Abstract
Advancements in neonatal care over the past few decades have resulted in significant improvements in survival of increasingly preterm infants. This success in acute neonatal care requires increasing knowledge about the physiology of breathing in preterm infants both during wakefulness and sleep and the long-term implications of premature birth on sleep.
In this chapter, we will review the basics of ventilatory control with specific attention to the unique aspects in the preterm infant. Examination of how the preterm infant responds to changes in oxygen, carbon dioxide, and hydrogen ion homeostasis will facilitate an understanding of sleep-disordered breathing in this population. This chapter will focus on periodic breathing, apnea of prematurity, the relationship between gastroesophageal reflux and apnea, and sudden infant death syndrome, as well as discussion regarding the long-term implications of preterm birth on breathing during sleep.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Control of Ventilation
Case Vignette
An infant born at 31 weeks gestation required continuous positive airway pressure (CPAP) for 36 h and then was weaned to room air. On day of life 3, the infant was noted to have apneic events with associated bradycardia and desaturation, often requiring stimulation to recover his oxygen saturation. These events occurred during wakefulness and sleep. After alternative causes of apnea, for example, infection and anemia were ruled out, a diagnosis of apnea of prematurity was made, and the infant was loaded with caffeine and commenced on maintenance caffeine therapy.
Definitions
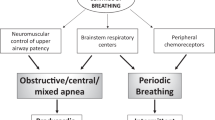
Please see Table 27.1 for additional information on the definitions which follow and others, which are instrumental to understanding the content of this chapter. Preterm birth is defined by the World Health Organization as “babies born alive before 37 weeks of pregnancy are completed” [1]. Additional categories of preterm infants are described in Table 27.1. Apnea, conceptually, is defined as absence of airflow as measured at the mouth or nares. This is further characterized based on the presence or absence of respiratory effort, as described in Table 27.1. Further, the American Academy of Sleep Medicine provides additional definitions of obstructive, central, and mixed apneas based on polysomnographic criteria [2].
Physiology and Control of Ventilation
Respiration is a complex physiologic process that begins in utero with fetal breathing movements, lasts the entire duration of one’s life, and ceases at the time of death. It is indeed an incredible phenomenon that relies on multiple sources of input, including behavioral cues, chemoreceptors, and pulmonary mechanoreceptors. Further elaboration on the role of chemoreceptors can be found in the sections which follow. Mechanoreceptors, as the name suggests, respond to mechanical stimuli. For example, stretch receptors provide information to respiratory control centers regarding distension of the airways to avoid, for example, overdistension of the lungs. Integration of these multiple sources of input results in uninterrupted respiration in order to maintain oxygen and carbon dioxide homeostasis (Fig. 27.1). In times of stress, the respiratory inputs may be altered with compensatory changes in respiration cadence, for example, during exercise or ascent to high altitude. However, both the respiratory rate and tidal volume are also under voluntary control to adapt to the environment and enable activities as required; for example, a diver is able to hyperventilate prior to a dive and then hold his breath while under water. Thus, the versatility offered by the respiratory system is the result of a complex, incompletely understood interaction between the various inputs responsible for respiration, integration of these inputs at a neural level, and the output of a respiratory rhythm that corresponds to one’s physiologic demands and voluntary desires.
In the following section, we will briefly review the neural structures involved in respiratory control, the basics of chemoreception, and ventilatory response to hypoxia and hypercapnia and then examine how these differ in preterm infants compared to term infants.
Central Pattern Generators and Respiratory Rhythm Generation
It has long been appreciated that locomotion in vertebrates is mediated by rhythmic activity that is not reliant on sensory input but rather generated by networks of neural structures collectively referred to as central pattern generators (CPGs) [3]. CPGs are responsible for a variety of motor functions ranging from locomotion to generation of rhythmic respiration [4]. What is unique to CPGs is that the output of motor activity remains rhythmic, without requiring rhythmic input. However, this rhythmic activity is not fixed, but rather flexible, and can be modified based on inputs to the system of the CPG [3]. In respiration, for example, changes in oxygen, carbon dioxide, and hydrogen ion levels in the blood result in changes in respiratory cadence and minute ventilation as outlined below. How this rhythm is generated is not entirely clear; possible explanations include the following [5]:
-
1.
Pacemaker neurons.
-
2.
Neural structures, as described below, which behave in a reciprocal inhibitory/excitatory fashion; although either structure on its own may not result in rhythmicity, they may function together to generate rhythmic activity.
-
3.
A combination of these mechanisms.
Neural Structures Involved in Control of Ventilation
The respiratory CPG is composed of two groups of neural structures: the pontine respiratory group and the medullary respiratory group (Fig. 27.2) [4]. The medullary respiratory group is the major location housing inspiratory and expiratory neural structures. The inspiratory neurons are located in the pre-Bötzinger complex and the rostral ventral respiratory group (VRG), and the expiratory neurons are located in the Bötzinger complex and the caudal VRG [6]. Although this is a somewhat simplified explanation, overall, these inspiratory and expiratory structures together behave in a reciprocal inhibitory/excitatory fashion so that when one group is active, it inhibits the other and vice versa, as a means of generating rhythmicity [5]. The pontine respiratory group is thought to communicate with the medullary respiratory groups in order to regulate the transition from inspiration to expiration [5]. Additional sites function to integrate, process, and then relay information from multiple sensory inputs to the medullary respiratory groups allowing modulation of respiration to meet the metabolic needs of the body [6]. Selected sensory inputs are described below. A discussion of the integration sites is beyond the scope of this text.
