Abstract
Asteroids and comets have continuously delivered organics to Earth and telluric planets since their formation ~4.55 Ga ago. Characterizing these organics and investigating their origin constitutes a major goal of astrobiology and planetary sciences. This chapter reviews past and current knowledge on the nature of the exogenous organics accreted by the Earth, their composition and structure and different issues regarding their origin and subsequent evolution through the effects of secondary processes in/on their original asteroidal or cometary parent bodies. Tertiary processes such as weathering and, in the case of dust, the heating and oxidation effects during atmospheric entry, are also discussed. The last section focuses on the nature and preservation of organics at the surface of Mars, in the context of the ExoMars and Mars2020 missions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Meteorites
- Exogenous Organics
- Interplanetary Dust Particles (IDPs)
- Antarctic Micrometeorites (AMMs)
- Stratospheric IDPs
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Asteroids and comets have continuously delivered organics to Earth and telluric planets since their formation ~4.55 Ga ago. Characterizing these organics and investigating their origin constitutes a major goal of astrobiology and planetary sciences. First and foremost, extraterrestrial organics could have been a source of prebiotic molecules from which life arose on Earth. In any case, they were a significant source of carbon to primitive telluric planets. However, they also testify to a complex abiotic chemistry and shed light on the astrophysical locations and conditions that led to the emergence of organic complexification. In this respect, they provide insightful evidence that helps investigate new paradigms in planetary habitability.
The inventory of the past and present delivery of extraterrestrial organics is a major field of investigation and relies on the determination of (1) the flux of cosmomaterials over time, including size and mass distributions; (2) their composition; (3) the effect of atmospheric entry; and (4) alteration processes at the surface of the planet (Earth or Mars). The objective of this chapter is to review past and current knowledge on these different issues, with emphasis on the organic inventory of the main cosmomaterials available on Earth and the detection and characterization of exogenous organic matter at the surface of Mars. The following paragraph concerns the cosmomaterials available in laboratories, such as meteorites collected on the ground, and micrometeorites and stratospheric Interplanetary Dust Particles (IDPs) from the Earth’s atmosphere. We then review the organic content in primitive meteorites and focussing on recent analytical developments and characterization, subsequently addressing organic matter in stratospheric IDPs and Antarctic micrometeorites (AMMs) that represent the dominant source of extraterrestrial organics on Earth and Mars. Finally, we discuss the origin of organic matter in chondrites and dust, and address the issue of the preservation and detection of allochthonous extraterrestrial organic matter at the surface of Mars, in the context of the Mars 2020 and ExoMars space missions. Given the broad scope of the present chapter, several points could not be exhaustively addressed. We refer the reader to excellent reviews by Sephton (2002) (overview of organics in chondrites), Burton et al. (2012) (amino acids and nucleobases in chondrites) and Alexander et al. (2017) (insoluble organic matter in chondrites).
2 Cosmomaterials Inherited by Telluric Planets
2.1 Past and Present Flux of Extraterrestrial Matter
Love and Brownlee (1993) estimated the pre-atmospheric dust flux (mass range 10−9 to 10−4 g) based on direct measurements of hypervelocity impact craters on the Long Duration Exposure Facility satellite. The meteoroid mass distribution peaks near 1.5 × 10−5 g—or roughly 200 μm in diameter—and the small particle mass accretion rate is 40 ± 20 ktons per year (Love and Brownlee 1993). Estimates of post-atmospheric flux are, however, slightly higher, but may not be significantly different, with values of 78 ± 30 ktons per year (Gabrielli et al. 2004) and of 64 ± 20 ktons per year (Lanci and Kent 2006). Most meteoritic material reaching the Earth system is in the form of meteoritic smoke made of nanometer-sized particles produced through recondensation of meteor ablated products (Hunten et al. 1980; Love and Brownlee 1991). Lanci and Kent (2006) used magnetic measurements of Greenland ices to estimate meteoritic smoke concentration and deduced an accretion rate assuming a given snow deposition rate. The estimates of Gabrielli et al. (2004) were based on measured concentrations of platinum and iridium Greenland ices during the Holocene epoch. Meteoritic smoke represents the main input of extraterrestrial matter to Earth but the comparison to pre-atmospheric flux shows that only a small fraction of extraterrestrial materials (and of the organics potentially within) survives entry to the Earth’s atmosphere.
Micrometeorites represent the major fraction of recoverable solid mass accreted to the Earth, with values of 2.7 ± 1.4 ktons per year estimated by Taylor et al. (1998) based on counting statistics of the South Pole water well (SPWW) collection and assuming that a similar fraction of unmelted micrometeorites is present in the SPWW and in Antarctic blue ice. The present flux of meteorites was determined by Halliday et al. (1984, 1989) to be 83 meteorites of mass equal or greater than 1 g per 106 km2 per year based on direct observations of fireballs by the Canadian camera network between 1974 and 1985 and on some assumptions made to estimate the proportion of surviving material. Bland et al. (1996) calculated between 36 and 116 meteorites >10 g per 106 km2 and per year based on counting statistics of meteorites on the ground of hot deserts and on an estimate of their preservation over time. This represents a total flux between 2900 and 7300 kg per year in the 10 g to 1 kg size range over the last 50,000 years.
Now let us look at whether the composition and intensity of the extraterrestrial flux to the Earth has changed over time. Access to several meteorite collection sites with slow weathering rates and the possibility of dating the terrestrial residence times of meteorites provides a time window of up to several millions years (e.g., terrestrial ages of up to two million years for meteorites in Antarctica; Jull 2006). A review by Zolensky et al. (2006a) concluded that there has been a significant variation of the flux of extraterrestrial material over time, with the present day flux being lower than the flux over the past few millions years by a factor of up to two. A higher past flux of extraterrestrial material to Earth has also been suggested from the study of fossil meteorites, which are traceable back to 470 Ma (Schmitz et al. 2001). Ordinary chondrites are the dominant form in both the modern and past flux of meteorites falling to Earth. Nevertheless, Zolensky et al. (2006a) also pointed out a variation in the types of meteorites, reaffirmed recently by Gattacceca et al. (2011), who focused on the San Juan meteorite field of the Atacama Desert (Chile). With terrestrial ages up to more than 40 ky included in that collection, they showed an overabundance of H chondrites and a shortage of LL chondrites in comparison with the population of modern falls. This suggests that there are short-term variations in the composition of meteorite flux, in agreement with observations made based on the Antarctic collection (e.g., Harvey and Cassidy 1989).
Collections of IDPs contain only contemporary material, but some chemical tracers of oceanic sediments (e.g., 3He; Farley 1995) make it possible to determine the flux of dust back through time. This is also possible from the study of micro-xenoliths (i.e., inclusions having a different origin from the host meteorites) that can be considered as fossil micrometeorites (Gounelle et al. 2003; Briani et al. 2012). Gounelle et al. (2003) noted that the ancient micrometeorite flux is dominated by C2-like matter, comparable to the present micrometeorite flux. Further investigations would be required to characterize a potential compositional variation.
The prerequisite conditions to find meteorites on planetary surfaces are (1) the presence of a sufficiently dense atmosphere to decelerate the projectiles to a low impact speed to prevent total vaporization and melting of material and (2) slow weathering to allow accumulation over time. These conditions exist in at least certain locations on Earth and Mars.
2.2 Cosmomaterials in Earth Collections
Cosmomaterials available for laboratory studies include extraterrestrial rocks and interplanetary dust particles collected on the ground as micrometeorites (e.g., in the blue ices and snowfields of Antarctica) and in the atmosphere of Earth as stratospheric IDPs. They also comprise materials collected in situ on the Moon, in the coma of comet Wild 2 (the Stardust mission) and on the asteroid Itokawa (the Hayabusa-1 mission). Meteorites are rocks derived from asteroids, although a cometary origin has been proposed for several of them (Gounelle et al. 2006). They are named falls when their fall is certified by an eyewitness and the material is recovered shortly afterwards. Should this not be the case, they are considered as finds. Although fresh falls are the preferred objects, as they largely escape terrestrial organic contamination and oxidation, they are rare. Indeed, collections mostly contain finds collected in Antarctica and to a lesser extent in hot deserts. Meteorites (in particular metal phases and organics) are better preserved in Antarctica than in hot deserts (Ash and Pillinger 1995; Alexander et al. 2007).
Meteorites are subdivided into two main groups: originating from undifferentiated (chondrite) or differentiated (achondrite, stony-irons and irons) parent bodies. The second group may contain some carbonaceous material but mostly in inorganic form (e.g., graphite, diamonds). Thus, hereafter we focus on chondrites. Chondrites are classified into three categories, ordinary, carbonaceous and enstatite meteorites, according to their bulk composition, mineralogy and petrology (Krot et al. 2014). Among the meteorites collected in Antarctica, 87% are classified as ordinary, 5% as carbonaceous and 1% as enstatite chondrites, the rest comprising not chondritic objects (Grady 2000). This distribution does not reflect the relative abundances of their presumed parent bodies: S-type (for OCs), C-type (for CM carbonaceous chondrites) and possibly D- and P-type asteroids (likely unrepresented by chondrites, but possibly by micrometeorites) (DeMeo and Carry 2013). This suggests that the dynamics of delivery to Earth-crossing orbits might favour OCs over other types, however carbonaceous chondrite material is also expected to be more friable than OC material and could therefore be more represented in micrometeorite collections.
Stratospheric IDPs and micrometeorites are micrometer-sized particles (2–60 μm for IDPs, Rietmeijer 1998; 30–1000 μm for Antarctic micrometeorites (AMMs), Genge et al. 2008), collected in the high stratosphere and in Antarctic and Greenland ice and snow and oceanic sediments, respectively. IDPs are classified according to their bulk composition (chondritic versus non-chondritic, carbon abundance), their morphology (compact or porous) and the dominant silicate, such as olivine and pyroxenes (anhydrous IDPs) or phyllosilicates (hydrated IDPs). Compact porous CP-IDPs generally have an anhydrous mineralogy and are believed to have a cometary origin (Rietmeijer 1998; Bradley 2014). In contrast, hydrated compact IDPs resemble CI, CM and CR chondrites and may be related to asteroids. However, the source of a large fraction of these particles is still controversial because of the anhydrous mineralogy of some asteroids, the probable existence of a comet-asteroid continuum, the hotly discussed origin of zodiacal cloud dusts, and the effects of atmospheric heating that remain only partly understood (e.g., Dermott et al. 2002; Genge et al. 2008; Nesvorný et al. 2010; Vernazza et al. 2012).
