ventilation to previously collapsed regions of the lung.
CT scans—apex, hilum, and base—in ARDS from sepsis. Images were taken at 5 cm H2O end-expiratory pressure. The CT scans show heterogeneous disease and both the craniocaudal and sternovertebral gradients (From Gattinoni et al. (2001))
Prone positioning may have a variable effect on improving oxygenation depending on the underlying recruitment status of the patient’s alveolar units. Recruitment normally progresses from ventral to dorsal and from cephalad to caudal (Puybasset et al. 1998). In light of this, one might imagine that prone positioning is less likely to have the same benefit in a derecruited lung as it might in a recruited lung. One study recently examined the effect of prone positioning on alveolar recruitment and oxygenation in a cohort of adult patients with ARDS (Guerin et al. 1999). The investigators found a correlation between change in oxygenation and the recruited lung volume. They hypothesized that one of the mechanisms by which prone positioning improved oxygenation was recruitment. Another study examined the effect of prone positioning on enhancing the outcome of recruitment maneuvers in patients with ARDS. The authors concluded that cyclical sustained inflations during ventilation in the prone position may produce optimal lung recruitment (Pelosi et al. 2003b). Oczenski and colleagues demonstrated a prolonged improvement in oxygenation after performing a recruitment maneuver in patients who were maintained in the prone position for 6 h (Oczenski et al. 2005). These studies support the hypothesis that redistribution of ventilation and enhanced alveolar recruitment is the mechanism of improvement in oxygenation in patients with ARDS. This may have important implications in patients with extrapulmonary ARDS, whose lungs may be more responsive to standard recruitment measures (Lim et al. 2003; Pelosi et al. 2003a). Because patients with secondary ARDS may have more recruitable lungs than those with primary ARDS, they are more likely to respond to prone positioning.
In supine patients, the anterior chest wall is normally more compliant than the posterior chest wall as there is little impediment to anterior movement of the rib cage. When patients are transitioned to the supine position, this anterior chest wall compliance is impeded by the patient’s bed, and the posterior chest wall has a relatively improved compliance. In a study of 16 adults with ARDS, Pelosi and colleagues proposed that these relative differences in chest wall compliance explain the improved alveolar ventilation of dependent lung areas when patients are supine (Pelosi et al. 1998).
Another potential benefit of utilizing the prone position in ARDS is that of limiting ventilator-induced lung injury (VILI). VILI involves various mechanisms, including overdistention through excessive volume delivery (“volutrauma”) or transpulmonary pressure (“barotrauma”) and the phenomenon of alveolar collapse and reinflation (“atelectrauma”), which may lead to a local and systemic inflammatory reaction (Slutsky 1999). In a recent animal model, Valenza and colleagues demonstrated a delay in the progression of VILI with prone positioning (Valenza et al. 2005). Recent animal studies with preinjured lungs ventilated with large tidal volumes demonstrated less histopathologic injury in the prone versus the supine position (Broccard et al. 1997, 2000). In addition, they found that in the supine position, lung injury was primarily located in the dependent lung areas, while in the prone position, lung injury was more homogeneously distributed. These preclinical studies uniformly suggest that the prone position may act to protect the lung from developing VILI, as well as to slow the progression of existing lung injury.
4 Effectiveness and Outcome
Most of the large clinical trials of prone positioning in ARDS have been performed in the adult population. In the neonatal and pediatric populations, several investigators have examined small cohorts of patients. These studies as well as a recently published randomized, controlled trial in pediatric patients are summarized in Table 23.1. The initial reports of prone positioning in pediatrics were limited by nonrandom assignment of prone positioning for variable time periods in a heterogeneous population. These limited reports, along with a lack of uniform guidelines to perform prone positioning safely and effectively, led to reluctance among pediatricians to perform this maneuver. Yet, these initial reports did describe dramatic improvements in oxygenation soon after children with ALI/ARDS were placed in the prone position. The improvement in oxygenation and the resultant potential to reduce inspired oxygen concentrations as well as airway pressures have spurred continued study of prone positioning in pediatrics.
