Abstract
To date, epilepsy surgery has been largely underutilized by the medical community. In recent years, surgical intervention for medically refractory epilepsy has been bolstered by promising results demonstrating improved seizure control and/or freedom as compared to continued medical therapy. The cumulative side effects of chronic epileptic seizures and multiple antiepileptic medications over years lead to substantial medical, cognitive, and behavioral declines in this patient population, not to mention the risks of “sudden unexplained death in epilepsy,” or SUDEP. In patients with medically refractory epilepsy, it is estimated that nearly 50% will benefit from invasive intracranial monitoring of electroencephalographic activity, in both lesional and non-lesional cases. In this context, this chapter focuses on the topic of invasive subdural electroencephalography, including clinical and technical considerations, in addition to reviewing related complications and special considerations in the pediatric population.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Invasive electroencephalography
- EEG monitoring
- Refractory epilepsy
- Subdural grids
- Depth electrodes
- Seizure
- Pediatric neurosurgery
- Grid complications
9.1 Introduction and Background
It is estimated that approximately 50 million people world-wide suffer from epilepsy [1]. Recent North American statistics have estimated that at least 1 in 10 adults will experience a seizure event in their lifetime, with at least 1–2% of adults and children developing chronic, persistent, and recurrent seizure activity (i.e., epilepsy) [1, 2]. Medically refractory epilepsy has been recently defined by the International League Against Epilepsy (ILAE) as the “failure of adequate trials of two tolerated and appropriately chosen and used anti-epileptic drug schedules , whether as monotherapies or in combination, to achieve sustained seizure freedom” [3]. In this context, there has been an emphasis on earlier referrals, particularly in childhood and early adulthood, to surgical epilepsy centers to assess potential candidacy for surgical intervention [4].
To date, surgery for epilepsy has been largely underutilized by the medical community despite mounting evidence advocating for earlier surgical intervention [5]. The latter has been bolstered by promising results demonstrating higher incidences for seizure control and/or freedom as compared to continued medical therapy across several well-designed trials in the literature [6,7,8,9]. Indeed, the cumulative side effects of chronic epileptic seizures and multiple antiepileptic medications over years lead to substantial medical, cognitive, and behavioral declines in this patient population. Moreover, epilepsy patients face an estimated fourfold higher risk for injury and 12% all-cause mortality within the first 2 years of diagnosis [10]. This is in addition to an ever-present risk for “sudden unexplained death in epilepsy” or SUDEP (estimated at 9% per decade per patient) [11]. Of course, the impact of epilepsy extends beyond the patient, affecting families and society as a whole. Surgical intervention, which often relies on invasive electroencephalography for mapping out the epileptogenic zone prior to resection or ablation, therefore continues to gain interest for its cost-effective approach to delivering an improved quality of life for many patients suffering from intractable epilepsy.
9.2 General Indications for Invasive Electroencephalography
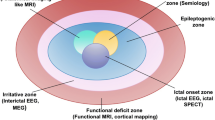
The standard preoperative evaluation for potential epilepsy surgery begins with noninvasive testing. The goal of this comprehensive evaluation is to lateralize and localize the epileptogenic zone, i.e., the regional site of seizure onset and networks implicated in the early spread of seizure activity in the hopes of identifying a suitable target amenable to resection, ablation, or disconnection. This hypothesis-driven approach typically begins with obtaining a detailed clinical history (including review of clinical semiology and assessment of seizure burden and prior treatments), ambulatory and video electro-encephalography (EEG) , 3T-magnetic resonance imaging (MRI) , and formal neuropsychological testing . Additional tests, as warranted, may include positron emission tomography (PET) , single-photon emission computed tomography (SPECT) , magnetoencephalography (MEG) , functional MRI , and Wada testing . For any given patient, his/her clinical profile and collective set of results are then reviewed by a multidisciplinary team encompassing neurologists, neurosurgeons, radiologists, nuclear medicine specialists, neuropsychologists, nurses, EEG technicians, and, on occasion, pathologists. It is here that a hypothesis is made toward localizing the epileptogenic zone, and an all-important decision focuses on the further necessity for invasive implantation of intracranial electrodes, depending on the congruence of the noninvasive results obtained up to that point. In general, of the nearly one third of patients with refractory epilepsy thought to be potential surgical candidates, it is estimated that between 25% and 50% will ultimately undergo invasive EEG monitoring as a means of better characterizing their epileptogenic zone [4, 12].