Antarctic micrometeorites are classified into four groups: fine-grained, crystalline, scoriaceous and cosmic spherules (Engrand and Maurette 1998; Genge et al. 2008). This classification mostly reflects the degree of heating during atmospheric entry. The respective abundances of the four groups in the CONCORDIA collection (Duprat et al. 2007) are 28%, 8%, 35% and 29%, respectively. The majority of the fine-grained class is comprised of 20% of fine-grained compact types and 8% of fine-grained fluffy types, among which 4% are the rare Ultra Carbonaceous AMMs (UCAMMs). UCAMMs share similarities with anhydrous stratospheric IDPs and cometary grains (Dobrica et al. 2009).
IDPs and AMMs have spiralling orbits due to Poynting-Robertson drag, limiting gravitational bias. They more likely sample friable and fragile objects that spread into micrometer-sized dust. Fine-grained and scoriaceous AMMs are carbon-rich and are related to carbonaceous chondrites, though they are not strictly similar to CI, CM and CR matrices. A fraction of cosmic spherules may be related to ordinary chondrites (e.g., Suavet et al. 2011; Rudraswamy et al. 2016).
2.3 Post-Accretion Processes on the Parent Body
Post-accretion—or secondary—processes refer to physical and chemical modifications occurring during and/or after parent body accretion. This includes geological processes such as aqueous alteration (AA), thermal metamorphism (TM), hydrothermalism, shock metamorphism, and space weathering of the surface and subsurface generated by micrometeorite impacts and solar wind irradiation (Huss et al. 2006). All of these processes have potential effects on the formation or evolution of organic matter.
The extent of thermal metamorphism and aqueous alteration is evaluated by the so-called petrologic type of a meteorite (see review by Huss et al. 2006). A number from 3 to 6 reflects increasing thermal metamorphism and from 3 to 1 an increasing degree of aqueous alteration. In practice, TM and AA are not independent and several chondrites show evidence of the combined effects of both these processes (e.g., the type 3 COs and oxidized CVs). Some C1 and C2 chondrites have experienced short periods of thermal metamorphism, possibly generated by impacts or solar heating (Tonui et al. 2014).
3 Diversity and Complexity of Organics in Meteorites
The search for organics started soon after meteorites were recognized as extraterrestrial rocks (Berthelot 1868). However, this field of research remained uncertain until the 1970s, when a new era was opened by the discovery of extraterrestrial amino acids and other compounds in the Murchison fresh fall (a CM2 chondrite, Kvenvolden et al. 1970). Earlier studies were limited by analytical capacities and by the difficulty to discriminate the contribution of terrestrial contamination.
Organic matter in meteorites is generally described as soluble and insoluble fractions. Soluble organic matter (SOM) comprises molecules that are extracted using common solvents (water, methanol, dichloromethane, toluene, etc). Insoluble organic matter (IOM) is the organic residue recovered after SOM extraction and acid (HF, HCl or CsF) digestion of most minerals. IOM is a polyaromatic organic solid that resembles, in many respects, type III terrestrial kerogens. The respective abundance of SOM versus IOM is unclear and depends upon the chemical class and secondary processes. Older publications (e.g., Hayes 1967) reported a value of 30 wt.%, but they mention neither the experimental protocols nor the chondrites that were used to derive this value.
3.1 Soluble Organic Matter
The Murchison CM2 chondrite has been extensively investigated, due to both its high available mass (100 kg) and the fact that it has undergone very minor terrestrial contamination. It is certainly the most well known chondrite in terms of soluble organics and, as such, is often used as a proxy for comparison with other chondrites. The fraction of molecules with C1–C10 carbon atoms can be classified into 25 groups (Fig. 2.1). Species with C, H and O atoms are the dominant fraction (72 wt.%), followed by C, H, O, N compounds (20 wt.%), C, H, O, S compounds (7 wt.%), and others (1 wt.%). Carboxylic acids are the main compounds, accounting for 43% of the whole SOM content. Gaseous species, amino acids, sugar-related compounds, urea, dicarboximides and sulfonic acids comprise 38%, while other compounds account for 19%.
Top: Abundances of molecular groups (C1–C10) present in SOM extracts of the Murchison CM2 chondrite (plotted with data compiled in Sephton et al. 2002). Bottom: SOM model based on NMR and FT-ICR analysis of methanolic extracts. Source: Adapted from Hertkorn et al. (2015) and Schmitt-Koplin et al. (2010)
Amino acids are of primary interest in astrobiology and have been extensively studied by GC-MS and HPLC combined with fluorescence detection (FD) and mass spectrometry (MS), as well as with dedicated setups for analysing chirality and stable isotopic composition (see Burton et al. 2012 for a thorough review). So far, 80 amino acids have been detected in Murchison, with the following characteristics: (1) carbon numbers ranging between 2 and 9; (2) abundance decreasing with increasing number of carbon atoms; (3) all isomers present for C3–C5 acids; (4) mono-amino acids in the major form with few di-amino acids and N, N acids; (5) racemic composition with some exceptions for most of the amino acids; and (6) D- and 15N-enrichments showing average solar 13C/12C ratios but with significant variations from one compound to another. Racemic mixtures and the D- and 15N-enrichments are convincing evidence of the extraterrestrial origin of these compounds and ended the long-standing debate on a potential contribution of terrestrial contamination.
Carboxylic acids form the dominant group of soluble organics (Sephton 2002). Apart from their interest to astrobiologists, some of these compounds have been detected in the Interstellar Medium (ISM), including formic and acetic acids (Zuckerman et al. 1971), and they are suspected to be present at the surface of comet 67P/Churyumov-Gerasimenko (Capaccioni et al. 2015).
Monocarboxylic acids share common characteristics with amino acids, such as (1) all isomers present in the C3–C5 range, with equal concentration of straight and branched chains, and (2) decreasing abundance with increasing number of carbon atoms (Yuen et al. 1984). Dicarboxylic and hydroxycarboxylic acids have also been detected, related to the most abundant corresponding monocarboxylic acids (Peltzer and Bada 1978). In the bulk rocks, carboxylic acids have been detected as carboxylates and a fraction of these compounds may be present in this form. Carbon and hydrogen isotopic compositions of short carboxylic acids point to an extraterrestrial origin, and high D-enrichments reflect the formation of either precursors or the whole molecule under cold conditions, presumably in the ISM. Other molecules of interest to astrobiology are aldehydes, sugar precursors, alcohols and nucleobases. The reader is referred to Sephton (2002) and Burton et al. (2012) for thorough reviews of these compounds.
Schmitt-Kopplin et al. (2010) reported the first high-resolution FT-ICR spectroscopy of SOM extracted from Murchison with different polar and apolar solvents. The thorough analysis of spectra collected from methanolic extracts with negative and positive electron spray ionization (ESI) led to the identification of 10,299 compositions over the mass range 150–1000 m/z (C10–C50). The main conclusions of this study are the following: (1) a molecular complexity exceeding that of terrestrial organic matter, including that of degradative origin as in soils and sediments; (2) a wide range of aliphatic and aromatic chemical composition including contributions of heteroatoms (O, N, S); (3) the presence of the chemical groups CH2, COO, OH, SH, SO3, NH and very weak N and NH2; (4) the presence of nitrogen as amide or heterocyclic groups; (5) all isomers of each composition; and (6) a global abiotic sulfurization process that occurred subsequent to the formation of the CHO and CHON compounds, presumably in the parent body, by fluid circulation during aqueous alteration. The whole elemental composition of the methanolic extract was estimated to be C100H155O20N3S3 and the total number of soluble molecules in Murchison is estimated to be several million. This is strikingly larger than the ~500 species detected in the C1–C50 range and is evidence of the amazing complexity of SOM.
1D and 2D H and 13C NMR, combined with FT-ICR, provided further insights into the chemical structure of Murchison SOM (Hertkorn et al. 2015). This study proposed a model structure consisting of a molecular center with high chemical diversity and complexity onto which chains formed by aliphatic and carboxylic groups are branched. This peripheral network is readily evidenced in NMR spectra thanks to its simple structure.
In contrast, the molecular center displays high molecular diversity and complexity, combining aromatic species and the heteroatoms O, N and S through a complex linkage that includes C3–C5 aliphatic groups with a statistical branching ratio. Overall, Murchison SOM is a highly aliphatic material with only 5–7% of aromatic species, unlike IOM which is highly aromatic (>60% of aromatic carbons). Note that the polar fraction analysed by ESI FT-ICR contains a large aromatic abundance (~27%) with respect to the entirety of SOM (Schmitt-Kopplin et al. 2010). A long-standing issue has been the origin and formation processes of SOM. The isotopic composition , chirality and high number of isomers for each elemental composition point to a complex chemistry that does not select specific chemical routes. This chemistry presents similarities with ISM chemistry, in particular at low temperatures in order to account for D-enrichments. The detection of amino acids and/or their isomers in comets Wild 2 and 67P/Churyumov-Gerasimenko (Elsila et al. 2009; Altwegg et al. 2016) thus support a pre-accretion origin. Experimental simulations, such as synthesis in cold plasma or radiolysis of ices, show that amino acids can indeed be formed in the ISM or under protosolar disk conditions (Elsila et al. 2007; Horst et al. 2012). The contribution of aqueous chemistry in the parent body has been proposed to account for the synthesis of amino acids through Strecker synthesis (see review by Burton et al. 2012). The location and physical conditions of this chemistry, either in the ISM or in the protosolar disk, is not presently clear.
3.2 Insoluble Organic Matter
The first analysis of IOM (often referred to as ‘organic polymers’ at the time) reported a highly aromatic and condensed material, containing carbonyl, aliphatic and carboxylic chemical groups (Bandurski and Nagy 1976 and references therein). This study also noted similarities with coals, kerogens, humic acid, lignine and laboratory polymers formed by Fischer-Tropsch reactions.
In the following years, several degradative techniques of analysis were successfully applied: gradual, stepwise and flash pyrolysis, hydrous pyrolysis, and combustion and oxidation (Hayatsu et al. 1977, 1980; Hayatsu and Anders 1981; Komiya and Shimoyama 1996; Alexander et al. 1998; Sephton et al. 2000; 2004; Sephton and Gilmour 2001; Wang et al. 2005; Remusat et al. 2005a,b). These experiments revealed highly substituted small-sized polyaromatic units (1–4 rings), an aliphatic linkage composed of short chains, bridged with oxygenated species, such as ester and ether groups, confirming the presence of carboxyl and ketone groups. A 13C NMR study by Cronin et al. (1987) pointed to the presence of aromatic/olefinic and aliphatic carbons in IOMs extracted from Orgueil, Murchison and Allende. Subsequent CP-MAS 13C NMR analyses by Gardinier et al. (2000) revealed and determined the abundance of several chemical groups such as CH3 branched on aliphatics and aromatics, CH2, aliphatic carbons bonded to heteroatoms, protonated and non-protonated aromatic carbons, carboxyls, and carbonyls. The aromatic abundance was found to range between 61–67% for Murchison and 69–78% for Orgueil. The single pulse (SP) and CP-MAS measurements of Cody et al. (2002) confirmed the aromatic content of Murchison IOM, the presence of oxygenated species and the highly branched aliphatic linkage. They also ruled out the presence of large polyaromatic units. Infrared spectroscopy pointed to the presence of aromatic carbons, carbonyl, hydroxyl and aliphatic groups and determined the CH2/CH3 ratio (Hayatsu et al. 1977; Gardinier et al. 2000; Kebukawa et al. 2011; Orthous-Daunay et al. 2013). Insights into the speciation of minor elements were provided by 15N NMR and UV resonant Raman spectroscopy, which identified pyrrholes, indole and carbazole groups in the CI1 Orgueil and cyanide in the CI1 Alais meteorites, respectively (Remusat et al. 2005b; Dobrica et al. 2009). A broad range of sulfur speciation was determined by XANES spectroscopy (Remusat et al. 2005b; Orthous-Daunay et al. 2010).