Key components to consider in the studies on pediatric prone positioning to date include patient selection, the timing and duration of prone positioning, and outcome measurement. Patient selection for prone positioning is challenging and may be integral to understanding the true benefit and correct application of the intervention. One important factor in patient selection is that acute lung injury from different etiologies does not share the same morphological, mechanical, and clinical characteristics. For years, investigators of adult ALI and ARDS have debated the clinical significance between pulmonary and extrapulmonary etiologies of lung injury (Callister and Evans 2002; Pelosi and Gattinoni 2001). Some assert that the two conditions are different entities, with different pathophysiology, radiographic appearance, and respiratory mechanics (Pelosi et al. 2003a). The two entities may also have different responses to prone positioning. Indeed, Pelosi and colleagues have reported that secondary ARDS, with associated diffuse atelectasis, may be more responsive to prone positioning (Pelosi et al. 2002). Primary ARDS, characterized by altered lung elastance, may not benefit from strategies such as increased PEEP, lung recruitment, and prone positioning, compared to patients with secondary, or extrapulmonary, ARDS, which may be characterized more by altered chest wall elastance. It is important to note, however, that the difference in the two conditions in terms of the impact of different therapies is unknown. Indeed, a recent study revealed no difference in lung functional assessment 6 months after hospital discharge in adult patients with pulmonary versus extrapulmonary ARDS (Suntharalingam et al. 2001).
The overall length of mechanical ventilation is important when considering patient selection in studies on prone positioning. In 1997, Numa and colleagues examined the effect of prone position on functional residual capacity (FRC), oxygenation, and respiratory mechanics in mechanically ventilated children with restrictive and obstructive lung disease and in controls (Numa et al. 1997). The investigators found a pattern of increased FRC in the prone position in each of the three subgroups, though this improvement did not reach statistical significance. There was no significant improvement in oxygenation in any of the groups. One important caveat to the study is that the authors enrolled patients after prolonged periods of mechanical ventilation (up to 2 weeks), and this may have influenced the physiologic effects that prone positioning had on this population.
Outcome measures are important to consider when evaluating studies on ARDS. Traditionally, the degree of oxygenation has been the main surrogate of the severity of lung injury in studies on ARDS. This holds true in studies evaluating the role of prone positioning. Pediatric studies evaluating the role of prone positioning in ARDS have used indices of oxygenation such as the oxygenation index (OI) and the PaO2/FiO2 (P/F) ratio as outcome measures (Casado-Flores et al. 2002; Curley et al. 2000; Kornecki et al. 2001). In a prospective case series of 25 pediatric patients with ARDS, an immediate and cumulative improvement in oxygenation was demonstrated after prone positioning (Curley et al. 2000). The study included patients from 2 months to 17 years that were enrolled within 19 h of meeting entry criteria. Patients were placed prone for 20 h each day and returned to the supine position for 4 h, accounting for 47 % of the time on mechanical ventilation. Indices of oxygenation, including P/F ratios and OI, are shown in Fig. 23.2 at four time points in the first 24 h of prone positioning. Both P/F ratio and OI significantly improved at both 1 h and up to 19 h after prone positioning. Based on the response to prone positioning in the first 24 h, the investigators then classified patients as immediate responders (n = 11, 44 %) and immediate nonresponders (n = 14, 56 %). In the immediate responders, oxygenation improves early and is sustained during the prone period (Fig. 23.3). Even after this subgroup of patients (immediate responders) returned to the supine position, they exhibited preserved improvement in oxygenation. Nonresponders were found to have a delayed and non-sustained improvement in oxygenation. The authors reported an overall 84 % rate of response to prone positioning in this population of children. Casado-Flores and colleagues also showed an improvement in oxygenation with prone positioning in a population of 23 children with ARDS (Casado-Flores et al. 2002). In contrast to the previously summarized study, they showed that the improvement in oxygenation was not sustained after patients returned to the supine position. Overall, studies using oxygenation as an outcome for response to prone positioning have shown variability in response and an inability to predict which patients might respond to the intervention.
