Abstract
In last few years, cancer became one of the leading cause of death in humans. There are several factors associated with the cancer initiation and progression including heavy metals. Several heavy metals including arsenic, cadmium, uranium, lead, mercury etc. and heavy metal-containing compounds are toxic to the humans and have been reported to induce mutations in human genome which further leads to the carcinogenesis. This chapter provides the detail understanding of molecular mechanisms and pathway analysis to heavy metal toxicity in human carcinogenesis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Heavy metal is simple collaboration of two entirely different words i.e. heavy and metal or we can say the metals which are heavy. The definition says that density of metal is high but actually this physical quantity is quite useless in the cases of plants and other living organisms (Jaishankar et al. 2014). Plants and living organisms do not deal with metals or we can say that these are not accessible to them in their elemental form (Jaishankar et al. 2014).
If we want to define these two words then heavy means something that have weight and metals means substances or elements which can conduct heat and electricity and have properties like ductility, malleability and luster (Aziz et al. 2008; Florea et al. 2012). The metals having a property i.e. temperature dependent conductivity which differentiate it from non-metals and metalloids (Jaishankar et al. 2014). Therefore heavy metals can simply be defined as elements those have a density i.e. 5 times greater than the specific density of water. The specific gravity of water is found to be 1 at a temperature of 4 °C (39 °F) (Ilyin et al. 2004). Well, we can describe specific density as a ratio in between the density of any substances to the density of some other substances considered as standard under specified conditions of pressure and temperature.
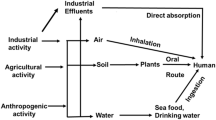
In case of liquids and solids we can consider water as standard and in case of gases hydrogen or air is considered as a standard (Aziz et al. 2008; Brochin et al. 2008). It is symbolized as sp.gr. This quantity is dimensionless and therefore not expressed in units. The heavy metals are chemical components or elements that are mostly found in the earth crust (Järup 2003; Mamtani et al. 2011. There are a specific place and description of each and every heavy metal in the periodic table. It is believed that there must be a correlation in between “toxicity” and “heaviness” (Fig. 1).
Human beings are blessed with power to understand things in a far better way than other living organisms. Humans, instead of using this power as an asset they are deteriorating and destroying the environment in which they live. Modernization has involved the use of toxic metals more than its limit value which is being causing a variety of health hazards (Kim et al. 2015).
There are basically two types of Health Hazards: (1) Hazards associated with most target organ effects, (2) Hazards associated with cancer and mutagenic effects. Inappropriate and over exploitation of our resources has a big hand in problems caused due to heavy metals (Kim et al. 2015). Various heavy metals like cadmium, mercury, arsenic, lead, etc. are being recklessly used in manufacture of items we use in our daily lives. In addition to this, the food we eat insecticides which contains Arsenic, mercury etc. chemicals in high amounts (Kawada 2016). Cadmium, a heavy metal is known to cause endometrial cancer (Mazariegos et al. 2010). Almost every heavy metal can cause cancer. Among these heavy metals few are needed by our body for good metabolism and possess various other functions in our body. Reactive oxygen species (ROS) causes oxidative stress that is proven to be a reason behind most of diseases caused by heavy metals (Grimsrud and Peto 2006). Cadmium, nickel, chromium and arsenic fall in 1st category according to International Agency for Research on Cancer (Su et al. 2007). Several reports show that vulnerability to these heavy metals causes interference in tumor suppressor gene expression, ruins repair processes and enzymatic agitated in metabolism by oxidative harm (Zhang et al. 2007). Screening our soil with these harmful heavy metals can contaminate the vegetation and can result in oral cancer. The revolution of heavy metals in our bio-system results in concentration of high amount of toxic metal (Wen et al. 2005). Today presence of tremendous amount of biological data is an outcome of the increased attention towards heavy metal and its carcinogenetic impact on health. So the data mining is very essential method and can be counted into our major concern (Lee et al. 2016). Chemical/Gene peculiar pathways that are actually very complex can be understood with the help of the pathways studio databases as it dispense drawings for the pathways by using data that are collected from variety of sources (Yuan et al. 2011). We can use and analyze the pathways because it can give a better and more comparative view on carcinogenesis, diseases and marker proteins that are related with heavy metals (Khatri et al. 2012). Further, more coordinate network between marker proteins and cell forms adds to the expectation of carcinogenesis particular protein markers. Damages/Harm or hazards that are instigated by metal could be prevented and detoxified by engaging different inter-cellular chelation procedures and cancer prevention or anti-oxidants (Lobo et al. 2010). Resistance against metal poisoning is developed by combing metal ions with the phytochelatins (phytochelatins known as the chelating agents in plants). Oxidative damages are overcome by the complex formed when molecules of anti-oxidants interacts with free radicals (“and dusts,” n.d.). Utilization of phytochemicals from cell reinforcement substances from plants can aid the cell reinforcement related detoxification processes.
2 Why It Is Important to Discuss Metal Toxicity?
Heavy metals can be further categorized into beneficial heavy metals and toxic heavy metals. Here, we lay our focus on the toxic heavy metals since they are of much concern for us (Hodson 2004; Jan et al. 2015; Patil et al. 2013). Out of 35 metals that are needed to be concerned, 23 are heavy metals small amounts of these metals are essential for good health but large intake is hazardous and can cause acute or chronic toxicity. Excessive intake causes damage to mental and central nervous function, lower energy levels, damaged blood composition, lungs, kidneys, liver and other vital organs (Arif et al. 2016; Singh et al. 2011). Long-term exposure to such metals can result in-slow progression in cancer, physical muscular and neurological degenerative processes, Alzheimer’s disease, Parkinson’s disease, muscular dystrophy, multiple sclerosis etc.
3 Heavy Metals not Associated with Cancer
As earlier stated heavy in small quantities are very essential and beneficial for health. They exist in various compounds and unimolecular forms and in various food stuffs.
They have various medical and industrial applications
-
1.
Indirect injection of gallium in the radiological procedure
-
2.
In x-ray equipment ‘lead’ is used as a radiation shield.
-
3.
In manufacturing pesticides, batteries, alloys, electroplated metal parts, textile dyes etc.
-
4.
They also constitute important role in products used in our homes.
4 Toxic Heavy Metals
Heavy metals when not metabolized act as toxic heavy metals resulting in their accumulation in the soft tissues (Goyer et al. 2004). Their intake/pathways into the human body are through food, water, air, or adsorption by the skin in case of pharmaceutical or industrial setting (Barra et al. 2006; Mamtani et al. 2011. Their intake varies to different age groups such that adults develop metal toxicity generally through Industrial Exposure whereas in children it develops by eating toxic metals (Prüss-Ustün et al. 2011; Village 2005) in form of some substance or also from hand to mouth activity of small children when they touch dirty and contaminated soils etc.
Other common routes of exposure to toxicity are during a radiological procedure, from inappropriate doing during parental nutrition, from parental nutrition, from broken thermometers. People residing in older homes with lead painted or old plumbing develop contamination (Length 2007; Morin et al. 2007; OEHHA 2001). Inhalation or skin contact with dust, fumes or vapor causes acute poisoning (Kazemipour et al. 2008; Sankhla et al. 2016). The agency for toxic substances and disease registry in Atlanta, Georgia in cooperation with U. S. Environmental Protection Agency has compiled a priority list named “The top 20 hazardous substances” in 2001. The heavy metals 1 Arsenic, 2 Lead, 3 Mercury, 7 Cadmium occur in this list.
4.1 Arsenic
Chronic exposure to Arsenic can result in damage to central nervous system, excessive skin darkening (hyperpigmentation) in areas not exposed to sunlight, excessive formation of skin on soles and palms (hyperkeratosis), or white bands of arsenic deposits across the fingernails (visible after 4–5 weeks of exposure) (Tchounwou et al. 2004). Cardiovascular changes are often diagnosed earlier which later can lead to cardiovascular collapse (Chioma et al. 2017; Soignet et al. 2001). Table 1 summarizing the sources of arsenic and associated factors.
