Abstract
Monoclonal gammopathy of unknown significance (MGUS) as one of the most common premalignant disorders and smoldering multiple myeloma (sMM) are both caused by a proliferation of monoclonal plasma cells leading to a detectable serum monoclonal protein and/or excess of plasma cells in the bone marrow. Prerequisite for the diagnosis is that plasma cell disease does not cause clinical symptoms. Cytogenetic aberrations are detectable in the majority of patient in the clonally expanded plasma cells. MGUS consistently proceeds symptomatic MM. The lifetime risk of progression into symptomatic multiple myeloma lies between 15% and 59% for patients with MGUS or sMM. Prognostic parameters for development of symptomatic multiple myeloma from MGUS or sMM are concentration of monoclonal protein, bone marrow plasmocytosis, a non- IgG subtype and an abnormal free-light chain ratio. Detection of more than 1 focal lesion in whole body MRI, 95% or more of bone marrow plasma cells displaying an aberrant phenotype in flow cytometry and an evolving clinical course in two consecutive follow-up visits are additional prognostic parameters for sMM. Currently there is no accepted secondary prevention strategy available for sMM and MGUS progression. Future studies are required to combine increasing knowledge on risk factors and molecular pathogenesis with targeted agents to prevent progression.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Multiple Myeloma
- Olmsted County
- Monoclonal Protein
- Bone Marrow Plasma Cell
- International Myeloma Working Group
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Definition of Monoclonal Gammopathy of Undetermined Significance (MGUS) and Smoldering Multiple Myeloma (sMM)
Monoclonal gammopathy of undetermined significance (MGUS) is defined by the detection of a monoclonal protein in serum or urine at a concentration of 30 mg/l or below in protein electrophoresis or free-light-chain (FLC) assay, the presence of <10% of plasma cells in the bone marrow and no evidence of end organ damage (Kyle et al. 2010).
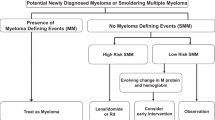
Smoldering (asymptomatic) multiple myeloma (sMM) is defined by the presence of a monoclonal protein level of 30 g/l or more or 10% or more of clonal plasma cells in the bone marrow but no end organ damage (Kyle et al. 2010; Blade et al. 2010) as summarized above and in Tables 6.1–6.3. The Mayo group has further clarified that for these criteria the monoclonal protein has to be of IgG or IgA and plasma cells need to be clonal (Kyle and Rajkumar 2009). Fifteen to twenty percent of newly diagnosed multiple myeloma patients are classified as sMM (Weber et al. 1997).
2 Prevalence of MGUS
MGUS is one of the most common premalignant disorders. Kyle et al. found an age-adjusted prevalence of MGUS in residents of Olmsted county in Minnesota (USA) of 4.0% in men versus 2.7% in women (Kyle et al. 2006). Other studies have clearly demonstrated that there are ethnic differences in the MGUS prevalence. The overall prevalence of MGUS in the Japanese population is lower than in western population with a prevalence of 2.8% in men versus 1.6% in women (Iwanaga et al. 2007). The highest overall prevalence reported so far was 5.84% among men in Ghana (Landgren et al. 2007). These results were confirmed by a comparative analysis of the MGUS prevalence among African Americans and white veterans in the United States showing an age-adjusted prevalence ratio of 3.0 in African Americans compared to white veterans (Landgren et al. 2006).
Furthermore, there is a well-known correlation between age and the occurrence of MGUS. In the Japanese population, the prevalence increases with age in both sexes: from 1% in participants aged 42–49 years, 1.9% in those 50–59 years, 2.6% in those 60–69 years, 3% in those 70–79 years, and 4.4% in those 80 years and older (Iwanaga et al. 2007). Similar data with age related increase of incidence and prevalence are available from the United States (Olmsted county) with 5.3% in persons 70 years or older and 7.5% in those 85 years and older, respectively with a preference of men (Kyle et al. 2006).
Recently, Dispenzieri et al. have presented the most extensive investigation on the condition of light-chain MGUS by analyzing blood/serum samples from the Olmsted county cohort (Dispenzieri et al. 2010). Light-chain MGUS was defined as an abnormal protein electrophoresis with no IgH expression, plus increased concentration of the involved light chain. Whereas the overall prevalence of MGUS in this population was 3.3%, 0.8% of patients fulfilled the criteria for light-chain MGUS. Progression into plasma cell-disorders were approximately 1% per year for conventional and only 0.3% for light-chain MGUS. Of note, progression of light chain MGUS was always into light-chain myeloma. Importantly, the risk of renal diseases was increased in conventional and light-chain MGUS and 23% of light-chain MGUS had renal disease that was not recognized as being related to a plasma cell disorder.
3 Differential Diagnosis and Diagnostic Assessment
Monoclonal immunoglobulins can be associated with other lymphoproliferative disorders like AL-amyloidosis, Waldenström’s disease (in case of a monoclonal IgM) or POEMS-syndrome, and patients should be evaluated for these entities. Figure 6.1 demonstrates the frequency of distinct monoclonal plasma cell-diseases in 1,684 consecutive cases of a Mayo Clinic population in 2006 (Kyle and Rajkumar 2007).
Distribution of incidence of monoclonal plasma cell disorders (Kyle and Rajkumar 2007)
3.1 Initial Diagnostic Assessment
Recently expert panels have reviewed the initial diagnostic work-up of patients with monoclonal gammopathy (Kyle et al. 2010; Berenson et al. 2010; Blade et al. 2010). The first step is a complete medical history and physical examination. Laboratory assessment includes quantification of the M-protein in serum and urine by electrophoresis. Most experts recommend 24-h-urine collection and analysis of M-protein and total protein for all patients at initial diagnosis. Some experts consider it sufficient for patients with expected MGUS that presence of M-protein should initially be investigated in a regular urine specimen and – in case of positive result – have a follow-up investigation using a 24-h-urine specimen. Further recommended laboratory tests are serum electrolytes, blood count, and routine chemistry in particular to determine the renal function.
Finally serum FLC should be performed as an additional tool to assess risk for development of Multiple Myeloma (see below). Serum chemistry and hematology lab data particularly focus on the question if any of the “CRAB”-criteria relevant for the diagnosis of symptomatic multiple myeloma according to the International Myeloma Working Group are met (Calcium elevation (>2.75 mmol/l), Renal dysfunction (creatinine >173 μmol/l), Anemia (hemoglobin <100 g/l), and Bone disease) (Kyle et al. 2010) (Tables 6.1–6.3).