P/F ratio, OI, and PaCO2 at four time points in first 24 h of prone positioning. Day 1 PaO2/FiO2 ratio, OI, and PaCO2. Top: The PaO2/FiO2 ratios among the four data collection points were significantly different (p = 0.006). The PaO2/FiO2 ratio significantly increased (p = 0.04) from 143 ± 10 mmHg at baseline in the supine position to 173 ± 14 mmHg after 1 h in the prone position. It continued to increase significantly (p = 0.005) to 194 ± 15 after 19 h in the prone position. The PaO2/FiO2 ratio at hour 21 in the supine position (150 ± 11 mmHg) was not significantly different from that at baseline. Middle: The OIs among the four data collection points were significantly different (p = 0.01). The OI significantly decreased (p = 0.05) from a baseline value in the supine position of 15.7 ± 1.7–13.6 ± 1.6 after 1 h in the prone position and continued to decrease significantly (p = 0.008) to 10.9 ± 1.5 after 19 h in the prone position. The OI at hour 21 in the supine position (14 ± 1.9) was not significantly different from that at baseline. Bottom: PaCO2 values were not significantly different among the four data collection points (From Curley et al. (2000))
The PaO2/FiO2 ratio was significantly different between the two subgroups (p = 0.05) over the four data collection points (p = 0.003) and between the two subgroups over the four data collection points (p = 0.03). Immediate responder group (•): The PaO2/FiO2 ratio significantly increased (p = 0.003) from a baseline value in the supine position of 134 ± 11–213 ± 21 mmHg after 1 h in the prone position. Significant (p = 0.02) but not cumulative increases in oxygenation were seen after 19 h in the prone position (220 ± 25 mmHg). The PaO2/FiO2 ratio at hour 21 in the supine position (170 ± 12) was significantly better than that at baseline in the supine position (p = 0.02). Immediate nonresponder group (■): The PaO2/FiO2 ratio did not significantly increase from a baseline value in the supine position of 152 ± 16–141 ± 13 mmHg after 1 h in the prone position. Significant (p = 0.02) and cumulative increases in oxygenation were seen after 19 h in the prone position (173 ± 15 mmHg). The PaO2/FiO2 ratio at hour 21 in the supine position (135 ± 15 mmHg) was not significantly better than that at baseline in the supine position. Note that with the exception of increasing the FiO2 to keep Spo2 at >85 %, ventilator settings were held constant during the 1-h supine-to-prone and prone-to-supine repositioning (From Curley et al. (2000))
It has been demonstrated in a number of clinical trials in adults with ARDS that improved oxygenation is not associated with improved clinical outcomes (ARDSnet 2000; Brower et al. 2004; Doyle et al. 1995). The only randomized, controlled trial of the prone position in pediatric patients that evaluated relevant clinical outcomes as well as gas exchange was published in 2005 (Curley 2005). The study population included 102 patients, aged 2 weeks to 18 years, from seven pediatric intensive care units who were enrolled within 48 h of meeting criteria for ALI or ARDS. To ensure uniform co-interventions among the patients, the two groups were managed using identical ventilator protocols, extubation readiness testing, sedation protocols, and guidelines related to hemodynamics, nutrition, and skin care. Patients in the prone positioning group spent 20 h/day in the prone position, for a maximum of 7 days. The primary outcome was ventilator-free days, and the secondary outcomes included mortality, time to recovery from lung injury, and organ-failure-free days. The authors did not demonstrate significant differences in either the primary outcome or any of the secondary outcome variables. The study was designed to enroll patients early in their presentation and to follow them throughout the acute phase, with patients placed prone on average of 28 h after fulfilling eligibility requirements and being treated for 79 % of the acute phase of the illness. Consistent with an earlier study on the prone position (Curley et al. 2006), this study demonstrated that 90 % of patients managed in the prone position were categorized as responders, defined by an improvement in P/F ratio or OI. Despite a robust study design, which included early enrollment, algorithms for all relevant interventions likely to affect the primary outcome, and a high rate of compliance with all treatment protocols, the study failed to show a clinically relevant benefit of prone position in the treatment of pediatric ARDS or ALI.
A recent meta-analysis of studies on prone positioning in both adult and pediatric ARDS revealed no significant improvement in mortality (Abroug et al. 2008). A meta-analysis of adult trials also demonstrated no significant overall improvement in mortality, though there was a significantly improved mortality in patients with higher severity of illness (Alsaghir and Martin 2008). No studies have demonstrated that prone positioning in patients with ARDS improves mortality, though one recent trial has shown a trend toward improved 60-day survival in adults (Fernandez et al. 2008).
Another important consideration when evaluating pediatric studies on prone positioning is the duration of prone positioning. The timing of prone positioning ranges from a single 30-min prone maneuver in one study to other studies that place patients prone from 8 to 24 h a day (Murdoch and Storman 1994). Some studies report early improvement in hypoxemia in patients who respond to prone positioning. Relvas and colleagues performed a retrospective analysis of patients with ARDS placed in the prone position and compared the OI prior to the intervention to OI values after brief (6–10 h) and prolonged (18– 24 h) periods of prone ventilation (Relvas et al. 2003). The patients placed in a prone position for a prolonged period of time demonstrated a more pronounced and stable reduction in OI than did patients placed prone for brief periods (Curley et al. 2006; Kornecki et al. 2001).
5 Indications and Contraindications
Because prone positioning has not been shown to have a positive impact on clinical outcomes, it should not routinely be used in the care of pediatric patients with ALI and ARDS. Prone positioning may be used in patients with ARDS and refractory hypoxemia as a recruitment maneuver. Particularly in patients with extrapulmonary ARDS, whose lungs may be more “recruitable,” prone positioning may prove helpful. The duration of prone positioning may be limited by practical considerations that include patient size and clinical status. Patients who do not show an immediate oxygenation response may not be good candidates for continued prone positioning.