4.2 Lead
Chronic exposure to lead leads to birth defects, mental retardation, autism, psychosis, allergies, dyslexia, hyperactivity, weight loss, shaky hands and muscular weakness (Stohs and Bagchi 1995; Tchounwou et al. 2012a, b). Children are often sensitive to lead toxicity. Symptoms of chronic lead exposures include—colic, allergies, autism, hyperactivity, mood swings, nausea, numbness, and lack of concentration (Kaul et al. 1999; Yedjou et al. 2010). Table 2 summarizng the sources of lead and associated factors.
4.3 Mercury
Mercury exists in three forms: Elemental mercury, organic and inorganic mercury.
Liquid mercury is more likely to be ingested by the children because of its beautiful colors and unique behavior when spilled. Common sources of liquid mercury are broken thermometer, or drinking medicine containing mercury (Rooney 2007). Chronic exposure to mercury can cause permanent problems to central nervous system and kidneys. Mercury is capable of entering into the placenta and accumulation of which can cause, mental retardation, brain damage, blindness, cerebral palsy, inability to speak. Table 3 summarizng the sources of mercury and associated factors.
4.4 Cadmium
Chronic exposure to cadmium causes hazardous disease resulting in chronic obstructive lung diseases renal disease and fragile bones. It has been concluded by various research studies especially performed on Egyptian females that in case of breast cancer the levels of cadmium and copper were found to be increased and levels of iron was reduced. Table 4 summarizng the sources of cadmium and associated factors.
4.5 Aluminum
Aluminum studies show that long-term exposure to aluminum could be a reason for developing Alzheimer’s disease since the Alzheimer’s patients were found with the significant amount of aluminum in their brain tissues (Al-fartusie and Mohssan 2017). Aluminum-based coagulants are also being used for water purification which has balanced its potential health concerns to much extent. Table 5 summarizng the sources of aluminum and associated factors.
4.6 Iron
Chronic overdose of iron causes its deposit in the heart which may cause death due to myocardial siderosis. Table 6 summarizng the sources of iron and associated factors. The high amount of ingestion of iron causes cellular toxicity and impaired oxidative phosphorylation and mitochondrial dysfunction resulting in cellular death (Griswold and Martin 2009).
4.7 Copper
Chronic exposure of copper causes damage to liver and kidney and destroys RBC’s. Acute (short term) effects of copper causes temporary gastrointestinal distress. Though copper is essential for body as it helps fight anemia and necessary for normal metabolic functions in Human. Deficiency of copper causes low numbers of white blood cells, osteoporosis in infants and children, and defects in connective tissue leading to skeletal problems. Table 7 summarizng the sources of copper and associated factors.
4.8 Nickel
Chronic Exposure to nickel causes lung cancer, nose cancer, larynx cancer and prostate cancer. Exposure to nickel and compounds containing nickel causes dermatitis known as “nickel itch” to sensitive people (Zofkova et al. 2017). Nickel is a micronutrient and essential for health. Possible sources for exposure of nickel to human are air we breathe, drinking water, eating food (vegetables contain nickel) or smoking cigarettes. Inhalation of nickel gas causes respiratory failure, lung embolism and chronic bronchitis. Table 8 summarizng the sources of nickel and associated factors.
4.9 Tin
Large amount of consumption of inorganic tin causes stomachaches, anemia, liver and kidney problems. Chronic poisoning to tin can result in neurological problems. Acute exposure can cause skin and eye irritation, respiratory irritation and gastrointestinal effect. High amount ingestion can be fatal. Table 9 summarizng the sources of tin and associated factors.
4.10 Uranium
Uranium (U) is the heaviest radioactive metal which occurs naturally in the environment. It has an atomic number, A, of 92. It is one of the actinide series elements which is well documented, extensively studied and highly explored by human beings. Its physical properties are given in Table 10. Natural uranium (Nat U) is a mildly radioactive element consists of three (radio) isotopes namely U-238, U-235 and U-234, which exist in almost a secular equilibrium with each other. It emanates mainly Alpha particles, beta particles and gamma rays. The most relevant isotopes and their half-lives are provided in Table 11 and the level of contamination of uranium in ground water in many countries given in Table 12. It is known that Alpha particles, which carry massive energy of 4–8 meV (Mega electron volts), pose almost no external hazard i.e. when it is present outside of the body. That is because its range in air is only a few centimeters and it can be stopped even by a piece of sheet. Alpha cannot even penetrate the dead layer of our skin. However, when ingested through water or food can cause of a lot of internal damage to the soft tissues.
United States Environmental Protection Agency (USEPA) has classified uranium as a confirmed human carcinogen (Group A) and has published guidelines to enforcement agencies to follow a Zero tolerance for its presence in drinking water, with a maximum permissible limit of 30 ppb. World Health Organization (WHO) has published a set of reports in which it has emphasized that limits of uranium in drinking water should be less than 15 ppb, which it later in 2011 changed to 30 ppb. In India Atomic Energy Regulatory Board (AERB) has fixed maximum permissible limits of 60 ppb (AERB 2004).
Since uranium it is a naturally occurring radionuclide with an estimated half-life of millions of year, it is present in varying proportions in Earth’s crust, seawater, surface waters, groundwater, plants and animals. While its concentration is reported around 3 ppb (part per billion or µg/L) in different water bodies, including seawater, it occurs in Earth’s crust at an average worldwide level of around 3 ppm (Hu and Gao 2008). As it is a naturally occurring and ubiquitously present radioactive element, it ingestion from food, drinking water and inadvertently from soil is a regular phenomenon. It has been estimated that an average adult human ingests (intakes) 1–2 µg/day of uranium from food and 1.5 µg/day from drinking water (Konietzka 2015; Singh et al. 1990). One gram of natural uranium having this relative isotopic abundance has an activity of 0.69 µCi (or 25,530 Bq as 1 Ci = 3.7 × 1,010 Bq). Of this 49.0% of the activity is attributable to U-234, 2.27% of the activity is attributable to U-235, and 48.7% of the activity is attributable to U-238 (Fig. 2).
Uranium when inhaled or ingested through air or food or water gets rapidly eliminated from the body. The maximum absorption in case of inhalation is 1–5% through lungs and in case of ingestion it is around 0.5–5%. Majority of inhaled uranium gets cleared from the lungs via mucociliary action or through fecal excretion of the swallowed sputum, however a very small portion of uranium may reside in the lungs for years. Ingested uranium (only 0.5–5%), on the other hand, gets absorbed in the blood from where it gets distributed initially to soft tissues and lymph nodes and then finally to kidney, liver and bone. Overall, most ingested uranium is excreted in feces and remainder in urine (Radespiel-Tröger and Meyer 2013).
Uranium, being an actinide series element can exist in +II, +III, +IV, +V and +VI oxidation states, however oxidation states of +IV and +VI are the ones which form a range of complex compounds in the environment and the only states which are abundant enough to study. The U(VI) oxidation states are mainly water-soluble compounds while +IV are otherwise and are abundantly found in soils and rocks. U(IV) is rather insoluble and exists in complex forms with inorganic ligands e.g. fluoride, chloride, sulphate and phosphate. U(VI), as uranyl (UO2++) complex is abundant in wet soils and water mediums (Gómez et al. 2006).
In 20th century which lead to discovery of fission of uranium, to produce vast amount of energy trapped in the nucleus, has led to slight increase in worldwide concentration of fallout uranium. Extensive uranium mining, atmospheric nuclear tests, nuclear fuel recycling, use of depleted uranium in armours and waste disposal are some of the anthropogenic sources which cause increase in Uranium content of the environment accessible to human beings.