In essence, general indications for invasive EEG monitoring via implantation of intracranial electrodes include lateralizing/localizing the epileptogenic zone and mapping functional (or eloquent) cortical and/or subcortical regions. More specifically, indications for considering intracranial electrodes include (i) ambiguous or discordant results obtained during the noninvasive work-up (e.g., based on EEG and imaging results); (ii) non-lesional temporal or extratemporal lobe epilepsy; (iii) bilateral temporal lobe epilepsy; and (iv) functional mapping of eloquent cortex and its relation to a potential epileptogenic zone [12,13,14,15]. Indeed, intracranial EEG captures refined signals free of external signal artifact and offers higher rates of success for accurately localizing an epileptogenic focus. Two conventional methodologies for performing invasive EEGs include the use of subdural electrodes and/or stereoelectroencephalography electrodes. Here, we present the subdural electrode strategy.
9.3 Subdural Grids
The “North American” approach to invasive EEG monitoring was popularized by Wilder Penfield and Herbert Jasper at the Montreal Neurological Institute (Quebec, Canada) [16], where they expanded intraoperative electrocorticography as a technique for localizing interictal epileptiform activity. Modern-day subdural electrodes evolved during the 1980s from the previous ball-tipped probes used by Penfield and Jasper into thin, flexible, customizable two-dimensional sheets (i.e., grids) or strip arrays of various configurations that could be applied to the surface of the brain [15, 17,18,19]. The principal indications for grid implantation include lateralizing/localizing the epileptogenic zone and mapping eloquent, functional regions to be spared in the subsequent resection or disconnection. As opposed to intraoperative electrocorticography , grid placement facilitates the collection of long-term, extraoperative EEG recordings of spontaneous seizure events over a period of days to weeks. During this interval, additional testing via safe, extra-operative electrical stimulation may be performed to assess for eloquent cortical tissue (e.g., motor, sensory, speech/language). In this fashion, a tailored cortical resection sparing eloquent cortex may be achieved while minimizing the risk for neurologic morbidity [19, 20]. Subsequently, a therapeutic resective or disconnective procedure is performed at the immediate time of grid removal, all within the same hospital admission.
9.3.1 Strategy and Protocol
9.3.1.1 Preoperative Considerations
Based on the hypothesis conceived for a given patient’s epileptogenic zone, appropriately sized and shaped grids (or strips) are selected preoperatively to ensure that the extent of cortical coverage is sufficient for adequate sampling of the region of interest. It is prudent to check with the intraoperative electrophysiologist or technician to ensure sufficient recording channels are available, keeping in mind that an additional number of ground electrode contacts are to be applied at the end of the case as an averaged reference. Conventionally, grids are implanted via a standard neurosurgical craniotomy in the operating room under general anesthesia. Standard intraoperative neuronavigation is also useful for planning purposes, to ensure that the craniotomy is centered on the region of interest. On occasion, our preference is to supplement with frameless stereotaxy for introducing a small number of depth electrodes into a desired target (e.g., hippocampus or amygdala), depending on that patient’s epilepsy hypothesis. Optimal head positioning, hyperventilation, mannitol dosing, and elevating the head of the bed all lead to brain relaxation, which is essential when placing subdural grids with minimal morbidity. The skin incision and underlying craniotomy are planned out beforehand, taking into account the extra room required for tunneling the electrode wires extracranially through the skin. Standard antibiotics and steroids (e.g., dexamethasone) are administered and a safety time-out is performed prior to starting.