In some patients, prone positioning may not be a practical intervention. This may include patients who are unable to be positioned due to body habitus (e.g., extreme obesity or recent abdominal surgery), patients unable to tolerate prone positioning (e.g., neurologic trauma), and patients undergoing therapies that make the changes in position challenging (e.g., extracorporeal membranous oxygenation or continuous venovenous hemofiltration). Compared to adults, pediatric patients may be more safely placed in the prone position due to their size (Fineman et al. 2006).
As noted above, prone positioning is unlikely to be beneficial in a patient whose lung is not recruited. Prior to placing a child in the prone position, clinicians should evaluate the patient for lung inflation and aim to achieve optimal lung recruitment. To assess the degree of recruitment, clinicians should rely on oxygenation and on measurement of lung mechanics to evaluate the response to recruitment maneuvers at the bedside.
6 Complications
Before a clinician makes the decision to employ prone positioning in a child with ARDS, one must weigh the benefits of this maneuver with its potential adverse effects. The potential complications associated with prone positioning include tissue injury and nerve injury at pressure areas (Casado-Flores et al. 2002; Winfree and Kline 2005), decreased venous return and dependent edema, changes in intraocular pressure (Hunt et al. 2004), problems accessing or positioning lines and tubes, difficulty accessing patients during resuscitation (Vollman 1997), risk of disconnection of lines and tubes, and delay in recognition of cardiorespiratory deterioration. Utilizing a well-designed algorithm along with well-trained personnel who are familiar with the maneuver can minimize the risks of these complications (Curley et al. 2006; Martin de la Torre Martin et al. 2000).
Dependent edema is a common complication of prone positioning. Many of the studies delineating complications of prone ventilation were performed in adults. In these patients, edemas of the face, eyelids, conjunctiva, lips, and tongue increase progressively after a few hours of prone positioning. Though the edema is reversible, it may be difficult to avoid, even with frequent changes in patient position (Chatte et al. 1997; Fridrich et al. 1996).
Dislodgement of invasive tubes and lines is an important potential complication of the prone positioning maneuver, though studies suggest that this complication is relatively uncommon if the position change is performed in a planned manner. In a study by Marcano and colleagues, the authors found that prone positioning of pediatric patients does result in cephalad movement of the endotracheal tube (Marcano et al. 2003). The study involved the review of 15 pairs of radiographs before and after prone positioning in 14 patients. The authors recommend placement of the tip of the endotracheal tube deeper than the level of one-third tracheal length to avoid potential cephalad movement and dislodgement. In the randomized trial summarized above involving 102 patients, none of the 51 patients placed prone were inadvertently extubated during the process of repositioning, and the rate of inadvertent extubation over the period of study was similar to that previously reported in supine children (Fineman et al. 2006). Haefner and colleagues described their institutional experience with prone positioning in patients receiving ECMO therapy (Haefner et al. 2003). In 962 position changes among 93 patients, no unplanned extubations, tube displacements, skin pressure ulcerations, or corneal abrasions were reported. Of particular importance to clinicians considering prone positioning in this patient population, the incidence of bleeding from cannula and tube insertion sites was no higher in this patient population than in other ECMO patients.
Skin breakdown and ulceration are other important concerns in patients placed in the prone position. Jolliet and colleagues reported pressure ulcers in 3 of 19 patients in their study which utilized a 12-h period of prone positioning (Jolliet et al. 1998). Willems and colleagues reported one subject that developed bilateral nipple ulcers after a 5-day period of being prone (Willems et al. 1998). In a study where head and arm position was changed every 2 h, Fridrich and colleagues reported minor skin changes but no pressure ulcers (Fridrich et al. 1996). Pediatric studies of prone positioning have reported similar potential complications. In the study by Casado-Flores and colleagues, children with ARDS were placed in the prone position and then repositioned to the supine position every 8 h for 10 days on average (Casado-Flores et al. 2002). The protocol included the application of pads and massage to pressure points. The authors reported complications of knee scarring in two patients and external ear necrosis in a single patient. In a series of 25 children managed with a protocol which utilized a prone positioning device which suspended the abdomen as well as pressure-relieving material at areas of patient contact, 24 % of the patients enrolled in the study developed stage II pressure ulcers, but none developed clinically relevant skin breakdown (Curley et al. 2006).