Apart from its presence in food, uranium gets inside human body through its presence in groundwater which is major source of drinking water worldwide. Slightly elevated levels of uranium in has been reported from all over the world including India. The reasons for elevated levels were previously attributed to leaching from soil due to excessive use of fertilizers or from fly-ashes produced from Thermal Power Plants operating on coal, which may be a case in few locations (Bajwa et al. 2017; Brindha and Elango 2013; Efstathiou et al. 2014; Liesch et al. 2015). However, recent developments and studies have found the source of uranium may be more of geogenic origin than anthropogenic (Liesch et al. 2015).
One of the peculiar phenomenon which occurs during the decay series of U-238 is production of a volatile gaseous daughter products, Radon-222 (Rn-222), which a half-life of 3.82 days. Rn-222 then diffuses out from the rocks, soil or aquifer sources to the atmosphere, dissolves in groundwater or sometime to crevices present in the nearby vicinity and the decays to its subsequent daughter products (Gokhale 2008). Although the half-life is in days, it is always found present in the environment as it is being continuously produced due to decay of natural uranium. Rn-222 is an alpha emitter with an energy of 5.5 meV and it is one the major cause of lung cancer in non-smokers. Rn-222 causes thousands of deaths worldwide because of its inhalation from milling, mining and cement and concrete materials which have elevated levels of natural uranium in it (Samet 2011). International Atomic Energy Agency (IAEA) has given a threshold limit for annual activity concentration of Rn-222 as 1,000 Bq/m3 for building with high occupancy factor (IAEA 2015).
5 Methodologies for Radiological and Chemotoxic Risk Assessment of Uranium
Human health effects due to exposure of uranium can be classified as radiological risk (ionizing radiation effects of uranium isotopes) and as chemotoxic risk being a heavy metal. The radiological risk factor can be evaluated based on the general USEPA standard method (Hartmann et al. 2000). Using the risk factor and uranium level in subsurface water, the excess cancer risk which an average individual faces due to presence of uranium in drinking water can be calculated from below given equations (Kumar et al. 2011).
where,
- Risk coefficient (RC):
-
1.19 × 10−9 Bq,
- Water Ingestion Rate (WIR):
-
4.05 L/day,
- Total Exposure Duration:
-
Avg. Life Expectancy (India, 63.7 years) × 365 = 23,250 days.
where,
- Uranium concentration (Bq/L):
-
Measured value (μg/L) × conversion factor (0.025 Bq/μg).
The chemotoxic risk can similarly be calculated based on the hazard quotient (HQ) and chemical toxicity risk in the form of Lifetime Average Daily Dose (LADD) were calculated through ingestion of groundwater by the following formula
where,
- Ci:
-
Concentration of U in subsurface water (μg/L),
- IR:
-
Ingestion rate (L/day),
- EF:
-
Exposure frequency (days/year),
- LE:
-
Life expectancy (years),
- AT:
-
Average Time (days),
- BW:
-
Bodyweight (kg),
- RfD:
-
Reference Dose (μg/kg/day),
- LADD:
-
Lifetime average daily dose, (μg/kg/day).
6 Clinical Effects of Various Toxic Metals
Arsenic: Arsenic causes perforation of nasal septum, respiratory cancer, peripheral neuropathy, dermatomes and skin cancer.
Cadmium: Cadmium causes proteinuria, glucosuria, osteomalacia, aminoaciduria, emphysemia.
Chromium: Chromium causes ulcer, perforation of nasal septum, respiratory cancer.
Manganese: Central and peripheral neuropathies.
Lead: Lead causes encephalopathy, peripheral neuropathy, Central nervous disorders, and anemia.
Nickel: Nickel causes cancer and dramatis.
Tin: Tin causes central nervous system disorders, visual defects and EEG changes and Pneumoconiosis.
Mercury: Mercury causes proteinuria.
7 Mechanism of Toxicity and Carcinogenicity of Some Specific Heavy Metals
7.1 Chromium
Main factors that determine the toxicity of chromium compounds are oxidation state and solubility. Chromium (VI) compounds are considered more toxic and irritation and corrosion. They are also better oxidizing agents (Mamtani et al. 2011; Dayan and Paine 2001). Inspite of the fact that biological mechanisms are not known, but the level of toxicity of various states of chromium can be explained as the more easily Cr(VI) can pass through cell membranes and further intracellular reduction to reactive intermediates (Adenocarcinoma et al. 2014). Therefore Cr(VI) is more toxic than Cr(III) i.e. poorly absorbed by any route.
Cr(VI) to Cr(III) extracellularly can help reducing in toxic effects of chromium. Cr(VI) form can be soaked up by the gastrointestinal tract, lungs and even up to certain extent by skin (Chioma et al. 2017; Odewabi and Ekor 2017; Stohs and Bagchi 1995). Cr(VI) can be reduced under physiological conditions by hydrogen peroxide (H2O2), glutathione (GSH) reductase, ascorbic acid and GSH to form reactive intermediates which includes Cr(V), Cr(IV), thiylradicals, hydroxyl radicals, and ultimately, Cr(III). Any of these species can attack/strike DNA, proteins, membrane lipids henceforth in disturbing cellular integrity and function (O’Brien et al. 2003).
7.1.1 Carcinogenesis Due to Chromium
According a report of epidemiological investigations workers are found suffering from respiratory cancer due to exposure to Cr(VI) containing compounds in their occupational environment (Clarkson 1993; O’Brien et al. 2003; Tchounwou et al. 2012a, b). Oxidative damage is considered as hidden cause of the genotoxic effects which include chromosomal abnormalities and DNA stand breaks (Dayan and Paine 2001) (Fig. 3). However recent studies show a biological relevance of non-oxidative mechanisms in Cr(VI) carcinogenesis. Carcinogenicity seems to be linked with inhalation of less soluble ore insoluble Cr(VI) compounds. Cr(VI) isn’t toxic in its elemental form. Toxicity shows a vast variation to different Cr(VI) compounds (Clarkson 1993; Dayan and Paine 2001). Epidemiological evidence state Cr(VI) as a factor in Carcinogenesis (Goulart et al. 2005).
The figure is obtained from KEGG databases showing mechanism of Carcinogenesis induced by chromium metal. The oxidised and reduced forms of chromium metal act as a genotoxic carcinogens which leads to the formation of a DNA adduct and non genotoxic carcinogens which activate a transcription factor that causes DNA damage respectively. Further altogether these processes results into NSCLC and SCLC
Solubility and other properties of chromium such as size, crystal modification, surface charge and the ability to be phagocytized could be significant in determining cancer risk (IARC 2006; Yamashoji and Isshiki 2001). Hypothetical concepts have been proposed to explain the carcinogenicity of chromium and its salts, although there have some issues from initial when discussing metal carcinogenesis because its different compounds have different potencies. Due to exposure of multiple chemicals in the industries thus it would be hard to conclude the carcinogenicity from any single metal (Browning et al. 2014; Duffus 2002). Hence carcinogenic risk is said to be caused not because of any single metal. Thus, carcinogenic risk often can be said to cause not because of any single but due to some group of metals.
7.2 Arsenic
Solubility, oxidation state as well as several other extrinsic and intrinsic parameters strongly affects the toxicity. In case of Arsenic toxicity these factors in addition with many other factors that are reported by several research conducts lie frequency and time-period, exposure dose, gender and age, the biological species, along with genetic and nutritional parameters and person susceptibilities play a key role in regulating the toxicity levels (Puccetti et al. 2000). Exposure to inorganic As (arsenic) can be considered as a reason for a large number of human toxicity caused by the Arsenic.
In comparison to pentavalent arsenate As(V), the inorganic trivalent arsenite As(III) possess 2–10 times more toxicity (Hong et al. 2014; Velma and Tchounwou 2011). The As(III) attacks on the sulfhydryl groups or thiol of the protein and form a complex with them. By this way it can stop the activity of more than 200 enzymes (Jhaa et al. 1992). This mechanism is mainly responsible for the arsenic toxicity, effects of which can widely observed on various organ systems (Dong 2002; Mumtaz et al. 2002; Walker et al. 2010). Whereas pentavalent arsenate As(V) induces an exchange of phosphate group which in implicated in various biochemical pathways (Basu et al. 2001; Length 2007; Mamtani et al. 2011. The uncoupling of oxidative phosphorylation and the suppression of many mitochondrial enzymes causes impairment of cellular respiration through this mechanism arsenic impart its toxic effects (Fig. 4).