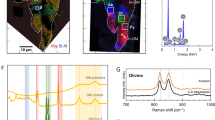
Raman micro-spectroscopy is vibrational spectroscopy operating at the micrometric scale. As a technique that is relatively easy to implement and essentially non-destructive, it has been used in several studies and can be combined with Secondary Ion Mass Spectroscopy (SIMS) and FTIR measurements. A typical Raman spectrum of IOM consists of two peaks at ~1600 and 1350 cm−1, termed the G- and D-bands, respectively. These features provide information on the structure of the carbonaceous material in terms of the degree of disorder and are particularly powerful for discriminating and classifying carbonaceous materials. Raman spectroscopy is basically a resonant process when applied to carbonaceous materials, as they absorb visible and UV photons. The Raman cross-sections of the G- and D-bands are therefore enhanced by several orders of magnitude with respect to those of free molecules and minerals. As a result, Raman measurements are less sensitive to minerals and SOM and are most useful for probing the IOM polyaromatic network. The first Raman spectroscopic studies of meteorites were published in the 1980s (Michel-Lévy and Lautie 1981), but this technique only became more widely used in the 2000s. Measurements on primitive (unmetamorphosed) chondrites have revealed a polyaromatic structure with a high degree of disorder, pointing to a kerogen-like structure in accordance with the results of NMR and degradative techniques (Quirico et al. 2003, 2005, 2009, 2014; Matrajt et al. 2005; Busemann et al. 2007; Starkey et al. 2013). The use of multi-wavelength Raman spectroscopy suggested that the IOM in primitive chondrites experienced some heating in the protosolar disk prior to accretion, although irradiation processes could not be fully excluded (Quirico et al. 2014).
Stable H, C and N isotopic compositions have been extensively investigated since the 1980s. Robert and Epstein (1982) reported large D-enrichments in several primitive carbonaceous chondrites and a large 15N-excess in Renazzo (CR2). These values ruled out IOM synthesis from Fischer-Tropsch or Miller-Urey reactions . The authors suggested synthesis in cold conditions, presumably in the local ISM. A systematic survey of stable isotopic compositions in chondrites from the main groups later confirmed large D-enrichments in the most primitive chondrites, both D- and 15N-enrichments in CR chondrites, and a solar C isotopic composition (Alexander et al. 1998, 2007, 2010). Large variations among chondrites were also observed and were partly attributed to the effects of parent processes. Apart from high enrichments of D- and 15N, a major characteristic of IOM is the heterogeneity of its isotopic composition. Busemann et al. (2006) showed that very high D- and 15N-enrichments are present in micrometric/sub-micrometric areas referred to as hot spots. Hydrous pyrolysis showed that part of the IOM is extractable with water and characterized by 13C- and 15N-enrichments, while the non-extractable, more refractory component is 13C- and 15N-depleted (Sephton et al. 2003). Kerridge et al. (1987) proposed the presence of three isotopically distinct components: aliphatic 13C- and D-enriched, 13C-depleted, D-enriched and 13C-depleted, D-depleted aromatic components. Okumura and Mumura (2011) also pointed out a 13C- and D-enriched aliphatic component, but a single 13C- and D-depleted aromatic component. Wang et al. (2005) reported individual hydrogen isotopic compositions of pyrolysates of several chondrites and confirmed isotopic heterogeneity at the molecular level. Their study also evidenced similar patterns across several primitive chondrites although there were some differences particularly for the C2 Tagish Lake chrondrite, which experienced hydrothermalism at higher temperatures than the more primitive C1 and C2 chondrites. Remusat et al. (2006) combined pyrolysis and ruthenium oxide combustion on IOM extracted from the Orgueil chondrite and attempted to reconstruct the isotopic heterogeneity in the IOM molecular structure prior to degradation. Their study reports a selective deuteration of 1250, 550 and 150‰ in benzylic, aliphatic and aromatic carbons, respectively. The lower deuteration in aromatics with respect to aliphatics is consistent with the results from Okumura and Mimura (2011). The origin of this isotopic heterogeneity is, however, unclear and different mechanisms have been suggested: ion-reactions with H3+ in the upper layers of the protosolar disk (Remusat et al. 2006), interstellar heritage (Okumura and Mimura 2011) or radiolysis-induced fractionation (Le Guillou et al. 2012; Laurent et al. 2014). Note however that this radiolytic process is inhibited at low temperature and that these conclusions are derived from the study of only one chondrite (Orgueil). The selective deuteration in other chondrites is known to be different (Wang et al. 2005). Finally, note also that nitrogen fractionation remains unexplained by these interpretations.
IOM paramagnetic centers have been investigated with Electron Paramagnetic Spectroscopy (EPR). Binet et al. (2002, 2004) showed that Orgueil IOM, unlike type III kerogens and coals, contained paramagnetic radicals heterogeneously distributed in the material. The concentration of radicals also displays dramatic change with temperature, pointing to the occurrence of diradicaloids hosted in ~10-ring polyaromatic units at temperatures below 150 K. Gourier et al. (2008) showed that these paramagnetic centers host large D-enrichments (up to 100,000‰), just one order of magnitude below the highest fractionation reported in dense cores (Parise et al. 2006). Based on these results, Remusat et al. (2009) suggested that the deuterium hot spots in chondritic IOMs were coincident with paramagnetic centers. Delpoux et al. (2011) investigated their electronic structures and showed that they were dominated by biradicals and biradicaloids. They developed a model that suggested that this electronic structure was directly connected to the IOM structure and concluded that C–H breaking and D-enrichments were subsequent to IOM formation. This model, however, does not account for the C and N isotopic compositions.
A major finding in the last twenty years is the dramatic difference in the composition and structure of chondrite groups and between chondrites within the same group. Post-accretion processes have a major impact on IOM, which (1) urges us to select the most primitive objects in order to obtain insights into the protosolar disk and/or presolar cloud chemistry and (2) offers the opportunity to characterize the nature and extent of parent body processes. The effect of thermal metamorphism is very important and has been evidenced through variations in elemental composition (Alexander et al. 1998, 2007; Naraoka et al. 2004; Oba and Naraoka 2009), chemical composition (Kitajima et al. 2002; Cody and Alexander 2005; Wang et al. 2005; Yabuta et al. 2005, 2010; Cody et al. 2008; Alexander et al. 2014) and polyaromatic structure (Vis et al. 2002; Quirico et al. 2003, 2009, 2011, 2014; Bonal et al. 2006, 2007, 2016; Le Guillou et al. 2012; Busemann et al. 2007; Cody et al. 2008). The most common evolution results in the loss of H and heteroatoms as O, N and S (carbonization), accompanied by aromatization and an increase of structural order. The highest-order stage is a metastable O-rich carbonaceous material and, ultimately, the whole IOM is destroyed, at least in carbonaceous and ordinary chondrites (Alexander et al. 2007; Cody et al. 2008; Quirico et al. 2009). The degree of advancement of this transformation was found to be useful in rating the intensity of thermal metamorphism and to separate petrologic types. The determination of peak temperature has been proposed (Busemann et al. 2007; Cody et al. 2008), but these thermometric approaches have been considered as unreliable by other authors (see discussion in Bonal et al. 2016).
The impact of aqueous alteration on IOM is unclear even though it is a major issue given that it could blur valuable pre-accretion molecular or isotopic features for investigating the origin of IOM. Cody and Alexander (2005) proposed an oxidizing process that would lead to the loss of aliphatic groups in type 1 and 2 chondrites, based on four chondrites (Orgueil, Murchison, EET 92042, Tagish Lake). These effects were not confirmed by the subsequent studies of Orthous-Daunay et al. (2013) and Quirico et al. (2014), who observed alteration only in chondrites having experienced short duration thermal metamorphism. Herd et al. (2011) reported significant differences among different lithologies of the aqueously altered Tagish Lake, although it appears that this material experienced hydrothermalism at various elevated temperatures (Alexander et al. 2014). The alteration of the isotopic composition by fluid circulation is the subject of divergent conclusions (Alexander et al. 2010; Bonal et al. 2013; Piani et al. 2015; Remusat et al. 2016), although similar issues investigated in the field of terrestrial organic matter indicate H–D transfer between water and kerogens (Schimmelmann et al. 2006). To conclude, there is, to date, no consensus on the effect of low-temperature aqueous alteration and further studies, including laboratory experiments, will be required to reach firmer conclusions.
4 Organic Matter in Stratospheric IDPs and AMMs
Organics in IDPs and AMMs are less well-known than those in primitive chondrites. The very low mass of these particles (~1–100 ng) severely restricts the number of analytical techniques. In addition, the separation of organics from minerals and into soluble and insoluble fractions is extremely difficult to perform on these small particles and has been done only for a few cases. So far, most studies have focused on bulk raw grains.
The bulk carbon abundance in IDPs is higher than that of carbonaceous chondrites, reaching 45 wt.% and a mean value of ~15 wt.% (Thomas et al. 1993; Rietmeijer 1998). The carbon abundance in AMMs can reach even higher values (up to ~90 wt.% in UCAMMs) (Dartois et al. 2013). XANES spectroscopy at the C and, less frequently, O and N K-edges has provided valuable insights into the compositions of such organic material. This technique operates at a sub-micrometric spatial resolution (down to ~50 nm). Moreover, it can be combined with Transmission Electron Microscopy (TEM) and thus preserves the petrologic context. Electron Energy Loss Spectroscopy (EELS) is also reported in some studies, providing similar information, however sample stability under the electron beam is a very critical issue. Flynn et al. (2003) and Keller et al. (2004) report the characterization of 21 stratospheric IDPs, including 7 hydrated IDPs. They show that almost all particles contain aromatic rings and ketone groups (C=O). A reappraisal of the interpretation of their XANES spectra based the approach of Le Guillou et al. (2014) suggests that carboxylic groups, COOH, are also present. These XANES spectra share similarities with the spectra of chondritic IOM extracted from the Murchison chondrite, as they contain the same three peaks at 285, 286 and 289 eV. However, they display a broader range of variations that can be interpreted as (1) variations in the composition and structure of the IOM component and/or (2) the combined contribution of both IOM and SOM components. The second point is supported by XANES data collected on bulk matrices from Orgueil (CI) and Murchison (CM), which point to large spectral variations (Le Guillou et al. 2014), and by the infrared spectra of CI, CM and CR matrices (Beck et al. 2010; Bonal et al. 2013).