Chemoreception: Oxygen and Carbon Dioxide
Oxygen
The main site of oxygen chemoreception is the peripheral chemoreceptors located in the carotid body [7]. Hypoxia results in activation of the carotid chemoreceptors, which in turn triggers the release of excitatory neurotransmitters causing an increase in minute ventilation [7]. This increase can be divided into two phases: (1) an initial rapid increase in minute ventilation, followed by (2) a subsequent decline to a new baseline minute ventilation. This new baseline remains higher than the initial minute ventilation in room air [8].
The ventilatory response to hypoxia, although largely controlled by carotid bodies, does vary with carbon dioxide (CO2) levels; specifically, the ventilatory response to declining inspired oxygen levels in the presence of hypercapnia is much more pronounced than during normocapnia as described in more detail below [9].
Carbon Dioxide
In both adults and children, increasing CO2/H+ results in an increase in minute ventilation [7] through stimulation of central and peripheral chemoreceptors [4]. Centrally, the main site of chemoreception for CO2/H+ is located in the rostral ventrolateral medulla [10]. Peripherally, the carotid bodies appear to have a role in CO2/H+ chemoreception, despite the historical belief that they were exclusively oxygen sensors [4].
Ventilatory Control in the Preterm Infant
Preterm infants have long been observed to demonstrate ventilatory instability, related to immaturity of the neural structures involved in control of ventilation [7]. What would otherwise be perceived as a relatively innocuous stress to a term infant, for example, changes in body temperature, can result in ventilatory instability and apnea in the vulnerable preterm infant [8, 10] as described below.
Oxygen Chemosensitivity of the Preterm Infant
As mentioned above, the mature response to hypoxia is biphasic, but the net effect is an increase in ventilation from baseline [9]. In the fetus, when oxygen delivery via the placenta is reduced, there is a reduction in respiratory movements by the fetus [11]. This response likely serves as a protective mechanism to limit energy utilization in times of hypoxia. However, when the fetus is born, if this response persists, it becomes less functional and potentially detrimental [11] and may be particularly harmful in the preterm infant.
Rigatto et al. studied moderate to late preterm infants (33–37 weeks gestation) exposed to hypoxia (fractional inspired oxygenation [FiO2] of 15%) and found that they demonstrated an initial increase in ventilation with a subsequent reduction below the baseline ventilation observed in room air [12]. This response is similar to that in adults in that it is biphasic, but notably different in that after the initial rise in ventilation, there is a fall below the baseline ventilation in room air. When more significantly preterm infants were studied, specifically <1500 g with a mean gestational age of 29 weeks, Alvaro et al. demonstrated a response to hypoxia (FiO2 of 15%) similar to that of the fetus. In these infants, hypoxia resulted in only an immediate and sustained reduction in ventilation [13]. This finding was attributed to the central depressive effect that hypoxia has on respiration in preterm infants [14]. These findings collectively demonstrate the destabilizing effect of hypoxia on the respiration of preterm infants, termed hypoxic ventilatory depression. Preterm infants, with a small functional residual capacity (FRC) and increased metabolic demand and oxygen consumption, are at particular risk of hypoxia. At the end of a tidal breath, the neonate is at a reduced FRC compared to older children and adults. Thus, a neonate requires only a brief pause in respiration to result in hypoxia, with hypoxia having a destabilizing effect on the control of breathing, although the underlying precise mechanisms are not well understood. Conversely, hyperoxia may have a stabilizing effect on the respiratory pattern in preterm infants. Weintraub et al. assessed the effects on respiration when infants of gestational age 27–31 weeks were exposed to incremental increases in FiO2 from room air to 40% oxygen [14]. In room air, all infants had periodic breathing and apneas. As the FiO2 was increased, these infants were noted to have a normalization of their respiratory pattern and a reduction in apneas, while the minute ventilation remained unchanged [14].
Carbon Dioxide Chemosensitivity in the Preterm Infant
Adults, children, and older infants all respond to hypercapnia with an increase in ventilation, and this response is mediated both at a central and peripheral level [15]. Preterm infants also tend to increase their ventilation in response to hypercapnia, but their response is blunted when compared to adults, children, and term and even late preterm infants [16,17,18]. Frantz et al. demonstrated that preterm infants (29–32 weeks) had a marked reduction in ventilatory response to hypercapnia which improved with post-natal age when compared to late preterm infants (33–36 weeks) [16]. In one study, infants born preterm (mean gestational age of 30.2 weeks) with apnea showed a reduced ventilatory response to CO2 than gestational age and birth weight-matched controls who did not have apnea [19]. This blunted response, then, appears to represent a risk factor for the development of irregular breathing and apnea. In summary, the ventilatory response to changes in oxygen and carbon dioxide levels documented in preterm infants places them in a position of vulnerability for respiratory instability and apnea. During sleep, with a reduction in ventilation, hypoxemia, and oscillations in CO2, the preterm infant is at particular risk for worsening respiratory instability with resultant apnea and periodic breathing [18].