Corneal edema and ulcerations are serious complications that have been described in association with prone positioning. They have the potential to cause loss of vision if not discovered and treated early. Stocker and colleagues reported a case report of a patient who developed corneal ulceration that ultimately required corneal transplantation (Stocker et al. 1997).
Prone positioning may have effects on intra-abdominal pressure and may thereby affect renal function and patient hemodynamics. Hering and colleagues reported the effect that prone positioning had on intra-abdominal pressure, renal function, and cardiovascular function in mechanically ventilated adults (Hering et al. 2001). The authors reported an improvement in systemic oxygenation with no change in hemodynamic status or renal function and perfusion. Intra-abdominal pressure increased by 12–14 mmHg in the prone patients, though no attempts were made to suspend the abdomen.
7 Practical Steps for Staff
In order to minimize the risk of iatrogenic injury due to prone positioning, an institutional protocol for standardization is recommended. Appendix 23.1 includes the protocol for prone positioning utilized by in a previously summarized trial (Curley 2005). The protocol begins with a checklist that helps the practitioner anticipate and plan for potential problems that might arise during a turning maneuver. An important part of safely turning a patient is having adequate personnel participating in the turning. The clinician should anticipate and be ready to intervene if any changes in the patient’s respiratory mechanics are detected after the position change. Prior to repositioning, a team member should check and secure all invasive lines and tubes. To prevent damage to the skin, face, and eyes, the team should use pressure-relieving positioning aides. Any new protocols should include the involvement of the pediatric physical therapist. After the actual position change, the bedside team should monitor the patient closely for pressure ulcers or corneal injury.
In patients who are maintained on high-frequency oscillatory ventilation (HFOV) in the prone position, we have noted another important safety issue. Current HFOV ventilators do not have safety alarms for detecting decreased tidal volume. Clinicians often rely on chest movement or the “wiggle factor” to determine the efficacy of oscillations. A transition to the prone position can impair the amplitude of oscillations and therefore result in rapidly increasing hypercapnia. This potential side effect should be anticipated and documented with early arterial blood gases after repositioning.
8 The Future
As noted above, no studies to date have demonstrated that improved oxygenation decreases mortality in patients with ARDS. Future studies should focus on the use of prone positioning in select subsets of patients with ARDS, such as those with higher severity of illness or those with extrapulmonary ARDS. In addition, studies should focus on long-term outcomes rather than short-term physiologic endpoints or short-term mortality. Indeed, studies investigating long-term outcome in adults with ARDS have revealed that they experience significant pulmonary morbidity, including persistent oxygen requirement, and non-pulmonary morbidity, including muscle fatigue, atrophy, and weakness (Herridge et al. 2003). Assessments of long-term outcome of therapeutic interventions in ARDS should monitor, quantify, and evaluate the effect on functional outcomes, as well as mortality.
9 Summary and Conclusion
Prone positioning has been well described as a maneuver to improve oxygenation in patients with ARDS and ALI. Through a variety of physiologic mechanisms, most importantly an improvement in lung recruitment and V/Q matching, application of mechanical ventilation to patients in the prone position may have an immediate and prolonged effect on oxygenation. Though no studies have shown that prone positioning has a significant effect on clinical outcomes, it is a safe maneuver that may be employed in select populations of children with ARDS. However, in the absence of proven improvements in clinical outcomes, prone positioning should not be a part of the routine management of these patients.
Essentials to Remember
-
Prone positioning improves oxygenation in diseased lungs through improvement in lung recruitment, better matching of ventilation and perfusion, and changes in chest wall compliance.
-
Clinical studies evaluating the use of prone positioning for ARDS and ALI in pediatric patients, including a recent multicenter randomized controlled trial, have failed to show significant improvements in clinical outcomes.
-
With attention to safe turning and to interventions to minimize possible complications, prone positioning may be safely employed in children with ARDS or ALI, but should not be routinely used in this patient population.
