Abstract
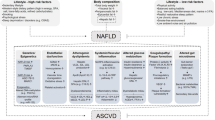
Recent evidence suggests that non-alcoholic fatty liver disease (NAFLD) is a risk factor for the development of cardiovascular disease (CVD). Although in the past few decades, there has been a decline in CVD age-adjusted mortality rates in the developed world, the recent increase in prevalence of metabolic diseases such as NAFLD and type 2 diabetes that are both strongly associated with central obesity and metabolic syndrome, is likely to slow, or even reverse, this decline in the near future. This chapter describes: (a) the current evidence for NAFLD as an independent risk factor for CVD; (b) some of the liver-centred mechanisms in NAFLD, by which NAFLD may contribute to the pathogenesis of CVD. Mechanisms that are discussed include the following: (1) hepatic ‘selective insulin resistance’ with consequent reduction of nitric oxide production leading to endothelial dysfunction; (2) development of non-cirrhotic portal hypertension associated with left ventricular dysfunction; (3) de novo lipogenesis and its association with atherogenic dyslipidaemia; (4) hepatokine production and effects on the vasculature; (5) coagulation factor production and effects on the atherothrombotic process; and (6) the effect of PNPLA3 I148M genotype and associations with vascular disease. Additionally, (c) we discuss the potential benefits on the vasculature of the Mediterranean diet and statins, as well as two agents (pioglitazone and vitamin E) that have recently been recommended for the treatment of non-alcoholic steatohepatitis (NASH).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cardiovascular disease
- Selective insulin resistance
- Hepatokines
- Pioglitazones
- Non-cirrhotic portal hypertension
9.1 Introduction
Cardiovascular disease (CVD) is an umbrella term used to describe a cluster of disorders of heart and blood vessels, and include among others: hypertension, coronary heart disease, arrhythmias, cerebrovascular disease, peripheral vascular disease, heart failure and cardiomyopathies. Despite a marked reduction in the rate of age-standardised CVD death over the past 30 years, the burden of CVD remains high [1, 2]. According to the WHO, CVD is the most common cause of death in the Westernised countries (35% of all deaths) and by 2030, almost 23.6 million people will die from CVD, mainly from heart disease and stroke. Due to the high morbidity, mortality and healthcare costs associated with CVD, it is crucial to investigate the effects of non-alcoholic fatty liver disease (NAFLD) on the development of cardiovascular events in order to organise an efficient health prevention and treatment programme to identify the risk of developing CVD in patients with NAFLD. Currently, it is difficult to prove an independent role for NAFLD in the development of CVD as this liver condition is often embedded in a more complex metabolic syndrome involving insulin resistance, dyslipidaemia, central adipose tissue dysfunction and gut microbiota alteration. In this chapter, we aim to explain some of the liver-centred mechanism associated with CVD that may explain why NAFLD is a risk factor for CVD. We describe the role of: (1) hepatic ‘selective insulin resistance’ with consequent reduction of nitric oxide production leading to endothelial dysfunction; (2) hepatic structural changes and the development of non-cirrhotic portal hypertension associated with left ventricular dysfunction; (3) increases in de novo lipogenesis and its association with atherogenic dyslipidaemia; (4) liver hepatokines which are associated with CVD; (5) coagulation factors that have a role in the thrombotic process and (6) PNPLA3 I148M genotype and its association with ischaemic heart disease.
9.2 Epidemiology
In the past few decades, there has been a decline in age-standardised CVD mortality rates worldwide [3]. From 1990 to 2013, the annual age-adjusted cardiovascular mortality rates have declined, falling by 22% in nearly all regions of the world, especially in high-income North America, Western Europe, Japan, Australia and New Zealand [1, 3,4,5]. The age-standardised rates of death due to CVD fell 15.6%, whereas, global CVD deaths rose by 12.5% between 2005 and 2015. These age-standardised rates of death reductions were largely driven by declining mortality rates due to cerebrovascular disease (i.e. stroke; decreased by 21.0%) since 2005 [6]. Most of the epidemiological studies on CVD morbidity and mortality used the IMPACT Coronary Heart Disease Model that is a statistical model employed to examine the relative contributions of medical and surgical interventions for coronary heart disease versus preventive strategies that target the reduction of major coronary heart disease risk factors [1, 7,8,9]. Using this model, Ford et al. were able to estimate that approximately 47% of the decline in coronary heart disease mortality rate was attributable to changes in medical and surgical treatments including secondary preventive therapies. Whereas, risk-factor changes accounted for approximately 44% of the decrease in deaths and was attributed to primary prevention with changes in risk factors, including reductions in total cholesterol (24%), systolic blood pressure (20%), smoking prevalence (12%) and physical inactivity (5%) [10]. In another study on Swedish population, Björck et al. reported that 75% of the mortality reduction came from primary prevention and that the major contributors to the mortality reduction were dietary changes [11].
However, despite a decline in CVD mortality in the first half of twentieth century, the increase in prevalence of obesity, metabolic syndrome, NAFLD, and type 2 diabetes is likely to be responsible for a slowing in the decline of CVD mortality rates. Ford et al. showed that the increased prevalence in BMI and type 2 diabetes accounted for an increase in CVD mortality of 8% and 10%, respectively [10]. A recent meta-analysis conducted by Targher et al. investigated the association between NAFLD and risk of incident CVD [12]. The presence of NAFLD was associated with an increased risk of a fatal and non-fatal CVD events such as myocardial infarction, angina, stroke, or coronary revascularisation [12]. Based on 16 observational prospective and retrospective studies comprising 34,043 adult individuals (36.3% with NAFLD), patients with NAFLD were found to have a higher risk of fatal and/or non-fatal CVD events considered together (random effect OR 1.63, 95% CI 1.06–2.48, I2 = 83%; p = 0.02) than those without NAFLD. Additionally, presence of more severe NAFLD with fibrosis was associated with an increased risk of CVD mortality (random effect OR 3.28, 95% CI 2.26–4.77, I2 = 0) as well as an increased risk of fatal and non-fatal CVD events considered together (random effect OR 1.94, 95% CI 1.17–3.21, I2 = 23%) [12]. In a recent cross-sectional study in South Korean population, Lee et al. investigated the influence of NAFLD on subclinical coronary atherosclerosis detected by coronary computed tomography angiography in an asymptomatic population. This study showed that patients with NAFLD (diagnosed by ultrasound) had a higher coronary calcium score than those without NAFLD (p < 0.001) [13]. In addition, the odds ratios adjusted for cardiovascular risk factors (age, sex, obesity, diabetes mellitus, hypertension, hyperlipidaemia, current smoking, family history of CAD and hs-CRP) for any atherosclerotic plaque was 1.18; 95% CI 1.03–1.35; p = 0.016 and for non-calcified plaque was 1.27; 95% CI 1.08–1.48; p = 0.003 with NAFLD [13]. This is the largest study to date to describe the association between NAFLD and atherosclerotic plaque. In a retrospective single-centre study, Pais et al. presented a cross-sectional and longitudinal evidence that NAFLD is an important risk factor for the development of early carotid atherosclerosis [14]. The authors examined the impact of steatosis (diagnosed with the fatty liver index − FLIFootnote 1 [15]) on the presence and progression of carotid intima-media thickness and carotid plaques. They found that steatosis independently predicted carotid intima-media thickness (p = 0.002) after adjustment for metabolic syndrome and cardiovascular risk factors. Steatosis at baseline predicted carotid plaque occurrence (OR = 1.63, 95% CI 1.10–2.41, p = 0.014), independently of age, sex, type-2 diabetes, tobacco use, C-reactive protein, hypertension, and carotid intima-media thickness. Interestingly, in a post-hoc analysis of a prospective Japanese cohort study where NAFLD was diagnosed by ultrasound the adjusted hazard ratios for incident CVD were 10.4 (95% confidence interval 2.61–44.0, P = 0.001) in non-overweight with NAFLD, 1.96 (0.54–7.88, P = 0.31) in overweight without NAFLD and 3.14 (0.84–13.2, P = 0.09) in overweight with NAFLD [16]. However, there was a 12-year gap between the enrolment and the post-hoc analysis without an update on more recent information leading to possible bias in the analysis. In addition, the diagnosis of CVD was made by self-administered questionnaire and there was no information on dietary habits and genetic polymorphisms. Nevertheless, this study showed a potential role of NAFLD on CVD not associated with obesity. Thus, the majority of current evidence suggest that there is an independent association between NAFLD and CVD.