Micro-infrared spectroscopy measurements have been reported in several studies. Spectra show a broad peak around 2800–2900 cm−1, with components that are due to the symmetric and anti-symmetric stretching modes of CH2 and CH3 functional groups (Flynn et al. 2003; Keller et al. 2004; Matrajt et al. 2005; Munoz Caro et al. 2006; Merouane et al. 2014). The CH2/CH3 ratio derived from these features is higher than that in the 3.4 μm band observed in the Diffuse Interstellar Medium, putting into questioning at which step of stellar evolution the IDP organics were formed (molecular cloud, protostar envelope or protosolar disk). The CH2/CH3 ratio is also found to be different from that of IOM extracted from primitive chondrites (which fits the 3.4 μm band observed in the Diffuse Interstellar Medium) and is, in fact, more consistent with thermally processed chondrites (Flynn et al. 2003; Kebukawa et al. 2011; Orthous-Daunay et al. 2013). As mentioned above for XANES data, the interpretation of such similarities and differences is potentially biased by the fact that SOM and IOM are measured together in the case of IDPs, and by the fact that organics in IDPs could be modified during atmospheric entry. Matrajt et al. (2005) report measurements on carbon-enriched IDPs in which HF treatment was used to dissolve the silicates. The infrared spectra of these residues point to the presence of ketone C=O groups and likely carboxylic groups. The spectra also present differences with IOM extracted from primitive chondrites regarding the CH2/CH3 ratio, as well as the position and intensities of the bands in the range 1800–1000 cm−1. Note that this procedure does not fully remove soluble molecules and that these spectra do not represent the insoluble fraction only of the studied IDPs.
The first Raman study on stratospheric IDPs focused on 20 particles, both anhydrous and hydrated, which were classified into six groups (Wopenka 1988). At the time, the low sensitivity of spectrometers produced spectra with low signal-to-noise ratios, which could not be fitted. The spectra were therefore analysed by eye. In addition, the acquisition conditions probably led to sample annealing (compared to current studies, the power applied to the sample was 30× higher and the collection times were 10× higher). This six-group classification scheme was not confirmed by subsequent studies based on 60 IDPs and 40 AMMs, which pointed to (1) the large internal structural heterogeneity within particles, sometimes encompassing the inter-particle heterogeneity, (2) the low degree of structural order (except for 3 of the 60 particles, whose provenance was likely thermally metamorphosed chondrites) and (3) structural signatures dissimilar to that of type 1 and 2 carbonaceous chondrites of CI, CM and CR types (Quirico et al. 2005; Bonal et al. 2006; Busemann et al. 2009; Dobrica et al. 2011; Starkey et al. 2013; Merouane et al. 2014). The structural differences between IDPs/AMMs and carbonaceous chondrites may point to different organics accreted by the parent body, but the effect of heating/oxidation during atmospheric entry and irradiation in space cannot be ruled out. Firm conclusions would require experimental simulations in order to determine the consequences of atmospheric heating.
Raman data collected with a single wavelength excitation (generally 514 or 532 nm) can be enhanced using spectra collected with other wavelengths. Due to the resonance effect, different excitation wavelengths probe different fractions of the samples and improve discrimination. This Multi-Wavelength Raman has been applied to IDPs and resulted in low dispersion of the G-band, i.e., 0.08 cm−1/nm (Starkey et al. 2013). This low dispersion is consistent with values measured in carbonaceous chondrites, but is inconsistent with most amorphous carbons formed in the laboratory and might point to a heating event experienced by insoluble organics trapped in these IDPs (Quirico et al. 2014). Raman spectra collected with a 244 nm excitation have shown clear structural differences between an IDP, a UCAMM and primitive carbonaceous chondrites and have made it possible to identify the –CN cyanide chemical group (Dobrica et al. 2011). Overall, these data suggest that the insoluble component of organics in IDPs/AMMs might be inherited from a heating event. As organics are very similar in anhydrous and hydrated IDPs (Flynn et al. 2003; Merouane et al. 2014), this heating may have occurred prior to accretion in the inner region of the protosolar disk and hydrothermalism on the parent body may not have been involved in their formation.
The isotopic and elemental compositions of bulk organics (H, C and N) in IDPs and AMMs at the micrometric and sub-micrometric scales have been determined by SIMS. The C/H ration was found to range between 1 and 3 in IDPs (Aléon et al. 2001) and between 2 and 6 in UCAMMs (Duprat et al. 2010). Note however that bias due to instrumental fractionation might be serious in the case of the H/C ratio. The C/N ratio also shows variations at the micrometric scale, with areas characterized by N abundances as high as 10–20 wt.% (Aléon et al. 2003), while in UCAMM a high N/C ratio is observed across a large area (Dartois et al. 2013). In IDPs, as in IOM extracted from chondrites, the isotopic composition is heterogeneous at the micrometer and sub-micrometer scales, with hot spots that show very high D- and 15N-enrichments (Messenger 2000, 2002; Aléon et al. 2001, 2003; Keller et al. 2004; Floss et al. 2006; Busemann et al. 2006, 2009). UCAMMs also show very high D-enrichments across larger areas and not restricted to tiny hot spots (Duprat et al. 2010). The D-enrichments have been interpreted as a fingerprint of low-temperature chemistry and ion-molecule reactions, which occurred either in the local ISM or in the protosolar disk. Due to the small size of IDPs and AMMs, several techniques—such as EPR—could not be applied and it is therefore unclear whether these high D-enrichments correspond to radicals, diradicals and diradicaloids as observed in the IOM extracted from the CI Orgueil chondrite. Lastly, AIB amino acids were identified in AMMs by Brinton et al. (1998) and Matrajt et al. (2004). These amino acids have been proved to be endogenous.
5 Origin and Formation of Organics in Chondrites and Dust
The origin of IOM in chondrites remains a debated issue. In the 1970s, formation in the protosolar disk through Fischer-Tropsch reactions was the favoured mechanism of production due to the similarities with laboratory analogs (Hayatsu et al. 1977) and to the general context of a fully gaseous solar nebula with a full reset of interstellar conditions (e.g., Grossman and Larimer 1974). This view was severely challenged with the discovery of large D-enrichments that pointed to a cold chemistry, presumably in the ISM (Robert and Epstein 1982). Fischer-Tropsch reactions were not able to provide the large H isotopic fractionations and phyllosilicates that were advocated to catalyse those reactions were formed in the parent body and not in the protosolar disk (Alexander et al. 1998). Concurrently, the spectral match of the aliphatic peak at 3.4 μm in the infrared spectra of Orgueil IOM and the diffuse ISM (collected towards the Galactic center) supported an interstellar origin (Ehrenfreund et al. 1991). This view was challenged around the mid-2000s. The 3.4 μm feature of diffuse ISM has since been assigned to hydrogenated amorphous carbon that is not similar to IOM (Pendleton and Allamandola 2002; Dartois et al. 2004, 2007). The cometary grains brought back by the Stardust mission confirmed observations made by the satellite ISO in the 1990s, i.e., crystalline minerals formed in the inner hot region of the protosolar disk are present in comets (Crovisier et al. 1997; Zolensky et al. 2006b). To date, estimating the abundance of amorphous versus crystalline minerals in comets remains difficult and the proportions of presolar versus protosolar materials is unclear.
The interpretation of the elevated D/H ratio in IOM in terms of ISM heritage has also been challenged. A debate has emerged about whether the conditions in the protosolar disk were favourable to ion-molecule reactions (Henning and Semenov 2013). The selective deuteration of molecular groups of IOM has, for instance, been interpreted by ion-molecule reactions in cold regions of the protosolar disk (Remusat et al. 2006, 2009; Delpoux et al. 2011), but other studies argue that the penetration of Galactic Cosmic Rays within a protoplanetary disk is shielded against by stellar winds (Cleeves et al. 2014). Recent publications have also experimentally demonstrated that radiolytic processes can produce moderate deuterium enrichment in organic solids, but these processes seem to be inhibited at low temperature and cannot account for the high values reported for hot spots (Laurent et al. 2014, 2015). On the other hand, the main carbon reservoirs in diffuse ISM are polycyclic aromatic hydrocarbons (PAHs) and hydrogenated amorphous carbons (HACs), which both formed in hot circumstellar environments. Therefore, they are not expected to bear deuterium enrichments. In conclusion, deuteration does not appear to be a clear and unambiguous tracer of IOM origin.
The distinction between solar versus interstellar is also somewhat simplistic and artificial. The evolution of diffuse ISM towards planets is actually a continuous succession of varying environments with overlaps of conditions between subsequent stages. PAHs and HACs are not recovered directly in meteorites. They may have been transformed to various extents either in molecular clouds through the action of GCRs or possibly in the inner hot region of the protosolar disk (Alexander et al. 2010; Quirico et al. 2014). To date, the respective effects of ion irradiation and thermal processing on these solids have not been clearly established (Bernstein et al. 2003; Brunetto et al. 2009) and require more observational and experimental investigations. Note that an alternate view proposes the formation of IOM from chondrites and IDPs through thermal processes within the parent body through the carbonization of H2CO precursors in aqueous conditions (Cody et al. 2011; Kebukawa et al. 2013). This scenario is however inconsistent with the lack of liquid water in comets in the case of IDPs and it does not satisfactorily account for the IOM isotopic heterogeneity.
Organics in IDPs and AMMs are much less known, as outlined above, but comparison with chondritic organic matter sheds light on their origin. First, they share a number of similarities: (1) functional groups such as aliphatic, carboxyl, ketone and aromatic groups; (2) a disordered polyaromatic structure (with a weakly dispersive G-band position); (3) large and heterogeneous D- and 15N-enrichments and (4) soluble and insoluble components. These similarities support the view that organics in meteorites and dusts were synthesized under fairly similar conditions. In this respect, solving the puzzle of the origin of chondritic organics might help resolve that of organics in dusts as well. However, this interpretation may not hold for the N-rich UCAMMs that might have been formed at the surface of TNOs or cometary nuclei in the Oort belt (Dartois et al. 2013).
In the near future, several complementary approaches should provide new clues and push the debate forward. Firstly, new techniques can simultaneously characterize the SOM and IOM at the sub-micrometer scale within their petrologic context, for instance XANES microscopy combining measurements at the C, N and O K-edge. The development of ultra-spectroscopy (infrared and Raman spectrometers coupled to an Atomic Force Microscope) should even provide insights into the composition at a spatial scale <10 nm (Dominguez et al. 2014). The development of protocols for characterizing SOM from micrometer-sized particles with High Resolution Mass Spectrometry and isolation of IOM from chondritic (small) clasts and IDPs and AMMs should soon provide very valuable data. Lastly, the ALMA and JWST instruments will provide insights into the composition of protoplanetary disks with an unprecedented angular resolution. This will provide important information on the chemical and isotopic heterogeneity in protoplanetary disks and thus on the extent to which an ISM-like chemistry can be induced.