Although some experts have questioned the relevance of bone evaluation for low-risk MGUS patients, the authors clearly recommend to assess bone disease at least with plain X-ray evaluation as part of the initial work-up (Berenson et al. 2010). For patients with bone pain or unclear results of the plain bone X-ray additional imaging techniques as MRI or CT are indicated (Bäuerle et al. 2009; Hillengass et al. 2010). Due to the prognostic impact and the possibility to recognize potentially clinical relevant lesions authors nowadays recommend a spine/pelvis MRI as part of initial work-up.
In addition, for initial work-up of IgM MGUS to investigate for lymphoproliferative disease an abdominal imaging technique is recommended at least as an abdominal ultrasound or CT of the abdomen (Weber et al. 2003).
Bäuerle et al. have demonstrated that 39% of MGUS and asymptomatic myeloma patients with normal bone skeletal survey had lesions in the axial skeleton and 37% in the extra-axial skeleton. Lesions in this group of patients can be clinically relevant as 13% of lesions violated the cortical bone implying an increased risk of fracture. Moreover MGUS patients in initial work-up need to be distinguished from solitary plasmocytoma which sometimes is difficult if the solitary plasmocytoma is not visible in the plain X-ray but produces an M-Protein sufficient to be detected by Immunofixation/protein electrophoresis. For these reasons, whole body MRI has to be considered superior to spinal MRI in initial work-up. The analysis by Bäuerle et al. did not reveal an alternative clinical or laboratory parameter that would predict the presence of lesions or even clinically relevant lesions in MGUS patients.
In summary, MRI of pelvis and spine are recommended in case of symptomatic MGUS/sMM patients. In addition, recent publications have recommended MRI of pelvis and spine for sMM and MGUS even in asymptomatic patients as MRI has overall prognostic implications and can reveal lesions that can lead to local clinical symptoms in the near future (e.g., fracture, extramedullary disease) (Blade et al. 2010; Kyle et al. 2010).
For patients with suspected osteopenia as per conventional X-ray skeletal status or in a CT a dual energy X-ray absorptiometry (DXA) scan is recommended. As described below (paragraph 6.5.5) in more detail MGUS and sMM patients with asymptomatic osteopenia can be considered for bisphosphonate treatment in case of significant osteopenia.
The plasma cell labeling index and flow-cytometric analysis of circulating plasma cells are possible additional investigations (Nowakowski et al. 2005). In the study by Perez-Persona a prognostic score for MGUS/sMM patients was developed using multicolor flow cytometry of bone marrow plasma cells to detect percentage of abnormal plasma cells. Immunoparesis and DNA ploidy status will be discussed later in this chapter in the sMM part (Perez-Persona et al. 2010).
Although cytogenetic evaluation has brought a wealth of data to support a sub-categorization of MGUS as described below, up to now there is no clear prognostic evidence for MGUS patients (Ross et al. 2010). Although cytogenetic investigation will be an important analysis in future clinical studies in MGUS, there is no general recommendation outside of clinical studies to perform those analyses using conventional cytogenetics or FISH (fluorescence in situ-hybridization) techniques.
3.2 Follow-up Recommendations
A first follow-up investigation should be performed 3–6 months after first diagnosis of MGUS/sMM. This visit should be focused on comparing the paraprotein in serum and urine with analysis obtained at first visit as well as renal function if no other clinical aspects occurred in the meantime. Further management of MGUS patients is dependent on the risk assessment.
Patients with low-risk MGUS (for risk factors see below) can be followed once a year and if stable in 2–3-year intervals. Patients with intermediate and high risk MGUS should receive follow-up investigation 3–6 months after first diagnosis and subsequently annually for lifetime. Bone marrow and imaging are not routinely performed on these follow-up visits but would be recommended if clinical evaluation or laboratory values indicate disease progression.
Bianchi et al. have recently investigated the relevance of regular long-term follow-up (Bianchi et al. 2010). Surprisingly, myeloma was diagnosed only in 16% of patients as a consequence of the routine follow-up whereas in 45% as a result of serious MM-related complication. In 25% MM was diagnosed as a result of less serious symptoms, during work-up of unrelated medical conditions (11%) or unknown (3%).
4 Risk Factors for Progression
4.1 Prognostic Factors for Progression for Patients with MGUS
In a prospective long-term study, Landgren et al. recently showed that among 77,469 Healthy donors 71 developed a MM and that in all cases a MGUS was present before, indicating that MM is consistently proceeded by MGUS (Landgren et al. 2009).
For monitoring the disease and future therapeutic options it is important to assess the risk of progression from MGUS into a clinical MM. The International Myeloma Working Group has summarized the existing research and identified five predictors to estimate the risk of progression into MM: (1) size of the M-protein; (2) type of paraprotein; (3) degree of plasma cell infiltration in bone marrow; (4) free-light-chain ratio in serum; and (5) flow-cytometric and cytogenetic characteristics.
Kyle et al. found that patients with a paraprotein level of 25 g/dl or higher had a risk of 49% to develop multiple myeloma or related disorder (Kyle et al. 2002). This related to a 14% risk of progression for patients with a paraprotein level lower than 5 g/dl. The relevance of paraprotein concentration as a strong predictor for progression was confirmed in subsequent studies. The type of immunoglobulin is relevant as IgM or IgA monoclonal protein is associated with a higher risk compared to IgG. The same group recently updated their recommendations and published relative risk according to the three risk factors M-Protein level, immunoglobulin subtype, and FLC ratio (Kyle et al. 2010) (Table 6.4). For IgA monoclonality this was shown earlier by Blade et al. (Blade et al. 1992). Regarding the bone marrow infiltration with plasma cells it was reported in 2002 that a percentage of more than 5% plasma cells in bone marrow is a risk factor for progression, but due to the introduction of the entity “smoldering myeloma” patient with more than 10% plasma cells in bone marrow are classified as smoldering myeloma anyway (Cesana et al. 2002). Rajkumar et al. showed that an abnormal free-light-chain ratio in serum predicts for a higher risk of progression as well as the presence of aberrant plasma cells in bone marrow (assessed by flow cytometry) in combination with their ploidity-status. Table 6.4 summarizes the risk-stratification model to predict progression of MGUS to multiple myeloma or related disorders.