The obtained figure is from KEGG database showing mechanism of carcinogenesis induced by arsenic metal. The reduction and oxidation phenomena of arsenic metal is responsible for the activation of lipid transcription factor and formation of two products i.e. monomethylarsonic acid and dimethylarsinic acid respectively that causes DNA damage and finally leads to the occurrence of different types of skin, lung and liver cancers
Enzymes like thiolase and dihydrolipoyl dehydrogenase becomes inactive when sulfhydryl groups of protein react with arsenic as result of which the processes of oxidation and beta oxidation of pyruvate and fatty acids respectively gets inhibited. In humans methylation is main metabolic pathway for inorganic arsenic (Basu et al. 2001; Sankhla et al. 2016; Tchounwou et al. 2004). Through a Non-enzymatic process, the Arsenic trioxide gets methylated to two main metabolites. Firstly it gets converted into monomethylarsonic acid (MMA) and before the discharge in the Urine it again enzymatically methylated to dimethyl arsenic acid (DMA) (Chioma et al. 2017; Soignet et al. 2001). According to recent research it has been found that methylater metabolites can be more hazardous in comparison to arsenite if they possess arsenic trivalent forms (Stevens et al. 2010; Takahashi et al. 2002). Arsenic compounds have ability that they can restrict the process of DNA repair, can bring out chromosomal aberrations, replacement in between sister-chromatid and also causes organization of micronuclei in both rodent cells in culture and humans and in exposed human cells (Khoury et al. 2015; Liu et al. 1996; Odewabi and Ekor 2017; Verin et al. 1998).
7.3 Cadmium
Cadmium is an extreme pneumonic and gastrointestinal aggregation, which can be lethal if breathed in or ingested (Chioma et al. 2017; Zhang et al. 2004). Side-effects like muscle cramps, vertigo, stomach pain, burning sensations, spewing, sickness (nausea), lack of consciousness, shock and convulsions are typically observed within 15–30 min if taken in small amounts. It’s consumption or intake in small amount can also lead to problem like disintegration of gastrointestinal tract, hepatic or renal, pneumatic damage and chronic unconsciousness i.e. coma and is totally based upon the course (routes) of poisoning (Kippler et al. 2012; Odewabi and Ekor 2017). A negative Impact has been observed on the serotonin, acetylcholine and norepinephrine levels upon the persistent exposure to chromium.
Pulmonary adenocarcinomas can be caused by chronic inhalation of cadmium and it is proved by the experiments or research works conducted upon the rodents (Mumtaz et al. 2002; Skipper et al. 2016; Yedjou and Tchounwou 2007). Systematic or direct subjection can also be a factor for the prostatic proliferate lesions that in turn contain adenocarcinomas. In spite of the fact that here we have an inadequate information about the mechanism of cadmium toxicity it has been observed that the reason of cell destruction is mainly the production of ROS, which in turns leads to destruction of the single stranded DNA and distorted synthesis of proteins and nucleic acid (Adenocarcinoma et al. 2014; Length 2007; Mumtaz et al. 2002; Skipper et al. 2016; Yedjou and Tchounwou 2007). Against cadmium exposure many stress response system are activated e.g. Heat shock, oxidative stress, cold shock, stringent response, SOS etc. and it is proved by using 2-D gel-electrophoresis studies. According to in vitro studies cadmium concentrations from 0.1 to 10 µm can induce free radical dependent DNA destruction and cytotoxic effect.
Cadmium being a weak mutagen alters signal transduction induces production of inositol polyphosphate, high amounts in cytosolic free calcium level in different cell types and restricting calcium channels. At lesser concentration (1–100 µm) cadmium sticks to protein leading to 70 protein degradation, poor DNA repair, and increases the cytokines and proto-oncogenes as for c-hyc, c-jun, c-fos and gear ups expression of various gene consisting of metallothioneins glutathione transfers heat shock protein, heme oxygenase, acute-phase reactants and DNA polymerase β (Kazemipour et al. 2008; Morin et al. 2007). At a concentration of 4 mg/kg body weight, male reproduction changes as in mice model. Cadmium is considered to be a human carcinogen as it had been found that people suffering from lung cancer were exposed to cadmium also that data shows pulmonary systems, as the primary target site of exposure to cadmium. All the cancerous heavy metals are found to cause DNA damage through base pair mutation, deletion, or oxygen radical attack on DNA (Aziz et al. 2008; Kazemipour et al. 2008; Morin et al. 2007; Skipper et al. 2016).
7.4 Mercury
The molecular system of toxicity of mercury are relied upon its biological characteristics and chemical activities which refer that oxidative stress is responsible in its toxicity (Clarkson and Magos 2006). Oxidative stress of mercury has exhibited that mechanisms of sulfhydryl reactivity. Hg2+ and MeHg make covalent bonds with cysteine residues of protein and consume cellular anti-oxidants. Consumption of mercury compounds causes oxidative damage by gathering reactive oxygen species (ROS) which generally gets removed by cellular anti-oxidants (Clarkson and Magos 2006; Goyer et al. 2004; Jan et al. 2015; Patil et al. 2013; Singh et al. 2011). In eukaryotes, the synthesis of ROS is done in the mitochondria by normal metabolism (Clarkson and Magos 2006; Goyer et al. 2004; Jan et al. 2015; Patil et al. 2013; Singh et al. 2011; Stohs and Bagchi 1995). Inorganic mercury cause increase in synthesis of ROS through inducing glitch in oxidative phosphorylation and electron transport. Mercury causes underdeveloped shedding of electrons to molecular oxygen which results in an increase in production of ROS by increasing of electron transfer in electron transport frame (Chioma et al. 2017; Odewabi and Ekor 2017).
Organic mercury compounds are found to cause growth in intracellular calcium by advancing the influx of calcium against extracellular medium and mobilizing intracellular stores. Mercury compounds causes increased level of 3-4 methylenedioxyamphetamine (MDA) in livers, kidney, and lungs. Carcinogenesis is found to have its stages viz. initiation, promotion, progression, followed by metastasis. Exposure to mercury has been a doubtful topic (Clarkson 1993; Khoury et al. 2015; Yedjou et al. 2015). There are some studies which assure the genotoxic potential of mercury while other deny (Puccetti et al. 2000; Tchounwou et al. 2003). Mercury causes production of ROS which is known to lead to DNA damage in cells (Al-azzawie et al. 2013), a method that is known to lead to the carcinogenesis procedure. Though mercury and its compounds are not mutagenic in bacterial assays, inorganic mercury is found to cause mutational events in eukaryotic cells (Schurz et al. 2000). People consuming contaminated fish which is intoxicated by methyl mercury are found to have higher Glutathione levels. Despite of all, the studies show the chronic intake of mercury causing DNA damage also it can be cell specific as well as species specific.
7.5 Lead
Lead toxicity possess a lot of severed unfavorable impacts in both in adults and children’s populations (Goyer 1993; Kaul et al. 1999). In children’s it causes blood poisoning and diminished intelligence, hindered neurobehavioral development, diminished hearing sharpness, discourse and dialect handicaps, development implement, poor capacity to focus, and hostile to social and persistent practices (Alghazal et al. 2008; IARC 2006). In grown up population defects like diminished Sperm check in Men, abortions or pre-nature births in Women are caused by high lead exposure (Yedjou and Tchounwou 2007).