6 Dust and Meteorites at the Surface of Mars
6.1 Flux of Exogenous Matter on Mars
The amount of extraterrestrial input to the surface of Mars has been constrained by several studies. In particular, Flynn and McKay (1990) concluded that fine-grained meteoritic material (60–1200 μm) is a major contributor to the exogenous input on Mars. They advocated the return of Mars soil samples in which the sampling of micrometeorites might reveal a complementary picture to micrometeorites on Earth. Most particles larger than 100 μm are melted on entry into the Earth’s atmosphere, but given the lower density and lower gravity of the Martian atmosphere, 90% of these particles would survive Martian atmospheric entry without significant melting. For particles of 1000 μm diameter, the estimated 30% survival percentage is much higher than that on Earth. Flynn and McKay (1990) estimated the planet-wide meteoritic mass influx on Mars to be between 2700 and 59,000 t/year; the large range being related to the uncertainty in the terrestrial flux. With a production rate of Martian soil of 1 m per billion years, they estimated the concentration of micrometeorites ranging from 2% to 29% by mass (Flynn and McKay 1990).
In a complementary study, Bland and Smith (2000) focused on meteorite accumulation on Mars. Entry speed (from 5 to 30 km s−1, mean value of 10.2 km s−1), initial mass at the top of the atmosphere of Mars (10–130 g) and ablation rate are some of the main input parameters. Based on experimental studies, it is assumed that 1.6 km s−1 is the upper limit of survivability for stony meteorites impacting Mars. To estimate the impact rate of meteorites on Mars, a scaling factor of 2.6 compared to the present flux of meteoroids reaching Earth (based on crater statistics and reflecting for example the closer proximity of Mars to the asteroid belt) was considered. Bland and Smith (2000) then computed the proportions of meteoroids of a given entry mass surviving impact on Mars. They showed that a narrow range of small masses (10–50 g) should survive. Bland and Smith (2000) further calculated (in what they deemed a conservative estimation) a flux of 44–176 meteorites in the mass range 10–50 g at the surface of Mars, per 106 km2 per year. Chemical and physical weathering operating on Mars is rather limited: oxidation would indeed occur on a timescale of 109 years, rather than 105 to 106 years as observed on Earth and based on analyses of meteorites from Antarctica. This absence of significant chemical alteration is confirmed by the remote study of iron meteorites found on Mars, in which iron oxides do not dominate (Schröder et al. 2008; Ashley et al. 2011). Bland and Smith (2000) thus concluded that meteorite accumulation on Mars could possibly be quite significant.
Finally, Davis (1993) demonstrated that the number of large impacts (>10 kg) should be similar to that on the Moon, with ~100 events per year.
This non-negligible contribution of meteoritic material to the surface of Mars is in agreement with analytical data. For example, based on X-Ray fluorescence data, Boslough (1988) suggested that the soils analysed by Viking are consistent with a mixture of 60% basaltic rock fragments and 40% meteoritic material. More recently, elemental analysis of siderophile elements (Ni, Ir) in the Martian meteorite NWA 7533 were explained by admixing up to 5% of meteoritic material in Martian regolith (Humayun et al. 2013). The composition of the Moon is also informative. Without an atmosphere, there is no deceleration to free-fall velocity of the impacting material. As a consequence, only a few projectiles survive as un-melted fragments on the Moon. This is consistent with only a few rare millimeter-diameter fragments of meteorites recovered in lunar soil samples (e.g., Rubin 1997). Nevertheless, extraterrestrial matter contributes to the lunar regolith as indicated, for example, by the abundance of iridium in lunar soils (Anders et al. 1973).
To conclude, there might be a substantial input and preservation of extraterrestrial matter in Martian soils. Considering that this implies the presence of significant exogenous organic matter, several questions are raised concerning the preservation of organics once on Mars given the absence of clear detection of relatively abundant organics by the various rovers on Mars.
6.2 Exogenous Organics on Mars: Detection and Preservation
Mars’ proximity to the asteroid belt may lead to sampling of different proportions of asteroidal material when compared with Earth. Moreover, meteorites arriving at the surface of Mars impact at a higher speed: their survival will thus depend on sample consistency and cohesive strength and whether the timescales of weathering are longer than on Earth. If this is the case, meteorites on Mars may reveal a slightly different history (older) to Earth (Flynn and McKay 1990). However, in the absence of reliable constraints, we here consider similar compositions for the material accreted by Mars and Earth.
In what follows, we discuss only meteoritic organics and do not consider carbonaceous molecules that could derive from Martian reservoirs (e.g., lithospheric magmatic carbon, weathering of carbonates in the hydrosphere, etc.). Since the meteoritic mass accreted by Mars is largely dominated by the flux of dust (see Sect. 2.6), we will neglect the flux of meteorites (Bland and Smith 2000) and large objects (Davis 1993) for the evaluation of exogenous organic matter. Taking into account (1) the present flux of dust at Mars (Flynn and McKay 1990), (2) the fraction of particles not heated above the pyrolysis temperature of carbonaceous matter (Flynn 1996) and (3) and assuming an average carbon content of 10 wt.% in IDPs (Thomas et al. 1993), the accretion rate of unaltered meteoritic carbon by Mars is estimated to be 2.4 × 105 kg/year (Flynn 1996).
Assessing past habitability and detecting potential preserved biosignatures are some of the major scientific goals of current and future missions on/to Mars. Models of dust flux on Mars clearly indicate that there is a non-negligible input of organic-containing extraterrestrial matter. Thus the detection, up to now, of only of a few organic molecules at low abundance is quite puzzling. This can either be interpreted as being due to (1) measurement biases and/or a low instrumental sensitivity or (2) the absence of organics in the analysed soil samples, explained for example by environmental conditions not in favour of the preservation of organics.
Our current knowledge concerning the organic compounds that are present in Martian soils has been derived only from molecular analysis from three experiments on board Martian landers: (1) Gas Chromatograph and Mass Spectrometer (GC-MS) experiments on board the Viking Lander (Biemann et al. 1977) and (2) on board MSL with the SAM instrument (Mahaffy et al. 2012) and (3) the Thermal and Evolved Gas Analyser (TEGA) on board Phoenix (Ming et al. 2009). These instruments require pyrolysis of the samples as a first step for analysis. In the case of Mars, clear difficulties were revealed in comparison with experiments conducted in laboratories on Earth, in particular for the interpretation of the data as the heating procedures induce an oxidation of the organics by the soils (Navarro-González et al. 2006; Steininger et al. 2012; Freissinet et al. 2015). The presence of perchlorates at the Martian surface (Hecht et al. 2009; Glavin et al. 2013) does not necessarily preclude the detection of organics by gas chromatography, but it clearly complicates data interpretation (e.g., Freissinet et al. 2015; Millan et al. 2016), for instance by the formation of new organic molecules through the decomposition of perchlorates (e.g., chlorohydrocarbons; Millan et al. 2016; Steininger et al. 2012) or by the combustion of organics through the release of O2. Moreover, according to Benner et al. (2000), upon pyrolysis, meteoritic organic compounds are most likely to be converted to carboxylic acid derivatives under Martian conditions and these would be generated with variable yields, in addition to the generation of non-volatile components, carbon dioxide, carbon monoxide and water. All but the non-volatile material would be detectable by GC-MS, but since they are also components of the Martian atmosphere, data interpretation is more difficult (Benner et al. 2000). The identification of chlorinated organic compounds required numerous laboratory analogue studies, but it has finally been concluded that they originate from Martian organic carbon (as opposed to terrestrial contamination by the instrument) (Freissinet et al. 2015). Viking data have been reconsidered and may reflect a few ppm of organic material in Martian soils (Navarro-González et al. 2006, 2010; Navarro-González and McKay 2011), although even this is debated (Biemann and Bada 2011).
In addition to analytical issues, the environmental conditions on Mars may induce some modification of the exogenous organic input through photochemical (UV irradiation by sunlight and by galactic and solar energetic particles) and oxidation processes, which are characterized by distinct kinetics and scales of interaction. In particular, oxidation may be effective from a few meters to possibly hundreds of meters in depth (Davila et al. 2008), whereas UV radiation can interact only with the surface (a few mm) of the regolith. Solar energetic particles and galactic cosmic rays have much higher energy than UV photons, but with a much lower flux. These energetic particles may degrade organics on a timescale of hundreds of millions of years (e.g., Kminek and Bada 2006), but UV photons act much more rapidly, typically in a few days to months (e.g., Poch et al. 2014).
Experimental simulations of Martian soils processes have focused on UV irradiation and oxidative processes. Although indispensable for improving our understanding of the on-going processes operating in Martian soils and their consequences for organics, laboratory experiments simulate relatively simple systems for which the results are not easily directly applicable to Martian conditions. For example, several studies have focused only on simple organic molecules, while the most common organic matter in micrometeorites and IDPs a complex macromolecule. The regeneration of Martian soils is rarely taken into account. Mineral grains that potentially play a shielding role are present in only a few recent studies (e.g., Poch et al. 2015). Nevertheless, even though they might not be directly applicable to natural Martian conditions, these experiments are informative and imply that there is modification but not total destruction of the organics (e.g., Burns and Fisher, 1993; Stoker and Bullock 1997; Moores and Schuerger, 2012; Poch et al. 2013, 2014, 2015—a non-exhaustive list of references). The absence of detection of high abundances of organic compounds in Martian soils does not necessarily imply the absence of exogenous organics on Mars. The use of different analytical techniques that do not requiring pyrolysis as a first step will undoubtedly shed new light on the question of organics on Mars. This is the hope placed in the SuperCam instrument on board the Mars2020 rover, which combines Laser Induced Breakdown Spectroscopy, Raman spectroscopy, time-resolved fluorescence and visible and near-infrared spectroscopy (Maurice et al. 2015).