4.2 Prognostic Factors for Progression of sMM
In the past 25 years, several authors have investigated risk factors for progression of sMM to myeloma. Initial publication contained “lytic bone lesions” as a strong risk factor but as nowadays osteolytic lesions are always considered a feature of symptomatic myeloma more recent publications excluded the group of asymptomatic patients with osteolyses from the analysis (Alexanian et al. 1988; Dimopoulos et al. 1993; Wisloff et al. 1991). Importantly, these publications already recognized additional risk factors that were confirmed subsequently as degree of infiltration by bone marrow plasma cells, concentration of M-protein and concentration of Bence–Jones proteinuria. More recently this important question was reevaluated as described in the following paragraph (Facon et al. 1995; Weber et al. 1997) (Table 6.5).
Kyle et al. investigated a cohort of 276 patients with sMM and 163 patients (59%) developed symptomatic multiple myeloma or AL-amyloidosis during follow-up (Kyle et al. 2007). For the first 5 years the risk of progression was 10% per year with approximately 3% per year for the next 5 years and 1% for the last 10 years of follow-up. The cumulative probability of progression into active multiple myeloma or AL-Amyloidosis was 51% at 5 years, 66% at 10 years and 73% at 15 years. The median time to progression was 4.8 years. Of the patients developing progressive disease 79% developed multiple myeloma. At diagnosis, significant risk factors for progression included the serum level and type of monoclonal protein, the presence of urinary light chains, the extent and pattern of bone marrow involvement and the reduction in uninvolved immunoglobulins. The concentration of serum monoclonal protein and percentage of plasma cells in bone marrow were the most important factors for progression. Therefore a predictive model with three groups was formed: group 1: BMPC <10%, M-Protein ≥3 g/dl, group 2: BMPC >10%, M-protein <3 g/dl, group 3: BMPC >10%, M-Protein ≥3 g/dl.
Subsequently, Dispenzieri described that the free-light-chain ratio is an independent additional risk factor for progression. Hemoglobin level, type of heavy chain and other factors were investigated as well but were not significant (Dispenzieri et al. 2008). Incorporating FLC ratio at a breakdown lower than 0.126 or higher than 8 resulted in an improvement of the prognostic classification with an even more balanced distribution (Table 6.5). The low (0–1 risk factor), intermediate (two risk factors), and high (three risk factors) risk group showed a probability of progression at 5 years of follow-up of 25%, 51%, and 71%, respectively (Table 6.5).
Rosinol et al. have confirmed in their study what is also clinical knowledge of many years and described that patients with progressive increase in the paraprotein (“evolving”: increase of the M-protein in two of the consecutive follow-up visits) have a significant worse prognosis with a time to progression of 1.3 years compared to 3.9 years for the evolving and non-evolving types, respectively (Rosinol et al. 2003).
There are several different areas of research that add to the established risk factors as described above.
4.2.1 Immunophenotyping and Immunoparesis
Perez-Persona et al. have shown that the presence of an aberrant phenotype defined as the over expression of CD56 and CD19 with CD45 negativity and/or decreased CD38 reactivity in ≥95% of BMPC was a powerful predictor of early progression from sMM to active MM. The cumulative progression rate at 5 years was 64% versus 8% for the patients with ≥95% of aberrant BMPC or <95%, respectively. In this study the detection of immunoparesis as the decrease in one or two uninvolved immunoglobulins was also identified as a significant prognostic factor in multivariate analysis. Based on these two factors a prognostic stratification of sMM could be performed in three groups with a cumulative probability of progression at 5 years of 4%, 46%, and 72% when none, one, or two factors were present (Perez-Persona et al. 2010).
4.2.2 Role of Imaging in Prognostic Evaluation of sMM
Clinical studies investigating cross-section imaging as low dose computed tomography of the skeletal system, whole body or spinal MRI, and positron emission tomography have delivered data that have either revealed organ complications which were not detected with conventional staging procedures or revealed predictive (related to treatment indication) or prognostic relevance for symptomatic myeloma patients (Walker et al. 2007; Hillengass et al. 2010). Dimopolous et al. and Mariette et al. were among the first groups to describe the prognostic implication of MRI of the spine in asymptomatic myeloma/stage I Durie/Salmon (Dimopoulos et al. 1993; Mariette et al. 1999). More recently our group confirmed and extended on these earlier findings in 149 patients with asymptomatic multiple myeloma and found that 28% of patients with sMM had focal lesions (FL) typical for myeloma in whole body MRI. The presence of FL and more than one FL were strongest adverse prognostic factors for progression into MM in multivariate analysis. A diffuse infiltration pattern in MRI, a monoclonal protein of 40 g/l or greater, and bone marrow plasma cell infiltration of 20% or greater were other adverse prognostic factors for progression in univariate analysis.
It has been suggested to integrate MRI findings into the staging of multiple myeloma and the so-called Durie/Salmon PLUS classification was proposed (Table 6.6) (Baur et al. 2002; Durie Hematol J 2003). However, further prospective analysis of MRI is needed to find appropriate thresholds for the different stages of disease especially because rapid technical development leads to the possibility to perform total skeletal or whole body MRI. The imaging techniques and their application in multiple myeloma are also described in detail on page 133.
4.3 Genetic Risk Stratification
The risk stratification of patients according to GEP profiles is an aim for future clinical studies. As the costs for this analysis are expected to substantially decrease and the standardization has greatly improved it is possible that in 5–10 years from now this technique will be available for routine work-up if prospective studies support the clinical value (Hose et al. 2009; Zhan et al. 2002).
5 Etiology and Pathogenesis of MGUS and sMM and Considerations Regarding Primary Prevention
5.1 Population-Based Studies
An important tool to further investigate the etiologic factors of MGUS and myeloma are population-based studies.
Large population-based prevalence studies were performed with the aim to assess the risk for MGUS and MM of relatives of patients with plasma cell-disorders. A study among Swedish residents showed that relatives of MGUS patients had increased risk for developing MGUS (RR = 2.8; 1.4–5.6), MM, lympho-plasmocytic lymphoma/Waldenström’s macroglobulinemia and chronic lymphocytic leukemia. Vachon et al. confirmed, among residents of the Olmsted county, Minnesota, USA, that the risk of first-degree relatives from MGUS and MM patients to develop a plasma cell-disorder is increased by 2.6-fold. The prevalence of MGUS increased with age compared to patients from unaffected families starting with 1.6% in the age group of 40–49 up to 21% for the age group ≥81 years. Interestingly, the risk of MGUS or myeloma was seen among relatives of MM (RR, 2.0; 95% CI, 1.4–2.8) and MGUS patients (RR, 3.3; 95% CI, 2.1–4.8).