In acute exposure, Lead can caused damage to kidney, brain, and various gastrointestinal disease and it’s chronic exposure through an adverse impact Vitamin D metabolism, blood pressure and CNS (Awasthi et al. 1996; Heipieper et al. 1996). Lead having an ability that it can mimic or inhibit the action of calcium by the way it can incorporate itself in place of calcium inside the skeleton and then interact with various biological molecules like proteins etc. and by acquiring a number of mechanics it interrupts their function. When amide and sulfhydryl groups of enzyme from a complex with lead it changes their configuration and decreased their actions or activities (Awasthi et al. 1996; Heipieper et al. 1996; Village 2005). In human externalization of phosphatidylserine and turn on of caspase-3, damage of DNA, transcriptional turn on of stress genes, oxidative stress and cell deaths are events that are associated with many cellular and molecular processes observed apoptosis and toxicity and are involved due to lead as reported by various research studies (Kazemipour et al. 2008; Patil et al. 2013; Village 2005).
8 Special Considerations
8.1 Children
Children are at much higher risk of being caught by environmental hazards. The possible reasons for this are—drinking more water, breathing more air, eating more food per unit weight also they are more in contact with the floor and they touch and put it in their mouth which seems them attractive (Hotz et al. 1999). The major difference between a children mechanism than the adults is their immune system. While adults have much developed immune system than children thus they are more prone to be caught up by the diseases. Children playing outside are often found to suffer more from air pollution (Alfvén et al. 2000).
Mercury is present in high amount in fishes of fresh water and ocean through disposal of mercury in water. Thus, consumption of fishes can damage the brain effect the memory of a person. This can be illustrated by a case in Minamata Bay, Japan in 1960s where discharge of large amount of mercury in the bay caused contamination of the fishes and ingestion of those fishes by pregnant women resulted in death of 41 infants and 30 found to be born with brain injury hereby, certifying the bad impacts of mercury on a child’s health (Jarup et al. 1995). Children don’t have developed blood-barrier like as in adults. So, the inorganic lead can pass through the blood-barrier in children making them exposed to the diseases caused by lead toxicity.
Cadmium has a half-life of 10–30 years in bones and kidneys thus children suffer more from cadmium toxicity from its exposure (Nishijo et al. 1995). Pregnant women who smoke causes serious threat to their infants since tobacco and tobacco smoke contain cadmium which can also cause cancer at its highest exposure (Duruibe et al. 2007). Thus children are at much higher risk of being exposed to toxic metals thus immediate prevention from them is the time’s need. Along with all these environmental and parental factors poverty is also a major factor since children do not get proper nutrition, proper medication, and healthy environment thereby leading to chronic exposure to metal toxicity. Soil contains traces of many metals along with pesticides and many other toxic materials (Ayandiran et al. 2009). Therefore, its consumption is very unsafe to health. Many children develop habit of eating soil which if ignored can cause serious threats to life (Morin et al. 2007).
8.2 Challenges Ahead
Exposure to toxic metals not only causes serious illness and even deaths. Inadequate services, unawareness of people about the diseases from metal toxicity has made it much serious case which is needed to handled immediately (Appenroth 2010). Almost all the metals on their high exposure show similar symptoms. It is a big challenge in front to identify all the factors which make people especially children exposed of metal toxicity. This subject is not given its needed priority in medical and nursing schools as a result there are very less doctors who have intense knowledge to this subject. Thus, an urgent concern over this subject is the needed. The factors, the relation between metal exposure and risk of disease caused are the important matters to be understood in depth. Factors like smoking and obesity require a more deep inspection (Al-fartusie and Mohssan 2017; Jaishankar et al. 2014). One of the biggest challenges is to understand the carcinogenetic impacts of some heavy metals on their severe exposure. Monitoring and establishing the measures to control over metal toxicity is a big challenge and will require additional resources and inter sectoral collaboration.
8.3 Eco-Friendly Ways to Remove Heavy Metal Toxicity
Water is life-essential resource. Water is being used by each and every living-organism that is present on this earth because of this element (Paknikar et al. 2003; Volesky et al. 1995). Chemically it is oxygen and hydrogen but its application is very broad. Because of a poor life-style and management this resource is being polluted day by day. One of the reasons which need our strong concern is the heavy metal toxicity in water bodies. Lead, chromium, mercury, uranium, selenium, zinc, arsenic, cadmium, silver, gold and nickel are the metals considered as threat if occurs in a large quantity in the living organisms (Paul et al. 2006; Yan and Viraraghavan 2000). In the natural environment the sediment and ores are primary sites of the heavy metals where these are found in immobilized form. However, we have observed an increment in the levels of heavy metals that are depositing itself in our aquatic and terrestrial environment and the reason behind this is the several human undertakings (Wilke et al. 2006; Duruibe et al. 2007; Morin et al. 2007). For example, industrial activities and ore mining that has disturbed the natural biogeochemical circle. When these pollutants are liberated out in the absence of a regular treatment causes a trouble for natural system as well as for the public health (Ayandiran et al. 2009; Wilke et al. 2006). These heavy metals are non-biodegradable and remains as it is or constant with the passage of time. Metals can enter into the food web by a process known as leaching in which the metals are extracted away from the dumped waste materials, polluted soils and water. This process leads to another important phenomenon that is bio-magnification where these toxic metals get incorporated in food chains.
We can also use a word bio-accumulation in which certain substances or chemical gets deposited inside an organism or plants (Paul et al. 2006; Yan and Viraraghavan 2000). These Heavy metals have ability that they can bind with protein molecules and can restrict the process of DNA replication which further blocks the process of cell-division. Therefore, to prevent this health risk we need to discard these toxic metals from waste water/polluted water before its further disposal. Heavy metals should be removed from the waste water before their disposal in order to prevent health related risks. The various sources of heavy metal poisoning are urban industrial aerosols, solid wastes from animals, mining activities, industrial and agricultural chemicals. Acid rain and break down of soil and rock into water also contaminates the water (Paknikar et al. 2003; Volesky et al. 1995).
To purify the contaminated water resources, there are several technologies viz. reverse osmosis, electro dialysis, ultra-filtration, ion-exchange, chemical precipitation, phytoremediation etc. However, these methods are not subjected for total removal of metal removal (Duruibe et al. 2007). Since the present technologies have various disadvantages therefore we need some cost-effective alternatives technologies. Recently Biomass has been emanated as another waste water treatment process and it is a cost-effective and eco-friendly method. Biosorption is defined as “a non-directed phyicochemical interaction” which can occur in midst metal and microbial cells. It can be used to treat contaminated water also it has several pros over other methods like chemical/biological sludge economical, regeneration of biosorbent making it is possible to take out metal from contaminated water.
Solvent is attracted and bounded to sorbate with various mechanisms since the sorbent has a higher affinity. This process keeps on going until equilibrium is established among quantity of solid-bound sorbate species and its part left in the solution (Yan and Viraraghavan 2000).
A fine biosorbent leads to fruitful biosorption (Table 13). However starting from selection of types of biomass followed by prior treatment confinement is done so as to gain productivity of metal uptake and hence removing the adsorbed metal, by desorption process so as the biosorbent can be reiterated for other operations (Paknikar et al. 2003).
8.4 Toxic Heavy Metals and Undeclared Drugs
Asian Herbal Medicines (AHMs) are becoming more pronounced in most the developed countries (Ko 1999). AHM’s are not supplied as medicine since because of proper information about pharmacology and toxic properties are disguised (Cosyns et al. 1999; Napolitano 2001). It is a crucial matter to be looked as AHM’s contain heavy metals or undeclared drugs. In India, a case revealed that out of 12 cases of poisoning in drug in taking, 9 were caused due to herbal medicines which contained inappropriate amount of heavy metals. A recent report by Indian authors brought out that 31 ayurvedic traditional medicines contained mercury, out of which 30 contained it in amount more than as set up by the standards i.e. 1 ppm. These data bring up about the real picture of herbal Ayurvedic medicines in India. Thus, it should be over looked.