9.3 Aetiology and Pathogenesis
The liver is anatomically linked to the cardiovascular system through the hepatic veins which drain blood into the inferior vena cava. In the presence of lipid accumulation in the hepatocytes, the liver undergoes structural changes depending on the degree of severity of liver disease and the presence of fibrosis or ballooning. These changes affect the structure not only of the hepatocytes that become swollen due to lipid accumulation and inflammation (ballooning), but there is also a change in the structure of hepatic sinusoids, bile ducts, hepatic arterioles and the space of Disse [17]. These structural changes along with the liver dysfunction with the production of hepatokines and dysregulation of glucose and lipid metabolism might contribute to the pathogenesis of CVD.
9.3.1 Selective Insulin Resistance and Structural Changes in the Liver
Endothelial dysfunction is the primary cause of vascular dysfunction, and it is one of the earliest markers of atherosclerosis. Recent studies showed that endothelial dysfunction, which is potentially responsible for CVD development, and increased risk of incident hypertension were associated with NAFLD [18,19,20,21,22,23,24]. The mechanism underlying the correlation between NAFLD and endothelial dysfunction is not completely understood. One possible mechanism associated with endothelial dysfunction in patients with NAFLD could be the presence of ‘selective hepatic insulin resistance’, affecting both the liver and the vasculature. With insulin resistance there are two effects: (a) insulin fails to suppress gluconeogenesis as well as lipogenesis and (b) there is an impaired production of nitric oxide leading to endothelial dysfunction [25] (see Fig. 9.1a,b). The liver expresses both insulin receptors IRS1 and IRS2. IRS2 expression is regulated by insulin levels in fasting and post-meal state, whereas IRS1 expression is not affected by insulin and therefore remains unaltered in both fasting state and immediately after food intake. The required condition for the development of ‘selective insulin resistance’ is the presence of an altered ratio between IRS1 and IRS2 with a reduced expression of IRS2 and increase expression of IRS1. Research studies show that increased liver fat is associated with both increased expression of IRS1 and impaired insulin clearance contributing to the development of hepatic insulin resistance [26]. In the physiological state, insulin is involved in cardiac metabolism, promoting glucose uptake, protein synthesis, regulation of long-chain fatty acid metabolism, and vascular tonicity. Moreover, insulin has opposing haemodynamic actions on blood vessels as it regulates the endothelial vasoconstriction and vasodilation in two ways: (1) via the phosphorylation of the IRS2 and activation of phosphatidyl inositol 3-kinase (PI3K)/Akt pathway, responsible for the nitric oxide production [27]; and (2) via phosphorylation of the IRS1 the activation of mitogen-activated protein kinase (MAPK) pathway, regulating the secretion of endothelin-1 [25], see Fig. 9.1a. Therefore, insulin regulates the balance between nitric oxide-mediated vasodilation and endothelin-1-mediated vasoconstriction. In the first pathway, the activation of (PI3K)/Akt in endothelial cells leads to phosphorylation of endothelial nitric oxide synthase that in turn synthesises nitric oxide from the guanidine group of arginine (L-arginine) and O2. This pathway regulates the expression of nitric oxide synthase, vascular endothelial growth factor, antioxidant haeme oxygenase-1, and vascular cell adhesion molecule-1. The action of nitric oxide on the endothelium is primarily mediated via reductions in intracellular calcium concentrations promoting vasodilation. In the second pathway, the activation of Grb2 and Shc causes a cascade of phosphorylation to activate the MAPK pathway with subsequent secretion of endothelin-1 [25]. Endothelin-1 plays an important role in vascular function through its action on vascular smooth muscle cells, oxidative stress proliferation and apoptosis [28, 29]. Reduced nitric oxide production and/or bioavailability are associated with hypertension, atherosclerosis and angiogenesis-associated disorders. A recent study from Persico et al. showed that nitric oxide synthase phosphorylation was reduced in liver samples obtained from both NASH and NAFLD patients, compared to liver samples from healthy control subjects [30]. These authors also found that endothelial dysfunction measured with flow-mediated dilation of the brachial artery was reduced according to liver disease severity. In the absence of nitric oxide signalling, there is a disturbance in vascular homeostasis, triggering a series of events leading to pathologies such as hypertension, renal vascular insufficiency and chronic heart failure [31, 32], see Fig. 9.1b.
Insulin signalling in endothelial cells. (a) Insulin has opposing haemodynamic actions in blood vessels. (1) Pro-atherogenic action: Insulin regulates endothelial vasoconstriction via phosphorylation of insulin receptor 1 (IRS1) and the activation of mitogen-activated protein kinase (MAPK) pathway, regulating the secretion of endothelin-1 (ET-1) and plasminogen activator inhibitor-1 (PAI). (2) Anti-atherogenic action: insulin affects vasodilation via the phosphorylation of insulin receptor 2 (IRS2) and activation of the phosphoinositide 3-kinase (PI3K)/Akt pathway, responsible for nitric oxide production (NO). (b) Selective insulin resistance. Reduced expression of IRS2 leads to a selective inhibition of the (PI3K)/Akt pathway causing a deterioration of intracellular signalling that reduces NO synthesis. High extracellular concentration of glucose increases the synthesis of superoxide (O−2) dependent of NAD(P)H oxidase, which reacts with NO to generate peroxynitrite (ONOO−), contributing to endothelial cell dysfunction. IRS1 expression is unchanged or increased therefore the (MAPK) pathway is not inhibited resulting in enhanced expression of endothelin-1 and proliferation of vascular smooth muscle cells with pro-atherosclerotic action. In addition, impaired insulin signalling causes a reduction in outward potassium (K+) currents causing abnormal repolarisation in cardiomyocytes. IRS1 and IRS2 insulin receptors 1 and 2, MAPK mitogen-activated protein kinase, ONOO− peroxynitrite, NO nitric oxide, PI3K phosphatidylinositol-4,5-bisphosphate 3-kinase; nitric oxide synthase, O−2 superoxide, K+ potassium, ET-1 endothelin-1, PAI plasminogen activator inhibitor-1
In the presence of endothelial insulin resistance, the (PI3K)/Akt pathway and (MAPK) pathway are selectively impaired resulting in a ‘selective insulin resistance’ state. In this state, there is a selective inhibition of the (PI3K)/Akt pathway causing a deterioration of intracellular signalling that reduces the L-arginine transport with consequent reduction of NO synthesis. By contrast the (MAPK) pathway is not inhibited [33] resulting in enhanced expression of endothelin-1 and proliferation of vascular smooth muscle cells with pro-atherosclerotic action [34, 35]. Multiple pathophysiological stimuli typical of NAFLD such as increased production of inflammatory cytokines, hyperglycaemia, high levels of asymmetric dimethylarginine [36], hypoadiponectinemia [37] and increased release of free fatty acids can also cause a selective inhibition of the (PI3K)/Akt pathway with consequent reduction of NO production. In addition, high extracellular concentrations of D-glucose increase synthesis of O−2 dependent of NAD(P)H oxidase, which reacts with the NO to generate ONOO−, contributing to endothelial dysfunction [32], see Fig. 9.1b.
Insulin has also a direct effect on cardiomyocytes, modulating cardiac contractility and affecting cardiac output. Moreover, insulin mediates the cellular hypertrophy and generates an antiapoptotic effect on cardiomyocytes by activating other intermediary intracellular signalling pathways that affect potassium currents [38, 39]. Impaired insulin signalling causes a reduction in the outward K+ currents causing abnormal repolarisation in cardiomyocytes [40]. The arrhythmogenic potential of altered outward K+ currents can contribute to an increase in the incidence of heart failure [41]. Several studies have assessed the association between NAFLD and left ventricular dysfunction and hypertrophy [42]. In a multicentre community-based Coronary Artery Risk Development in Young Adults (CARDIA) study, VanWagner et al. have performed a cross-sectional analysis of 2713 participants with imaging-diagnosed NAFLD. Theses authors showed that NAFLD was independently associated with left ventricular systolic and diastolic dysfunction and myocardial remodelling [43]. In a recent cross-sectional study during a health screening programme, 3300 subjects underwent echocardiography and hepatic ultrasonography. In this study, the presence of NAFLD was independently associated with a 68% increase in the risk of left ventricular diastolic dysfunction. After adjusting for age, sex and waist circumference, the risk of diastolic dysfunction incrementally increased according to the severity of fibrosis. After stratifying the population according to BMI, the association between NAFLD with fibrosis and LV diastolic dysfunction was significant only in non-obese subjects [21].