References
Abroug F et al (2008) The effect of prone positioning in acute respiratory distress syndrome or acute lung injury: a meta-analysis. Areas of uncertainty and recommendations for research. Intensive Care Med 34(6):1002–1011
Alsaghir A, Martin C (2008) Effect of prone positioning in patients with acute respiratory distress syndrome: a meta-analysis. Crit Care Med 36(2):603–609
ARDSnet (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342(18):1301–1308
Blair E, Hickam JB (1955) The effect of change in body position on lung volume and intrapulmonary gas mixing in normal subjects. J Clin Invest 34(3):383–389
Broccard AF et al (1997) Influence of prone position on the extent and distribution of lung injury in a high tidal volume oleic acid model of acute respiratory distress syndrome. Crit Care Med 25(1):16–27
Broccard A et al (2000) Prone positioning attenuates and redistributes ventilator-induced lung injury in dogs. Crit Care Med 28(2):295–303
Brower RG et al (2004) Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 351(4):327–336
Bryan AC (1974) Conference on the scientific basis of respiratory therapy. Pulmonary physiotherapy in the pediatric age group. Comments of a devil’s advocate. Am Rev Respir Dis 110(6 Pt 2):143–144
Callister ME, Evans TW (2002) Pulmonary versus extrapulmonary acute respiratory distress syndrome: different diseases or just a useful concept? Curr Opin Crit Care 8(1):21–25
Casado-Flores J et al (2002) Pediatric ARDS: effect of supine-prone postural changes on oxygenation. Intensive Care Med 28(12):1792–1796
Chatte G et al (1997) Prone position in mechanically ventilated patients with severe acute respiratory failure. Am J Respir Crit Care Med 155(2):473–478
Christie C, Beams A (1933) The estimation of normal vital capacity with special reference to the effect of posture. Arch Intern Med 30:34–39
Cox RG, Ewen A, Bart BB (2001) The prone position is associated with a decrease in respiratory system compliance in healthy anaesthetized infants. Paediatr Anaesth 11(3):291–296
Curley M (2005) Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA 294(2):229–237
Curley MA, Thompson JE, Arnold JH (2000) The effects of early and repeated prone positioning in pediatric patients with acute lung injury. Chest 118(1):156–163
Curley MA et al (2006) Clinical trial design–effect of prone positioning on clinical outcomes in infants and children with acute respiratory distress syndrome. J Crit Care 21(1):23–32; discussion 32–37
Douglas WW et al (1977) Improved oxygenation in patients with acute respiratory failure: the prone position. Am Rev Respir Dis 115(4):559–566
Doyle RL et al (1995) Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med 152(6 Pt 1):1818–1824
Fernandez R et al (2008) Prone positioning in acute respiratory distress syndrome: a multicenter randomized clinical trial. Intensive Care Med 34(8):1487–1491
Fineman LD et al (2006) Prone positioning can be safely performed in critically ill infants and children. Pediatr Crit Care Med 7(5):413–422
Fridrich P et al (1996) The effects of long-term prone positioning in patients with trauma-induced adult respiratory distress syndrome. Anesth Analg 83(6):1206–1211
Froese AB, Bryan AC (1974) Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 41(3):242–255
Gattinoni L et al (2001) What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med 164(9):1701–1711
Gerhardt T, Bancalari E (1980) Chestwall compliance in full-term and premature infants. Acta Paediatr Scand 69(3):359–364
Glenny RW, Robertson HT (1991) Fractal modeling of pulmonary blood flow heterogeneity. J Appl Physiol 70(3):1024–1030
Glenny RW et al (1991) Gravity is a minor determinant of pulmonary blood flow distribution. J Appl Physiol 71(2):620–629
Guerin C et al (1999) Effects of prone position on alveolar recruitment and oxygenation in acute lung injury. Intensive Care Med 25(11):1222–1230
Haefner SM et al (2003) Complications of intermittent prone positioning in pediatric patients receiving extracorporeal membrane oxygenation for respiratory failure. Chest 123(5):1589–1594
Heldt GP, McIlroy MB (1987) Distortion of chest wall and work of diaphragm in preterm infants. J Appl Physiol 62(1):164–169
Hering R et al (2001) The effects of prone positioning on intraabdominal pressure and cardiovascular and renal function in patients with acute lung injury. Anesth Analg 92(5):1226–1231
Herridge MS et al (2003) One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348(8):683–693
Hunt K et al (2004) Changes in intraocular pressure in anesthetized prone patients. J Neurosurg Anesthesiol 16(4):287–290
Hurtado A, Frey W (1933) Studies of total pulmonary capacity and its subdivisions. III: changes with body posture. J Clin Invest 12:825–831