8.5 Chinese Herbal Ayurvedic Medicines
In China, from time to time, various case and series of incidents of heavy metals related to use of traditional Chinese medicine have been published. In California various Chinese herbal medicines have been banned in the retail stores. However, heavy metals are not only contaminant present in herbal remedies; they are even associated with contaminants like herbicides, pesticides, micro-organisms or mycotoxins, insects or undeclared herbal constituents. In Belgium contamination of heavy metals because of plants of Aristolochia species resulted in plague of subacute intestinal nephropathy which caused kidney transplantation of many of the patients (Ernst 2002; Ko 1999; Koneman and Roberts 2002; Saper et al. 2008). In various case reports published by different countries viz. Australia, Belgium, China, Netherlands, New Zealand, UK and USA states that adulteration of TCM’s with some synthetic drug causes health problems to user some of which are fatal (Barnes 2003; Cosyns et al. 1999; Ernst 2004; Keane et al. 1999; Linde et al. 2001). The symptoms to these altered herbal remedies may appear or may not.
8.6 Concern About Safety of Asian Herbal Medicines
The above data reveals the critical situation of present which could even become worse if not handled today. The herbal medicine that we take to cure the disease is itself causing diseases because of being altered by various adulterants. Thus the current need is to restrict the supply of contaminated herbal medicines (Barnes 2003; Keane et al. 1999).
8.7 Measures to Be Taken by Every Patient with Reference to Use of Herbal Medicine
-
The Herbal remedies must be regarded as medicines.
-
The Herbal remedies should be in taken by doctor’s prescription and dosages should also be followed.
-
Long term use of these medicines should be prevented.
-
If some undesired symptoms are observed after ingestion to the herbal medicine. Immediately stop its use and report it to your doctor.
-
Be careful of the adulterated herbal medicine.
-
Buy it only from reputed stores.
-
Pregnant women and young children should not in take herbal medicine.
9 Conclusion
Some of the heavy metals are directly associated with cancer initiation and progression through suppressing immune system and altering cancer signaling pathways. Heavy metals which are associated with cancer are: arsenic, uranium mercury, lead, cadmium and aluminum etc. These deadly and silent invaders cause suppression and/or deregulation of the immune system, leading to cancer initiation and progression. Heavy metals are also linked to increased free-radical activity, DNA damage, apoptosis, cell damage, cell death, ROS and NOS generation and oxidation processes that promote cancer initiation and progression. Understanding the molecular mechanisms of heavy metal toxicity in cancer initiation and progression would be helpful to find effective therapeutic intervention for the cancer specifically induced by heavy metals.
References
Adenocarcinoma B, Tchounwou CK, Yedjou CG, Farah I, Tchounwou PB (2014) NIH Public Access 601:156–160
AERB (2004) Drinking water specifications in India. Retrieved from Mumbai: www.aerb.gov.in
Al-azzawie HF, Umran A, Hyader NH (2013) Oxidative stress, antioxidant status and DNA damage in a mercury exposure workers. Br J Pharmacol Toxicol 4:80–88
Al-fartusie FS, Mohssan SN (2017) Essential trace elements and their vital roles in human body. Indian J Adv Chem Sci 5(3):127–136
Alfvén T, Elinder CG, Carlsson MD, Grubb A, Hellström L, Persson B, Järup L (2000) Low-level cadmium exposure and osteoporosis. J Bone Miner Res 15(8):1579–1586
Alghazal MA, Lenártová V, Holovská K, Sobeková A, Falis M, Legáth J (2008) Activities of antioxidant and detoxifying enzymes in rats after lead exposure. Acta Vet Brno 77(3):347–354
Appenroth K (2010) Definition of “ Heavy Metals” and their role in biological systems. In: Soil heavy metals. Soil biology, vol 19. Springer, Berlin, pp 19–30
Arif N, Yadav V, Singh S, Singh S, Ahmad P, Mishra RK et al (2016) Influence of high and low levels of plant-beneficial heavy metal ions on plant growth and development. Front Environ Sci 4:69
Awasthi S, Awasthi R, Pande VK, Srivastav RC, Frumkin H (1996) Blood lead in pregnant women in the urban slums of Lucknow, India. Occup Environ Med 53(12):836–840
Ayandiran TA, Fawole O, Adewoye SO, Ogundiran M (2009) Bioconcentration of metals in the body muscle and gut of Clarias gariepinus exposed to sublethal concentrations of soap and detergent effluent. J Cell Anim Biol 3(8):113–118
Aziz HA, Adlan MN, Ariffin KS (2008) Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: post treatment by high quality limestone. Biores Technol 99(6):1578–1583
Bajwa BS, Kumar S, Singh S, Sahoo SK, Tripathi RM (2017) Uranium and other heavy toxic elements distribution in the drinking water samples of SW-Punjab, India. J Radiat Res Appl Sci 10(1):13–19
Barnes J (2003) Quality, efficacy and safety of complementary medicines: fashions, facts and the future. Part 1: regulation and quality. Br J Clin Pharmacol 55:226–233
Barra R, Colombo JC, Eguren G, Gamboa N, Jardim WF, Mendoza G (2006) Persistent organic pollutants (POPs) in eastern and western South American countries. In: Reviews of environmental contamination and toxicology. Springer, New York, NY, pp 1–33
Basu A, Mahata J, Gupta S, Giri AK (2001) Genetic toxicology of a paradoxical human carcinogen, arsenic: a review. Mutat Res Rev Mutat Res 488(2):171–194
Brindha K, Elango L (2013) Occurrence of uranium in groundwater of a shallow granitic aquifer and its suitability for domestic use in southern India. J Radioanal Nucl Chem 295(1):357–367
Brochin R, Leone S, Phillips D, Shepard N, Zisa D, Angerio A (2008) The cellular effect of lead poisoning and its clinical picture. Georgetown Undergraduate J Health Sci 5(2):1–8
Browning C, The T, Mason M, Wise JP (2014) Titanium dioxide nanoparticles are not cytotoxic or clastogenic in human skin cells. J Environ Anal Toxicol 4(6):1–15
Chioma O, Emmanuel A, Peter A (2017) Accumulation and toxicological risk assessment of Cd, As, Pb, Hg, and Cu from topsoils of school playgrounds at Obio-Akpor LGA Rivers State Nigeria. Int J Sci World 5(1):38
Clarkson TW (1993) Molecular and ionic mimicry of toxic metals. Annu Rev Pharmacol Toxicol 33(1):545–571
Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36(8):609–662
Cosyns JP, Jadoul M, Squifflet JP, Wese FX, De Strihou CVY (1999) Urothelial lesions in Chinese-herb nephropathy. Am J Kidney Dis 33(6):1011–1017
Dayan AD, Paine AJ (2001) Mechanisms of chromium toxicity, carcinogenicity and allergenicity: review of the literature from 1985 to 2000. Hum Exp Toxicol 20(9):439–451
Dong Z (2002) The molecular mechanisms of arsenic-induced cell transformation and apoptosis. Environ Health Perspect 110(SUPPL. 5):757–759
Duffus JH (2002) “Heavy Metals”—a meaningless term? (IUPAC technical report). Pure Appl Chem 74(5):793–807 [National Representatives: Z. Bardodej (Czech Republic) J. Park (Korea) F. J. R. Paumgartten (Brazil)]
Duruibe JO, Ogwuegbu MO, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2(5):112–118
Efstathiou M, Aristarchou T, Kiliari T, Demetriou A, Pashalidis I (2014) Seasonal variation, chemical behavior and kinetics of uranium in an unconfined groundwater system. J Radioanal Nucl Chem 299(1):171–175
