Abstract
Current rates of economic development are interrelated with an increase in environmental pollution. Among different contamination agents, modern insecticides such as neonicotinoids (NNIs) require precise attention in evaluation of losses and benefits. NNIs is relatively new class of systemic insecticides, being in use for about 20 years and embracing around 25% of global pesticide market. Currently there are several methods to apply NNIs to plants such as foliar sprays, soil drenches and seed treatments, and in recent years there has been a global shift towards seed treatment (seed dressing) rather than aerial spraying. The discovery of NNIs was considered as a milestone in the research on insecticides. Possessing chemical structure similar to nicotine and acting as agonists at insects’ acetylcholine receptors, NNIs demonstrate selective toxicity to invertebrates versus vertebrates. In addition, toxicity of NNIs in mammals is between one to three orders of magnitude lower than the toxicity caused by their predecessors: organophosphates, carbamates and pyrethroids. However, NNIs are mobile contaminants that can be transferred from plants to soils and water and induce diverse array of toxic effects in non-target organisms, even affecting animals not in contact with them directly. Surface- and groundwater may also act as vector for the transport of NNIs to untreated locations. The presence of NNIs in water bodies might facilitate their uptake by non-target plants present in littoral and riparian zones, with the potential threat to herbivorous insects. Leaching of NNIs to groundwater may imply their further distribution to other matrices, potentially leading to undesirable environmental issues. Pollinators and aquatic insects appear to be especially susceptible to these insecticides and chronic sublethal effects tend to be more prevalent than acute toxicity. Although a complete knowledge of the fate of NNIs in the environments is missing, authorities are starting to react to the threat they pose by limiting their use and application. Relevant improvements have been made in the field of the toxicity to non-target organisms. Studies that include factors such as mixture toxicity, field or semi-field exposures can make significant contribution to the further evaluating of costs-benefits of neonicotinoids.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Agricultural pollution
- Aquatic insects
- Pollinators
- Predators
- Neonicotinoids
- Non-target organisms
- Systemic insecticides
1 Introduction
Current rates of industrial and agricultural development have inevitable side effects and one of those is environmental contamination (Grossman and Kruger 1995). Persistent organic pollutants (POPs), petroleum hydrocarbons, heavy metals, plastic pollutants and pesticides are some of the most common contaminants affecting terrestrial and aquatic ecosystems in a daily routine. Their massive or continuous release can provoke ecological disasters, causing acute toxicity to many different organisms. However, inconspicuous, hidden or extended consequences can be even more dangerous. Not resulting in immediate alarming effects, they may go unnoticed for a long time before the critical moment comes. In addition, complex interactions between climate change and pollution can alter physical and chemical stressors, starting to be even more problematic for the organisms, living at the edge of their tolerance (Noyes et al. 2009). Finally, the ongoing environmental contamination and related ecosystem change lead to widespread species extinction and biodiversity loss (Butchart et al. 2010; Hooper et al. 2012).
Terrestrial and aquatic ecosystems are closely interrelated. One of the major land-water linkages in the biosphere is gravitational movement of material in drainage waters (Likens and Bormann 1974). Some contaminants entering soil may cause pronounced effects to organisms living in water. Holistic approach regarding land-air-water interactions is required for intelligent management of landscapes.
Regarding pesticide pollution, the case of neonicotinoid insecticides is one of the most pressing issues nowadays. Neonicotinoids (new nicotine-like insecticides—NNIs) are a family of toxic substances, including imidacloprid, acetamiprid, dinotefuran, nitenpyram, thiamethoxam, thiacloprid and clothianidin, are used to kill agricultural pest insects (Simon-Delso et al. 2015). Also, they are in use in veterinary medicine for controlling parasitic insects such as ticks or fleas. Neonicotinoids are relatively new class of systemic insecticides, being in active use for only two decades. NNIs possess chemical structure similar to nicotine and act as agonists at insects’ acetylcholine receptors (nAChRs), exhibiting selective toxicity to invertebrate versus vertebrate species (Matsuda et al. 2001). The approval of the first neonicotinoid, imidacloprid, was granted in 1994 in USA and in 2005 in the EU. During the following years, the other neonicotinoids were developed and approved to be used in the market. These were nitenpyram, thiamethoxam, clothianidin, dinotefuran, thiacloprid and acetamiprid.
Many recent studies confirmed toxic effects of NNIs to non-target organisms (Beketov and Liess 2008; Lever et al. 2014; Addy-Orduna et al. 2019). Meanwhile, it is still necessary to summarise and understand general linkages of neonicotinoid pollution between terrestrial and aquatic ecosystems. Therefore, here we aimed to review current state of knowledge about neonicotinoid contamination in terrestrial and aquatic ecosystems and the toxic effects of NNIs to non-target organisms, living in these ecosystems.