Sleep, Normal Respiratory Variants, and Disease States in Preterm Infants
Sleep Architecture in Preterm Infants
There are three types of sleep recognized in the newborn: quiet sleep (QS) (analogous to NREM sleep), active sleep (AS) (equivalent to REM sleep), and indeterminate sleep. QS is characterized by minimal muscle movements and regular breathing cycles. During AS, sucking motions, twitches, smiles, frowns, irregular breathing, and gross limb movements are observed. Indeterminate sleep is the period of sleep that cannot be defined by polysomnogram as either active or quiet sleep.
Sleep organization in preterm infants undergoes significant development between birth and reaching term gestation. Active sleep decreases, and quiet sleep and waking states increase, with gestational age [20,21,22,23]. Breathing is more regular in QS, the percentage of AS with rapid eye movements decreases, and awakenings become longer [20,21,22]. Similar changes are observed in the early weeks after term gestation [20, 24]. Infants born prematurely sleep less and display more alertness and activity at each adjusted age than those born at term. They also have longer sustained episodes of quiet sleep but more body movements and rapid eye movements associated with active sleep [20, 24].
Sleeping and waking patterns of preterm infants have been associated with developmental outcomes. Different measures of sleep-wake states during the preterm period, i.e., the amount of crying, amount of REM during active sleep, sleep cycle length, and amount of nighttime sleep, predict cognitive and motor development at 1 year of age [25,26,27]. Prematurely born children who show a more rapid decrease in active sleep in the preterm period have higher intelligence quotients and better language and fine motor abilities at 3 years [28].
Mother-infant skin-to-skin contact (kangaroo care) has been shown to change sleep architecture in preterm infants. Infants who received kangaroo care have longer periods of deep-sleep and quiet-awake state and less time in light sleep or drowsy state [29].
Sleep-Disordered Breathing and Preterm Birth
Periodic Breathing
The occurrence of some periodic breathing (PB) is considered normal in almost all newborns [30]. A widely accepted definition of PB is at least three sequential central apneas of at least 3 s duration, with less than 20 s of regular breathing between [31]. PB is hypothesized to be the result of dominant peripheral chemoreceptor activity responding to fluctuations in arterial oxygen tension [32, 33]. Physiological models of PB demonstrate a natural distinction between transient and sustained oscillations, and PB represents high gain in the control loop [34,35,36]. The “high gain” observed in neonates is the result of chemoreceptor sensitivity to changes in blood oxygen and carbon dioxide levels that leads to sustained oscillations between breathing and apnea, especially during quiet sleep [30, 37,38,39]. At birth the peripheral chemoreceptors are desensitized by the acute rise in blood oxygen content during the fetal to neonatal transition. The chemoreceptor sensitivity is gradually reset during the first week of life [40]. Therefore, PB rarely occurs in the first 48 h of life and is not considered a precursor to significant apnea [40]. In infants of 32-week gestational age, the fraction of time spent in PB peaks at 7–14 days after birth at 6.5% of the total daily time [41].
Apnea of prematurity (AOP) and PB are distinct entities that differ in character, gestational age, and time of onset and resolution (Table 27.2). Hypoxia may trigger or exacerbate PB and AOP [42], and oxygen administration to preterm infants can decrease both PB and AOP [14, 43].
PB is thought to be benign, but a recent longitudinal study in former preterm infants followed during their first 6 months post-term showed that PB persisted in 40% [44]. Although in most infants PB was not associated with significant falls in SaO2, several infants had significant oxygen desaturations and reduced cerebral oxygenation, especially during active sleep [44]. However, the clinical significance of these desaturations associated with PB on neurodevelopmental outcome is unknown.
Apnea of Prematurity
Apnea of prematurity (AOP) is seen as a breathing disorder distinct from periodic breathing, which occurs in infants born before 34 weeks gestational age and usually resolves by 36–40 weeks postmenstrual age (Table 27.2). AOP is demonstrated by short respiratory pauses associated with chronic intermittent hypoxia (CIH) and bradycardia. The frequency of CIH tends to increase after the first week of life and may be sustained over many subsequent weeks [45]. AOP is very common and occurs in 75% of all infants born at less than 32 weeks gestation. Moreover, 90% of infants born before 28 weeks gestation have AOP that usually resolves by 44 weeks postmenstrual age only [46,47,48].
The pathogenesis of AOP is due to immaturity of the respiratory control characterized by abnormal ventilatory responses to hypoxia and CO2 as well as immature reflexes and upper airway instability [49]. Apnea is classified as central, obstructive, or mixed depending on the presence of continued inspiratory efforts and upper airway obstruction. In central apnea (CA), there is no inspiratory effort. During an obstructive apnea, inspiratory efforts persist but are ineffective in the presence of upper airway obstruction. During a mixed apnea, there is upper airway obstruction with inspiratory efforts that precedes or follows a central apnea.
Most apneic spells in preterm infants are mixed (50%) or central (40%) [50]. Longer episodes are more likely to be mixed apnea as opposed to short respiratory pauses, which are classified as central apnea.