9.3.1.2 Intraoperative Procedure
After a standard craniotomy is made, the dura is opened widely in C-shaped fashion to permit access to the cortical surface (Fig. 9.1). In the case of redo procedures the dura may be tightly adherent, in which case microsurgical technique under microscopic or loupe magnification is used to carefully dissect the dura off while sparing the cortical surface and vessels beneath. Following exposure, based on direct visual inspection of anatomic landmarks and concomitant intraoperative neuronavigation, correlation is made between the patient’s anatomy, MR imaging, and the anticipated region to be electrographically sampled. In the event that depth electrodes are to be placed, frameless stereotaxy is conventionally used for accurate placement of a limited number of electrodes into specific targets to be sampled (for example into the depth of a sulcus or into the hippocampus or amygdala). It is conventionally easier to perform the depth electrode placement early on, prior to covering the cortical surface with a grid that may interfere with accurate placement in this regard. The pia is gently incised with a 15-blade knife or microscissors instrument, and the depth electrodes are delivered to specific targets using preoperatively defined trajectories under standard neuronavigation.
Representative dual-pathology case of medically refractory epilepsy in a 42-year-old male patient with a left temporal low-grade glioma and left-sided hippocampal sclerosis. Axial (a) and sagittal (b) contrast-enhanced T1-weighted MR images confirmed a nonenhancing, low-grade brain mass situated within the left posterior temporal lobe. (c) Coronal T1-FLAIR imaging revealing increased signal within the lesion. (d) Implantation map showing the selection of two grids for coverage of the left temporal lobe, with the larger grid strategically positioned over Wernicke’s area to assist with language mapping. Note the depth electrode, labelled as B′, targeting the head of the hippocampus. (e) Open left temporal craniotomy window revealing the implantation of both grid electrodes and a smaller depth electrode. Each electrode has been sutured to the dural edge to minimize displacement prior to externalization through the scalp. Of note, during EEG monitoring the hippocampal depth electrode was not involved in the epileptogenic zone. With this confirmation and subsequent to language mapping, direct lesionectomy (i.e., tumor resection) was therefore performed, resulting in dramatic seizure improvement for the patient
Measurements are then taken using a soft, flexible ruler, and the chosen grids (or strips) are conformed appropriately such that they lie flat along the cortical surface. Rough grid edges are carefully trimmed to smooth them out, thereby minimizing the chance for cortical laceration. In some cases, large grids must be cut into individual strips while preserving the internal grid circuitry, facilitating placement over a large cortical surface. Ultimately, the grids are gently placed using bayonet forceps under plentiful irrigation, taking care not to damage the grid contacts or wires therein. In many instances, the grid can slide along the cortical surface to be placed beyond the margins of the craniotomy along the convexity or into the basal frontal and/or temporal regions and even into the interhemispheric fissure itself. Great care is taken to protect superficial draining veins, particularly the veins of Labbé or Trolard in addition to the venous sinus system, since venous injury or compression can lead to significant congestion-related morbidity, which often extends beyond the exposed cortical region in view. Regions of adherence may hint at underlying bridging veins, which must be either carefully protected or disconnected. When appropriate individual wires attached to the electrodes are then sutured against the dural margins to reinforce the location of the grid(s) and to prevent against pull-out or shift. Digital photographs are taken to corroborate the relation of the grid(s) to the underlying cortical anatomy. Intra-operative consultation is also sought with the neurology team to confirm proper hardware placement/orientation prior to closure.