Steatosis, inflammation and fibrosis cause significant structural changes in the liver that might explain the endothelial and myocardial dysfunction described in this metabolic liver condition. Recent evidence showed that hepatic parenchymal alterations are responsible for the biomechanical and rheological changes in patients with NAFLD [17]. Hepatocyte enlargement due to hepatocellular lipid accumulation and ballooning may cause changes in the hepatic microvasculature [44] with sinusoidal compression, sinusoidal space restriction, distortion of the sinusoidal pattern (reducing the sinusoidal space by as much as 50% compared with normal liver) [45], compression of sinusoids and loss of fenestrae resulting in impaired sinusoidal flow with increase in intrahepatic resistance causing an increase in portal venous pressure [46, 47]. In this condition, there is a disruption of sinusoidal flow starting in zone 3 of the liver (from the central vein) and then expanding in through the entire lobule. With these structural and functional changes, liver sinusoidal endothelial cells become defenestrated and deposit extracellular matrix within the space of Disse causing relative hypoxia [45]. Experimental studies in steatotic animal models indicate that moderate steatosis reduces sinusoidal blood flow by approximately half because of distortion of the sinusoids by fat-filled hepatocytes [48]. These alterations are associated with increase in intrahepatic resistance responsible of post-sinusoidal non-cirrhotic portal hypertension [49]. Franque et al. studied the portal pressure in 50 patients with non-alcoholic fatty liver disease using transjugular liver–vein catheterisation and biopsy. They found that the hepatic venous pressure gradient was ≥5 mmHg; the threshold indicating sinusoidal portal hypertension in about one-third of the study population, and that this portal hypertension was related to the steatosis grade and not to the presence of extensive fibrosis or cirrhosis [50]. In another study, Chung et al. showed that NAFLD was associated with a 29% increase in the risk of diastolic dysfunction compared with controls. In addition, the authors found that in non-obese subjects, the risk to develop diastolic dysfunction increased incrementally according to fibrosis grade [21].
These hepatic haemodynamic changes in patients with NAFLD suggest that there could be a reduction in hepatic arterial flow [51] with a consequent decrease in cardiac preload resulting in early asymptomatic cardiovascular alterations [52]. This would have an effect on the micro and macro circulation with possible increase in vascular calcifications and atherosclerosis formation [53], endothelial dysfunction [30] and increase in intima-media thickness [54] and myocardial dysfunction [42, 55, 56].
9.3.2 Lipid Metabolism and Atherosclerosis
Several studies have shown that the process of atherogenesis is initiated by two main mechanisms: (1) endothelial injury and/or (2) accumulation of low-density lipoproteins (LDL) within the arterial wall, which are generally prone to oxidisation [57, 58].
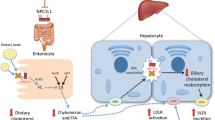
The liver plays a major role in regulating lipid metabolism by the combined action of de novo lipogenesis and lipid oxidation, as well as uptake and secretion of lipoproteins. Liver fat accumulation is associated with an imbalance in hepatic fatty acid uptake, endogenous lipid synthesis, lipid oxidation and very-low-density lipoprotein production [59, 60]. NAFLD is associated with hepatic insulin resistance and induces hepatic VLDL production via changes in the rate of apo B synthesis [61] and stimulation of de novo lipogenesis [62]. In the presence of hepatic insulin resistance, there is an increased expression of sterol regulatory element binding protein 1c (SREBP1c) that leads to the activation of key enzymes for de novo lipogenesis [63]. Moreover, carbohydrate responsive-element binding protein (ChREBP) is also stimulated by hyperglycaemia contributing to the activation of lipogenesis [64].
In the liver, lipid droplets are stored in the endoplasmic reticulum of the hepatocytes where VLDL particles are assembled. Subsequently, apolipoprotein B-containing VLDL particles are secreted into the circulation. Increased circulating levels of VLDL particles can lead to the generation of small, dense LDL that are highly atherogenic. In the circulation, LDL can enter the artery wall and be oxidised by vascular cells (endothelial cells, smooth muscle cells and macrophages) with oxidising enzymes including lipoxygenase and myeloperoxidase. Oxidation of LDL can occur in two ways: (a) mild oxidation of LDL, with absence of changes or little changes in apolipoprotein B100 (this mild oxidised LDL retains its affinity for the LDL receptor); (b) mild oxidised LDL can be further oxidised leading to a loss of recognition by the LDL receptor and a shift to recognition by scavenger receptors [58]. Oxidised LDL activates the conversion of monocytes to macrophages foam cells with subsequent formation of the fatty streak. In addition, the reduction of NO bioavailability (described previously) with consequent increase in the production of reactive oxygen species such as O−2 and ONOO− contribute to the oxidative modification of LDL and the development of atherosclerosis. The accumulation of subendothelial atherogenic apolipoprotein B-containing low-density and very-low-density lipoproteins and chylomicrons plus monocytes activation and migration through the endothelial wall into the vascular smooth muscle cells layer of the intimal media contribute to the formation of the atherogenic streak. Several studies have showed an association between NASH and an altered LDL profile [59, 60]. Chalasani et al. showed a significant association between NASH and increased levels of oxidised LDL compared with controls. This was in line with other studies conducted previously by Sanyal and MacDonald where they found an association between lipid peroxidation and severity of liver disease. Alkhouri et al. showed that in patients with NAFLD, the histologic severity of liver disease was strongly associated with an increased level of triglycerides and low-density lipoprotein and a decrease in high-density lipoprotein [65]. In addition, in a large, multi-ethnic, sex-balanced cohort, CT-diagnosed NAFLD was associated with atherogenic dyslipidaemia defined as low HDL-cholesterol and high triglycerides and a triglycerides/HDL ratio greater than 3 [60].
9.3.3 Hepatokines
The ectopic accumulation of lipids in the liver is associated with the infiltration and activation of immune cells and production of pro-atherogenic and pro-inflammatory cytokines known as hepatokines. Hepatokines are proteins that influence metabolism and inflammatory pathways by affecting insulin sensitivity, homeostasis and cardiovascular health [66]. The liver secretes numerous hepatokines; however, the specific role of these hepatokines is not been completely elucidated. Some of them have been associated with NAFLD and CVD although the exact role has not been clarified. Fetuin-A (also known as α2-HS-glycoprotein), that is primarily synthesised by hepatocytes, is a natural inhibitor of the insulin receptor tyrosine kinase. Several lines of evidence showed that fetuin-A is a potent inhibitor of calcification. Fetuin-A binds with bioactive Ca2+ suggesting its potential role in the inhibition of systemic calcification by protecting VSMC from the detrimental effects of Ca2+ overload and subsequent calcification [67, 68]. However, the role of fetuin-A in NAFLD and CVD seems to be complex and controversial as it seems to be modulated by various independent pathogenetic mechanisms such as inflammation and insulin resistance. Sato et al. showed that serum fetuin-A concentration was negatively correlated with platelet count, NAFLD fibrosis score and mean IMT [69]. In contrast, Celebi et al. observed no difference in plasma levels of fetuin-A between NASH and NAFLD groups. Moreover, the authors did not find any association of circulating fetuin-A with liver histology and insulin resistance in subjects with NAFLD [70]. By contrast, some studies showed high plasma levels of fetuin-A with insulin resistance and hepatic steatosis and increased risk of myocardial infarction and ischemic stroke [71]. Kahraman et al. described high plasma concentrations of fetuin-A in patients with NASH; this result was confirmed by mRNA and protein expression of fetuin-A in liver tissue [72]. Fetuin-A could represent a possible biomarker to detect CVD in patients with NAFLD; however, further studies are needed to clarify its metabolic function and its association with liver disease, atherosclerosis and vascular calcification.
Fibroblast growth factor 21 is another hepatokine secreted mainly by the liver and is regulated by several transcription factors including peroxisome proliferator-activated receptor α (PPAR- α), PPARγ, ChREBP and SREBP [73]. Fibroblast growth factor 21 has been shown to have beneficial effects on energy homeostasis, glucose and lipid metabolism. Emerging evidence suggests that fibroblast growth factor 21 is also a physiological protector of vascular functions via two major mechanisms: (1) indirectly via inducing expression and secretion of adiponectin that in turn leads to the production of NO in endothelial cells [74, 75]; and (2) directly by inhibiting the hepatic cholesterol biosynthesis by suppressing SREBP [76]. However, in contrast to this evidence, recent studies showed that high levels of fibroblast growth factor 21 are associated with NAFLD and atherosclerosis [77, 78].