The management of AOP includes the correction of contributing factors such as hypoglycemia, hypocalcaemia, metabolic alkalosis, anemia, arterial hypotension, hypoxemia, and any other factor that increases work of breathing. Respiratory stimulants, such as caffeine and aminophylline, are effective in reducing the incidence of apnea and periodic breathing but rarely eliminate it. Both therapies are effective, but caffeine is preferred for its oral route of administration, longer half-life, and wider therapeutic range [51]. Concerns regarding potential long-term neurodevelopmental side effects prompted larger, prospective trials that showed no significant side effects. The use of caffeine in preterm infants was related to a decrease in the incidence of patent ductus arteriosus (PDA), decreased duration of noninvasive positive pressure ventilation, decreased incidence of bronchopulmonary dysplasia (BPD), and better neurodevelopmental outcomes at 2 years of age [52,53,54,55]. Recent studies have explored the effectiveness and safety of short courses of higher doses (20 mg/kg/day vs. 5 mg/kg/day) of caffeine citrate to ensure successful extubation after 48 h of mechanical ventilation in infants less than 30 weeks gestational age [56]. Extubation failure rates in the treatment group was half that of the placebo group, and duration of mechanical ventilation in infants less than 28 weeks gestation receiving the high dose of caffeine was decreased significantly. No difference in adverse effects was detected in terms of mortality, major neonatal morbidity, death, or severe disability at 12 months [56]. Similar results were seen in a recent study that used even higher doses (40 mg/kg/day loading dose and 20 mg/kg/day maintenance) prior to extubation [57].
In follow-up studies, Marcus et al. studied the long-term effects of neonatal caffeine use on sleep architecture and breathing during sleep in former preterm infants now aged 5–12 years [58]. They found no difference in total sleep time, sleep efficiency, or incidence of obstructive sleep apnea (OSA) between groups randomized to caffeine versus placebo [58].
Finally, in refractory cases of apnea, respiratory support involving nasal continuous positive airway pressure (CPAP), high-flow nasal cannula, or invasive mechanical ventilation may be required to prevent severe hypoxic events and their consequences.
The Relationship Between Gastroesophageal Reflux and Apnea
Apnea of prematurity and gastroesophageal reflux (GER) are both common occurrences in preterm infants, and their causal relationship has been widely debated [59]. GER is the result of transient relaxation of the lower esophageal sphincter. It is physiologically plausible that reflux can trigger apnea as a protective reflex [60, 61]. This hypothesis is supported by a study of Omari and colleagues that showed that a decrease in lower esophageal pressure is associated with the onset of apnea in some neonates [62]. Conversely, animal studies have shown that apnea can trigger GER in turn [63]. Figure 27.3 illustrates the hypothesized cycle of apnea and GER.
However, clinical studies designed to confirm this causal relationship have reported conflicting results. This may be in part because earlier studies only used pH monitoring to diagnose GER which is less sensitive as it does not detect nonacid reflux. Recent studies that included multiple intraluminal impedance monitoring in addition to esophageal pH, designed to capture evidence of nonacid reflux as well, did not consistently demonstrate a causal relationship between apnea and GER. A recent review of published literature found that both GER and apnea occur frequently in premature infants, but less than 3% of cardiorespiratory events actually follow a GER event, and GER is not associated with increased duration or severity of cardiorespiratory events [64]. Indeed, it is more common for an episode of GER to follow a cardiorespiratory event (9%) [64].
The role of pharmacotherapy in GER is still debated; more recent studies have highlighted the lack of efficacy of gastric acid inhibitors (GAIs), e.g., proton-pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2RAs), and prokinetics, while other studies raised concern about associated side effects [65,66,67]. A case-control study of very low birth weight infants showed histamine-2 antagonist (H2RA) use was associated with higher rates of necrotizing enterocolitis [65]. Another neonatal study showed an increased risk of bloodstream infections (relative risk = 4.2) and candidemia (odds ratio = 2.44) with H2RA exposure [68]. Although, GAIs have been well tolerated by infants, recent studies showed a harmful association between the use of GAIs and the development of bacterial enteric infection and community-acquired pneumonia in infants and young children [69, 70].
Pharmacotherapy should only be considered for infants who have evidence of pathological GER with severe symptoms refractory to non-pharmacological treatment, and treatment should be monitored closely and only continued in those with clear benefit.
Sudden Infant Death Syndrome and Prematurity
Sudden infant death syndrome (SIDS) is defined as the sudden and unexplained death of an infant less than 1 year, with the onset of the lethal episode apparently occurring during sleep. A death classified as SIDS remains unexplained after a thorough investigation including a review of the circumstances of death, a clinical history, and a complete autopsy [71]. More than 90% of SIDS deaths occur in the first 6 months of life, with a peak incidence between 2 and 4 months [72]. Although the incidence of SIDS has declined since the introduction of the Back to Sleep campaign in 1994, it remains the leading cause of post-neonatal mortality in North America at approximately 0.5 per 100 live births [73]. Some concern has been raised by the observed increased incidence of plagiocephaly following the introduction of the Back to Sleep campaign [74, 75]. In the only prospective study as of yet, eight factors were associated with an increased risk of deformational plagiocephaly at 7 weeks of age: male gender, first-born birth rank, positional preference when sleeping, head to the same side on chest of drawers, only bottle feeding, positioning to the same side during bottle feeding, tummy time when awake <3 times per day, and slow achievement of motor milestones [76].
SIDS is considered multifactorial in origin, and a “triple risk hypothesis” has been proposed to organize current knowledge. This hypothesis proposes that SIDS results when three factors coincide: a vulnerable infant, a critical developmental period in homoeostatic control, and an external stressor [77]. The final pathway to SIDS is believed to involve immature cardiorespiratory autonomic control, together with a failure of arousal responsiveness from sleep. Several genetic polymorphisms, as well as clinical and environmental risk factors, have been identified [72, 73].