References
Aléon J, Engrand C, Robert F et al (2001) Clues to the origin of interplanetary dust particles from the isotopic study of their hydrogen-bearing phases. Geochim Cosmochim Acta 65:4399–4412
Aléon J, Robert F, Chaussidon M et al (2003) Nitrogen isotopic composition of macromolecular organic matter in interplanetary dust particles. Geochim Cosmochim Acta 67:3773–3783
Alexander CMO’D, Russell SS, Arden JW et al (1998) The origin of chondritic macromolecular organic matter: a carbon and nitrogen isotope study. Meteorit Planet Sci 33:603–622
Alexander CMO’D, Fogel M, Yabuta H et al (2007) The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim Cosmochim Acta 71:4380–4403
Alexander CMO’D, Newsome SN, Fogel ML et al (2010) Deuterium enrichments in chondritic macromolecular material – implications for the origin and evolution of organics, water and asteroids. Geochim Cosmochim Acta 74:4417–4437
Alexander CMO’D, Cody GD, Kebukawa Y et al (2014) Elemental, isotopic and structural changes in Tagish Lake insoluble organic matter produced by parent body processes. Meteorit Planet Sci 49:503–525
Alexander CMO’D, Cody GD, De Gregorio BT et al (2017) The nature, origin and modification of insoluble organic matter in chondrites, the possibly interstellar source of Earth’s C and N. Chem Erde 77:227–256
Altwegg K, Balsiger H, Bar Nun A et al (2016) Prebiotic chemicals amino acid and phosphorus in the coma of comet 67P/Churyumov-Gerasimenko. Sci Adv 2:e1600285
Anders E, Ganapathy R, Krähenbühl U et al (1973) Meteoritic material on the Moon. The Moon 8:3–24
Ash RD, Pillinger CT (1995) Carbon, nitrogen and hydrogen in Saharan chondrites: the importance of weathering. Meteoritics 30:85–92
Ashley JW, Golombek MP, Christensen PR et al (2011) Evidence for mechanical and chemical alteration of iron-nickel meteorites on Mars: process insights for Meridiani Planum. J Geophys Res 116:E00F20
Bandurski EL, Nagy B (1976) The polymer-like organic material in the Orgueil meteorite. Geochim Cosmochim Acta 40:1397–1406
Beck P, Quirico E, Montes-Hernandez G (2010) Hydrous mineralogy of CM and CI chondrites from infrared spectroscopy and their relationship with low albedo asteroids. Geochim Cosmochim Acta 74:4881–4892
Benner SA, Devine KG, Matveeva LN et al (2000) The missing organic molecules on Mars. Proc Natl Acad Sci USA 97:2425–2430
Bernstein MP, Moore MH, Elsila JE et al (2003) Side group addition to the polycyclic aromatic hydrocarbon coronene by proton irradiation in cosmic ice analogs. Astrophys J 582:L25–L29
Berthelot P (1868) Cosmologie – Sur la matière charbonneuse des météorites. C R Hebd Seances Acad Sci 67:849
Biemann K, Bada JL (2011) Comment on “Reanalysis of the Viking results suggests perchrlorate and organics at midlatitudes on Mars” by Rafael Navarro-Gonzáles et al. J Geophys Res 116:E12001
Biemann K, Oro J, Toulmin P III et al (1977) The search for organic substances and inorganic volatile compounds in the surface of Mars. J Geophys Res 82:4641–4658
Binet L, Gourier D, Derenne S et al (2002) Heterogeneous distribution of paramagnetic radicals in insoluble organic matter from the Orgueil and Murchison meteorites. Geochim Cosmochim Acta 66:4177–4186
Binet L, Gourier D, Derenne S et al (2004) Occurence of abundant diradicaloid moieties in the insoluble organic matter from the Orgueil and Murchison meteorites: a fingerprint of its extraterrestrial origin? Geochim Cosmochim Acta 68:881–891
Bland PA, Smith TB (2000) Meteorite accumulation on Mars. Icarus 144:21–26
Bland PA, Smith TB, Jull AJT et al (1996) The flux of meteorites to the Earth over the last 50000 years. Mon Not R Astron Soc 283:551–565
Bonal L, Quirico E, Bourot-Denise M (2006) Determination of the petrologic type of CV3 chondrites by Raman spectroscopy of included organic matter. Geochim Cosmochim Acta 70:1849–1863
Bonal L, Bourot-Denise M, Quirico E et al (2007) Organic matter and metamorphic history in CO chondrites. Geochim Cosmochim Acta 71:1605–1623
Bonal L, Alexander CMO’D, Huss GR et al (2013) Hydrogen isotopic composition of the water in CR chondrites. Geochim Cosmochim Acta 106:111–113
Bonal L, Quirico E, Flandinet L et al (2016) Thermal history of type 3 chondrites from the Antarctic meteorite collection determined by Raman spectroscopy of their polyaromatic carbonaceous matter. Geochim Cosmochim Acta 189:312–337
Boslough MD (1988) Meteoritic enrichment of Martian regolith. Abstracts of the Lunar and Planetary Science Conference 19:120
Bradley JP (2014) Early Solar Nebula grains – interplanetary dust particles. In: Davis AM (ed) Meteorites and cosmochemical processes. Volume 1 of Treatise on geochemistry, 2nd edn. Elsevier, Amsterdam, pp 287–308
Briani G, Gounelle M, Bourot-Denise M et al (2012) Xenoliths and microxenoliths in H chondrites: sampling the zodiacal cloud in the asteroid Main Belt. Meteorit Planet Sci 47:880–902
Brinton KLF, Engrand C, Glavin DP et al (1998) A search for extraterrestrial amino acids in carbonaceous Antarctic micrometeorites. Orig Life Evol Biosph 28:413–424
Brunetto R, Pino T, Dartois E, Cao A-T, d'Hendecourt L, Strazzulla G, Bréchignac P (2009) Comparison of the Raman spectra of ion irradiated soot and collected extraterrestrial carbon. Icarus 200:323–337
Burns RG, Fisher DS (1993) Rates of oxidative weathering on the surface of Mars. J Geophys Res 98(E2):3365–3372
Burton AS, Stern JC, Elsila JE et al (2012) Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem Soc Rev 41:5459–5472
Busemann H, Young A, Alexander CMO’D et al (2006) Interstellar chemistry recorded in organic matter from primitive meteorites. Science 312:727–730
Busemann H, Alexander CMO’D, Nittler LR (2007) Characterization of insoluble organic matter in primitive meteorites by microRaman spectroscopy. Meteorit Planet Sci 42:1387–1416
Busemann H, Nguyen AN, Cody GD et al (2009) Ultra-primitive interplanetary dust particles from the comet 26P/Grigg-Skjellerup dust stream collection. Earth Planet Sci Lett 288:44–57
Capaccioni F, Coradini A, Filacchione G et al (2015) The organic-rich surface of comet 67P/Churyumov-Gerasimenko as sees by VIRTIS/Rosetta. Science 347:aaa0628
Cleeves LI, Bergin EA, Alexander CMO’D et al (2014) The ancient heritage of water ice in the solar system. Science 345:1590–1593
Cody GD, Alexander CMO’D (2005) NMR studies of chemical structural variation of insoluble organic matter from different carbonaceous chondrite groups. Geochim Cosmochim Acta 69:1085–1097
Cody GD, Alexander CMO’D, Tera F (2002) Solid state (1H and 13C) NMR spectroscopy of the insoluble organic residue in the Murchison meteorite: a self-consistent quantitative analysis. Geochim Cosmochim Acta 66:1851–1865
Cody GD, Alexander CMO’D, Yabuta H et al (2008) Organic thermometry for chondritic parent bodies. Earth Planet Sci Lett 272:446–455
Cody GD, Heying E, Alexander CMO’D et al (2011) Establishing a molecular relationship between chondritic and cometary organic solids. Proc Natl Acad Sci USA 108:19171–19176
Cronin JR, Pizzarello S, Frye JS (1987) 13C NMR spectroscopy of the insoluble carbon of carbonaceous chondrites. Geochim Cosmochim Acta 51:299–303
Crovisier J, Leech K, Bockelée-Morvan D et al (1997) The spectrum of comet Hale-Bopp (C/199501) observed with the Infrared Space Observatory at 2.9 astronomical units from the Sun. Science 275:1904–1907
Dartois E, Marco O, Munoz-Caro GM et al (2004) Diffuse interstellar medium organic polymers. Astron Astrophys 423:L33–L36
Dartois E, Geballe TR, Pino T et al (2007) IRAS 08572+3915: constraining the aromatic versus aliphatic content of interstellar HACs. Astron Astrophys 46:635–640
Dartois E, Engrand C, Brunetto R et al (2013) Ultracarbonaceous Antarctic micrometeorites, probing the solar System beyond the nitrogen snow-line. Icarus 224:243–252
Davila AF, Fairen AG, Gago-Duport L et al (2008) Subsurface formation of oxidants on Mars and implications for the preservation of organic biosignatures. Earth Planet Sci Lett 272:456–463
Davis PM (1993) Meteoroid impacts as seismic sources on Mars. Icarus 105:469–478
Delpoux O, Gourier D, Vezin H et al (2011) Biradical character of D-rich carriers in the insoluble organic matter of carbonaceous chondrites: a relic of the protoplanetary disk chemistry. Geochim Cosmochim Acta 75:326–336
DeMeo FE, Carry B (2013) The taxonomic distribution of asteroids from multi-filter all-sky photometric surveys. Icarus 226:723–741
Dermott SF, Durda DD, Grogan K et al (2002) Asteroidal dust. In: Bottke WF Jr, Cellino A, Paolicchi P et al (eds) Asteroids III. University of Arizona Press, Tucson, pp 423–442
Dobrica E, Engrand C, Duprat J et al (2009) Connection between micrometeorites and Wild 2 particles from Antarctic snow to cometary ices. Meteorit Planet Sci 44:1643–1661
Dobrica E, Engrand C, Quirico E et al (2011) Raman characterization of carbonaceous matter in CONCORDIA Antarctic micrometeorites. Meteorit Planet Sci 46:1363–1375
Dominguez G, McLeod AS, Gainsforth Z et al (2014) Nanoscale infrared spectroscopy as a non-destructive probe of extraterrestrial samples. Nat Commun 5:5445
Duprat J, Engrand C, Maurette M et al (2007) Micrometeorites from Central Antarctic snow: the CONCORDIA collection. Adv Space Res 39:605–611
Duprat J, Dobrica E, Engrand C et al (2010) Extreme deuterium excesses in ultracarbonaceous micrometeorites from central Antarctic snow. Science 328:742–745
Ehrenfreund P, Robert F, d’Hendecourt L et al (1991) Comparison of interstellar and meteoritic organic-matter at 3.4 μm. Astron Astrophys 252:712–717
Elsila JE, Dworkin JP, Berstein MP et al (2007) Mechanisms of amino acid formation in interstellar ice analogs. Astrophys J 660:911–918
Elsila JE, Glavin DP, Dworkin JP (2009) Cometary glycine detected in samples returned by Stardust. Meteorit Planet Sci 44:1323–1330
Engrand C, Maurette M (1998) Carbonaceous micrometeorites from Antarctica. Meteorit Planet Sci 33:565–580