Selenoprotein P is a secretory protein primarily produced and released by the liver, and it is responsible for transporting selenium from the liver to extrahepatic tissues. Selenoprotein P is upregulated in the liver of patients with NAFLD [79], type 2 diabetes [80] and CVD [81]; however, there have been very few studies that have investigated the relationship between selenoprotein P and CVD. The mechanism by which selenoprotein P causes CVD is not clear; one possible mechanism is through its effect on insulin resistance. However, further studies are needed to identify the independent relationship between selenoprotein P and CVD and to clarify the underlying mechanism linking selenoprotein P and CVD.
Hepatokines that are mainly secreted from the liver have para- and endocrine effects and are known to directly affect inflammation, and glucose and lipid metabolism. Although accumulating evidence shows that hepatokines play an important role in modulating inflammatory processes that in turn affect atherosclerotic process, it remains controversial whether there is an independent effect of these hepatokines to affect the pathogenesis of CVD.
9.3.4 Prothrombotic Factors
The liver synthesises several coagulation factors including fibrinogen, and plasminogen activator inhibitor-1 (PAI-1), which may have important roles in the development of CVD. In addition, insulin has also been shown to increase the expressions of PAI-1, through the MAPK pathways [33]. In a large community-based, prospective observational study of CVD risk, increasing PAI-1 levels were associated with an adverse cardiovascular risk profile [82].
Kotronen et al. showed an independent association between increased activities of coagulation factors (FVIII, FIX, FXI and FXII) and NAFLD (liver fat diagnosed by MRS) compared with controls [83]. This study was in accordance with Tripodi et al. showing that plasma from patients with NAFLD was characterised by a procoagulant imbalance that progressed with increasing severity of disease from simple steatosis to cirrhosis [84].
By contrast, Verrijken et al. studied a large cohort of overweight/obese patients who underwent a clinical assessment for coagulation factors, and metabolic and liver disease. In this study, severity of liver histology was associated with a significant and independent increase in PAI-1. Whereas, other metabolic features (but not NAFLD) were associated with an increase in fibrinogen, factor VIII and von Willebrand factor, antithrombin III was decreased [85]. Similar results were found in other research studies in adults and children where increased PAI-1 levels were associated with NAFLD severity and CVD [86,87,88]. PAI-1 is the primary inhibitor of the endogenous fibrinolytic system, and it is responsible for reducing fibrinolytic activity and plays a key role in the atherothrombotic process [86, 89, 90]. Increased PAI-1 plasma levels would reduce the capacity of the fibrinolytic system to prevent fibrin deposition in vessel walls and thrombus formation [91].
9.3.5 PNPLA3 I148M Genotype
The relationship between liver fat content, NAFLD and ischaemic heart disease (IHD) has recently been investigated in a Mendelian randomisation and meta-analysis of 279,013 individuals [92]. In a cohort study of the Danish general population (n = 94,708/IHD = 10,897), the authors tested whether a high liver fat content or a diagnosis of NAFLD was associated with IHD. The authors then tested whether a genetic variant in the gene encoding the protein patatin-like phospholipase domain containing 3 proteins (PNPLA3), I148M (rs738409) (a strong and specific cause of high liver fat content and NAFLD) was causally associated with the risk of IHD.
As expected from existing evidence, the authors found that the risk of IHD increased stepwise with increasing liver fat content (in quartiles) up to an odds ratio (OR) of 2.41 (1.28–4.51) (P-trend = 0.004). The corresponding OR for IHD in individuals with vs. without NAFLD was 1.65 (1.34–2.04) (P = 3 × 10–6), which is in keeping with existing evidence. PNPLA3 I148M was associated with a stepwise increase in liver fat content of up to 28% in MM vs. II-homozygotes (P-trend = 0.0001) and with ORs of 2.03 (1.52–2.70) for NAFLD (P = 3 × 10–7), 3.28 (2.37–4.54) for cirrhosis (P = 4 × 10–12) and 0.95 (0.86–1.04) for IHD (P = 0.46). In the meta-analysis (N = 279,013/IHD = 71,698), the OR for IHD was 0.98 (0.96–1.00) per M-allele vs. I-allele. The OR for IHD per M-allele for higher genetically determined liver fat content was 0.98 (0.94–1.03) vs. an observational estimate of 1.05 (1.02–1.09) (P for comparison = 0.02).
Therefore, despite confirming the known observational association of hepatic fat content (and NAFLD) with the risk of prevalent IHD in this analysis, the authors suggested that fatty liver due to PNPLA3 variant is not causally associated with IHD [92]. Although, these data are undoubtedly thought provoking, we believe that it is important to be cautious about the interpretation of these data for the following reasons. (a) Based on the ICD-8 codes (and computed tomography scanning), the prevalence of NAFLD (i.e. 0.7% of the whole cohort; 633 out of 94,708) was extraordinarily low, and it is also quite possible that there was contamination bias (with up to 25–30% of subjects in the reference group possibly having undiagnosed NAFLD). (b) Using a Mendelian randomisation approach, the authors failed to show any increase in the risk of prevalent IHD with the presence of the PNPLA3 148M allele in a subgroup of 1439 individuals in whom liver fat content was detected by computed tomography scanning. It is important to note that many subjects in this analysis did not have NAFLD (because liver fat percentage was <5.6%), and it is also noteworthy that the mean liver fat percentage was extremely low and similar in all three PNPLA3 genotypes (II = 5.1%, IM = 6.0% and MM = 6.5%, respectively). (c) The authors also tested whether the PNPLA3 genotype was associated with risk of prevalent IHD in the whole cohort, of whom nearly 99% did not have known NAFLD. Since PNPLA3 148 MM was associated with a tiny increase in liver fat percentage in people with imaging-diagnosed NAFLD, it is perhaps not surprising that in the general population without NAFLD, PNPLA3 148 MM was not associated with IHD. Although a subsequent meta-analysis also confirmed the lack of a significant association between this genetic variant and IHD, again no information was available about NAFLD status in the CARDIOGRAMplusC4D consortium.
To date, a consensus is emerging that there are at least two distinct forms of NAFLD, i.e. the obese/metabolic NAFLD and the PNPLA3-associated NAFLD, which may have different consequences for risk of IHD [12, 93,94,95]. Less than 5–6% of European individuals with NAFLD carry the PNPLA3 148MM genotype, and this genotype is neither sufficient nor necessary to cause non-alcoholic steatohepatitis, cirrhosis or primary liver cancer. The contribution of genetic polymorphisms to inter-individual variation in NAFLD phenotype is relatively small, and the role of the PNPLA3 148M allele in the general population without NAFLD is far from clear.
Thus, we consider that further research is urgently needed to test the effect of PNPLA3 148 MM genotype on risk of incident cardiovascular outcomes in cohorts with proven NAFLD. Since undiagnosed NAFLD is very common in the general ‘healthy’ population, it is also important to know that the control/reference population does not have NAFLD.
9.4 Treatments
Since NAFLD is associated with extrahepatic complications such as type 2 diabetes (T2DM) and chronic kidney disease that also increase risk of cardiovascular disease (CVD) [12, 96,97,98], effective treatment strategies are urgently required [94]. Crucially, similar proportions of people with NAFLD die from CVD as from liver disease [94] and when patients with NAFLD develop type 2 diabetes, the presence of diabetes further increases risk of CVD, creating a vicious spiral of potential ill health [99]. Consequently, an ideal effective treatment for NAFLD might therefore be expected to not only reduce risk of chronic liver disease-related complications but also to decrease risk of type 2 diabetes and CVD.
In 2017, the comparative benefits and harms of different interventions using standard Cochrane methodology were evaluated [100]. These authors concluded that due to the very low-quality evidence, there was current uncertainty about the effectiveness of pharmacological treatments for people with NAFLD including those with NASH. Importantly as stated, further well-designed randomised clinical trials with sufficiently large sample sizes are necessary. Nevertheless, that said, the purpose and focus of this section are to discuss the existing evidence for potential diets and pharmacological treatments for NAFLD which also have beneficial effects on CVD and CVD risk factors.