Premature birth is one of most significant risk factors for SIDS. In 1987, the risk of SIDS was 2.32 times greater for extremely premature infants compared with term infants, and although the overall incidence has subsequently decreased, the adjusted odds ratio for SIDS among the most preterm infants (24–28 weeks gestation) is two and a half times higher than for term infants. The risk for SIDS is also increased by 56% in infants weighing less than 2500 g and also for growth restricted, small for gestational age infants [73].
Although for many years, apnea was thought to be the precursor of SIDS, results of studies such as the Collaborative Home Infant Monitoring Evaluation have shown that it neither precedes nor predicts SIDS [49]. However, concerns have been raised about the possible association between SIDS and PB. Kelly et al. were the first to document excessive PB in cases of “near SIDS” (now known as apparent life-threatening events or ALTEs) and in the siblings of infants who died of SIDS [78, 79]. In a more recent case, excessive PB (30% of total recorded time) was observed in a patient who subsequently died of SIDS [41]. The authors developed a method to quantify PB in large numbers of patients over long periods. Their method can distinguish PB from irregular apnea clusters and may be a helpful method to study the possible link between excessive PB in the newborn and future risk for SIDS [41].
Long-Term Implications of Prematurity on Sleep and Breathing
Many factors contribute to neurodevelopmental outcomes in premature infants, and the impact of AOP on long-term neurological outcome has not been studied systematically. However, available data suggests an increased risk for neurological impairment (defined as a Bayley Scales of Infant Development or psychomotor index score <70, or cerebral palsy or blindness at 3 years of age) in preterm infants less than 32 weeks gestation who required more mechanical ventilation days and had more apnea days (total number of days with at least one apnea recorded per day) after extubation [80]. Existing studies of long-term effects of prematurity on sleep are reviewed in Table 27.3.
The need for further studies is highlighted by recent observations that former preterm infants are more likely to have sleep-disordered breathing (SDB) in later childhood [58, 81,82,83,84]. Marcus et al. found a prevalence of OSA of 9.6% in 201 former preterm infants now aged 5–12 years compared with 1–4% of the general population [58]. Although earlier studies raised concerns regarding past exposure to xanthines such as caffeine and theophylline for AOP as a risk for SDB in childhood [83], the aforementioned study by Marcus and colleagues showed no difference in the prevalence of OSA between those who received caffeine and those who were randomized to the placebo arm [58]. Of further interest, the periodic limb movement index was increased in the caffeine group compared with the placebo group, but sleep efficiency was the same in both groups.
Sleep problems other than SDB in the first 6 months seem less common in preterm than full-term infants [85]. Sleep patterns and incidence of sleep problems in the first 10 years of life in historical cohorts did not differ between those born at term or preterm [86]. Even in young adults born prematurely, sleep quality and amount seemed unchanged despite the fact that they were at greater risk for SDB [81, 87]. Interestingly, a more recent study described significantly earlier bed and wake times and fewer arousals in adolescents born prematurely [88].
Summary and Conclusions
Prematurity leads to a period of respiratory instability, especially during sleep. It is important that health-care professionals involved in the care of preterm infants understand not only the normal respiratory physiology and breathing of these preterm infants but also abnormal breathing states that may predispose to short- and long-term morbidity, including poorer neurocognition if left untreated. In particular, increasing evidence suggests that preterm birth may be associated with an increased prevalence of sleep-disordered breathing, specifically OSA. As such, the authors would recommend that preterm children should be screened for sleep disorders in early childhood and those with a high index of suspicion for sleep-disordered breathing, referred to a pediatric sleep center.
Future Directions
Future research is needed to understand the exact mechanisms predisposing to an increased risk of sleep disorders that may further predispose to adverse neurocognition. It would be helpful to strategize about preventative factors limiting risks of sleep-disordered breathing in this already vulnerable population. Specifically, optimizing respiratory status early on in the lives of these infants may be associated with a reduction in risk for sleep disorders.
Abbreviations
- BPD:
-
Bronchopulmonary dysplasia
- BSID:
-
Bayley Score of Infant Development
- CI:
-
Confidence interval
- GA:
-
Gestational age
- IVH:
-
Intraventricular hemorrhage
- PSG:
-
Poysomnography
- RCT:
-
Randomized controlled trial
- Ref:
-
Reference number
- SCU:
-
Special care unit
- SD:
-
Standard deviation
- SDB:
-
Sleep-disordered breathing
- VLBW:
-
Very low birth weight
References
World Health Organization. Preterm birth fact sheet. Reviewed Nov 2016. http://www.who.int/mediacentre/factsheets/fs363/en/.
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619.
Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52(5):751–66.
Carroll JL, Agarwal A. Development of ventilatory control in infants. Paediatr Respir Rev. 2010;11(4):199–207.
Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond Ser B Biol Sci. 2009;364(1529):2577–87.
Kline DD, King TL, Austgen JR, Heesch CM, Hasser EM. Sensory afferent and hypoxia-mediated activation of nucleus tractus solitarius neurons that project to the rostral ventrolateral medulla. Neuroscience. 2010;167(2):510–27.
Matthews EE, Aloia MS. Cognitive recovery following positive airway pressure (PAP) in sleep apnea. Prog Brain Res. 2011;190:71–88.