2 History of Neonicotinoids
The discovery of NNIs was considered as a milestone in the research on insecticides (Tomizawa and Casida 2011). Their solubility in water, together with their low toxicity to humans and other species of mammals made them a great choice among the available plant protection products. The insecticide market slowly switched from organophosphates, carbamates and pyrethroids to NNIs (Wood and Goulson 2017). Comparing the oral LD50 in rats (available in Yu 2015), it can be concluded that the toxicity of NNIs is between one and three orders of magnitude lower than the toxicity caused by their predecessors, such as organophosphates, carbamates and pyrethroids (Yu 2015).
However, NNIs were not as safe as it seemed for other organisms. Already during the registration process, imidacloprid was found highly toxic to three species of bees, with a LD50 of 0.0439 µg/bee (USEPA 1992). In 2008, an accidental release of clothianidin affected 11,000 hives and resulted in a massive death of bees in Germany (BVL 2008). The EU commission requested a conclusive assessment to the European Food Safety Authority (EFSA) on the risk of three NNIs (imidacloprid, thiamethoxam and clothianidin) to bees (EFSA 2012).
EFSA presented evidence of sublethal toxic effects in bees resulting from the exposure to these insecticides (EFSA 2013a, b, c). As the EU member states did not come to an agreement about the banning of these substances (some state members did not agree and some others such as Finland abstained), the European commission adopted a proposal (Regulation No. 485/2013) that prompted the restriction in the use of three NNIs for the period of two years, until new scientific evidence was gathered. The restriction started on December 1st, 2013, and it applied to three members of the neonicotinoid family, imidacloprid, clothianidin and thiamethoxam. Additionally, during the following years, several emergency authorizations were granted to a total of seven member states despite to the ban (e.g.: EFSA 2018a, b). Authorizations were granted due to the lack of alternative measures or products against specific pests.
In addition, the five NNIs approved for use in the EU were selected in 2016 to complete the First Watch list for emerging water pollutants (European Commission 2015). EFSA examined the new evidence related to the risk assessment of the three NNIs involved and after long deliberations, the EU commission implemented the regulations that ban the outdoor use of imidacloprid, clothianidin and thiamethoxam in May 2018 (Regulations (EU) 2018/783, 2018/784 and 2018/785, respectively). It must be highlighted that the regulations do allow the use of the three compounds indoors (e.g.: in greenhouses), what may protect bee populations but also may cause NNIs to leach to water bodies. It is also important to remark that the ban does not affect the use of other NNIs such as acetamiprid and thiacloprid. At first these two compounds were found to be less toxic to bees than imidacloprid, thiamethoxam and clothianidin (EFSA Panel PPR 2012), but they are highly toxic to freshwater invertebrates (Raby et al. 2018a) and therefore a threat for the future of aquatic organisms and their surrounding ecosystems.
In the US, the environmental protection agency (US-EPA), released NNIs assessments for public comment in 2017 and will come to a definitive conclusion during 2019, aiming at reducing risk. The NNIs involved in these procedures are imidacloprid, thiamethoxam, clothianidin, dinotefuran and acetamiprid. It is worth noting that thiacloprid permission was voluntarily cancelled by the manufacturer already in 2014.
3 Neonicotinoids in the Environments
Neonicotinoids are systemic insecticides: the active compounds distribute to all plant tissues and therefore provide a more complete protection to pests that would feed on any part of the plant. Due to the unique properties of NNIs such as increased intrinsic acute and residual activity for many agricultural pests (whiteflies, Colorado potato beetles, aphids etc.) and their systemic properties, they are applicable to many different crops: rape, corn, cotton, potatoes, sugar beet, tobacco, cereals, pome fruits (Jeschke and Nauen 2008).
Currently there are several methods to apply NNIs to plants, including foliar sprays, soil drenches and seed treatments (Bonmatin et al. 2015). In recent years there was a global shift towards widespread application of these insecticides as a seed treatment rather than aerial spraying (Hladik et al. 2018). The so called “seed dressing” initiates the protection of the plant at the seed stage and, due to the solubility of NNIs, helps to the distribution of the insecticides to all plant tissues. Flowers and pollen would therefore contain the applied neonicotinoid(s) even if application methods such as foliar spray are avoided. Seed dressing additionally minimizes the loading of pesticides to the surrounding environment and reduces the occupational exposure of farmers compared to spraying or foliar treatments.