The ability to diagnose NASH and monitor NASH is crucial for the testing of therapeutic interventions for NASH and to evaluate their effectiveness on CVD and cardiac complications of NAFLD.
Currently, the only investigation with acceptable sensitivity and specificity for diagnosing and monitoring NASH is liver biopsy and histological examination; and this is the current ‘gold standard’ that has undoubtedly hampered the testing of drug effectiveness in NASH [101]. Despite this caveat, current guidelines have concluded that there is sufficient evidence to consider the use of a Mediterranean diet (MD) [102] and pioglitazone or vitamin E therapy in the treatment of NASH [102,103,104].
Weight loss is the most effective way to promote liver fat removal, and several controlled studies have confirmed that an intense approach to lifestyle changes, carried on along the lines of cognitive-behaviour treatment, is able to attain the desired 7–10% weight loss, associated with reduced liver fat, non-alcoholic steatohepatitis (NASH) remission and also reduction of fibrosis [105]. Even larger benefits have been reported after bariatric surgery in NAFLD, where 80% of subjects achieve NASH resolution at 1-year follow-up [105].
The major focus of this section will be to discuss the potential CVD benefits of the MD diet as well as pioglitazone and vitamin E as this diet and these two agents have recently been recommended by the Guidelines discussed above for NAFLD. We will also discuss the role of statins as these agents have been used for many years to lower low-density lipoprotein (LDL-C) and decrease CVD risk.
9.4.1 Mediterranean Diet (MD)
The benefits of the MD as the diet of choice for NAFLD have recently been discussed in an excellent review of the subject [106]. The individual components of the MD such as olive oil, fish, nuts, whole grains, fruits and vegetables have been shown to beneficially affect or negatively correlate with NAFLD. Additionally, an MD contains lower amounts of dietary components that are thought to be potentially harmful for obesity, NAFLD and CVD, such as fructose, refined carbohydrates, trans fatty acids and red meats and therefore an MD diet tends to comply with current guidelines to reduce the risk of CVD [107]. In June 2017, the American Heart Association’s presidential advisory on dietary fats stated that replacing saturated fat with polyunsaturated vegetable oil reduces the incidence of CVD by ~30% [108]. Importantly, this shift towards more unsaturated fats occurs when a Westernised diet containing processed foods is replaced by the Mediterranean diet (MD) [108]. It is beyond the scope of this chapter to discuss the many potential mechanisms of benefit by which a MD may benefit NAFLD and CVD. However, we have briefly summarised the key components of the diet and the key factors that may be favourably affected in reducing risk of CVD in NAFLD in Fig. 9.2.
Potentially beneficial effects of the Mediterranean diet in NAFLD. The Mediterranean diet (MD) contains a variety of nutrients that have the potential for affecting improvements in vascular risk factors. The MD may have stimulatory effects in the intestine to promote favourable changes in gut microbiota and intestinal function with beneficial effects for liver disease in NAFLD. BCAAs branched chain amino acids, DAGs di-acylglycerols, di-P PA di-palmitolyl phosphatidic acid, LCFAs long-chain fatty acids, LPS lipopolysaccharide, SCFAs short chain fatty acids, TAGs tri-acylglycerols, TMA trimethylamine
Data from three small, brief duration randomised trials have suggested a potential beneficial effect of the MD in NAFLD [109,110,111]. We believe that longer-term RCTs are needed, preferably with histological liver outcomes to test whether there is any benefit on NASH and/or liver fibrosis. It has to be stressed that in most cases any form of healthy diet, which leads to caloric reduction and is acceptable to the patient, should be encouraged for patients with NAFLD. For the patient who finds caloric restriction difficult, changing dietary composition without necessarily reducing caloric intake could offer a more feasible alternative although the benefit on liver health is not as marked as weight reduction alone [102, 105]. The importance of weight loss has been highlighted in patients with NASH, where weight loss per se is able to induce NASH resolution, without any worsening of fibrosis [112].
9.4.2 Thiazolidienediones (Pioglitazone) and Vitamin E
Recently as a result of several randomised placebo-controlled trials in patients with NASH, three key international bodies (National Institute for Health and Care Excellence (NICE), the Joint European Societies (Diabetes, Hepatology and Obesity) and the American Association for the Study of Liver Diseases) have recently also recommended the use of pioglitazone for the treatment of NASH [102,103,104].
9.4.2.1 Pioglitazone
Thiazolidienediones (TZDs) are well known to lower plasma glucose concentrations over many years of treatment, and these drugs (rosiglitazone and pioglitazone) have been licensed for the treatment of type 2 diabetes for almost 20 years. TZDs are potent peroxisome proliferator-activated receptor gamma (PPARγ) agonists that target both adipose tissue metabolism and also inflammation. The first available TZD was troglitazone which was rapidly withdrawn (in the UK in 1997, and in the USA in 2000) because of toxic side effects. In the UK rosiglitazone was withdrawn as a result predominantly of concerns raised about possible increased cardiovascular risk in a meta-analysis published in 2007 [113]. Yet despite those concerns, in 2013 the US Food and Drug Administration (FDA) lifted the final regulatory restrictions on rosiglitazone in 2013, stating that ‘we have continued monitoring these medicines and identified no new pertinent safety information. As a result, we have determined the risk evaluation and mitigation strategy is no longer necessary to ensure that the benefits of rosiglitazone medicines outweigh their risks’. Since most of the available evidence with this class of drug in NASH exists for pioglitazone, because of these problems with rosiglitazone, we will focus discussion on the evidence with pioglitazone treatment.
Pioglitazone treatment results in histological resolution of NASH in ~50% of patients regardless of diabetes status [114,115,116]. The mean effect for response to pioglitazone defined as resolution of NASH from three key trials [114,115,116] is 51% (95% CI 42, 60), and a recent meta-analysis of pioglitazone treatment in NASH has concluded that thiazolidinediones significantly improve ballooning degeneration, lobular inflammation, steatosis and combined necroinflammation in patients with NASH and that pioglitazone may improve fibrosis [117].
Extensive use of pioglitazone to treat T2DM has established its safety and generic pioglitazone costs to the UK NHS are only ~£1.15 (1.31 Euros or 1.51 USD in June 2018); per patient per month. Importantly since patients with NAFLD are also at increased risk of type 2 diabetes and CVD, RCT evidence also shows that treatment with pioglitazone also decreases risk of developing type 2 diabetes [118], myocardial infarction [119] and stroke [120, 121]. For all of these additional benefits of treatment with pioglitazone, the magnitude of the benefit of treatment with pioglitazone is a reduction in risk of between 16 and 72%. For example in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events) [119], the main secondary end point was the composite of all-cause mortality, non-fatal myocardial infarction and stroke. 301 patients in the pioglitazone group and 358 in the placebo group reached this end point, and there was a significant 16% decrease in risk of this end point with pioglitazone treatment. In order to examine whether pioglitazone can reduce the risk of type 2 diabetes mellitus in adults with impaired glucose tolerance, a total of 602 patients were randomly assigned to receive pioglitazone or placebo. After a median follow-up of 2.4 years, compared with placebo, pioglitazone reduced the risk of conversion of impaired glucose tolerance to type 2 diabetes by 72% (95% confidence interval, 0.16–0.49; P < 0.001) [118]. Similarly, for the primary [120] and secondary [122] prevention of stroke, there was a similar magnitude of benefit with pioglitazone treatment. IRIS (Insulin Resistance Intervention after Stroke) was a primary prevention trial [120]. 3876 patients who had had a recent ischemic stroke or transient ischaemic attack, subjects received either pioglitazone (target dose, 45 mg daily) or placebo. Eligible patients did not have diabetes but were found to have insulin resistance on the basis of a score of more than 3.0 on the homeostasis model assessment of insulin resistance (HOMA-IR) index. The primary outcome was fatal or non-fatal stroke or myocardial infarction. By 4.8 years of follow-up, a primary outcome had occurred in 175 of 1939 patients (9.0%) in the pioglitazone group and in 228 of 1937 (11.8%) in the placebo group (hazard ratio in the pioglitazone group, 0.76; 95% confidence interval [CI], 0.62–0.93; P = 0.007). Diabetes developed in 73 patients (3.8%) and 149 patients (7.7%), respectively (hazard ratio, 0.48; 95% CI, 0.33–0.69; P < 0.001). Importantly, overall safety and tolerability was good with no change in the safety profile of pioglitazone identified. For example, in the high-risk PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events) [119], whilst there was a very slight increase in the well-recognised side effect of cardiac failure 6% versus 4% of those in the pioglitazone versus placebo groups; mortality rates from heart failure did not differ between groups. Thus, in summary in NAFLD, treatment with pioglitazone may directly benefit the liver and decrease risk of type 2 diabetes, myocardial infarction and stroke (Fig. 9.3).