Cardot V, Chardon K, Tourneux P, Micallef S, Stephan E, Leke A, et al. Ventilatory response to a hyperoxic test is related to the frequency of short apneic episodes in late preterm neonates. Pediatr Res. 2007;62(5):591–6.
Easton PA, Slykerman LJ, Anthonisen NR. Ventilatory response to sustained hypoxia in normal adults. J Appl Physiol (1985). 1986;61(3):906–11.
Chardon K, Telliez F, Bach V, Leke A, Delanaud S, Bouferrache B, et al. Effects of warm and cool thermal conditions on ventilatory responses to hyperoxic test in neonates. Respir Physiol Neurobiol. 2004;140(2):145–53.
Poets CF. Apnea of prematurity: what can observational studies tell us about pathophysiology? Sleep Med. 2010;11(7):701–7.
Rigatto H, Brady JP, de la Torre Verduzco R. Chemoreceptor reflexes in preterm infants: I. The effect of gestational and postnatal age on the ventilatory response to inhalation of 100% and 15% oxygen. Pediatrics. 1975;55(5):604–13.
Alvaro R, Alvarez J, Kwiatkowski K, Cates D, Rigatto H. Small preterm infants (less than or equal to 1500 g) have only a sustained decrease in ventilation in response to hypoxia. Pediatr Res. 1992;32(4):403–6.
Weintraub Z, Alvaro R, Kwiatkowski K, Cates D, Rigatto H. Effects of inhaled oxygen (up to 40%) on periodic breathing and apnea in preterm infants. J Appl Physiol (1985). 1992;72(1):116–20.
Gauda EB, Miller MJ, Carlo WA, Difiore JM, Johnsen DC, Martin RJ. Genioglossus response to airway occlusion in apneic versus nonapneic infants. Pediatr Res. 1987;22(6):683–7.
Frantz ID 3rd, Adler SM, Thach BT, Taeusch HW Jr. Maturational effects on respiratory responses to carbon dioxide in premature infants. J Appl Physiol. 1976;41(1):41–5.
Duffin J. Measuring the ventilatory response to hypoxia. J Physiol. 2007;584(Pt 1):285–93.
Darnall RA. The role of CO(2) and central chemoreception in the control of breathing in the fetus and the neonate. Respir Physiol Neurobiol. 2010;173(3):201–12.
Gerhardt T, Bancalari E. Apnea of prematurity: I. Lung function and regulation of breathing. Pediatrics. 1984;74(1):58–62.
Holditch-Davis D, Scher M, Schwartz T, Hudson-Barr D. Sleeping and waking state development in preterm infants. Early Hum Dev. 2004;80(1):43–64.
Scher MS, Johnson MW, Holditch-Davis D. Cyclicity of neonatal sleep behaviors at 25 to 30 weeks’ postconceptional age. Pediatr Res. 2005;57(6):879–82.
Giganti F, Ficca G, Cioni G, Salzarulo P. Spontaneous awakenings in preterm and term infants assessed throughout 24-h video-recordings. Early Hum Dev. 2006;82(7):435–40.
Foreman SW, Thomas KA, Blackburn ST. Individual and gender differences matter in preterm infant state development. J Obstet Gynecol Neonatal Nurs. 2008;37(6):657–65.
Davis DH, Thoman EB. Behavioral states of premature infants: implications for neural and behavioral development. Dev Psychobiol. 1987;20(1):25–38.
Ednick M, Cohen AP, McPhail GL, Beebe D, Simakajornboon N, Amin RS. A review of the effects of sleep during the first year of life on cognitive, psychomotor, and temperament development. Sleep. 2009;32(11):1449–58.
Gertner S, Greenbaum CW, Sadeh A, Dolfin Z, Sirota L, Ben-Nun Y. Sleep-wake patterns in preterm infants and 6 month’s home environment: implications for early cognitive development. Early Hum Dev. 2002;68(2):93–102.
Arditi-Babchuk H, Feldman R, Eidelman AI. Rapid eye movement (REM) in premature neonates and developmental outcome at 6 months. Infant Behav Dev. 2009;32(1):27–32.
Holditch-Davis DBM, Edwards LJ. Prediction of 3-year developmental outcomes from sleep development over the preterm period. Infant Behav Dev. 2005;28(2):118–1.
Bastani F, Rajai N, Farsi Z, Als H. The effects of Kangaroo Care on the sleep and wake states of preterm infants. J Nurs Res. 2016;25(3):231–9.
Rigatto H. In: Matthew O, editor. Respiratory controls and disorders in the newborn. Boca Raton: CRC Press; 2003.
Barrington KJ, Finer NN, Wilkinson MH. Progressive shortening of the periodic breathing cycle duration in normal infants. Pediatr Res. 1987;21(3):247–51.
Rigatto H, Brady JP. Periodic breathing and apnea in preterm infants. I. Evidence for hypoventilation possibly due to central respiratory depression. Pediatrics. 1972;50(2):202–18.
Rigatto H, Brady JP. Periodic breathing and apnea in preterm infants. II. Hypoxia as a primary event. Pediatrics. 1972;50(2):219–28.
Ben-Tal A, Smith JC. Control of breathing: two types of delays studied in an integrated model of the respiratory system. Respir Physiol Neurobiol. 2010;170(1):103–12.