The dura is then closed in water-tight fashion. Care is taken to ensure that the wires can easily pass through an open burr hole or exposed bony margin, thereby minimizing the chance for lead kinking and/or fracture prior to securing the bone flap with sutures or plates. Attention is paid to leaving at least one or two borders of the bone flap free to permit outward displacement in the rare event of underlying cerebral edema. The wires are typically externalized through the scalp at a safe distance away from the incision line. These are then secured using purse-string sutures to prevent leakage of CSF fluid. The color coding and numbering of each lead is documented and relayed to the neurology team, again in reference to that grid’s position. The scalp flap is approximated in typical fashion and a subgaleal drain is left behind. It is at this time that two to four external ground leads are applied to the scalp prior to careful dressing of the wound and coverage with a formal head wrap. The electrodes are carefully brought out of the wrap and secured within a plastic bag, facilitating access by the EEG technician and neurology teams.
9.3.1.3 Postoperative Considerations
Postoperatively, the patient is taken to recovery and subsequently to the intensive care unit (ICU), where imaging is acquired to confirm the placement of the grid(s). This typically includes skull X-rays, but most importantly CT and MR imaging studies, which are subsequently used for three-dimensional reconstruction purposes. The wires are attached to EEG equipment, permitting real-time EEG analysis and monitoring, and antiepileptic medications are weaned according to the neurology team’s protocol. Assuming the first night is uneventful, the patient is then taken to the Epilepsy Monitoring Unit for ongoing monitoring and stimulatory testing purposes, where warranted. Postoperative antibiotics are administered, and in many centers these antibiotics are continued during the monitoring interval [21, 22]. Similarly, postoperative steroids (e.g., dexamethasone) are administered and weaned over several days. The head dressing may be changed sporadically under sterile technique to inspect the lead sites for CSF leakage and/or wound infection, taking care to protect the electrode lead wires during this process.
9.3.1.4 Grid Removal
Once sufficient EEG data are collected and following all stimulatory testing, the patient is returned to the operating room for grid removal. At this time, depending on review again at the Epilepsy Conference, the decision may also be made for concomitant resection. Regardless, the prior surgical incision is opened and great care is taken to avoid shifts in grid placement, as this may serve as the cortical reference or map for resection guided by the seizure data collected for that patient. The grids are delicately removed, again paying close attention to venous preservation, and ample irrigation is applied to aid with this. The wires are disconnected and can be pulled out of the field at this time (provided they have been released at the scalp). The resection may then proceed accordingly, or alternatively in the event of failed localization the craniotomy is simply closed in standard fashion. At the end of the case, care is taken to ensure that all lead exit sites at the scalp have also been closed in order to minimize the chance for CSF leak and infection.
9.3.2 Complications: Avoidance and Management
In general, the overall risk of implanting subdural grids can be as high as 9–22% in some series if not higher, with a separate risk of 5–6% for depth electrode placements [15, 19, 23,24,25]. Focusing specifically on the craniotomy procedure for grid and/or depth electrode placement, there are a number of inherent risks to be discussed beforehand with the patient and his/her family. A discussion of these risks follows.
9.3.2.1 CSF Leakage and Infections
Leakage of CSF is one established risk factor for infection, namely for meningitis, which must be dealt with promptly at the time of identification. Reported risks for CSF leaks range anywhere from 0.5–2% to as high as 30% in some series [21, 23]. Typically, CSF may be noted to be leaking at electrode exit sites, in which case suture reinforcement and subsequent wrapping of the lead sites with betadine-soaked gauze may help minimize the risk for subsequent infection. In cases of persistent leakage, placement of a temporary lumbar drain for CSF diversion may be beneficial.
In most modern hospitals, the general incidence of infection associated with cranial procedures, as with other types of surgical procedures, is estimated to be approximately 0.5–2%. However, in terms of grid and/or depth electrode implantations (with electrodes externalized through the scalp), the risk of infection rises, given the context of implanted foreign bodies. Thus, the risk for clinically relevant infection is expectedly higher, and based on recent series has been estimated to be approximately 4–5% [19, 21, 23, 25]. These infections may include any of the following: superficial wound infection; meningitis; epi- or subdural abscess; osteomyelitis; and intraparenchymal brain abscess. Other factors identified as contributing to the risk of infection include length of implantation (i.e., days with electrodes in situ) and increased numbers of electrodes owing to increased lead exit sites in the scalp [19, 21].