When the plant has flowered, NNIs are distributed throughout all the plant tissues. Different species of invertebrate and vertebrate pollinators are in contact with NNIs through flowers and pollen and may uptake them. Neonicotinoids are present at every parts of plants growing from treated seeds: in stem, leaves, nectar and pollen, and it is generally assumed that from 2 to 20% of pesticide’s coating is absorbed by plants’ tissues (Alford and Krupke 2017; Hladik et al. 2018).
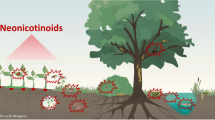
There are several routes of environmental exposure of NNIs from treated seeds to terrestrial and aquatic ecosystems (Fig. 1). Seeds can be ingested by birds and some small terrestrial mammals. NNIs are highly persistent in soils and are characterized by a high runoff and leaching potential to surface and groundwater (Bonmatin et al. 2015). Penetrating aquatic environments, they become a threat to larvae of aquatic insects and fish.
4 NNIs Contamination: Links Between Ecosystems
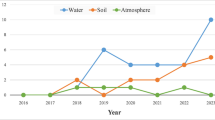
Despite the good intentions of the seed dressing treatments, up to 95% of the NNIs that are coating the seeds leach to the surrounding soils (Sur and Stork 2003). In soils, NNIs are persistent, with DT50’s reaching thousands of days (Goulson 2013). If the DT50 reach over one year, the compounds are accumulating in the soil and most likely their concentrations increase with time. Neonicotinoids are prone to leaching if the right conditions are met. Usually, rainfalls (Hladik et al. 2014) or even melting snow (Main et al. 2016) can provoke a rise in neonicotinoid concentrations in the surrounding water bodies. Thus, it has been common to find NNIs in water bodies all around the world in concentrations ranging from the low ng L−1 to the hundreds µg L−1 (Morrissey et al. 2015), and often surpassing the concentrations set as quality guidelines for e.g.: imidacloprid. In addition to the findings in surface water bodies, NNIs have been detected also in groundwater (Bradford et al. 2018).
Surface- and groundwater may act as vector for the transport of NNIs to untreated locations. Two key aspects related to this transport have not been extensively investigated and are important when considering the whole neonicotinoid cycle since they enter the environment. First, the presence of NNIs in water bodies might facilitate their uptake by non-target plants present in littoral and riparian zones, with the potential threat to herbivorous insects. A similar scenario was first presented by Goulson (2013), who suggested that NNIs might be available from soils to non-target flora in areas near treated fields, what has been recently reviewed in Wood and Goulson (2017). Second, the leaching of NNIs to groundwater may imply their further distribution to other matrices, potentially leading to undesirable environmental issues (Huseth and Groves 2014). If groundwater is used as irrigation, NNIs may recirculate to the same crop they were originally applied to (Huseth and Groves 2014).
It is still unknown whether lotic waters (surface- and groundwaters) can transport NNIs to areas far away from the agricultural fields where they were originally applied to. In addition, it would be interesting to know whether non-target plants or organisms uptake NNIs from these lotic water bodies.
Most likely, pollinators are the vector of transport of NNIs to their predators, as for example thiacloprid and imidacloprid have been found in the blood of the European honey buzzard (Byholm et al. 2018), as one of its major food sources are pollinating bumble bees. The transfer to honey buzzards may be reinforced by the fact that bees that have uptaken NNIs are less likely to avoid predators (Tan et al. 2014).
Contrarily to what occurs with other hydrophobic contaminants such as PCBs, it is not expected that the concentrations of neonicotinoids increase along the trophic chain (biomagnification), due to their solubility in water and therefore their excretion in urine. However, the finding of imidacloprid and thiacloprid in this long distance migrating raptor opens more questions about the presence of NNIs in other animals and the toxic effects that they may cause. Unfortunately, the aforementioned study is not the only one that has recently reported the presence of NNIs in birds. Additionally to the honey buzzard, NNIs have been found in other birds such as the Eurasian eagle owl (Taliansky-Chamudis et al. 2017), hummingbirds (Bishop et al. 2018) and quail (Turaga et al. 2016). The ingestion of NNIs by birds may result in toxic effects. It has been found that the South America eared dove would need to eat as less as 1.7 g of seeds to reach the LD50 of imidacloprid (Addy-Orduna et al. 2019). Importantly, a recent article reported a reduction in the migration ability of songbirds (Eng et al. 2017), finding that opens questions about the effects of NNIs in other migrating species such as the European honey buzzard.
Additional to the toxic effects caused by direct ingestion or contact with NNIs present in seeds, prey or pollen, indirect effects caused by other factors may also affect bird populations (Gibbons et al. 2015). Factors as declining populations of invertebrate prey are important when considering the whole consequences in the use of NNIs and other pesticides. A decrease in insectivorous birds has been associated with higher concentrations of imidacloprid in surface water of The Netherlands (Hallmann et al. 2014), what constitutes a dramatic example of indirect effects to entire bird populations.