Genetic abnormalities were described that correlate with the risk of MGUS/MM: an analysis of germ line mutations in families with a high incidence showed that a mutation of CDKN2A increased the susceptibility for MM but also for melanoma and pancreatic cancer. Sandström et al. found in a family with congenital dyserythropoietic anemia type III an abnormal prevalence of MGUS and MM.
5.2 Concept of Chronic Antigenic Stimulation
Grass et al. recently demonstrated in sporadic and familial MGUS/MM that a frequent target of the paraprotein in MM and MGUS patients is a hyperphosphorylated form of paratarg-7, a protein with unknown function, which is expressed in all human tissues. Only sporadic or familial forms of myeloma with hyperphosphorylated paratarg-7 had a paratarg-7 specific paraprotein (Grass et al. 2010; Grass et al. 2009; Preuss et al. 2009). This finding suggests that hyperphosphorylation of paratarg-7 can cause autoimmunity and chronic antigenic stimulation leading to MGUS and multiple myeloma. Also Jego et al. have reported on mechanisms by which chronic antigen stimulation might contribute or lead to clonal proliferation (Jego et al. 2006). This research group demonstrated that an abnormal response to antigenic stimulation mediated by aberrant expression of Toll-like receptors and overexpression of interleukin 6 (IL-6) receptors can be a survival factor for myeloma cell lines and primary human myeloma cells.
These and other reports support the hypothesis that a proportion of MGUS and MM might arise from chronic (self) antigen stimulation. Removal of the antigen might therefore be one strategy to counteract MGUS while another consideration is to abort the abnormal immune response (Rajkumar 2009).
5.3 Molecular Genetics and Cytogenetics
Interestingly two types of primary cytogenetic abnormalities are detected in the majority of MGUS patients: hyperdiploidy (in approximately 50% of patients) or immunoglobulin heavy chain (IgH) translocations (in approximately 50% of patients) (Fig. 6.2). Only in a small proportion of MGUS patients hypodiploidy or no specific cytogenetic abnormality is found (Brousseau et al. 2007; Ross et al. 2010).
In the group of “IHT (IgH-translocation)-MGUS” IgH translocation commonly involve recurrent partner chromosome loci: 4p16.3 (FGF-R3 and MMSET), 6p21 [CCND3 (cyclin D3 gene)], 11q13 [CCND1 (cyclin D1)], 16q23 (c-maf) and 2βq11 (mafB) (Chng et al. 2007). Therefore at least six MGUS subentities: hyperdiploidy and the five most common primary IgH translocations have to be distinguished and considered for future primary intervention studies (Rajkumar 2009). It is likely that age, racial disparities, and environmental influences will have different impact on the various MGUS forms; therefore, future studies will need to examine the cytogenetic types separately. Importantly, cytogenetic abnormalities in MGUS do not necessarily have the same prognostic implications as the same translocation or abnormality in myeloma patients. Recently Ross and colleagues investigated cytogenetic abnormalities involving the MAF pathway in 2,207 patients with plasma cell dyscrasias including 148 patients with sMM and 193 patients with MGUS. None of the investigated abnormalities (t14; 20) and t14; 16] predicted for a higher risk for progression (Ross et al. 2010).
From the above mentioned analysis and review age, hormonal factors, family history, immunosuppression, and exposure to certain pesticides have to be considered as risk factors for the development of MGUS and sMM.
5.4 Concepts for Secondary Prevention of Progression to Multiple Myeloma and Other Lymphoproliferative Diseases
Whereas the initiation of MGUS follows a cumulative damage model, the molecular pathogenesis leading from MGUS to MM has been a somewhat controversial topic. Research of several groups has demonstrated that over-expression or aberrant expression of cyclin-dependent kinases are hallmarks of plasma cell disease (Bergsagel and Kuehl 2003; Hose 2010). Importantly, MGUS and sMM clonal plasma cells often harbor cytogenetic aberrations that are present in symptomatic myeloma as well (Magrangeas et al. 2005). Later during disease progression in MM additional cytogenetic and molecular changes occur (Cremer 2005) (Fig 6.2). It is currently undoubted that later molecular and genetic changes contribute to more aggressive multiple myeloma or increased resistance to therapy but it is not confirmed that additional molecular changes are a prerequisite for a transition from MGUS to myeloma (for details regarding molecular pathogenesis please see chapter 3). Many lines of evidence point to the concept that transition from MGUS to myeloma in the majority of patients could be the result of a progressive accumulation of plasma cells in the bone marrow with a consecutive remodeling of the bone marrow microenvironment including activation of osteoclasts. Final confirmation of the time dependent “accumulation” model or the “second genetic hit” model could come from genome wide screening for myeloma specific mutations.
Several potentially pathogenetic genetic abnormalities have been described as “second hits”: ras, p53, myc mutations, p16 methylation, and secondary translocations. The described genetic changes not only change the metabolism of the affected plasma cell clone but as a consequence induce paracrine loops involving IL-6 and other growth factors and a remodeling of the bone marrow microenvironment. The consequences including the increase of bone marrow angiogenesis are described in more detail in Chap. 4. The main regulator of IL-6 signaling in myeloma is the transducer and activator of transcription-3 (STAT3) (Bharti et al. 2004). Although an emerging ability of clonal plasma cells to induce osteolytic bone disease belongs to the stepwise process of malignancy. An increase in receptor activator of nuclear factor kB ligand (RANKL)-expression by osteoblasts (and possibly plasma cells) accompanied by reduction of its decoy receptor, osteoprotegerin are relevant (Roodman 2002). In addition, it was shown that increased levels of MIP-1a (macrophage inflammatory protein), IL-3, and IL-6 result in osteoclast activation (Lee et al. 2004; Tsubaki et al. 2007). The result of these changes and in particular the increase in the RANKL/OPG ratio leads to osteoclast maturation, activation, and increased bone resorption.
5.5 Summary of Clinical Studies to Halt Progression
To interfere with the progression of early asymptomatic plasma cell-disease MGUS and sMM have attracted a lot of interest and the evidence is summarized herein.