Ernst E (2002) Adulteration of Chinese herbal medicines with synthetic drugs: a systematic review. J Intern Med 252(2):107–113
Ernst E (2004) Prescribing herbal medications appropriately. J Fam Pract 53(12):985–988
Florea AM, Büsselberg D, Carpenter D (2012) Metals and disease. J Toxicol 2012:2012–2014
Gokhale BSL (2008) Groundwater Radon-222 concentrations in Antelope Creek, Idaho: measurement and interpolation. Open Environ Bio Monit J 3:12–20
Gómez P, Garralón A, Buil B, Turrero MJ, Sánchez L, de la Cruz B (2006) Modeling of geochemical processes related to uranium mobilization in the groundwater of a uranium mine. Sci Total Environ 366(1):295–309
Goulart M, Batoréu MC, Rodrigues AS, Laires A, Rueff J (2005) Lipoperoxidation products and thiol antioxidants in chromium exposed workers. Mutagenesis 20(5):311–315
Goyer RA (1993) Lead toxicity: current concerns. Environ Health Perspect 100:177–187
Goyer R, Golub M, Choudhury H, Hughes M, Kenyon E, Stifelman M (2004) Issue paper on the human health effects of metals. In: US environmental protection agency risk assessment forum, vol 1200
Grimsrud TK, Peto J (2006) Persisting risk of nickel related lung cancer and nasal cancer among clydach refiners. Occup Environ Med 63(5):365–366
Griswold W, Martin S (2009) Human health effects of heavy metals. Environ Sci Technol 15:1–6
Hartmann HM, Monette FA, Avci HI (2000) Overview of toxicity data and risk assessment methods for evaluating the chemical effects of depleted uranium compounds. Hum Ecol Risk Assess 6(5):851–874
Heipieper HJ, Meulenbeld G, Van Oirschot Q, De Bont JAM (1996) Effect of environmental factors on the trans/cis ratio of unsaturated fatty acids in pseudomonas putida S12. Appl Environ Microbiol 62(8):2773–2777
Hodson ME (2004) Heavy metals—geochemical bogey men? Environ Pollut 129(3):341–343
Hong YS, Song KH, Chung JY (2014) Health effects of chronic arsenic exposure. J Prev Med Public Health 47(5):245–252
Hotz P, Buchet JP, Bernard A, Lison D, Lauwerys R (1999) Renal effects of low-level environmental cadmium exposure: 5-year follow-up of a subcohort from the cadmibel study. Lancet 354:1508–1513
Hu Z, Gao S (2008) Upper crustal abundances of trace elements: a revision and update. Chem Geol 253(3):205–221
IAEA (2015) Protection of the public against exposure indoors due to radon and other natural sources of radiation. In: IAEA safety standards for protecting people and the environment, vol SSG-32. IAEA, Vienna, p 112
IARC (2006) Inorganic and organic lead. IARC monographs on the evaluation of carcinogenic risks to humans, 87
Ilyin I, Berg T, Dutchak S, Pacyna J (2004) Heavy metals. EMEP assessment part I European perspective. Norwegian Meteorological Institute, Oslo, Norway, pp 107–128
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdisc Toxicol 7(2):60–72
Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QMR (2015) Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 16(12):29592–29630
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Jarup L, Persson B, Elinder CG (1995) Decreased glomerular filtration rate in solderers exposed to cadmium. Occup Environ Med 52(12):818–822
Jhaa N, Noditi M, Nilsson R, Natarajana T (1992) Genotoxic effects of sodium arsenite on human cells. Mutat Res 284(2):215–221
Kaul B, Sandhu RS, Depratt C, Reyes F (1999) Follow-up screening of lead-poisoned children near an auto battery recycling plant, Haina, Dominican Republic. Environ Health Perspect 107(11):917–920
Kawada T (2016) Predictive validity of a specific questionnaire for psychiatric morbidity and suicidal ideation. J Formos Med Assoc 115(11):1019–1020
Kazemipour M, Ansari M, Tajrobehkar S, Majdzadeh M, Kermani HR (2008) Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond, pistachio shell, and apricot stone. J Hazard Mater 150(2):322–327
Keane FM, Munn SE, du Vivier AW, Taylor NF, Higgins EM (1999) Analysis of Chinese herbal creams prescribed for dermatological conditions. BMJ (Clin Res Ed) 318(7183):563–564
Khatri P, Sirota M, Butte AJ (2012) Ten years of pathway analysis: current approaches and outstanding challenges. PLoS Comput Biol 8(2):e1002375
Khoury EDT, Da Silva Souza G, Da Costa CA, De Araújo AAK, De Oliveira CSB, De Lima Silveira LC, Da Conceição NPM (2015) Somatosensory psychophysical losses in inhabitants of riverside communities of the Tapajós River Basin, Amazon, Brazil: exposure to methylmercury is possibly involved. PLoS ONE 10(12):1–19
Kim HS, Kim YJ, Seo YR (2015) An overview of carcinogenic heavy metal: molecular toxicity mechanism and prevention. J Cancer Prev 20(4):232–240
Kippler M, Tofail F, Gardner R, Rahman A, Hamadani J, Bottai M, Vahter M (2012) Maternal cadmium exposure during pregnancy and size at birth: a prospective cohort study. Environ Health Perspect 120(2):284–289
Ko R (1999) Adverse reactions to watch for in patients using herbal remedies. West J Med (September):181–186
Koneman EW, Roberts GD (2002) Your lab focus. Lab Med 33(12):437–445
Konietzka R (2015) Gastrointestinal absorption of uranium compounds—a review. Regul Toxicol Pharmacol 71(1):125–133
Kumar A, Usha N, Mishra MK, Tripathi RM, Rout S, Jaspal S (2011) Risk assessment for natural uranium in subsurface water of Punjab State, India. Hum Ecol Risk Assess 17:381–393
Lee CP, Lee YH, Lian IB, Su CC (2016) Increased prevalence of esophageal cancer in areas with high levels of nickel in farm soils. J Cancer 7(12):1724–1730
Length F (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2(5):112–118
Liesch T, Hinrichsen S, Goldscheider N (2015) Uranium in groundwater—fertilizers versus geogenic sources. Sci Total Environ 536:981–995
Linde K, Vickers A, Hondras M, Ter Riet G, Thormählen J, Berman B, Melchart D (2001) Systematic reviews of complementary therapies—an annotated bibliography. Part 1: acupuncture. BMC Complement Altern Med 1:3
Liu Y, Guyton KZ, Gorospe M, Xu Q, Lee JC, Holbrook NJ (1996) Differential activation of ERK, JNK/SAPK and P38/CSBP/RK map kinase family members during the cellular response to arsenite. Free Radic Biol Med 21(6):771–781
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: impact on human health. Pharmacognosy Rev 4(8):118
Mamtani R, Stern P, Dawood I, Cheema S (2011) Metals and disease: a global primary health care perspective. J Toxicol 2011(319136):1–11
Mazariegos M, Hambidge KM, Westcott JE, Solomons NW, Raboy V, Das A, Krebs NF (2010) Neither a zinc supplement nor phytate-reduced maize nor their combination enhance growth of 6- to 12-month-old guatemalan infants. J Nutr 1–4(9):1041–1048
Morin S, Vivas-Nogues M, Duong TT, Boudou A, Coste M, Delmas F (2007) Dynamics of benthic diatom colonization in a cadmium/zinc-polluted river (Riou-Mort, France). Fundam Appl Limnol/Archiv Für Hydrobiol 168(2):179–187
Mumtaz MM, Tully DB, El-Masri HA, De Rosa CT (2002) Gene induction studies and toxicity of chemical mixtures. Environ Health Perspect 110(SUPPL.6):947–956
Napolitano V (2001) Complementary medicine use by Mexican migrants in the San Francisco Bay Area. West J Med 174(3):203–206
Nishijo M, Nakagawa H, Morikawa Y, Tabata M, Senma M, Miura K, Nogawa K (1995) Mortality of inhabitants in an area polluted by cadmium: 15 year follow up. Occup Environ Med 52(3):181–184
O’Brien TJ, Ceryak S, Patierno SR (2003) Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat Res Fundam Mol Mech Mutagenesis 533(1–2):3–36