5 Ecotoxicological Effects of NNIs in Non-target Species
Neonicotinoids specifically bind and continuously activate the insect nicotine acetylcholine receptor (nAChR), causing a series of disorders and finally leading to the death of an insect (Yu 2015). Certainly, there is no specific distinction between the binding capacities of NNIs to the nAChR in target vs. non-target insects. Thus, the high sensitivity of most insects to NNIs does not come as a surprise. The main use of NNIs has led to widespread detection of NNIs in the environment (in soil, water, pollen or honey). Pollinators and aquatic insects appear to be especially susceptible to these insecticides and chronic sublethal effects tend to be more prevalent than acute toxicity.
5.1 Neonicotinoids and Non-target Terrestrial Insects: Threats to Pollinators
When comparing agricultural pollution to other pollution types, environmental contamination by pesticides might appear relatively insignificant, however pesticide residues in soils might be toxic to soil microbial and invertebrate faunas (Iyaniwura 1991). Those insects, living in soil permanently or partly during some their stages facing this type of exposure. There are many of such examples, including ground-nesting bumble bees and wild solitary bees, spider wasps, larvae of predatory beetles or hoverflies. Many of those are important pollinators and natural predators, helping to control and reduce populations of pest insects. One extreme example of the threats posed by pesticides was the case of bees’ mass-dying occurred close to corn fields during sowing NNIs-treated seeds. That was due to acute intoxication via exposure to the dust clouds near sowing machines (Girolami et al. 2012). However, the effects of such sowing techniques on many other wild beneficial insects remains unknown.
Ongoing chemicalization of terrestrial ecosystems is one of the most severe danger for overall biodiversity and for resilience in important ecosystem services (ES), provided by insects. Insects are major agents for plant pollination, which is one of the most essential ES (Noriega et al. 2018). Indeed, the value pollination is widely accepted in financial, food security and health terms, as insect pollination services represent 9.5% of global crop production value (Gallai et al. 2009). Substantial loss of pollinators has been documented in many regions of the globe (Potts et al. 2010; Cameron et al. 2011; Lever et al. 2014). A horizon scan approach determined novel NNIs to be among six major issues of high priority, which will remain significant threats for pollinators in the nearest future (Brown et al. 2016).
Both commercial and wild pollinators such as honeybee, wild bees, bumblebees, beetles, wasps, ants and butterflies are in the high risk-zone. Honey bee (Apis mellifera) is the most studied non-target terrestrial invertebrate (Pisa et al. 2015). Because of the specific metabolic routes and target links to nervous system NNIs have direct effects on learning capacities, memory and behavior. Wild pollinators are also especially susceptible to neonicotinoid pesticides, which induce chronic sub lethal effects rather than acute toxicity (Hladik et al. 2018). Neonicotinoid pesticides are spreading from agricultural areas to neighboring wildflowers and make greater impact on wild pollinators than it was initially assumed (Botías et al. 2016). Because NNIs are systemic pesticides, pollinating insects are exposed to small amounts of insecticides each time, when they feed on pollen or nectar of treated plants (Godfray et al. 2014).
5.2 NNIs and Non-target Aquatic Organisms
Once the presence of NNIs in diverse aquatic ecosystems was confirmed, the question was whether the levels of the NNIs reported were a threat to the species living in these ecosystems. In order to comply with the protection of the aquatic environment and its organisms against chemical threats, threshold concentrations of NNIs were set by environmental agencies in different parts of the World. For imidacloprid, the threshold concentrations are as low as 0.23 µg L−1 for Canadian freshwaters (CCME 2007) and an annual average of 0.0083 µg L−1 for Dutch freshwaters (maximum acceptable concentrations of 0.2 µg L−1; RIVM 2014). In countless occasions these values are exceeded, with the consequent risk to populations of aquatic organisms.
The values for freshwater in The Netherlands are more complete, since they distinguish average and maximum concentrations. In the real environment, variable concentrations are usually found, especially in lotic water bodies near agricultural areas. Neonicotinoids may be detected in pulses after rain events in these water bodies (Hladik et al. 2014; Beketov and Liess 2008), due to their leaching potential. In lentic water bodies such as wetlands, low but more constant concentrations of NNIs may be the dominant trend (Maloney et al. 2018). Therefore, the patterns of exposure of organisms dwelling in lentic and lotic may be completely different (Raby et al. 2018a).