Cherniack NS, Longobardo G, Evangelista CJ. Causes of cheyne-stokes respiration. Neurocrit Care. 2005;3(3):271–9.
Fowler AC, Kalamangalam GP. The role of the central chemoreceptor in causing periodic breathing. IMA J Math Appl Med Biol. 2000;17(2):147–67.
Cherniack NS, Longobardo GS. Mathematical models of periodic breathing and their usefulness in understanding cardiovascular and respiratory disorders. Exp Physiol. 2006;91(2):295–305.
Edwards BA, Sands SA, Berger PJ. Postnatal maturation of breathing stability and loop gain: the role of carotid chemoreceptor development. Respir Physiol Neurobiol. 2013;185(1):144–55.
Pereira MR, Reis FC, Landriault L, Cates DB, Rigatto H. Profile of alveolar gases during periodic and regular breathing in preterm infants. Biol Neonate. 1995;67(5):322–9.
Barrington KJ, Finer NN. Periodic breathing and apnea in preterm infants. Pediatr Res. 1990;27(2):118–21.
Mohr MA, Fairchild KD, Patel M, Sinkin RA, Clark MT, Moorman JR, et al. Quantification of periodic breathing in premature infants. Physiol Meas. 2015;36(7):1415–27.
Al-Matary A, Kutbi I, Qurashi M, Khalil M, Alvaro R, Kwiatkowski K, et al. Increased peripheral chemoreceptor activity may be critical in destabilizing breathing in neonates. Semin Perinatol. 2004;28(4):264–72.
Simakajornboon N, Beckerman RC, Mack C, Sharon D, Gozal D. Effect of supplemental oxygen on sleep architecture and cardiorespiratory events in preterm infants. Pediatrics. 2002;110(5):884–8.
Decima PF, Fyfe KL, Odoi A, Wong FY, Horne RS. The longitudinal effects of persistent periodic breathing on cerebral oxygenation in preterm infants. Sleep Med. 2015;16(6):729–35.
Di Fiore JM, Bloom JN, Orge F, Schutt A, Schluchter M, Cheruvu VK, et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr. 2010;157(1):69–73.
Di Fiore JM, Martin RJ, Gauda EB. Apnea of prematurity – perfect storm. Respir Physiol Neurobiol. 2013;189(2):213–22.
Pillekamp F, Hermann C, Keller T, von Gontard A, Kribs A, Roth B. Factors influencing apnea and bradycardia of prematurity – implications for neurodevelopment. Neonatology. 2007;91(3):155–61.
Ramanathan R, Corwin MJ, Hunt CE, Lister G, Tinsley LR, Baird T, et al. Cardiorespiratory events recorded on home monitors: comparison of healthy infants with those at increased risk for SIDS. JAMA. 2001;285(17):2199–207.
Martin RJ, Wilson CG. Apnea of prematurity. Compr Physiol. 2012;2(4):2923–31.
Finer NN, Barrington KJ, Hayes BJ, Hugh A. Obstructive, mixed, and central apnea in the neonate: physiologic correlates. J Pediatr. 1992;121(6):943–50.
Henderson-Smart DJ, De Paoli AG. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst Rev. 2010;12:CD000140.
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–21.
Schmidt B. Methylxanthine therapy for apnea of prematurity: evaluation of treatment benefits and risks at age 5 years in the international Caffeine for Apnea of Prematurity (CAP) trial. Biol Neonate. 2005;88(3):208–13.
Dobson NR, Patel RM, Smith PB, Kuehn DR, Clark J, Vyas-Read S, et al. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J Pediatr. 2014;164(5):992–8.e3.
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357(19):1893–902.
Steer P, Flenady V, Shearman A, Charles B, Gray PH, Henderson-Smart D, et al. High dose caffeine citrate for extubation of preterm infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2004;89(6):F499–503.
Mohammed S, Nour I, Shabaan AE, Shouman B, Abdel-Hady H, Nasef N. High versus low-dose caffeine for apnea of prematurity: a randomized controlled trial. Eur J Pediatr. 2015;174:949–56.
Marcus CL, Meltzer LJ, Roberts RS, Traylor J, Dix J, D’Ilario J, et al. Long-term effects of caffeine therapy for apnea of prematurity on sleep at school age. Am J Respir Crit Care Med. 2014;190(7):791–9.
Suarez-Moran E, Morales-Fuentes GA, Inzunza-Gonzalez JA, Cedillo-Ley I, Gerardo-del Hoyo M, Silva-Ramirez H. Influence of central apnea in the preterm newborn with gastroesophageal reflux disease. Cir Cir. 2011;79(6):511–9.
Slocum C, Hibbs AM, Martin RJ, Orenstein SR. Infant apnea and gastroesophageal reflux: a critical review and framework for further investigation. Curr Gastroenterol Rep. 2007;9(3):219–24.
Davies AM, Koenig JS, Thach BT. Characteristics of upper airway chemoreflex prolonged apnea in human infants. Am Rev Respir Dis. 1989;139(3):668–73.
Omari TI. Apnea-associated reduction in lower esophageal sphincter tone in premature infants. J Pediatr. 2009;154(3):374–8.
Kiatchoosakun P, Dreshaj IA, Abu-Shaweesh JM, Haxhiu MA, Martin RJ. Effects of hypoxia on respiratory neural output and lower esophageal sphincter pressure in piglets. Pediatr Res. 2002;52(1):50–5.