It should be noted that the degree of subsequent intervention depends on the severity of infection. Superficial wound infections may be addressed by initiating antibiotic therapy for the duration of implantation. Deeper infections, however, must be dealt with by reoperation to remove the grids and/or depth electrodes, culture sampling of the collection, copious irrigation and debridement, and subsequent intravenous antibiotic therapy. In such cases, the treatment decision is delayed and/or modified, often delaying subsequent resection until the infection is resolved.
For chronic infections including those of the bone (e.g., osteomyelitis), these typically appear several weeks to months later with a 2–3% likelihood [15, 25]. In such cases, removal of the bone flap may be indicated followed by aggressive antibiotic therapy. A delayed procedure to replace the open craniotomy site may be pursued using a prosthetic substitute (e.g., titanium mesh or synthetic customized cranial implant).
9.3.2.2 Cerebral Edema
Local irritation and subsequent inflammatory cerebral edema in response to implanted hardware is another common complication associated with grid placement. According to the literature, the risk of clinically significant brain edema may occur in 2–3% of cases [23]. In such instances, the presenting symptoms are usually consistent with headaches and/or progressive neurologic deficits, including contralateral hemiparesis and/or speech and language disturbances, followed by eventual loss of consciousness and potentially fatal herniation. Pediatric patients, in particular, are generally more susceptible to developing cerebral edema than their adult counterparts. Attempts at minimizing the risk for cerebral edema include opening the dura widely during implantation, closing the bone flap in such a way that it is loosely hinged on one side (through the use of sutures rather than titanium plates), and the perioperative use of steroids. In the most severe cases of refractory brain edema resulting in neurologic compromise, the electrodes should be removed immediately and the patient closely monitored for subsequent resolution of his or her symptoms.
9.3.2.3 Hemorrhage
Intracranial hemorrhage poses another risk for refractory epilepsy patients undergoing grid and/or depth electrode implantation. The rate of radiographic hemorrhage (16%) is known to exceed that of clinically significant hemorrhage (7–8%) [20, 23]. The placement of grids predisposes to risks for cortical injury and venous occlusion/disruption, particularly in close proximity to the sinuses (e.g., in interhemispheric implantations); this can lead to subsequent brain edema and/or subdural hematoma formation [15, 24, 25]. The latter may arise either superficially or deep to the electrodes (directly overlying the cortical surface). In such instances, this may disrupt the acquisition of accurate intracranial EEG signals and could potentially result in mass effect and clinical decline [25].
9.3.2.4 Other Complications
On rare occasion, the implanted grid(s) may fail to capture epileptiform events in their entirety, with part of the seizure activity seen to extend beyond the edge of the hardware. In certain cases, consideration must be given to reoperation to either adjust the current grid(s) in place or perhaps introduce yet another one for more accurate coverage. Theoretically, multiple reopenings may increase the risk for wound infection and other surgical-related problems.
Moreover, as described above, once the grids and/or depth electrodes are placed, the leads are typically sutured at the dural edges before tunneling out through the scalp (where they are again secured at the surface). The intention of these steps is to prevent grid migration or electrode pull-out. Careful attention to electrode care and dressing changes by the nursing and EEG tech team helps to preserve the integrity of the hardware during the implantation phase. Nevertheless, there is a small but very real risk for displacement, removal, or fracture of the implanted electrodes. This is particularly the case for patients who are experiencing violent seizure events with a heavy motor component to their semiology, those in a state of postictal confusion, or at the time of reopening a craniotomy to remove the electrodes. In the latter case, care must be taken by the surgeon to confirm that the grids remain in place while the scalp layers, bone, and dura are reopened so that accurate correlation can be made between the electrode contacts and the electrographically-confirmed epileptogenic zone immediately prior to resection.