Farley KA (1995) Cenozoic variations in the flux of interplanetary dust recorded by 3He in a deep-sea sediment. Nature 376:153–156
Floss C, Stadermann FJ, Bradle JP et al (2006) Identification of isotopically primitive interplanetary dust particles: a NanoSIMS isotopic imaging study. Geochim Cosmochim Acta 70:2371–2399
Flynn GJ (1996) The delivery of organic matter from asteroids and comets to the early surface of Mars. Earth Moon Planets 72:469–474
Flynn GJ, McKay DS (1990) An assessment of the meteorite contribution to the Martian soil. J Geophys Res 95:14497–14509
Flynn GJ, Keller LP, Feser M et al (2003) The origin of organic matter in the solar system: evidence from the interplanetary dust particles. Geochim Cosmochim Acta 67:4791–4806
Freissinet C et al (2015) Organic molecules in the Sheepbed Mudstone, Gale Crater, Mars. J Geophys Res Planets 120:495–514
Gabrielli P, Barbante C, Plane JMC et al (2004) Meteorite smoke fallout over the Halocene epoch revealed by iridium and plantinum in Greenland ice. Nature 432:1011–1014
Gardinier A, Derenne S, Robert F et al (2000) Solid state CP/MAS C-13 NMR of the insoluble organic matter of the Orgueil and Murchison meteorites: quantitative study. Earth Planet Sci Lett 184:9–21
Gattacceca J, Valenzuela M, Uehara M et al (2011) The densest meteorite collection area in hot deserts: the San Juan meteorite field (Atacama Desert, Chile). Meteorit Planet Sci 46:1276–1287
Genge MJ, Engrand C, Gounelle M et al (2008) The classification of micrometeorites. Meteorit Planet Sci 43:497–515
Glavin DP, Freissinet C, Miller KE et al (2013) Evidence for perchlorates and the origin of chlorinated hydrocarbons detected by SAM at the Rocknest aeolian deposit in Gale Crater. J Geophys Res Planets 118:1955–1973
Gounelle M, Zolensky ME, Liou J-C et al (2003) Mineralogy of carbonaceous chondritic microsclasts in Howardites: identification of C2 fossil micrometeorites. Geochim Cosmochim Acta 67:507–527
Gounelle M, Spurny P, Bland PA (2006) The orbit and atmospheric trajectory of the Orgueil meteorite from historical records. Meteorit Planet Sci 41:135–150
Gourier D, Robert F, Delpoux O et al (2008) Extreme deuterium enrichment of organic radicals in the Orgueil meteorite: revisiting the interstellar interpretation? Geochim Cosmochim Acta 72:1914–1923
Grady MM (2000) Catalogue of meteorites. Cambridge University Press, Cambridge
Grossman L, Larimer JW (1974) Early chemical of the solar system. Rev Geophys Space Phys 12:71–101
Halliday I, Blackwell AT, Griffin AA (1984) The frequency of meteorite falls on the Earth. Science 223:1405–1407
Halliday I, Blackwell AT, Griffin AA (1989) The flux of meteorites on the Earth’s surface. Meteoritics 24:173–178
Harvey RP, Cassidy WA (1989) A statistical comparison of Antarctic finds and modern falls: mass frequency distributions and relative abundance by type. Meteoritics 24:9–14
Hayes JM (1967) Organic consistuents of meteorites – a review. Geochim Cosmochim Acta 31:1395–1440
Hayatsu R, Matsuoka S, Scott RG et al (1977) Origin of organic matter in earlt solar system. VII. The organic polymer in carbonaceous chondrites. Geochim Cosmochim Acta 41:1325–1339
Hayatsu R, Winans RE, Scott RG et al (1980) Phenolic esters in the organic polymer of the Murchison meteorite. Science 207:1202–1204
Hayatsu R, Anders E (1981) Organic compounds in meteorites and their origins. Top Curr Chem 99:3–37
Hecht MH, Kounaves SP, Quinn RC et al (2009) Detection of perchlorate and the soluble chemistry of Martian soil at the Phoenix Lander Site. Science 325:64–67
Henning T, Semenov D (2013) Chemistry in protoplanetary disks. Chem Rev 113:9016–9042
Herd CDK, Blinova A, Simku DN et al (2011) Origin and evolution of prebiotic organic matter as inferred from the Tagish Lake meteorite. Science 332:1304–1307
Hertkorn N, Harir M, Cawley KM et al (2015) Molecular characterization of dissolved organic matter from subtropical wetlands: a comparative study through the analysis of optical properties, NMR and FTICR/MS. Biogeosciences 13:2257–2277
Horst SM, Yelle RV, Buch A et al (2012) Formation of amino acids and nucleotides bases in a Titan atmosphere simulation experiment. Astrobiology 12:809–817
Humayun M, Nemchin A, Zanda B et al (2013) Origin and age of the earliest Martian crust from meteorite NWA 7533. Nature 503:513–516
Hunten DM, Turco RP, Toon OB (1980) Smoke and dust particles of meteoric origin in the mesosphere and stratosphere. J Atmos Sci 37:1342–1357
Huss GR, Rubin A, Grossman J (2006) Thermal metamorphism in chondrites. In: Lauretta DS, McSween HY Jr (eds) Meteorites and the early solar system II. University of Arizona Press, Tucson, pp 567–586
Jull AJT (2006) Terrestrial ages of meteorites. In: Lauretta DS, McSween HY Jr (eds) Meteorites and the early solar system II. University of Arizona Press, Tucson, pp 889–905
Kebukawa Y, Alexander CMO’D, Cody GD (2011) Compositional diversity in insoluble organic matter in type 1, 2 and 3 chondrites as detected by infrared spectroscopy. Geochim Cosmochim Acta 75:3530–3541
Kebukawa Y, Kilcoyne ALD, Cody GD (2013) Exploring the potential formation of organic solids in chondrites and comets through polymerization of interstellar formaldehyde. Astrophys J 771:19
Keller LP, Messenger S, Flynn GJ et al (2004) The nature of molecular cloud material in interplanetary dust. Geochim Cosmochim Acta 68:2577–2589
Kerridge J, Chang S, Shipp R (1987) Isotopic characterization of kerogen-like material in the Murchison carbonaceous chondrite. Geochim Cosmochim Acta 51:2527–2540
Kitajima F, Nakamura T, Takaoka N et al (2002) Evaluating the thermal metamorphism of CM chondrites by using the pyrolitic behavior of carbonaceous macromolecular matter. Geochim Cosmochim Acta 66:163–172
Kminek G, Bada JL (2006) The effect of ionizing radiation on the preservation of amino acids on Mars. Earth Planet Sci Lett 245:1–5
Komiya M, Shimoyama A (1996) Organic compounds from insoluble organic matter isolated from the Murchison carbonaceous chondrite by heating experiments. Bull Chem Soc Jpn 69:53–58
Krot AN, Keil K, Scott ERD et al (2014) Classification of Meteorites and their genetic relationships. In: Davis AM (ed) Meteorites and cosmochemical processes. Volume 1 of Treatise on geochemistry, 2nd edn. Elsevier, Oxford, pp 1–63
Kvenvolden K, Lawless J, Pering K et al (1970) Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite. Nature 228:923–926
Lanci L, Kent DV (2006) Meteoritic smoke fallout revealed by superparamagnetism in Greenland ice. Geophys Res Lett 33:L13308
Laurent B, Roskosz M, Remusat L et al (2014) Isotopic and structural signature of experimentally irradiated organic matter. Geochim Cosmochim Acta 142:522–534
Laurent B, Roskosz M, Remusat L et al (2015) The deuterium/hydrogen distribution in chondritic organic matter attests to early ionizing irradiation. Nat Commun 6:8567
Le Guillou C, Rouzaud J-N, Bonal L et al (2012) High resolution TEM of chondritic carbonaceous matter: metamorphic evolution and heterogeneity. Meteorit Planet Sci 47:345–362
Le Guillou C, Bernard S, Brearley AJ et al (2014) Evolution of organic matter in Orgueil Murchison and Renazzo during parent body aqueous alteration: In situ investigations. Geochim Cosmochim Acta 131:368–392
Love SG, Brownlee DD (1991) Heating and thermal transformation of micrometeoroids entering the Earth’s atmosphere. Icarus 89:26–43
Love SG, Brownlee DE (1993) A direct measurement of the terrestrial mass accretion rate of cosmic dust. Science 262:550–553
Mahaffy PR, Webster CR, Cabane M et al (2012) The sample analysis at Mars investigation and instrument suite. Space Sci Rev 170:401–478
Matrajt G, Pizzarello S, Taylor S et al (2004) Concentration and variability of the AIB amino acid in polar micrometeorites: implications for the exogenous delivery of amino acids to the primitive Earth. Meteorit Planet Sci 39:1849–1858
Matrajt G, Munoz-Caro GM, Dartois E et al (2005) FTIR analysis of the organics in IDPs: comparison with the IR spectra of the diffuse interstellar medium. Astron Astrophys 433:979–995
Maurice S, Wiens RC, Anderson R et al and the SuperCam team (2015) Science objectives of the SuperCam instrument for the Mars2020 rover. LPSC #2818
Merouane S, Djouadi Z, Le Sergeant d’Hendecourt L (2014) Relations between aliphatics and silicate components in 12 stratospheric particles deduced from vibrational spectroscopy. Astrophys J 780:174–186
Messenger S (2000) Identification of molecular-cloud material in interplanetary dut particles. Nature 404:968–971
Messenger S (2002) Deuterium enrichments in interplanetary dust. Planet Space Sci 50:1221–1225
Michel-Lévy MC, Lautie A (1981) Microanalysis by Raman spectroscopy of carbon in the Tieschitz chondrite. Nature 292:321–322
Millan M, Szopa C, Buch A et al (2016) In situ analysis of martian regolith with the SAM experiment during the first mars year of the MSL mission: identification of organic molecules by gas chromatography from laboratory measurements. Planet Space Sci 129:88–102
Ming DW, Lauer HV Jr, Archer PD Jr et al (2009) Combustion of organic molecules by the thermal decomposition of perchlorate salts: implications for organics at the Mars Phoenix scout landing site. LPSC#2241
Moores JE, Schuerger AC (2012) UV degradation of accreted organics on Mars: IDP longevity, surface reservoir of organics, and relevance to the detection of methane in the atmosphere. J Geophys Res 117:E08008
Munoz Caro GM, Matrajt G, Dartois E et al (2006) Nature and evolution of the dominant carbonaceous matter in interplanetary dust particles: effects of irradiation and identification with a type of amorphous carbon. Astron Astrophys 459:147–159
Naraoka H, Mita H, Komiya M et al (2004) A chemical sequence of macromolecular organic matter in the CM chondrites. Meteorit Planet Sci 39:401–406
Navarro-González R, McKay CP (2011) Reply to comment by Biemann and Bada on “Reanalysis of the Viking results suggests perchlorate and organics at midlatitudes on Mars”. J Geophys Res 116:E12002