Hyatt RE, Bar-Yishay E, Abel MD (1985) Influence of the heart on the vertical gradient of transpulmonary pressure in dogs. J Appl Physiol 58(1):52–57
Jolliet P, Bulpa P, Chevrolet JC (1998) Effects of the prone position on gas exchange and hemodynamics in severe acute respiratory distress syndrome. Crit Care Med 26(12):1977–1985
Kornecki A et al (2001) 4A randomized trial of prolonged prone positioning in children with acute respiratory failure. Chest 119(1):211–218
Lamm WJ, Graham MM, Albert RK (1994) Mechanism by which the prone position improves oxygenation in acute lung injury. Am J Respir Crit Care Med 150(1):184–193
Lim CM et al (2003) Effect of alveolar recruitment maneuver in early acute respiratory distress syndrome according to antiderecruitment strategy, etiological category of diffuse lung injury, and body position of the patient. Crit Care Med 31(2):411–418
Marcano BV, Silver P, Sagy M (2003) Cephalad movement of endotracheal tubes caused by prone positioning pediatric patients with acute respiratory distress syndrome. Pediatr Crit Care Med 4(2):186–189
Martin de la Torre Martin M et al (2000) Postural technique in prone position: hemodynamic and respiratory parameters and complications. Enferm Intensiva 11(3):127–135
Moreno F, Lyons HA (1961) Effect of body posture on lung volumes. J Appl Physiol 16:27–29
Murdoch IA, Storman MO (1994) Improved arterial oxygenation in children with the adult respiratory distress syndrome: the prone position. Acta Paediatr 83(10):1043–1046
Mutoh T et al (1992) Prone position alters the effect of volume overload on regional pleural pressures and improves hypoxemia in pigs in vivo. Am Rev Respir Dis 146(2):300–306
Nicolai T, Lanteri CJ, Sly PD (1993) Frequency dependence of elastance and resistance in ventilated children with and without the chest opened. Eur Respir J 6(9):1340–1346
Numa AH, Hammer J, Newth CJ (1997) Effect of prone and supine positions on functional residual capacity, oxygenation, and respiratory mechanics in ventilated infants and children. Am J Respir Crit Care Med 156(4 Pt 1):1185–1189
Oczenski W et al (2005) Recruitment maneuvers during prone positioning in patients with acute respiratory distress syndrome. Crit Care Med 33(1):54–61; quiz 62
Pappert D et al (1994) Influence of positioning on ventilation-perfusion relationships in severe adult respiratory distress syndrome. Chest 106(5):1511–1516
Pelosi P, Gattinoni L (2001) Acute respiratory distress syndrome of pulmonary and extra-pulmonary origin: fancy or reality? Intensive Care Med 27(3):457–460
Pelosi P et al (1998) Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. Am J Respir Crit Care Med 157(2):387–393
Pelosi P, Brazzi L, Gattinoni L (2002) Prone position in acute respiratory distress syndrome. Eur Respir J 20(4):1017–1028
Pelosi P et al (2003a) Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl 42:48s–56s
Pelosi P et al (2003b) Sigh in supine and prone position during acute respiratory distress syndrome. Am J Respir Crit Care Med 167(4):521–527
Piehl MA, Brown RS (1976) Use of extreme position changes in acute respiratory failure. Crit Care Med 4(1):13–14
Puybasset L et al (1998) A computed tomography scan assessment of regional lung volume in acute lung injury. The CT Scan ARDS Study Group. Am J Respir Crit Care Med 158(5 Pt 1):1644–1655
Relvas MS, Silver PC, Sagy M (2003) Prone positioning of pediatric patients with ARDS results in improvement in oxygenation if maintained >12 h daily. Chest 124(1):269–274
Sharp JT et al (1970) Total respiratory compliance in infants and children. J Appl Physiol 29(6):775–779
Slutsky AS (1999) Lung injury caused by mechanical ventilation. Chest 116(1 Suppl):9S–15S
Stocker R et al (1997) Prone positioning and low-volume pressure-limited ventilation improve survival in patients with severe ARDS. Chest 111(4):1008–1017
Stocks J (1999) Respiratory physiology during early life. Monaldi Arch Chest Dis 54(4):358–364
Suntharalingam G et al (2001) Influence of direct and indirect etiology on acute outcome and 6-month functional recovery in acute respiratory distress syndrome. Crit Care Med 29(3):562–566
Valenza F et al (2005) Prone position delays the progression of ventilator-induced lung injury in rats: does lung strain distribution play a role? Crit Care Med 33(2):361–367
Vollman KM (1997) Prone positioning for the ARDS patient. Dimens Crit Care Nurs 16(4):184–193
West J, Dollery C, Naimark A (1964) Distribution of blood flow in isolated lung; relation to vascular and alveolar pressures. J Appl Physiol 19:713–724
Wiener CM, Kirk W, Albert RK (1990) Prone position reverses gravitational distribution of perfusion in dog lungs with oleic acid-induced injury. J Appl Physiol 68(4):1386–1392
Willems MC, Voets AJ, Welten RJ (1998) Two unusual complications of prone-dependency in severe ARDS. Intensive Care Med 24(3):276–277