Finally, from a medical standpoint, patients implanted in the EMU are conventionally bed- or chair-bound while waiting for sufficient seizure events to be captured. In their state of limited mobility, their risk for deep venous thrombosis, pulmonary embolism, pneumonia, and other medical complications is likely higher than for the typical postoperative population. Careful observation, early diagnosis, and immediate therapeutic intervention help prevent and/or minimize the medical consequences of such events.
9.4 Special Pediatric Considerations
There are several considerations that are uniquely relevant to pediatric patients undergoing work-up and treatment for refractory epilepsy .
From a noninvasive standpoint, despite testing similar to that for adult patients, children require a compassionate and calm approach by trained pediatric-focused hospital staff. Parents, grandparents, and other family members are typically involved, and additional time is often warranted for them to explain each step to the patient in order to ensure more accurate results. In some cases, obtaining a high-resolution 3T-MRI study requires that younger patients be given a general anesthetic to minimize motion artifact-related effects. Interestingly, in certain fMRI studies performed under general anesthesia, it is possible to passively activate eloquent brain regions in the toddler group, thereby mapping the primary motor, sensory, or visual cortices [26]. In contrast, older children may benefit from careful advanced instruction and practicing simple fMRI-related tasks to improve the results obtained with this imaging modality.
With respect to surgical grid implantation for invasive EEG monitoring in pediatric patients, care is taken to use an appropriate head holder specifically designed for children. Examples include the standard use of a donut or horseshoe, or using the Mayfield clamp with age-appropriate pins in cases requiring fixation for image-guidance (such as for placement of depth electrodes). Smaller grids may be required in younger children, and therefore careful consideration for the number of channels and overall grid dimensions should be reviewed prior to each procedure by the neurosurgeon and epileptology team. Upon implantation and tunneling the leads out through the scalp, these must be secured with sutures and dressing material to minimize the chance of the pediatric patient actively reaching up and grasping the electrodes, potentially removing them and/or fracturing them in the process.
Following grid implantation, extraoperative testing for eloquent cortex must utilize age-appropriate language and motor tasks to improve the accuracy of testing results. The subsequent removal of hardware is typically performed in conventional fashion, often in the setting of concomitant surgical resection and/or disconnection of an identified epileptogenic zone.
Conclusions
Invasive EEG monitoring using implanted intracranial grid and/or depth electrode arrays may be safely used in pediatric patients with refractory epilepsy for the purposes of lateralizing and localizing the epileptogenic zone and for functional mapping of eloquent cortical and/or subcortical regions. The concept was expanded by Penfield and Jasper in the 1950s and has evolved into one of two modalities for invasive EEG (the other being stereoelectroencephalography, or SEEG ). There are multiple technical considerations to review in planning these procedures, particularly in the pediatric age group, and it is important for the treating physician to gain an appreciation for the utility of the technique and its inherent risks, as described in this chapter. When employed by a trained epilepsy team, grid implantation offers a higher chance for identifying the epileptogenic zone, thereby leading to a more successful postoperative outcome while minimizing the risk for postoperative morbidity.
References
Epilepsy: Fact Sheet. World Health Organization, 2016.
Annual estimates of the resident population by sex, age, race, and Hispanic origin for the United States, States, and Counties: April 1, 2010, to July 1, 2013. U.S. Census Bureau, Population Division. (Database online.).
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the Ad Hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–77.
Albert GW, Serletis D. Surgical management of epilepsy in adolescent patients. J Pediatr Epilepsy. 2015;4:102–8.
Engel J Jr. Why is there still doubt to cut it out? Epilepsy Curr. 2013;13:198–204.
Jeha LE, Najm IM, Bingaman WE, Khandwala F, Widdess-Walsh P, Morris HH, et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology. 2006;66:1938–40.
Engel J Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307:922–30.