PPARγ agonist treatment in NAFLD. NAFLD increases risk of type 2 diabetes, CVD and hepatocellular carcinoma. With the development of type 2 diabetes, there is a further increase in the risk of liver fibrosis. With development of more advanced forms of liver fibrosis (e.g. F3 and F4 fibrosis), there is a marked increase in the risk of hepatocellular carcinoma. In NASH, PPARγ agonist treatment has a beneficial effect to cause resolution of NASH in ~50% of patients (after 2 years of treatment). PPARγ agonist treatment may be also beneficial in reducing liver fibrosis (although further research is needed in this patient group). PPARγ agonist treatment decreases risk of type 2 diabetes (and lowers plasma glucose concentrations in patients who have established type 2 diabetes). PPARγ agonist treatment also decreases risk of myocardial infarction and stroke
Pioglitazone targets both adipose tissue metabolism and inflammation, acting through the transcription factor PPARγ. PPARγ has three splicing variant isoforms (1–3, and) that display differences in tissue localisation for each isoform: 1 (ubiquitous localisation), 2 (localised in adipose tissue) and 3 (localised in macrophages, colon and adipose tissue) [123]. PPARγ is predominantly expressed in adipocytes, immune cells including macrophages (and Kupffer cells) and hepatic stellate cells. PPARγ agonists activate PPARγ receptor function to decrease supply of fatty acids to the liver by promoting pre-adipocyte differentiation. Additionally in the liver PPARγ agonists activate Kupffer cells polarisation from a pro-inflammatory M1 to a pro-resolving M2 phenotype [124] and reverses hepatic stellate cell trans-differentiation to myofibroblasts [125].
Although it is uncertain whether pioglitazone has actions in the intestine, colonic epithelium expresses high levels of PPARγ3 receptors, and a high potency natural ligand for PPARγ3 receptors is butyrate [126]. Butyrate is produced locally in the large intestine from the gut microbiota-induced fermentation of carbohydrate. Thus, dysbiosis could adversely affect the integrity of intestinal permeability via a butyrate-PPARγ3-mediated effect, with a consequent increase in lipopolysaccharide concentrations in the portal circulation promoting the risk of NASH. Interestingly, in mice a high-fat diet modifies the PPAR-γ pathway leading to disruption of the microbial and physiological ecosystem in small intestine, and these effects were reversed by treatment with the PPARγ agonist rosiglitazone [127]. Since many patients with type 2 diabetes also have dysbiosis and NASH, PPARγ agonist drugs would seem an ideal form of treatment for this group of patients, particularly if it were possible to develop even better PPARγ agonists that retained the beneficial effects of the drugs without increasing the risk of known side effects associated with the class. The potential modes of action of PPARγ agonists in NAFLD are shown in Fig. 9.4.
Potential modes of action of PPARγ agonist effects to confer benefit in NAFLD. PPARγ agonist treatment acts via PPARγ2 receptors in pre-adipocytes to promote adipocyte differentiation, increase expandability of peripheral adipose tissue depots and increased adiponectin release from adipocytes. The increased expandability of peripheral adipose tissue depots decreases fatty acid flux to the liver with consequent decreased fluxes in acetyl Co-A for lipid synthesis. Increased adiponectin release potentially decreases inflammation and improves hepatic insulin sensitivity. PPARγ agonist treatment acts in liver via PPARγ3 receptors in macrophages (Kupffer cells) to decrease activation of macrophages and also acts in hepatic stellate cells to decrease activation of these matrix-producing cells. Although it is uncertain whether pioglitazone has actions in the intestine, colonic epithelium expresses high levels of PPARγ3 receptors and butyrate is a high-potency natural ligand for PPARγ3 receptors. Butyrate is produced locally in the large intestine from the gut microbiota-induced fermentation of carbohydrate. Thus improvements in dysbiosis, perhaps due to a Mediterranean diet (see Fig. 9.2), could improve intestinal permeability via a butyrate-PPARγ3-mediated effect. A potentially beneficial consequence of this effect would be reduced levels of lipopolysaccharide in the portal circulation, thus reducing the levels of this pro-inflammatory stimulus in the liver. DAGs di-acylglycerols, di-P PA di-palmitoyl phosphatidic acid, LCFAs long-chain fatty acids; PAI-1 plasminogen activator inhibitor-1, CRP C-reactive protein, TNF-alpha tumour necrosis factor, FGF-21 fibroblast growth factor, VLDL very-low-density lipoprotein, HDL-C high-density lipoprotein cholesterol, LDL-C low-density-lipoprotein cholesterol
There is considerable interest in determining whether it is possible to dissociate the benefits of pioglitazone from the side effects. In recent years the global usage of pioglitazone has plummeted, largely because of fears about side effects associated with this drug (such as increased risk of bone fracture, fluid retention and increases in body fat). It has been known for many years that post-translational modification (PTM) of the PPARγ in the form of altered phosphorylation status affecting functioning of the PPARγ receptor [128, 129] and PPARγ activity is also known to be regulated by other PTMs such as sumoylation and ubiquitinylation [130]. Kraakman et al. [131] have recently tested in mice whether another PTM, i.e. deacetylation of PPARγ, is able to dissociate the metabolic benefits of TZDs from their adverse effects. TZDs induce the deacetylation of PPARγ on K268 and K 293 to cause the browning of white adipocytes. By mutating these two lysine residues to arginine (2KR) there is constitutive deacetylation of PPARγ and increased energy expenditure protecting the mice from diet-induced obesity, glucose intolerance and hepatic steatosis. When 2KR mice were treated with the TZD rosiglitazone, they retained the beneficial response to the TZD without the evidence of the potentially harmful side effects of the drug such as bone demineralisation, fluid retention or fat deposition. Intriguingly, these data provide the first evidence that it is possible to dissociate benefits from harms with this class of drugs. The data also suggesting the following fascinating possibilities:
-
(a)
it may be possible to design more specific PPARγ drugs than current TZDs or even to combine PPARγ drugs with specific acetylation inhibitors;
-
(b)
variable acetylation status of the PPARγ receptor may be very important in normal physiological conditions to increase the risk of obesity and obesity-related conditions;
-
(c)
and maybe lower doses of currently available TZDs (pioglitazone) would still confer a benefit in NASH without increasing the risk of harmful side effects.
9.4.2.2 Vitamin E
Although vitamin E is recommended for consideration of treatment of NASH in the Guidelines discussed above [102, 104, 132], there is less convincing evidence that vitamin E treatment confers any benefit beyond the liver. Although several observational epidemiologic studies suggested that vitamin E supplementation might decrease the risk of developing CVD, these data were not substantiated by the results from RCTs testing the effects of vitamin E on a variety of CVD end points.
Vitamin E is a powerful antioxidant that has the potential for reacting with lipid peroxyl radical and over the last two decades a number of studies have tested whether vitamin E is beneficial for CVD [133]. There have been several RCTs that have tested the effects of vitamin E on a range of CVD-related end points. Of these studies, notably the Physicians Health Study of 14,641 men over the age of 50 years were randomised to receive vitamin E (400 IU/day) alternate days and vitamin C (500 mg/day) every day for 8 years [134]. During a mean follow-up of 8 years, there were 1245 confirmed major cardiovascular events. Compared with placebo, vitamin E had no effect on the incidence of major cardiovascular events (both active and placebo vitamin E groups, 10.9 events per 1000 person-years; hazard ratio [HR], 1.01 [95% confidence interval, 0.90–1.13]; P = 0.86), as well as total myocardial infarction (HR, 0.90 [95% CI, 0.75–1.07]; P = 0.22), total stroke (HR, 1.07 [95% CI, 0.89–1.29]; P = 0.45) and cardiovascular mortality (HR, 1.07 [95% CI, 0.90–1.28]; P = 0.43). Importantly, vitamin E was associated with an increased risk of haemorrhagic stroke (HR, 1.74 [95% CI, 1.04–2.91]; P = 0.04). Further longer-term follow-up of this cohort with over 11 years of follow-up in 2012 confirmed there was no CVD benefit of vitamin E treatment [135]. Furthermore, a meta-analysis of the dose–response relationship between vitamin E supplementation and total mortality using data from RCTs was reported in 2005 [136]. Data from 135,967 participants in 19 clinical trials were analysed. Of these trials, nine trials tested vitamin E treatment alone and 10 tested vitamin E combined with other vitamins or minerals. The dosages of vitamin E ranged from 16.5 to 2000 IU/day (median, 400 IU/day). Nine of 11 trials testing high-dosage vitamin E (≥400 IU/day) showed increased risk for all-cause mortality in comparisons of vitamin E versus control. The pooled all-cause mortality risk difference in high-dosage vitamin E trials was 39 per 10,000 persons (95% CI, 3–74 per 10,000 persons; P = 0.035). A dose–response analysis showed a statistically significant relationship between vitamin E dosage and all-cause mortality, with increased risk of dosages greater than 150 IU/day.