5.5.1 Bisphosphonates
The use of bisphosphonates in patients with MGUS but reduced bone density as determined by DXA scan was addressed in two clinical studies. Both studies could demonstrate that anti-resorptive therapy with intravenous zoledronate or oral alendronate improved the bone density. Neither study was powered to investigate fracture risk. Therefore, the use of bisphosphonates is finally an individual decision that can be justified in this situation.
Preclinical evidence of an anti-myeloma activity of bisphosphonates has led to clinical observations indicative of down-modulation of myeloma activity by bisphosphonate treatment (Corso et al. 2005). There are case reports describing a significant reduction of monoclonal protein in three patients with sMM (Dhodapkar et al. 1998). However, reduction of M-protein cannot be seen as regular response to bisphosphonates as a Spanish study investigating 12 patients with sMM treated with single agent pamidronate did not find any decrease in the M-protein level but could confirm a positive effect on bone formation (Martin et al. 2002). A large randomized Italian study showed a significantly reduced number of skeletal events but no prolongation of TTP or overall survival (Musto et al. 2003). While the potential toxicities of bisphosphonates as for example renal complications or osteonecrosis of the jaw have to be taken into consideration, treatment with bisphosphonates could be of benefit for patients with early bone disease such as MM-related osteopenia.
5.5.2 Alkylating Agents and Corticosteroids
Hjorth et al. performed a randomized study for sMM patients comparing immediate therapy with MP (melphalan/prednisone) versus observation until progression in a series of 50 patients (Hjorth et al. 1993, 1990; Hjorth et al. 1990). For the 25 patients allocated to the observation group the median time to progression was 12 months. The response rate to therapy in patient treated at diagnosis was similar to that of those who were observed initially and received therapy at the time of progression to active myeloma (52% vs. 55%). There was no significant difference in time to response or overall survival between the groups. Similar results were obtained in the studies by Grignani et al. and Riccardi et al. (Riccardi et al. 2000; Grignani et al. 1996).
5.5.3 Thalidomide
Up to now three studies have evaluated a potential role of Thalidomide in sMM. In a clinical phase II study with 29 patients initiated by Rajkumar et al., the rate of PR/CR was 34% and if minor responses were considered the ORR was 66% (Rajkumar et al. 2001, 2003). Three patients had progression while on treatment and the Kaplan–Meier estimates of progression-free survival were 80% at 1 year and 63% at 2 years follow-up. Similar results were reported by Weber et al. (Weber et al. 2003).
Recently Barlogie reported on the results of a study involving 76 sMM patients treated at an initial dose of 200 mg thalidomide per day. At 4 years of enrollment the ≥PR rate was 42% with a median time to response of 1–2 years. The median time to progression was 7 years (Barlogie et al. 2008). In all studies the thalidomide specific adverse events profile in particular the peripheral neuropathy was detected. All authors confirmed that Thalidomide can prolong the time-to-progression (TTP) but a clinical recommendation can only be made if a clinical benefit is confirmed in phase III randomized studies.
Based on the encouraging results regarding Thalidomide a Spanish group of investigators has started a phase III study comparing Lenalidomide/Dexamethasone (len/dex vs. observation) in high risk sMM patients. A similar study comparing Lenalidomide single agents with observation will be started by the ECOG (eastern cooperative oncology group). In addition, clinical studies using cyclooxygenase-2 inhibitors are currently underway.
5.5.4 Immunotherapy and Interference with Cytokine Network
Immunotherapy for MGUS/sMM has also raised interest as the immune system is intact for the majority of patients as a prerequisite to elicit an immune response against the plasma cell clone (Goodyear et al. 2008, 2005).
However, regarding the discouraging results for immunotherapy in patients with MM due to an impaired immune system and a large amount of malignant cells, patients with an early-stage plasma cell-disease might benefit from antitumor vaccination therapies before the MM-clone arises.
Hansson et al. vaccinated 28 patients with sMM (MM stage I/II) with autologous paraprotein combined with IL-12 or GM-CSF as adjuvants and were able to induce idiotype specific T-cell responses in a high proportion of patients (Hansson et al. 2007). This indicates that immunotherapy might be a promising approach to avoid a progression into MM. Furthermore, combination of vaccine strategies with immunomodulatory drugs as Thalidomide or Lenalidomide need to be considered as well to enhance the therapeutic effect of a specific immunotherapy.
A very interesting study was recently published by Lust et al., which was based on the earlier observation that serum levels of Interleukin-1 beta (IL1-beta) constitute a marker of progression in asymptomatic monoclonal gammopathies (Lust et al. 2009). In this trial, 47 patients with sMM were treated in this phase II study with an IL1-RA or observation. For patients with a submaximum IL6 suppression by IL1-RA alone dexamethasone was added to the therapy (53%). The median PFS for patients with a greater than 15% decrease in the 6-month high-sensitivity (hsCRP) level was 3 years (n = 35) compared to 6 months for the group without change (n = 10). In seven patients a decrease in plasma cell-labeling index paralleled the reduction in the hsCRP level. Further studies are therefore necessary to investigate this approach.
5.5.5 Summary and Brief Outlook Regarding Clinical Studies
Based on the improved knowledge about MGUS/sMM pathogenesis, the availability of novel agents and a better risk stratification concept, experts worldwide are currently reconsidering the concept of early therapeutic intervention.
The use of bisphosphonates for patients with decreased bone density on DXA scan is already an accepted approach. Furthermore therapeutic interventions with chemotherapeutic agents have not been successful to prevent progression or prolong OS survival of patients and therefore are in general not recommended. Ongoing and future studies will focus on patients at higher risk of progression including those patients for which evidence of progression becomes obvious because of consistently raising monoclonal protein level (“evolving type”).
6 Summary and Conclusions
MGUS and sMM are the most prevalent premalignant conditions in worldwide population. Active myeloma for nearly all patients is preceded by MGUS/sMM. This observation as well as molecular and cytogenetic research support the two-hit genetic model of myeloma development starting with hyperdiploidy or IgH translocation followed by additional genetic alterations as ras, myc, or p53 mutations. Overall six or more subcategories can be defined based on genetic information in MGUS and sMM, although currently not relevant for clinical decision making.