Odewabi AO, Ekor M (2017) Levels of heavy and essential trace metals and their correlation with antioxidant and health status in individuals occupationally exposed to municipal solid wastes. Toxicol Ind Health 33(5):431–442
OEHHA (Office of Environmental Health Hazard Assessment Agency California Environmental Protection) (2001) Public health goals for chemicals in drinking water: NICKEL (August)
Paknikar KM, Pethkar AV, Puranik PR (2003) Bioremediation of metalliferous wastes and products using inactivated microbial biomass. Indian J Biotechnol 2(3):426–443
Patil YP, Pawar SH, Jadhav S, Kadu JS (2013) Biochemistry of metal absorption in human body: reference to check impact of nano particles on human being. Int J Sci Res Publ 3(4):1–5
Paul S, Bera D, Chattopadhyay P, Ray L (2006) Biosorption of Pb (II) by Bacillus cereus M1 16 immobilized in calcium alginate gel. J Hazard Subst Res 5(1):2
Prüss-Ustün A, Vickers C, Haefliger P, Bertollini R (2011) Knowns and unknowns on burden of disease due to chemicals: a systematic review. Environ Health 10(1):9
Puccetti E, Güller S, Orleth A, Brüggenolte N, Hoelzer D, Ottmann OG, Ruthardt M (2000) BCR-ABL mediates arsenic trioxide-induced apoptosis independently of its aberrant kinase activity. Can Res 60(13):3409–3413
Radespiel-Tröger M, Meyer M (2013) Association between drinking water uranium content and cancer risk in Bavaria, Germany. Int Arch Occup Environ Health 86(7):767–776
Rooney JPK (2007) The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicology 234(3):145–156
Samet JM (2011) Radiation and cancer risk: a continuing challenge for epidemiologists. Environ Health 10(1):541–549
Sankhla MS, Kumari M, Nandan M, Kumar R, Agrawal P (2016) Heavy metals contamination in water and their hazardous effect on human health: a review. Int J Curr Microbiol Appl Sci 5:759–766
Saper RB, Phillips RS, Sehgal A, Khouri N, Davis RB, Paquin J, Kales SN (2008) Lead, mercury, and arsenic in US- and Indian-manufactured ayurvedic medicines sold via the internet. JAMA J Am Med Assoc 300(8):915–923
Schurz F, Sabater-Vilar M, Fink-Gremmels J (2000) Mutagenicity of mercury chloride and mechanisms of cellular defence: the role of metal-binding proteins. Mutagenesis 15:525–530
Singh NP, Burleigh DP, Ruth HM, Wrenn ME (1990) Daily U intake in Utah residents from food and drinking water. Health Phys 59(3):333–337
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an overview. Indian J Pharmacol 43(3):246
Skipper A, Sims JN, Yedjou CG, Tchounwou PB (2016) Cadmium chloride induces DNA damage and apoptosis of human liver carcinoma cells via oxidative stress. Int J Environ Res Public Health 13(1):1–10
Soignet BSL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, Warrell RP (2001) United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol Official J Am Soc Clin Oncol 19(18):3852–3860
Stevens JJ, Graham B, Walker AM, Tchounwou PB, Rogers C (2010) The effects of arsenic trioxide on DNA synthesis and genotoxicity in human colon cancer cells. Int J Environ Res Public Health 7(5):2018–2032
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18(2):321–336
Su C, Yang H, Huang S, Lian I (2007) Distinctive features of oral cancer in Changhua county: high incidence, buccal mucosa preponderance, and a close relation to betel quid chewing habit. J Formos Med Assoc 106(3):225–233
Takahashi M, Barrett JC, Tsutsui T (2002) Transformation by inorganic arsenic compounds of normal Syrian hamster embryo cells into a neoplastic state in which they become anchorage-independent and cause tumors in newborn hamsters. Int J Cancer 99(5):629–634
Tchounwou PB, Patlolla AK, Centeno JA (2003) Invited reviews: carcinogenic and systemic health effects associated with arsenic exposure—a critical review. Toxicol Pathol 31(6):575–588
Tchounwou PB, Centeno JA, Patlolla AK (2004) Arsenic toxicity, mutagenesis, and carcinogenesis—a health risk assessment and management approach. Mol Cell Biochem 255(1–2):47–55
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012a) Molecular, clinical and environmental toxicology, vol 101, Springer, Basel, pp 1–30
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012b) Heavy metal toxicity and the environment. In: Molecular, clinical and environmental toxicology. Springer, Basel, pp 133–164
Velma V, Tchounwou PB (2011) NIH Public Access 698:43–51
Verin AD, Cooke C, Herenyiova M, Patterson CE, Garcia JG (1998) Role of Ca2+/calmodulin-dependent phosphatase 2B in thrombin-induced endothelial cell contractile responses. Am J Physiol 275(4 Pt 1):L788–L799
Village G (2005) Lead exposure in children: prevention, detection, and management. Pediatrics 116(4):1036–1046
Volesky B, Holan ZR, About M, Article T (1995) Biosorption of heavy metals. Biotechnol Prog 11(3):235–250
Walker AM, Stevens JJ, Ndebele K, Tchounwou PB (2010) Arsenic trioxide modulates DNA synthesis and apoptosis in lung carcinoma cells. Int J Environ Res Public Health 7(5):1996–2007
Wen CP, Tsai SP, Cheng TY, Chen CJ, Levy DT, Yang HJ, Eriksen MP (2005) Uncovering the relation between betel quid chewing and cigarette smoking in Taiwan. Tobacco Control 14(SUPPL. 1):16–22
Wilke A, Buchholz R, Bunke G (2006) Selective biosorption of heavy metals by algae. Environ Biotechnol 2:47–56
Yamashoji S, Isshiki K (2001) Rapid detection of cytotoxicity of food additives and contaminants by a novel cytotoxicity test, menadione-catalyzed H2O2 production assay. Cytotechnology 37(3):171–178
Yan G, Viraraghavan T (2000) Effect of pretreatment on the bioadsorption of heavy metals on Mucor rouxii. WATER SA-PRETORIA 26(1):119–124
Yedjou CG, Tchounwou PB (2007) N-acetyl-l-cysteine affords protection against lead-induced cytotoxicity and oxidative stress in human liver carcinoma (HepG2) cells. Int J Environ Res Public Health 4(2):132–137
Yedjou CG, Milner JN, Howard CB, Tchounwou PB (2010) Basic apoptotic mechanisms of lead toxicity in human leukemia (Hl-60) cells. Int J Environ Res Public Health 7(5):2008–2017
Yedjou CG, Tchounwou HM, Tchounwou PB (2015) DNA damage, cell cycle arrest, and apoptosis induction caused by lead in human leukemia cells. Int J Environ Res Public Health 13(1):56
Yuan T, Lian I, Tsai K, Chang T, Chiang C, Su C, Hwang Y (2011) Science of the total environment possible association between nickel and chromium and oral cancer: a case—control study in central Taiwan. Sci Total Environ 409(6):1046–1052
Zhang YL, Zhao YC, Wang JX, Zhu HD, Liu QF, Fan YG, Fan TQ (2004) Effect of environmental exposure to cadmium on pregnancy outcome and fetal growth: a study on healthy pregnant women in China. J Environ Sci Health Part A 39(9):2507–2515
Zhang R, Ma A, Urbanski SJ, McCafferty DM (2007) Induction of inducible nitric oxide synthase: a protective mechanism in colitis-induced adenocarcinoma. Carcinogenesis 28(5):1122–1130
Zofkova I, Davis M, Blahos J (2017) Trace elements have beneficial, as well as detrimental effects on bone homeostasis. Physiol Res 66:391–402
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Singh, P., Tiwari, D., Mishra, M., Kumar, D. (2019). Molecular Mechanisms of Heavy Metal Toxicity in Cancer Progression. In: Kesari, K. (eds) Networking of Mutagens in Environmental Toxicology. Environmental Science and Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-319-96511-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-96511-6_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-96510-9
Online ISBN: 978-3-319-96511-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)