It has been proved that aquatic insects are more sensitive to NNIs than other taxa (Beketov et al. 2008; Raby et al. 2018a, b) in both laboratory and mesocosm experiments. Morrissey et al. (2015) comprehensively reviewed the toxicity of aquatic invertebrates to NNIs and determined that the orders Ephemeroptera (mayflies), Trichoptera (caddisflies) and Diptera (flies) are, in that order the most sensitive aquatic insects. The responses of different species over short or long-term studies may vary dramatically and a single pulse of one neonicotinoid can alter the abundance and taxa richness of invertebrates in streams (Beketov et al. 2008). Interestingly, recovery of some species of invertebrates occurs weeks after the contamination episode or under low concentrations, even after a pronounced initial decline (Beketov et al. 2008; Rico et al. 2018; Pickford et al. 2018). Multivoltine insects such as mayflies and chironomids can recover from stress episodes. The fact that these species have a short life cycle and produce several generations per year is an advantage compared to the univoltine species, which larvae develops at a slower pace (Beketov et al. 2008; Rico et al. 2018). Additionally, NNIs have been found to affect reproduction (Raby et al. 2018b) and metamorphosis (Raby et al. 2018b), for example ecdysis (Cavallaro et al. 2018) in emerging insects, which are key species in connecting aquatic and terrestrial ecosystems (Baxter et al. 2005), as we have suggested above.
At the molecular level, imidacloprid caused oxidative stress in the amphipod Gammarus fossarum (Malev et al. 2012). The concentrations at which the toxic effects were observed were over 100 µg L−1, what proves the decreased sensitivity in amphipods compared to insects.
Regarding to aquatic vertebrates, some signs of immuno- and genotoxicity have been reported in fish (Hong et al. 2018; Iturburu et al. 2017; Velisek and Stara 2018). Also, the responses of Wood frogs to predation were altered in frogs exposed to imidacloprid as tadpoles (Lee-Jenkins and Robinson 2018). However, the concentrations at which toxic effects are found are several times higher than the concentrations at which NNIs are toxic to insects. In addition, positive results are usually found at concentrations not environmentally relevant. However, Vieira et al. (2018) found a significant increase in DNA damage in erythrocytes of Prochilodus lineatus at 1.25 µg L−1 of imidacloprid and Velisek and Stara (2018) found changes in the levels of antioxidant enzymes in the early life stages of the common carp at 4.5 µg L−1.
It is plausible that NNIs are genotoxic or immunotoxic at high concentrations. However, this would not be evident in more sensitive organisms, as at high concentrations mortality would occur before any toxicity is observed at the molecular level. Although in organisms that are not as sensitive as e.g.: insects, NNIs may cause geno-, immunotoxicity or oxidative stress, the mechanisms and long-term consequences are still unknown.
6 Conclusions
Neonicotinoids are clear stressors of natural ecosystems. They are highly mobile insecticides that can be detected in areas where they were not applied to and in organisms that were not the target of the application. Although a complete knowledge of their fate in the environment is missing, authorities are starting to react to the threat they pose by limiting their use and application. Relevant improvements have been made in the field of the toxicity to non-target organisms with the aim of protecting natural ecosystems and pollination processes. However, testing the toxicity of NNIs at more environmentally relevant conditions is a priority for the complete assessment of the threats to terrestrial and aquatic ecosystems. Studies that include factors such as mixture toxicity (Kunce et al. 2015; Maloney et al. 2017, 2018; Cavallaro et al. 2018; Rico et al. 2018), field or semi-field exposures (Beketov et al. 2008; Cavallaro et al. 2018; Pickford et al. 2018) and pulse exposures (Beketov et al. 2008; Beketov and Liess 2008; Mohr et al. 2012; Raby et al. 2018a) are of great value and need to motivate the performance of new experiments. They may contribute to renew the existing risk assessment data on single compounds in laboratory conditions by expanding the analyses to multiple compounds and stressors.