Standard procedures for the diagnostic evaluation of MGUS are conventional X-ray techniques to assess impairment of the bone system, laboratory assessment in combination with bone marrow investigation to evaluate the influence on the hematopoietic system, and bone marrow involvement as well as renal function analysis. In addition, an MRI of spine and pelvis is recommended. Applying the results of these investigations to the IMWG staging system introduced in 2003 will lead to a distinction between MGUS, asymptomatic myeloma, and symptomatic myeloma based on the tumor mass and the presence or absence of end organ damage. For patients in whom categorization and indication for systemic therapy is unclear additional investigations as modern cross-section imaging can be helpful. In addition, symptoms as polyneuropathy and hyperviscosity may be the only symptoms of MM and may lead to a decision to start therapy in the absence of other myeloma related symptoms or organ damage.
Prognostic categorization of MGUS and sMM is considered important as high risk MGUS and sMM patients should be followed more frequently and might be candidates for early intervention clinical studies. Most important risk factors for MGUS are: BMPC >5%, M-Protein ≥1.5 g/dl, and abnormal FLC ratio. For sMM risk factors are: BMPC >10%, M-Protein >3 g/dl, and FLC <0.125/>8. Additional risk factors can be derived from quantification of BMPC with aberrant phenotype, analysis of decrease in uninvolved immunoglobulins, and follow-up information related to increase in tumor mass (“evolving course”).
No primary prevention strategy is currently available for prevention of MGUS and sMM. The use of bisphosphonates for MGUS/sMM patients with decreased bone density on DXA scan is accepted. Interventional studies applying novel agents for secondary prophylaxis in MGUS and SMM focusing on the high risk patients are currently under way.
References
Alexanian R, Barlogie B, Dixon D (1988) Prognosis of asymptomatic multiple myeloma. Arch Intern Med 148:1963–1965
Barlogie B, van Rhee F, Shaughnessy JD Jr, Epstein J, Yaccoby S, Pineda-Roman M, Hollmig K, Alsayed Y, Hoering A, Szymonifka J, Anaissie E, Petty N, Kumar NS, Srivastava G, Jenkins B, Crowley J, Zeldis JB (2008) Seven-year median time to progression with thalidomide for smoldering myeloma: partial response identifies subset requiring earlier salvage therapy for symptomatic disease. Blood 112:3122–3125
Bauerle T, Hillengass J, Fechtner K, Zechmann CM, Grenacher L, Moehler TM, Christiane H, Wagner-Gund B, Neben K, Kauczor HU, Goldschmidt H, Delorme S (2009) Multiple myeloma and monoclonal gammopathy of undetermined significance: importance of whole-body versus spinal MR imaging. Radiology 252:477–485
Baur A, Stabler A, Nagel D, Lamerz R, Bartl R, Hiller E, Wendtner C, Bachner F, Reiser M (2002) Magnetic resonance imaging as a supplement for the clinical staging system of Durie and Salmon? Cancer 95:1334–1345
Berenson JR, Anderson KC, Audell RA, Boccia RV, Coleman M, Dimopoulos MA, Drake MT, Fonseca R, Harousseau JL, Joshua D, Lonial S, Niesvizky R, Palumbo A, Roodman GD, San-Miguel JF, Singhal S, Weber DM, Zangari M, Wirtschafter E, Yellin O, Kyle RA (2010) Monoclonal gammopathy of undetermined significance: a consensus statement. Br J Haematol 150:28–38
Bharti AC, Shishodia S, Reuben JM, Weber D, Alexanian R, Raj-Vadhan S, Estrov Z, Talpaz M, Aggarwal BB (2004) Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood 103:3175–3184
Bianchi G, Kyle RA, Colby CL, Larson DR, Kumar S, Katzmann JA, Dispenzieri A, Therneau TM, Cerhan JR, Melton LJ III, Rajkumar SV (2010) Impact of optimal follow-up of monoclonal gammopathy of undetermined significance (MGUS) on early diagnosis and prevention of myeloma-related complications. Blood 116(12):2019–2025
Blade J, Lopez-Guillermo A, Rozman C, Cervantes F, Salgado C, Aguilar JL, Vives-Corrons JL, Montserrat E (1992) Malignant transformation and life expectancy in monoclonal gammopathy of undetermined significance. Br J Haematol 81:391–394
Blade J, Dimopoulos M, Rosinol L, Rajkumar SV, Kyle RA (2010) Smoldering (asymptomatic) multiple myeloma: current diagnostic criteria, new predictors of outcome, and follow-up recommendations. J Clin Oncol 28:690–697
Brousseau M, Leleu X, Gerard J, Gastinne T, Godon A, Genevieve F, Dib M, Lai JL, Facon T, Zandecki M (2007) Hyperdiploidy is a common finding in monoclonal gammopathy of undetermined significance and monosomy 13 is restricted to these hyperdiploid patients. Clin Cancer Res 13:6026–6031
Cesana C, Klersy C, Barbarano L, Nosari AM, Crugnola M, Pungolino E, Gargantini L, Granata S, Valentini M, Morra E (2002) Prognostic factors for malignant transformation in monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. J Clin Oncol 20:1625–1634
Chng WJ, Glebov O, Bergsagel PL, Kuehl WM (2007) Genetic events in the pathogenesis of multiple myeloma. Best Pract Res Clin Haematol 20:571–596
Corso A, Ferretti E, Lazzarino M (2005) Zoledronic acid exerts its antitumor effect in multiple myeloma interfering with the bone marrow microenvironment. Hematology 10:215–224
Dhodapkar MV, Singh J, Mehta J, Fassas A, Desikan KR, Perlman M, Munshi NC, Barlogie B (1998) Anti-myeloma activity of pamidronate in vivo. Br J Haematol 103:530–532
Dimopoulos MA, Moulopoulos A, Smith T, Delasalle KB, Alexanian R (1993) Risk of disease progression in asymptomatic multiple myeloma. Am J Med 94:57–61
Dispenzieri A, Kyle RA, Katzmann JA, Therneau TM, Larson D, Benson J, Clark RJ, Melton LJ III, Gertz MA, Kumar SK, Fonseca R, Jelinek DF, Rajkumar SV (2008) Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood 111:785–789
Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Melton LJ III, Colby CL, Therneau TM, Clark R, Kumar SK, Bradwell A, Fonseca R, Jelinek DF, Rajkumar SV (2010) Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet 375:1721–1728Durie BG, Kyle RA, Belch A, Bensinger W, Blade J, Boccadoro M, Child JA, Comenzo R, Djulbegovic B, Fantl D, Gahrton G, Harousseau JL, Hungria V, Joshua D, Ludwig H, Mehta J, Morales AR, Morgan G, Nouel A, Oken M, Powles R, Roodman D, San Miguel J, Shimizu K, Singhal S, Sirohi B, Sonneveld P, Tricot G, Van Ness B (2003) Scientific Advisors of the International Myeloma Foundation. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J. 4(6):379–398
Facon T, Menard JF, Michaux JL, Euller-Ziegler L, Bernard JF, Grosbois B, Daragon A, Azais I, Courouble Y, Kaplan G (1995) Prognostic factors in low tumour mass asymptomatic multiple myeloma: a report on 91 patients. The Groupe d’Etudes et de Recherche sur le Myelome (GERM). Am J Hematol 48:71–75
Goodyear O, Piper K, Khan N, Starczynski J, Mahendra P, Pratt G, Moss P (2005) CD8+ T cells specific for cancer germline gene antigens are found in many patients with multiple myeloma, and their frequency correlates with disease burden. Blood 106:4217–4224
Goodyear OC, Pratt G, McLarnon A, Cook M, Piper K, Moss P (2008) Differential pattern of CD4+ and CD8+ T-cell immunity to MAGE-A1/A2/A3 in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma. Blood 112:3362–3372
Grass S, Preuss KD, Ahlgrimm M, Fadle N, Regitz E, Pfoehler C, Murawski N, Pfreundschuh M (2009) Association of a dominantly inherited hyperphosphorylated paraprotein target with sporadic and familial multiple myeloma and monoclonal gammopathy of undetermined significance: a case-control study. Lancet Oncol 10:950–956
Grass S, Preuss KD, Pfreundschuh M (2010) Autosomal-dominant inheritance of hyperphosphorylated paratarg-7. Lancet Oncol 11:12
Grignani G, Gobbi PG, Formisano R, Pieresca C, Ucci G, Brugnatelli S, Riccardi A, Ascari E (1996) A prognostic index for multiple myeloma. Br J Cancer 73:1101–1107
Hansson L, Abdalla AO, Moshfegh A, Choudhury A, Rabbani H, Nilsson B, Osterborg A, Mellstedt H (2007) Long-term idiotype vaccination combined with interleukin-12 (IL-12), or IL-12 and granulocyte macrophage colony-stimulating factor, in early-stage multiple myeloma patients. Clin Cancer Res 13:1503–1510
Hillengass J, Fechtner K, Weber MA, Bauerle T, Ayyaz S, Heiss C, Hielscher T, Moehler TM, Egerer G, Neben K, Ho AD, Kauczor HU, Delorme S, Goldschmidt H (2010) Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol 28:1606–1610
Hjorth M, Hellquist L, Holmberg E, Magnusson B, Rodjer S, Westin J (1990) Initial treatment in multiple myeloma: no advantage of multidrug chemotherapy over melphalan-prednisone. The Myeloma Group of Western Sweden. Br J Haematol 74:185–191
Hjorth M, Hellquist L, Holmberg E, Magnusson B, Rodjer S, Westin J (1993) Initial versus deferred melphalan-prednisone therapy for asymptomatic multiple myeloma stage I–a randomized study. Myeloma Group of Western Sweden. Eur J Haematol 50:95–102
Hose D, Moreaux J, Meissner T, Seckinger A, Goldschmidt H, Benner A, Mahtouk K, Hillengass J, Reme T, De VJ, Hundemer M, Condomines M, Bertsch U, Rossi JF, Jauch A, Klein B, Mohler T (2009) Induction of angiogenesis by normal and malignant plasma cells. Blood 114:128–143
Iwanaga M, Tagawa M, Tsukasaki K, Kamihira S, Tomonaga M (2007) Prevalence of monoclonal gammopathy of undetermined significance: study of 52, 802 persons in Nagasaki City, Japan. Mayo Clin Proc 82:1474–1479
Jego G, Bataille R, Geffroy-Luseau A, Descamps G, Pellat-Deceunynck C (2006) Pathogen-associated molecular patterns are growth and survival factors for human myeloma cells through Toll-like receptors. Leukemia 20:1130–1137Kyle RA, Greipp PR (1980) Smoldering Multiple Myeloma.N Engl J Med 302:1347–1349
Kyle RA, Rajkumar SV (2007) Epidemiology of the plasma-cell disorders. Best Pract Res Clin Haematol 20:637–664
Kyle RA, Rajkumar SV (2009) Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 23:3–9
Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, Melton LJ III (2002) A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med 346:564–569
Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, Dispenzieri A, Katzmann JA, Melton LJ III (2006) Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 354:1362–1369
Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM, Larson DR, Plevak MF, Jelinek DF, Fonseca R, Melton LJ III, Rajkumar SV (2007) Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med 356:2582–2590
Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, Kroger N, Einsele H, Vesole DH, Dimopoulos M, San MJ, Avet-Loiseau H, Hajek R, Chen WM, Anderson KC, Ludwig H, Sonneveld P, Pavlovsky S, Palumbo A, Richardson PG, Barlogie B, Greipp P, Vescio R, Turesson I, Westin J, Boccadoro M (2010) Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 24:1121–1127
Landgren O, Gridley G, Turesson I, Caporaso NE, Goldin LR, Baris D, Fears TR, Hoover RN, Linet MS (2006) Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood 107:904–906
Landgren O, Katzmann JA, Hsing AW, Pfeiffer RM, Kyle RA, Yeboah ED, Biritwum RB, Tettey Y, Adjei AA, Larson DR, Dispenzieri A, Melton LJ III, Goldin LR, McMaster ML, Caporaso NE, Rajkumar SV (2007) Prevalence of monoclonal gammopathy of undetermined significance among men in Ghana. Mayo Clin Proc 82:1468–1473
Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, Dispenzieri A, Kumar S, Clark RJ, Baris D, Hoover R, Rajkumar SV (2009) Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood 113:5412–5417