Navarro-González R, Navarro KF, de la Rosa J et al (2006) The limitations on organic detection in Mars-like soils by thermal volatilization–gas chromatography-MS and their implications for the Viking results. Proc Natl Acad Sci USA 103:16089–16094
Navarro-González R, Vargas E, de la Rosa J (2010) Reanalysis of the Viking results suggests perchlorate and organics at midlatitudes on Mars. J Geophys Res 115:E12010
Nesvorný D, Jenniskens P, Levison HF et al (2010) Cometary origin of the Zodiacal Cloud and carbonaceous micrometeorites. Implications for hot debris disks. Astrophys J 713:816–836
Oba Y, Naraoka H (2009) Elemental and isotopic behavior of macromolecular organic matter from CM chondrites during hydrous pyrolysis. Meteorit Planet Sci 44:943–954
Okumura F, Mumura K (2011) Gradual and stepwise pyrolyses of insoluble organic matter from the Murchison meteorite revealing chemical structure and isotopic distribution. Geochim Cosmochim Acta 75:7063–7080
Orthous-Daunay FR, Quirico E, Lemelle L et al (2010) Speciation of sulfur in the insoluble organic matter from carbonaceous chondrites by XANES spectroscopy. Earth Planet Sci Lett 300:321–328
Orthous-Daunay FR, Quirico E, Beck P et al (2013) Mid-infrared study of the molecular structure variability of insoluble organic matter from primitive chondrites. Icarus 223:534–543
Parise B, Ceccarelli C, Tielens AGGM et al (2006) Testing grain surface chemistry: a survey of deuterated formaldehyde and methanol in low-mass class 0 protostars. Astron Astrophys 453:949–958
Peltzer ET, Bada J (1978) Alpha-hydroxycarboxylic acids in Murchinson meteorite. Nature 272:443
Pendleton YJ, Allamandola LJ (2002) The organic refractory material in the diffuse interstellar medium: mid-infrared spectroscopic constraints. Astrophys J Suppl 138:75–98
Piani L, Robert F, Remusat L (2015) Micron-scale D/H heterogeneity in chondrite matrices: a signature of the pristine solar system water? Earth Planet Sci Lett 415:154–164
Poch O, Noblet A, Stalport F et al (2013) Chemical evolution of organic molecules under Mars-like UV radiation conditions simulated in the laboratory with the “Mars organic molecule irradiation and evolution” (MOMIE) setup. Planet Space Sci 85:188–197
Poch O, Kaci S, Stalport F et al (2014) Laboratory insights into the chemical and kinetic evolution of several organic molecules under simulated Mars surface UV radiation conditions. Icarus 242:50–63
Poch O, Jaber M, Stalport F et al (2015) Effects of nontronite smectite clay on chemical evolution of several organic molecules under simulated Martian surface ultraviolet radiation conditions. Astrobiology 15:1–17
Quirico E, Raynal PI, Bourot-Denise M (2003) Metamorphic grade of organic matter in six unequilibrated ordinary chondrites. Meteorit Planet Sci 38:795–811
Quirico E, Borg J, Raynal P-I et al (2005) A micro-Raman survey of 10 IDPs and 6 carbonaceous chondrites 2005. Planet Space Sci 53:1443–1448
Quirico E, Montagnac G, Rouzaud J-N et al (2009) Precursor and metamorphic conditions effects on Raman spectra of poorly-ordered carbonaceous matter in chondrites and coals. Earth Planet Sci Lett 287:185–193
Quirico E, Bourot-Denise M, Robin C et al (2011) A reappraisal of the thermal history of EH3 and EL3 enstatite chondrites. Geochim Cosmochim Acta 75:3088–3102
Quirico E, Orthous-Daunay FR, Beck P et al (2014) Origin of insoluble organic matter in type 1 and 2 chondrites: new clues, new questions. Geochim Cosmochim Acta 136:80–99
Remusat L, Derenne S, Robert F et al (2005a) New pyrolytic and spectroscopic data on Orgueil and Murchison insoluble organic: a different origin than soluble? Geochim Cosmochim Acta 69:3919–3932
Remusat L, Derenne S, Robert F (2005b) New insight on aliphatic linkages in the macromolecular organic fraction of Orgueil and Murchison meteorites through ruthenium tetroxide oxidation. Geochim Cosmochim Acta 69:4377–4386
Remusat L, Palhol F, Robert F et al (2006) Enrichment of deuterium in insoluble organic matter from primitive meteorites: a solar system origin? Earth Planet Sci Lett 243:15–25
Remusat L, Robert F, Meibom A et al (2009) Protoplanetary chemistry recorded by D-rich organic radicals in carbonaceous chondrites. Astrophys J 698:2087–2092
Remusat L, Piani L, Bernard S (2016) Thermal recalcitrance of the organic D-rich component of ordinary chondrites. Earth Planet Sci Lett 435:36–44
Rietmeijer FJM (1998) Interplanetary dust particles. In: Papike JJ (ed) Planetary materials. Revs mineral, vol 36. Mineralogical Society of America, Washington, DC, pp 1–95
Robert F, Epstein S (1982) The concentration and isotopic composition of hydrogen, carbon and nitrogen in carbonaceous meteorites. Geochim Cosmochim Acta 46:81–95
Rubin AE (1997) The Hadley Rille enstatite chondrite and its agglutinate-like rim: impact melting during accretion to the Moon. Meteorit Planet Sci 32:135–141
Rudraswami NG, Shyam Prasad M, Jones RH et al (2016) In situ oxygen isotope compositions in olivines of different types of cosmic spherules: an assessment of relationships to chondritic particles. Geochim Cosmochim Acta 194:1–14
Schimmelmann A, Sessions A, Mastalerz M (2006) Hydrogen isotopic (D/H) composition of organic diagenesis and thermal maturation. Annu Rev Earth Planet Sci 34:501–533
Schmitt-Koplin P, Gabelica Z, Gougeon RD et al (2010) High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc Natl Acad Sci USA 107:2763–2768
Schmitz B, Tassinari M, Peucker-Ehrenbrink B (2001) A rain of ordinary chondrite meteorites in the early Ordovician. Earth Planet Sci Lett 194:1–15
Schröder C, Rodionov DS, McCoy TJ et al (2008) Meteorites on Mars observed with the Mars Exploration Rovers. J Geophys Res 113:E06S22
Sephton MA (2002) Organic compounds in carbonaceous meteorites. Nat Prod Rep 19:292–311
Sephton MA, Gilmour I (2001) Pyrolysis-gas chromatography-isotope ratio mass spectrometry of macromolecular material in meteorites. Planet Space Sci 49:465–471
Sephton MA, Pillinger CT, Gilmour I (2000) Aromatic moieties in meteoritic macromolecular materials: analyses by hydrous pyrolysis and δ13C of individual compounds. Geochim Cosmochim Acta 64:321–328
Sephton MA, Verchovsky AB, Bland PA et al (2003) Investigating the variations in carbon and nitrogen isotopes in carbonaceous chondrites. Geochim Cosmochim Acta 67:2093–2108
Sephton MA, Love GD, Watson JS et al (2004) Hydropyrolysis of insoluble carbonaceous matter in the Murchison meteorite: new insights into its macromolecular structure. Geochim Cosmochim Acta 68:1385–1393
Starkey NA, Franchi IA, Alexander CMO’D (2013) A Raman spectroscopic study of organic matter in interplanetary dust particles and meteorites using multiple wavelength laser excitation. Meteorit Planet Sci 48:1800–1822
Steininger H, Goesmann F, Goetz W (2012) Influence of magnesium perchlorate on the pyrolysis of organic compounds in Mars analogue soils. Planet Space Sci 71:9–17
Stoker CR, Bullock MA (1997) Organic degradation under simulated martian conditions. J Geophys Res 102:10881–10888
Suavet C, Cordier C, Folco L et al (2011) Non carbonaceaous chondrite-related large cosmic spherules from the Transantarctic Mountains. Geochim Cosmochim Acta 75:6200–6210
Taylor S, Lever JH, Harvey RP (1998) Accretion rate of cosmic spherules measure at the South Pole. Nature 393:899–903
Thomas KL, Blanford GE, Keller LP et al (1993) Carbon abundance and silicate mineralogy of anhydrous interplanetary dust particles. Geochim Cosmochim Acta 57:1551–1566
Tonui E, Zolensky M, Hiroi T et al (2014) Petrographic, chemical and spectroscopic evidence for thermal metamorphism in carbonaceous chondrites I: CI and CM chondrite. Geochim Cosmochim Acta 126:284–306
Vernazza P, Delbo M, King PL et al (2012) High surface porosity as the origin of emissivity features in asteroid spectra. Icarus 221:1162–1171
Vis RD, Mrowiec A, Kooyman PJ et al (2002) Microscopic search for the carrier phase Q of the trapped planetary noble gases in Allende, Leoville and Vigarano. Meteorit Planet Sci 37:1391–1399
Wang Y, Huang Y, Alexander CMO’D et al (2005) Molecular and compound-specific hydrogen isotope analyses of insoluble organic matter from 80 different carbonaceous chondrites groups. Geochim Cosmochim Acta 69:3711–3721
Wopenka B (1988) Raman observations on individual interplanetary dust particules. Earth Planet Sci Lett 88:221–231
Yabuta H, Naraoka H, Sakanishi K et al (2005) Solid-state 13C NMR characterization of insoluble organic matter from Antarctic CM2 chondrites: evaluation of the meteoritic alteration level. Meteorit Planet Sci 40:779–787
Yabuta H, Alexander CMO’D, Fogel ML et al (2010) A molecular and isotopic study of the macromolecular organic matter of the ungrouped C2 WIS 91600 and its relationship to Tagish Lake and PCA 91008. Meteorit Planet Sci 45:1446–1460
Yuen G, Blair N, DesMarias DJ et al (1984) Carbon isotope composition of low molecular weight hydrocarbons and monocarboxylic acids from Murchison meteorite. Nature 307:252
Zolensky ME, Bland PA, Brown P et al (2006a) Flux of extraterrestrial materials. In: Lauretta DS, McSween HY Jr (eds) Meteorites and the early solar system II. University of Arizona Press, Tucson, pp 869–888
Zolensky ME, Zega TJ, Yano H et al (2006b) Mineralogy and petrology of comet 81P/Wild 2 nucleus samples. Science 314:1735–1739
Zuckerman B, Ball JA, Gottlieb CA (1971) Microwave detection of interstellar formic acid. Astrophys J 163:L41–L45
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Quirico, E., Bonal, L. (2019). Organic Matter in Interplanetary Dusts and Meteorites. In: Cavalazzi, B., Westall, F. (eds) Biosignatures for Astrobiology. Advances in Astrobiology and Biogeophysics. Springer, Cham. https://doi.org/10.1007/978-3-319-96175-0_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-96175-0_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-96174-3
Online ISBN: 978-3-319-96175-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)