Di Fiore J, Arko M, Herynk B, Martin R, Hibbs AM. Characterization of cardiorespiratory events following gastroesophageal reflux in preterm infants. J Perinatol. 2010;30(10):683–7.
Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006;117(2):e137–42.
Orenstein SR, Hassall E, Furmaga-Jablonska W, Atkinson S, Raanan M. Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. J Pediatr. 2009;154(4):514–20.e4.
Terrin G, Passariello A, De Curtis M, Manguso F, Salvia G, Lega L, et al. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics. 2012;129(1):e40–5.
Saiman L, Ludington E, Pfaller M, Rangel-Frausto S, Wiblin RT, Dawson J, et al. Risk factors for candidemia in Neonatal Intensive Care Unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr Infect Dis J. 2000;19(4):319–24.
Canani RB, Cirillo P, Roggero P, Romano C, Malamisura B, Terrin G, et al. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117(5):e817–20.
Canani RB, Terrin G. Gastric acidity inhibitors and the risk of intestinal infections. Curr Opin Gastroenterol. 2010;26(1):31–5.
Kraus JF, Greenland S, Bulterys M. Risk factors for sudden infant death syndrome in the US Collaborative Perinatal Project. Int J Epidemiol. 1989;18(1):113–20.
Moon RY, Horne RS, Hauck FR. Sudden infant death syndrome. Lancet. 2007;370(9598):1578–87.
Hakeem GF, Oddy L, Holcroft CA, Abenhaim HA. Incidence and determinants of sudden infant death syndrome: a population-based study on 37 million births. World J Pediatr. 2015;11(1):41–7.
Branch LG, Kesty K, Krebs E, Wright L, Leger S, David LR. Deformational plagiocephaly and craniosynostosis: trends in diagnosis and treatment after the “back to sleep” campaign. J Craniofac Surg. 2015;26(1):147–50.
Turk AE, McCarthy JG, Thorne CH, Wisoff JH. The “back to sleep campaign” and deformational plagiocephaly: is there cause for concern? J Craniofac Surg. 1996;7(1):12–8.
van Vlimmeren LA, van der Graaf Y, Boere-Boonekamp MM, L’Hoir MP, Helders PJ, Engelbert RH. Risk factors for deformational plagiocephaly at birth and at 7 weeks of age: a prospective cohort study. Pediatrics. 2007;119(2):e408–18.
Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65(3–4):194–7.
Kelly DH, Walker AM, Cahen L, Shannon DC. Periodic breathing in siblings of sudden infant death syndrome victims. Pediatrics. 1980;66(4):515–20.
Kelly DH, Shannon DC. Periodic breathing in infants with near-miss sudden infant death syndrome. Pediatrics. 1979;63(3):355–60.
Janvier A, Khairy M, Kokkotis A, Cormier C, Messmer D, Barrington KJ. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24(12):763–8.
Paavonen EJ, Strang-Karlsson S, Raikkonen K, Heinonen K, Pesonen AK, Hovi P, et al. Very low birth weight increases risk for sleep-disordered breathing in young adulthood: the Helsinki Study of Very Low Birth Weight Adults. Pediatrics. 2007;120(4):778–84.
Rosen CL, Larkin EK, Kirchner HL, Emancipator JL, Bivins SF, Surovec SA, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142(4):383–9.
Hibbs AM, Johnson NL, Rosen CL, Kirchner HL, Martin R, Storfer-Isser A, et al. Prenatal and neonatal risk factors for sleep disordered breathing in school-aged children born preterm. J Pediatr. 2008;153(2):176–82.
Raynes-Greenow CH, Hadfield RM, Cistulli PA, Bowen J, Allen H, Roberts CL. Sleep apnea in early childhood associated with preterm birth but not small for gestational age: a population-based record linkage study. Sleep. 2012;35(11):1475–80.
Wolke D, Sohne B, Riegel K, Ohrt B, Osterlund K. An epidemiologic longitudinal study of sleeping problems and feeding experience of preterm and term children in southern Finland: comparison with a southern German population sample. J Pediatr. 1998;133(2):224–31.
Iglowstein I, Latal Hajnal B, Molinari L, Largo RH, Jenni OG. Sleep behaviour in preterm children from birth to age 10 years: a longitudinal study. Acta Paediatr. 2006;95(12):1691–3.
Strang-Karlsson S, Raikkonen K, Kajantie E, Andersson S, Hovi P, Heinonen K, et al. Sleep quality in young adults with very low birth weight – the Helsinki study of very low birth weight adults. J Pediatr Psychol. 2008;33(4):387–95.
Hibbs AM, Storfer-Isser A, Rosen C, Ievers-Landis CE, Taveras EM, Redline S. Advanced sleep phase in adolescents born preterm. Behav Sleep Med. 2014;12(5):412–24.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bola, S.S., Kritzinger, F.E., Narang, I. (2019). Prematurity. In: Accardo, J. (eds) Sleep in Children with Neurodevelopmental Disabilities. Springer, Cham. https://doi.org/10.1007/978-3-319-98414-8_27
Download citation
DOI: https://doi.org/10.1007/978-3-319-98414-8_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-98412-4
Online ISBN: 978-3-319-98414-8
eBook Packages: MedicineMedicine (R0)