Lee JW, Chung HY, Ehrlich LA, Jelinek DF, Callander NS, Roodman GD, Choi SJ (2004) IL-3 expression by myeloma cells increases both osteoclast formation and growth of myeloma cells. Blood 103:2308–2315
Lust JA, Lacy MQ, Zeldenrust SR, Dispenzieri A, Gertz MA, Witzig TE, Kumar S, Hayman SR, Russell SJ, Buadi FK, Geyer SM, Campbell ME, Kyle RA, Rajkumar SV, Greipp PR, Kline MP, Xiong Y, Moon-Tasson LL, Donovan KA (2009) Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc 84:114–122
Mariette X, Zagdanski AM, Guermazi A, Bergot C, Arnould A, Frija J, Brouet JC, Fermand JP (1999) Prognostic value of vertebral lesions detected by magnetic resonance imaging in patients with stage I multiple myeloma. Br J Haematol 104:723–729
Martin A, Garcia-Sanz R, Hernandez J, Blade J, Suquia B, Fernandez-Calvo J, Gonzalez M, Mateo G, Orfao A, San Miguel JF (2002) Pamidronate induces bone formation in patients with smoldering or indolent myeloma, with no significant anti-tumour effect. Br J Haematol 118:239–242
Musto P, Falcone A, Sanpaolo G, Bodenizza C, Carella AM (2003) Pamidronate for early-stage, untreated myeloma. J Clin Oncol 21:3177–3178
Nowakowski GS, Witzig TE, Dingli D, Tracz MJ, Gertz MA, Lacy MQ, Lust JA, Dispenzieri A, Greipp PR, Kyle RA, Rajkumar SV (2005) Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood 106:2276–2279
Perez-Persona E, Mateo G, Garcia-Sanz R, Mateos MV, de Las HN, de Coca AG, Hernandez JM, Galende J, Martin-Nunez G, Barez A, Alonso JM, Martin A, Lopez-Berges C, Orfao A, San Miguel JF, Vidriales MB (2010) Risk of progression in smoldering myeloma and monoclonal gammopathies of unknown significance: comparative analysis of the evolution of monoclonal component and multiparameter flow cytometry of bone marrow plasma cells. Br J Haematol 148:110–114
Preuss KD, Pfreundschuh M, Ahlgrimm M, Fadle N, Regitz E, Murawski N, Grass S (2009) A frequent target of paraproteins in the sera of patients with multiple myeloma and MGUS. Int J Cancer 125:656–661
Rajkumar SV (2009) Prevention of progression in monoclonal gammopathy of undetermined significance. Clin Cancer Res 15:5606–5608
Rajkumar SV, Dispenzieri A, Fonseca R, Lacy MQ, Geyer S, Lust JA, Kyle RA, Greipp PR, Gertz MA, Witzig TE (2001) Thalidomide for previously untreated indolent or smoldering multiple myeloma. Leukemia 15:1274–1276
Rajkumar SV, Gertz MA, Lacy MQ, Dispenzieri A, Fonseca R, Geyer SM, Iturria N, Kumar S, Lust JA, Kyle RA, Greipp PR, Witzig TE (2003) Thalidomide as initial therapy for early-stage myeloma. Leukemia 17:775–779
Rajkumar SV et al (2005) Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance (MGUS). Blood 106:812–817
Riccardi A, Mora O, Tinelli C, Valentini D, Brugnatelli S, Spanedda R, De PA, Barbarano L, Di SM, Giordano M, Delfini C, Nicoletti G, Bergonzi C, Rinaldi E, Piccinini L, Ascari E (2000) Long-term survival of stage I multiple myeloma given chemotherapy just after diagnosis or at progression of the disease: a multicentre randomized study. Cooperative Group of Study and Treatment of Multiple Myeloma. Br J Cancer 82:1254–1260
Roodman GD (2002) Role of the bone marrow microenvironment in multiple myeloma. J Bone Miner Res 17:1921–1925
Rosinol L, Blade J, Esteve J, Aymerich M, Rozman M, Montoto S, Gine E, Nadal E, Filella X, Queralt R, Carrio A, Montserrat E (2003) Smoldering multiple myeloma: natural history and recognition of an evolving type. Br J Haematol 123:631–636
Ross FM, Chiecchio L, Dagrada G, Protheroe RK, Stockley DM, Harrison CJ, Cross NC, Szubert AJ, Drayson MT, Morgan GJ (2010) The t(14;20) is a poor prognostic factor in myeloma but is associated with long-term stable disease in monoclonal gammopathies of undetermined significance. Haematologica 95:1221–1225
Tsubaki M, Kato C, Manno M, Ogaki M, Satou T, Itoh T, Kusunoki T, Tanimori Y, Fujiwara K, Matsuoka H, Nishida S (2007) Macrophage inflammatory protein-1alpha (MIP-1alpha) enhances a receptor activator of nuclear factor kappaB ligand (RANKL) expression in mouse bone marrow stromal cells and osteoblasts through MAPK and PI3K/Akt pathways. Mol Cell Biochem 304:53–60
Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD Jr, Epstein J, van Hemert R, Erdem E, Hoering A, Crowley J, Ferris E, Hollmig K, van Rhee F, Zangari M, Pineda-Roman M, Mohiuddin A, Yaccoby S, Sawyer J, Angtuaco EJ (2007) Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol 25:1121–1128
Weber DM, Dimopoulos MA, Moulopoulos LA, Delasalle KB, Smith T, Alexanian R (1997) Prognostic features of asymptomatic multiple myeloma. Br J Haematol 97:810–814
Weber D, Rankin K, Gavino M, Delasalle K, Alexanian R (2003) Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol 21:16–19
Wisloff F, Andersen P, Andersson TR, Brandt E, Eika C, Fjaestad K, Gronvold T, Holm B, Lovasen K, Tjonnfjord GE (1991) Incidence and follow-up of asymptomatic multiple myeloma. The myeloma project of health region I in Norway. II. Eur J Haematol 47:338–341
Zhan F, Hardin J, Kordsmeier B, Bumm K, Zheng M, Tian E, Sanderson R, Yang Y, Wilson C, Zangari M, Anaissie E, Morris C, Muwalla F, van Rhee F, Fassas A, Crowley J, Tricot G, Barlogie B, Shaughnessy J Jr (2002) Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood 99:1745–1757
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Hillengass, J., Moehler, T., Hundemer, M. (2011). Monoclonal Gammopathy and Smoldering Multiple Myeloma: Diagnosis, Staging, Prognosis, Management. In: Moehler, T., Goldschmidt, H. (eds) Multiple Myeloma. Recent Results in Cancer Research, vol 183. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-85772-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-540-85772-3_6
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-85771-6
Online ISBN: 978-3-540-85772-3
eBook Packages: MedicineMedicine (R0)