McGovern RA, McKhann GM 2nd. The ERSET trial of early surgery for mesial temporal lobe epilepsy: results and frustrations. Neurosurgery. 2012;70:N23–4.
Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness, efficiency of surgery for temporal lobe epilepsy study G. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8.
Jehi L. Neurology’s silent killer: drug-resistant epilepsy. Epilepsy Curr. 2016;16:232–3.
Jones LA, Thomas RH. Sudden death in epilepsy: insights from the last 25 years. Seizure. 2017;44:232–6.
Kuzniecky R, Devinsky O. Surgery insight: surgical management of epilepsy. Nat Clin Pract Neurol. 2007;3:673–81.
Serletis D, Bulacio J, Alexopoulos A, Najm I, Bingaman W, Gonzalez-Martinez J. Tailored unilobar and multilobar resections for orbitofrontal-plus epilepsy. Neurosurgery. 2014;75:388–97.
Serletis D, Bulacio J, Bingaman W, Najm I, Gonzalez-Martinez J. The stereotactic approach for mapping epileptic networks: a prospective study of 200 patients. J Neurosurg. 2014;121:1239–46.
Salazar F, Bingaman WE. Placement of subdural grids. In: Lüders HO, editor. Textbook of epilepsy surgery. London: CRC Press (Taylor & Francis Group); 2008. p. 931–7.
Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. London: J. and A. Churchill Ltd.; 1954.
Wyllie E, Luders H, Morris HH 3rd, Lesser RP, Dinner DS, Rothner AD, et al. Subdural electrodes in the evaluation for epilepsy surgery in children and adults. Neuropediatrics. 1988;19:80–6.
Levy WJ, Hahn JH, Lueders H, Lesser R. Chronic cortical electrode array for seizure investigation. Childs Brain. 1982;9:48–52.
Swartz BE, Rich JR, Dwan PS, DeSalles A, Kaufman MH, Walsh GO, et al. The safety and efficacy of chronically implanted subdural electrodes: a prospective study. Surg Neurol. 1996;46:87–93.
Behrens E, Zentner J, van Roost D, Hufnagel A, Elger CE, Schramm J. Subdural and depth electrodes in the presurgical evaluation of epilepsy. Acta Neurochir. 1994;128:84–7.
Wiggins GC, Elisevich K, Smith BJ. Morbidity and infection in combined subdural grid and strip electrode investigation for intractable epilepsy. Epilepsy Res. 1999;37:73–80.
Heuer GG, Bauman J, Storm PB, Baltuch GH. Invasive EEG studies: peg, strip, and grid implantation. In: Baltuch GH, Villemure J-G, editors. Operative techniques in epilepsy surgery. New York: Thieme; 2009. p. 20–4.
Schmidt RF, Wu C, Lang MJ, Soni P, Williams KA Jr, Boorman DW, et al. Complications of subdural and depth electrodes in 269 patients undergoing 317 procedures for invasive monitoring in epilepsy. Epilepsia. 2016;57:1697–708.
Rahman Z, Bleasel AF, Bartley M, Dexter M, Galea T, Gill D, et al. Reduced complications from intracranial grid insertion by using a small grid size and a precise protocol during monitoring. Acta Neurochir (Wien). 2016;158:395–403.
Lee WS, Lee JK, Lee SA, Kang JK, Ko TS. Complications and results of subdural grid electrode implantation in epilepsy surgery. Surg Neurol. 2000;54:346–51.
Farmer J-P, Atkinson J. Invasive monitoring in pediatric neurosurgery. In: Cohen AR, editor. Pediatric neurosurgery: tricks of the trade. New York: Thieme; 2016. p. 665–73.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Serletis, D. (2019). Invasive Electroencephalography in Epilepsy. In: Fountas, K., Kapsalaki, E. (eds) Epilepsy Surgery and Intrinsic Brain Tumor Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-95918-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-95918-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95917-7
Online ISBN: 978-3-319-95918-4
eBook Packages: MedicineMedicine (R0)