References
Addy-Orduna LM, Brodeur JC, Mateo R (2019) Oral acute toxicity of imidacloprid, thiamethoxam and clothianidin in eared doves: a contribution for the risk assessment of neonicotinoids in birds. Sci Total Environ 650:1216–1223
Alford A, Krupke CH (2017) Translocation of the neonicotinoid seed treatment clothianidin in maize. PLoS ONE 12(3):e0173836
Baxter, CV Fausch, KD Saunders, WC (2005) Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshwater Biol 50 (2):201–220
Beketov MA, Liess M (2008) Acute and delayed effects of the neonicotinoid insecticide thiacloprid on seven freshwater arthropods. Environ Toxicol Chem 27(2):461–470
Beketov MA, Schafer RB, Marwitz A, Paschke A, Liess M (2008) Long-term stream invertebrate community alterations induced by the insecticide thiacloprid: effect concentrations and recovery dynamics. Sci Total Environ 405(1–3):96–108
Bishop CA, Moran AJ, Toshack MC, Elle E, Maisonneuve F, Elliott JE (2018) Hummingbirds and bumble bees exposed to neonicotinoid and organophosphate insecticides in the Fraser Valley, British Columbia. Canada Environ Toxicol Chem 37(8):2143–2152
Bonmatin J-M, Giorio V, Girolami D, Goulson D, Kreutzweiser DP, Krupke C, Liess M, Long E, Marzaro M, Mitchell EAD, Noome DA, Simon-Delso N, Tapparo A (2015) Environmental fate and exposure: neonicotinoids and fipronil 22 (1): 35-67
Botías C, David A, Hill EM, Goulson D (2016) Contamination of wild plants near neonicotinoid seed-treated crops, and implications for non-target insects. Sci Total Environ 566:269–278
Brown M et al (2016) A horizon scan of future threats and opportunities for pollinators and pollination. PeerJ 4:e2249
Butchart SHM, Walpole M, Collen B et al (2010) Global biodiversity: indicators of recent declines. Science: 1187512
BVL, Federal Office of Consumer Protection and Food Safety, 2008 Background information: bee losses caused by insecticidal seed treatment in Germany in 2008. https://www.bvl.bund.de/EN/08_PresseInfothek_engl/01_Presse_und_Hintergrundinformationen/2008_07_15_hi_Bienensterben_en.html Last accessed 30.10.2018
Bradford BZ, Huseth AS, Groves RL (2018) Widespread detections of neonicotinoid contaminants in central Wisconsin groundwater. PLoS ONE 13(10):e0201753
Byholm P, Mäkeläinen S, Santangeli A, Goulson D (2018) First evidence of neonicotinoid residues in a long-distance migratory raptor, the European honey buzzard (Pernis apivorus). Sci Total Environ 639:929–933
Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, Griswold TL (2011) Patterns of widespread decline in North American bumblebees. PNAS 108(2):662–667
Canadian Council of Ministers of the Environment. 2007 Canadian water quality guidelines for the protection of aquatic life: imidacloprid. In: Canadian environmental quality guidelines, 1999, Canadian Council of Ministers of the Environment, Winnipeg
Cavallaro MC, Liber K, Headley JV, Peru KM, Morrissey CA (2018) Community-level and phenological responses of emerging aquatic insects exposed to 3 neonicotinoid insecticides: an in situ wetland limnocorral approach. Environ Toxicol Chem 37(9):2401–2412
Delfino Vieira CE, Perez MR, Acayaba RD, Montagner Raimundo CC, dos Reis Martinez CB (2018) DNA damage and oxidative stress induced by imidacloprid exposure in different tissues of the Neotropical fish Prochilodus lineatus. Chemosphere 195:125–134
European Food Safety Authority (EFSA) (2012) Statement on the findings in recent studies investigating sub-lethal effects in bees of some neonicotinoids in consideration of the uses currently authorised in Europe. EFSA J 10(6):2752
European Food Safety Authority (EFSA) (2013a) Conclusion on the peer review of the pesticide risk assessment for bees for the active substance clothianidin. EFSA J 11(1):3066
European Food Safety Authority (EFSA) (2013b) Conclusion on the peer review of the pesticide risk assessment for bees for the active substance thiamethoxam. EFSA J 11(1):3067
European Food Safety Authority (EFSA) (2013c) Conclusion on the peer review of the pesticide risk assessment for bees for the active substance imidacloprid. EFSA J 11(1):3068
European Food Safety Authority (EFSA) (2018a) Evaluation of the emergency authorisations granted by Member State Finland for plant protection products containing clothianidin or thiamethoxam. EFSA supporting publication 2018: EN-1419, 13 pp. https://doi.org/10.2903/sp.efsa.2018.en-1419