Winfree CJ, Kline DG (2005) Intraoperative positioning nerve injuries. Surg Neurol 63(1):5–18; discussion 18
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix 23.1: Prone Positioning Check Sheet
Appendix 23.1: Prone Positioning Check Sheet
Preparation (prior to getting help into room) | |
□ | Create cushions using egg crate material (head, chest, pelvic, distal femoral, and lower limb). |
□ | Insert transpyloric feed tube and check placement. |
□ | Check ETT on CXR—tip should be in the lower 1/3 of the thoracic trachea. |
□ | Assess the security of the ETT, vascular lines, and SpO2 probe and reinforce as necessary. |
Retape the ETT to the upper lip on the side of the mouth that will end in the “up” position. | |
Place a protective layer of plastic tape over the white adhesive tape holding the ETT. | |
□ | If cuffed ETT/trach, inflate cuff using minimal leak technique (cuff pressure under 25 mmHg). |
□ | Protect eyes if chemically paralyzed and/or open (cleanse, lubricate, cover with plastic wrap). |
□ | If HFOV, apply plastic film dressing over anterior bony prominences to avoid friction injury. |
□ | Move EKG electrodes to the lateral aspects of the upper arms and hips. |
□ | Remove clothing surrounding thorax and abdomen. |
□ | Coil then secure bladder catheter to inner thigh. |
□ | Suction the patient’s oropharynx. (If ETT suctioned, postpone turn until unit patient returned to pre-suctioning ventilator settings.) |
□ | Temporarily cap nonessential vascular lines and the patient’s NGT/JT. |
□ | Final Check—Review the start and end point of all that is left attached to the patient. Arrange the remaining vascular lines and Foley catheter tubing to prevent excessive tension. |
□ | Premedicate with comfort medications at the discretion of the bedside nurse. |
Turning | |
□ | Call for RT and at least one other nurse. |
□ | Preplan responsibility: RT, head/ETT; Nurse 1, chest/arms; Nurse 2, hips/legs. |
□ | Review technique: |
Infants/toddlers: Levitate—levitate up, turn 45°, pause/reassess, turn prone, and levitate up to place cushions under the subject. | |
School aged/adolescents: Mummy—using all bed linens, slide patient to the edge of the bed away from the ventilator and place new draw sheet over patient; position chest and pelvic cushions over draw sheet; place full sheet over entire patient; create a mummy effect by tucking the edges of the full sheet under patient; turn patient 45° toward ventilator, pause/reassess, and position patient prone on new linen and cushions/remove old linen. | |
□ | Keep head in alignment with body, avoid hyperextension, keep arms next to torso, and point toes of the upper leg in the direction of turn. |
□ | Turn toward the ventilator without disconnecting. (FiO2 may be manipulated to maintain target SpO2. All other ventilator settings remain constant until 1-h post turn ABG obtained.) |
□ | Talk the patient through the turn. |
Immediately after the turn | |
□ | Reassess the security and patency of all tubes/lines. |
□ | Reassess SpO2, blood pressure, cardiac rhythm, and breath sounds. |
□ | Reassess ETT/trach leak (may adjust cuff volume, head position, delivered Vt to assure adequate ventilation). |
□ | Uncap/reattach capped off lines/NGT/NJT. |
□ | Position the patient: |
Turn head to side and cushion head and ear with pressure-relieving material. | |
Place an absorbent diaper under the patient’s mouth. | |
Avoid excessive flexion/extension of the spine; cushion the upper chest and pelvis—check that the abdomen is unrestrained. In males, check that the penis and scrotum are unrestrained. In adolescent females, check that the nipples are away from chest rolls. | |
Flex arms up. | |
Position knees and feet off bed using a roll under the distal femur and lower leg. | |
Check that everything attached to the patient is not pressing against their skin (ETT balloon port) and that the patient’s skin in not pinched in any way (periumbilical area). | |
Return to supine | |
□ | Precautions and techniques described above apply. |
□ | Consider performing the patient’s daily suctioning procedure. |
□ | Turn patients away from the ventilator without disconnecting. |
□ | Position the patient: |
Cushion head using pressure-relieving materials (pillow, jell pillow, or Spenco pad). | |
Elevate the patient’s heels off the bed using an appropriate size pillow. | |
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Green, M.L., Curley, M.A.Q., Arnold, J.H. (2015). The Prone Position in Acute Lung Injury. In: Rimensberger, P. (eds) Pediatric and Neonatal Mechanical Ventilation. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-01219-8_23
Download citation
DOI: https://doi.org/10.1007/978-3-642-01219-8_23
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-01218-1
Online ISBN: 978-3-642-01219-8
eBook Packages: MedicineMedicine (R0)