European Food Safety Authority (EFSA) (2018b) Evaluation of the emergency authorisations granted by Member State Romania for plant protection products containing clothianidin, imidacloprid or thiamethoxam. EFSA supporting publication 2018: EN-1416, 16 pp. https://doi.org/10.2903/sp.efsa.2018.en-1416
EFSA Panel on Plant Protection Products and their Residues (PPR) (2012) Scientific opinion on the science behind the development of a risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J 10 (5):2668
Eng ML, Stutchbury BJM, Morrissey CA (2017) Imidacloprid and chlorpyrifos insecticides impair migratory ability in a seed-eating songbird. Sci Rep 7(1):15176
European Commission, Directorate General Joint Research Centre. Institute for Environment and Sustainability/H01-Water Resources Unit (2015) Development of the first watch list under the environmental quality standards directive. Publications Office of the European Union, Luxembourg, 166 pp. https://doi.org/10.2788/101376
Gallai N, Salles JM, Settele J et al (2009) Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econom 68:810–821
Gibbons D, Morrissey C, Mineau P (2015) A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ Sci Pollut Res 22(1):103–118
Girolami V, Marzaro M, Vivan L, Mazzon L, Greatti M, Giorio C, Marton D, Taparro A (2012) Fatal powdering of bees in flight with particulates of neonicotinoids seed coating and humidity implication. J Appl Entomol 136:17–26
Godfray HCJ, Blacquiere T, Field LM, Hails RS, Petrokofsky G, Potts SG, Raine NE, Vanbergen AJ, McLean AR (2014) A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc Royal Soc B 281:20140558
Goulson D (2013) Review: an overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol 50(4):977–987
Grossman GM, Kruger AB (1995) Economic growth and the environment. Q J Econ 110 (2,1):353–377
Hallmann CA, Foppen RPB, Turnhout CAM, van Kroon HD, Jongejans E (2014) Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511:7509
Hladik ML, Kolpin DW, Kuivila KM (2014) Widespread occurrence of neonicotinoid insecticides in streams in a high corn and soybean producing region, USA. Environ Pollut 193:189–196
Hladik ML, Main AR, Goulson D (2018) Environmental risks and challenges associated with neonicotinoid insecticides. Environ Sci Technol 52(6):3329–3335
Hong X, Zhao X, Tian X, Li J, Zha J (2018) Changes of hematological and biochemical parameters revealed genotoxicity and immunotoxicity of neonicotinoids on Chinese rare minnows (Gobiocypris rarus). Environ Pollut 233:862–871
Hooper DU, Adair EC, Cardinale BJ et al (2012) A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105–108
Huseth AS, Groves RL (2014) Environmental fate of soil applied neonicotinoid insecticides in an irrigated potato agroecosystem. PLoS ONE 9(5):e97081
Iturburu FG, Zoemisch M, Panzeri AM, Crupkin AC, Contardo-Jara V, Pflugmacher S, Menone ML (2017) Uptake, distribution in different tissues, and genotoxicity of imidacloprid in the freshwater fish Australoheros facetus. Environ Toxicol Chem 36(3):699–708
Iyaniwura TT (1991) Non-target and environmental hazards of pesticides. Rev Environ Health 9(3):161–176
Jeschke P, Nauen R (2008) Neonicotinoids—from ziro to hero in insecticide chemistry. Pest Manage Sci 64(11):1084–1098
Kunce W, Josefsson S, Örberg J, Johansson F (2015) Combination effects of pyrethroids and neonicotinoids on development and survival of Chironomus riparius. Ecotoxicol Environ Saf 122:426–431
Lee-Jenkins SSY, Robinson SA (2018) Effects of neonicotinoids on putative escape behavior of juvenile wood frogs (Lithobates sylvaticus) chronically exposed as tadpoles. Environ Toxicol Chem 9999:1–9
Lever JJ, van Nes EH, Scheffer M, Boscompte J (2014) The sudden collapse of the pollinator communities. Ecol Lett 17:350–359
Likens GE, Bormann FH (1974) Linkages between terrestrial and aquatic ecosystems. BioScience 24(8):447–456
Main AR, Michel NL, Cavallaro MC, Headley JV, Peru KM, Morrissey CA (2016) Snowmelt transport of neonicotinoid insecticides to Canadian Prairie wetlands. Agric Ecosyst Environ 215:76–84
Malev O, Klobučar RS, Fabbretti E, Trebše P (2012) Comparative toxicity of imidacloprid and its transformation product 6-chloronicotinic acid to non-target aquatic organisms: microalgae desmodesmus subspicatus and amphipod gammarus fossarum. Pestic Biochem Physiol 104(3):178–186
Maloney EM, Morrissey CA, Headley JV, Peru KM, Liber K (2018) Can chronic exposure to imidacloprid, clothianidin, and thiamethoxam mixtures exert greater than additive toxicity in Chironomus dilutus? Ecotoxicol Environ Saf 156:354–365
Maloney EM, Morrissey CA, Headley JV, Peru KM, Liber K (2017) Cumulative toxicity of neonicotinoid insecticide mixtures to Chironomus dilutus under acute exposure scenarios. Environ Toxicol Chem 36(11):3091–3101
Matsuda K, Buchingham SD, Kleier D, Rauh JJ, Grauso M, Satelle DB (2001) Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci 22(11):573–580
Mohr S, Berghahn R, Schmiediche R, Hübner V, Loth S, Feibicke M, Mailahn W, Wogram J (2012) Macroinvertebrate community response to repeated short-term pulses of the insecticide imidacloprid. Aquat Toxicol 110–111:25–36
Morrissey CA, Mineau P, Devries JH, Sanchez-Bayo F, Liess M, Cavallaro MC, Liber K (2015) Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ Int 74:291–303
Noriega JA, Hortal J, Azcarate FM et al (2018) Research trends in ecosystem services provided by insects. Basic Appl Ecol 26:8–23
Noyes PD, McElwee MK, Miller HD, Clark BW, Van Tiem LA, Walcott KC, Erwin KN, Levin ED (2009) The toxicology of climate change: environmental contaminants in a warming world. Environ Int 35(6):971–986
Pickford DB, Finnegan MC, Baxter LR, Bohmer W, Hanson ML, Stegger P, Hommen U, Hoekstra PF, Hamer M (2018) Response of the mayfly (Cloeon dipterum) to chronic exposure to thiamethoxam in outdoor mesocosms. Environ Toxicol Chem 37(4):1040–1050
Pisa LW, Amaral-Rogers V, Belzunces LP et al (2015) Effects of neonicotinoids and fipronil on non-target invertebrates. Environ Sci Pollut Res Int 22:68–102
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25(6):345–353
Raby M, Nowierski M, Perlov D, Zhao X, Hao C, Poirier DG, Sibley PK (2018a) Acute toxicity of 6 neonicotinoid insecticides to freshwater invertebrates. Environ Toxicol Chem 37(5):1430–1445
Raby M, Zhao X, Hao C, Poirier DG, Sibley PK (2018b) Chronic toxicity of 6 neonicotinoid insecticides to Chironomus dilutus and Neocloeon triangulifer. Environ Toxicol Chem 37(10):2727–2739
Rico A, Arenas-Sanchez A, Pasqualini J, Garcia-Astillero A, Cherta L, Nozal L, Vighi M (2018) Effects of imidacloprid and a neonicotinoid mixture on aquatic invertebrate communities under Mediterranean conditions. Aquat Toxicol 204:130–143
RIVM (2014) Water quality standards for imidacloprid: proposal for an update according to the water framework directive. In: Smit CE (ed) National institute for public health and the environment. Bilthoven, Netherlands
Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Furlan L, Gibbons DW et al (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res 22 (1):5–34
Sur R, Stork A (2003) Uptake, translocation and metabolism of imidacloprid in plants. Bullet Insectol 56(1):35–40
Taliansky-Chamudis A, Gómez-Ramírez P, León-Ortega M, García-Fernández AJ (2017) Validation of a QuECheRS method for analysis of neonicotinoids in small volumes of blood and assessment of exposure in Eurasian eagle owl (Bubo bubo) nestlings. Sci Total Environ 595:93–100
Tan K, Chen W, Dong S, Liu X, Wang Y, Nieh JC (2014) Imidacloprid alters foraging and decreases bee avoidance of predators. PLoS ONE 9(7):e102725
Tomizawa M, Casida JE (2011) Neonicotinoid insecticides: highlights of a symposium on strategic molecular designs. J Agric Food Chem 59(7):2883–2886
Turaga U, Peper ST, Dunham NR, Kumar N, Kistler W, Almas S, Presley SM, Kendall RJ (2016) A survey of neonicotinoid use and potential exposure to northern bobwhite (Colinus virginianus) and scaled quail (Callipepla squamata) in the Rolling Plains of Texas and Oklahoma. Environ Toxicol Chem 35(6):1511–1515
United States Environmental Protection Agency (USEPA) (1992) Memorandum NTN 33893 Data Submissions for pending registration, DP Barcode #D182987. USEPA Archive Document
Velisek J, Stara A (2018) Effect of thiacloprid on early life stages of common carp (Cyprinus carpio). Chemosphere 194:481–487
Wood TJ, Goulson D (2017) The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ Sci Pollut Res Int 24(21):17285–17325
Yu SJ (2015) Classification of insecticides. The toxicology and biochemistry of insects, 2nd edn. CRC Press, Boca Raton, Florida, USA, pp 31–102
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Carrasco-Navarro, V., Skaldina, O. (2019). Contamination Links Between Terrestrial and Aquatic Ecosystems: The Neonicotinoid Case. In: Kesari, K. (eds) Networking of Mutagens in Environmental Toxicology. Environmental Science and Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-319-96511-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-96511-6_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-96510-9
Online ISBN: 978-3-319-96511-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)