Although the generalisability of these findings to patients with NAFLD is uncertain, considering that high-dosage (≥400 IU/day) vitamin E supplements may increase all-cause mortality and the dose of vitamin E that has been tested in NASH was 800 IU/day [115]; in our opinion vitamin E treatment should not be considered in NAFLD.
9.4.3 Statins
The role of statins in liver disease has recently been reviewed [137]. Although there had previously been concern that this class of agents may be harmful in liver disease, these agents are now known to be safe in patients with NAFLD. Analysis of the Dallas Heart Study data in 2006 showed that in 2264 Dallas Heart Study participants who were using no lipid-lowering agent (n = 2124), or who were being treated with a statin for lipid management (n = 140), statin use was not associated with a greater frequency of hepatic steatosis (38% vs. 34%) or elevated serum ALT (15% vs. 13%) by a pair-matched analysis [138]. A Cochrane Systematic Review in 2013 concluded that trials with larger sample sizes and low risk of bias are necessary before it could be concluded that statins were an effective treatment for patients with NASH. However, it was stated that because statins can improve the adverse outcomes of other conditions commonly associated with NASH (for example, hyperlipidaemia, diabetes mellitus, metabolic syndrome), the use of statins in patients with NASH may be justified [139]. A recent systematic review in 2017 evaluated the effects of statins in chronic liver disease [140] and found that statin use is probably associated with lower risk of hepatic decompensation and mortality, and might reduce portal hypertension, in patients with CLDs. Thirteen studies (3 randomised trials, 10 cohort studies) were identified in adults with chronic liver diseases, reporting the association between statin use and risk of development of cirrhosis, decompensated cirrhosis, improvements in portal hypertension, or mortality. Among 121,058 patients with CLDs (84.5% with hepatitis C), 46% were exposed to statins. In patients with cirrhosis, statin use was associated with 46% lower risk of hepatic decompensation (4 studies; RR, 0.54; 95% CI, 0.46–0.62), and 46% lower mortality (5 studies; RR, 0.54; 95% CI, 0.47–0.61). In patients with CLD without cirrhosis, statin use was associated with a nonsignificant (58% lower) risk of development of cirrhosis or fibrosis progression (5 studies; RR, 0.42; 95% CI, 0.16–1.11). In three randomised controlled trials, statin use was associated with 27% lower risk of variceal bleeding or progression of portal hypertension (hazard ratio, 0.73; 95% CI, 0.59–0.91). Thus one can conclude that prospective observational studies and randomised controlled trials are needed to confirm this observation.
Although, other agents have been tested in patients with NAFLD which have effects on CVD risk factors such as liraglutide, obeticholic acid and omega-3 fatty acids, none of these agents are currently recommended in international guidelines for patients with NASH. Because of the limitations of space and the remit of this chapter, we have therefore not discussed the use of these agents in NASH.
In summary, we consider that pioglitazone treatment should be considered for all patients with NASH, regardless of whether they have type 2 diabetes, providing the drug is not contraindicated. In our opinion it is important to undertake a baseline diagnostic liver biopsy and a repeat follow-up biopsy after ~2 years to evaluate response to pioglitazone therapy. For patients who show improvement of NASH, pioglitazone should be continued, and for patients who have worsening of NASH or no improvement of liver disease, the drug should be withdrawn. In contrast, for patients with NASH who also have co-existing type 2 diabetes, pioglitazone treatment should be used unless contraindicated, specifically as an effective glucose-lowering agent in this patient group. For such patients, pioglitazone treatment is advocated primarily as a treatment for type 2 diabetes with the possibility that it may also benefit liver disease in NAFLD, and decrease risk of myocardial infarction and stroke. For all patients with NAFLD, CVD risk should be assessed using available CVD risk calculators.
9.5 Conclusions
Since NAFLD is embedded in a more complex metabolic disease, it is difficult to dissect the independent role of a metabolically dysfunctional liver on the development of CVD. Therefore, the design of future clinical studies should take account of metabolic confounders such as adipose tissue dysfunction, metabolic inflammation, gut dysbiosis, dyslipidaemia and hyperglycaemia. Secondly, the different methods used for the diagnosis of NAFLD add uncertainty as to the relationship between liver disease severity and any association with CVD. Finally, some of the prospective epidemiological cohort studies had a long period of follow-up, without repeat measurements during follow-up which could result in confounding by unmeasured factors. Therefore, we suggest that better designed epidemiological studies are needed to clarify the independence of the role of the liver in NAFLD in the development of CVD.
We consider that two key questions still require further research. Firstly, which patients with NAFLD are at higher risk of CVD and secondly, do patients with CVD need to be screened for NAFLD?
With regard to the first question, as described in this chapter, the early anatomical and structural changes in the liver due to lipid accumulation in hepatocytes can cause a disruption of the hepatic sinusoids leading to non-cirrhotic portal hypertension. This process occurs before hepatic collagen deposition and therefore before the development of NASH [50]. Therefore, measurement of the hepatic venous pressure during liver ultrasound could be an inexpensive strategy that might help to identify a subset of patients with NAFLD at higher risk of developing myocardial dysfunction. However, further evaluation is needed in patients with NAFLD because measuring hepatic venous pressure could be recommended in the evaluation of CVD risk. Other tests include measurement of carotid ultrasound for measuring carotid intima-media thickness, flow-mediated dilation for measuring endothelial function, echocardiography for identifying any myocardial dysfunction and high-resolution computed tomography for the estimation of coronary calcium score to detect early signs of coronary artery atherosclerosis, may have clinical utility in refining the estimation of cardiovascular risk in NAFLD, but discussion of their value in NAFLD is beyond the scope of this chapter.
With regard to the second question, we think that more research needs to be undertaken to identify the prevalence of NAFLD in patients with established CVD. Investigating abnormalities of simple liver function tests with measurement of liver fat with ultrasound, combined with the assessment of liver fibrosis with simple biomarker tests in those patients with diagnosed liver fat, would help the physician to identify NAFLD and assess liver disease severity in those patients with established CVD [141].
Notes
- 1.
FLI: = (e0.953 × loge(triglycerides) + 0.139 × BMI + 0.718 × loge(GGT) + 0.053 × waist circumference − 15.745)/ (1 + e0.953 × loge(triglycerides) + 0.139 × BMI + 0.718 × loge(GGT) + 0.053 × waist circumference − 15.745) × 100
References
Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120(2):366–80.
Fu M, Rosengren A, Thunstrom E, Mandalenakis Z, Welin L, Caidahl K, et al. Although coronary mortality has decreased, rates of cardiovascular disease remain high: 21 years of follow-up comparing cohorts of men born in 1913 with men born in 1943. J Am Heart Assoc. 2018;7(9):e008769.
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–71.
Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Murray CJ, et al. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the global burden of disease 2010 study. Circulation. 2014;129(14):1483–92.
Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132(17):1667–78.
GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–544.
Capewell S, Beaglehole R, Seddon M, McMurray J. Explanation for the decline in coronary heart disease mortality rates in Auckland, New Zealand, between 1982 and 1993. Circulation. 2000;102(13):1511–6.
Capewell S, Morrison CE, McMurray JJ. Contribution of modern cardiovascular treatment and risk factor changes to the decline in coronary heart disease mortality in Scotland between 1975 and 1994. Heart. 1999;81(4):380–6.
Unal B, Critchley JA, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation. 2004;109(9):1101–7.
Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356(23):2388–98.
Bjorck L, Rosengren A, Bennett K, Lappas G, Capewell S. Modelling the decreasing coronary heart disease mortality in Sweden between 1986 and 2002. Eur Heart J. 2009;30(9):1046–56.
Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589–600.
Lee SB, Park GM, Lee JY, Lee BU, Park JH, Kim BG, et al. Association between non-alcoholic fatty liver disease and subclinical coronary atherosclerosis: an observational cohort study. J Hepatol. 2018;68(5):1018–24.
Pais R, Giral P, Khan JF, Rosenbaum D, Housset C, Poynard T, et al. Fatty liver is an independent predictor of early carotid atherosclerosis. J Hepatol. 2016;65(1):95–102.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33.
Yoshitaka H, Hamaguchi M, Kojima T, Fukuda T, Ohbora A, Fukui M. Nonoverweight nonalcoholic fatty liver disease and incident cardiovascular disease: a post hoc analysis of a cohort study. Medicine. 2017;96(18):e6712.
Baffy G. Origins of portal hypertension in nonalcoholic fatty liver disease. Dig Dis Sci. 2018;63(3):563–76.
Thakur ML, Sharma S, Kumar A, Bhatt SP, Luthra K, Guleria R, et al. Nonalcoholic fatty liver disease is associated with subclinical atherosclerosis independent of obesity and metabolic syndrome in Asian Indians. Atherosclerosis. 2012;223(2):507–11.
Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42(2):473–80.
Vlachopoulos C, Manesis E, Baou K, Papatheodoridis G, Koskinas J, Tiniakos D, et al. Increased arterial stiffness and impaired endothelial function in nonalcoholic fatty liver disease: a pilot study. Am J Hypertens. 2010;23(11):1183–9.
Chung GE, Lee JH, Lee H, Kim MK, Yim JY, Choi SY, et al. Nonalcoholic fatty liver disease and advanced fibrosis are associated with left ventricular diastolic dysfunction. Atherosclerosis. 2018;272:137–44.
Ryoo JH, Suh YJ, Shin HC, Cho YK, Choi JM, Park SK. Clinical association between non-alcoholic fatty liver disease and the development of hypertension. J Gastroenterol Hepatol. 2014;29(11):1926–31.
Sung KC, Wild SH, Byrne CD. Development of new fatty liver, or resolution of existing fatty liver, over five years of follow-up, and risk of incident hypertension. J Hepatol. 2014;60(5):1040–5.
Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68(2):335–52.
King GL, Park K, Li Q. Selective insulin resistance and the development of cardiovascular diseases in diabetes: the 2015 Edwin Bierman Award Lecture. Diabetes. 2016;65(6):1462–71.
Honma M, Sawada S, Ueno Y, Murakami K, Yamada T, Gao J, et al. Selective insulin resistance with differential expressions of IRS-1 and IRS-2 in human NAFLD livers. Int J Obes. 2018;42(9):1544–55.
Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101(13):1539–45.
Muniyappa R, Yavuz S. Metabolic actions of angiotensin II and insulin: a microvascular endothelial balancing act. Mol Cell Endocrinol. 2013;378(1–2):59–69.
Khimji AK, Rockey DC. Endothelin—biology and disease. Cell Signal. 2010;22(11):1615–25.
Persico M, Masarone M, Damato A, Ambrosio M, Federico A, Rosato V, et al. Non alcoholic fatty liver disease and eNOS dysfunction in humans. BMC Gastroenterol. 2017;17(1):35.
Vanhoutte PM. Endothelial control of vasomotor function: from health to coronary disease. Circ J. 2003;67(7):572–5.
Dubo S, Gallegos D, Cabrera L, Sobrevia L, Zuniga L, Gonzalez M. Cardiovascular action of insulin in health and disease: endothelial L-arginine transport and cardiac voltage-dependent potassium channels. Front Physiol. 2016;7:74.
Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28(5):463–91.
Jiang ZY, He Z, King BL, Kuroki T, Opland DM, Suzuma K, et al. Characterization of multiple signaling pathways of insulin in the regulation of vascular endothelial growth factor expression in vascular cells and angiogenesis. J Biol Chem. 2003;278(34):31964–71.
Lesniewski LA, Donato AJ, Behnke BJ, Woodman CR, Laughlin MH, Ray CA, et al. Decreased NO signaling leads to enhanced vasoconstrictor responsiveness in skeletal muscle arterioles of the ZDF rat prior to overt diabetes and hypertension. Am J Physiol Heart Circ Physiol. 2008;294(4):H1840–50.
Xiong Y, Fu YF, Fu SH, Zhou HH. Elevated levels of the serum endogenous inhibitor of nitric oxide synthase and metabolic control in rats with streptozotocin-induced diabetes. J Cardiovasc Pharmacol. 2003;42(2):191–6.
Khan RS, Kato TS, Chokshi A, Chew M, Yu S, Wu C, et al. Adipose tissue inflammation and adiponectin resistance in patients with advanced heart failure: correction after ventricular assist device implantation. Circ Heart Fail. 2012;5(3):340–8.
Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109(5):629–39.
Riehle C, Abel ED. Insulin signaling and heart failure. Circ Res. 2016;118(7):1151–69.
Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146(12):5341–9.
Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2018;15(7):425–39.
Mantovani A, Ballestri S, Lonardo A, Targher G. Cardiovascular disease and myocardial abnormalities in nonalcoholic fatty liver disease. Dig Dis Sci. 2016;61(5):1246–67.
VanWagner LB, Wilcox JE, Colangelo LA, Lloyd-Jones DM, Carr JJ, Lima JA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: a population-based study. Hepatology. 2015;62(3):773–83.
Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat Rec. 2008;291(6):684–92.
Ijaz S, Yang W, Winslet MC, Seifalian AM. Impairment of hepatic microcirculation in fatty liver. Microcirculation. 2003;10(6):447–56.
Francque S, Laleman W, Verbeke L, Van Steenkiste C, Casteleyn C, Kwanten W, et al. Increased intrahepatic resistance in severe steatosis: endothelial dysfunction, vasoconstrictor overproduction and altered microvascular architecture. Lab Invest. 2012;92(10):1428–39.
Hirooka M, Koizumi Y, Miyake T, Ochi H, Tokumoto Y, Tada F, et al. Nonalcoholic fatty liver disease: portal hypertension due to outflow block in patients without cirrhosis. Radiology. 2015;274(2):597–604.
Seifalian AM, Piasecki C, Agarwal A, Davidson BR. The effect of graded steatosis on flow in the hepatic parenchymal microcirculation. Transplantation. 1999;68(6):780–4.
Sarin SK, Kapoor D. Non-cirrhotic portal fibrosis: current concepts and management. J Gastroenterol Hepatol. 2002;17(5):526–34.
Francque S, Verrijken A, Mertens I, Hubens G, Van Marck E, Pelckmans P, et al. Noncirrhotic human nonalcoholic fatty liver disease induces portal hypertension in relation to the histological degree of steatosis. Eur J Gastroenterol Hepatol. 2010;22(12):1449–57.
Yilmaz Y, Kurt R, Yonal O, Polat N, Celikel CA, Gurdal A, et al. Coronary flow reserve is impaired in patients with nonalcoholic fatty liver disease: association with liver fibrosis. Atherosclerosis. 2010;211(1):182–6.
Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol. 2014;20(37):13306–24.
Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56(2):605–13.
Fracanzani AL, Burdick L, Raselli S, Pedotti P, Grigore L, Santorelli G, et al. Carotid artery intima-media thickness in nonalcoholic fatty liver disease. Am J Med. 2008;121(1):72–8.
Bonapace S, Perseghin G, Molon G, Canali G, Bertolini L, Zoppini G, et al. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35(2):389–95.
Lee YH, Kim KJ, Yoo ME, Kim G, Yoon HJ, Jo K, et al. Association of non-alcoholic steatohepatitis with subclinical myocardial dysfunction in non-cirrhotic patients. J Hepatol. 2018;68(4):764–72.
Mitra S, Goyal T, Mehta JL. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovasc Drugs Ther. 2011;25(5):419–29.
Yoshida H, Kisugi R. Mechanisms of LDL oxidation. Clin Chim Acta. 2010;411(23–24):1875–82.
Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99(8):1497–502.
DeFilippis AP, Blaha MJ, Martin SS, Reed RM, Jones SR, Nasir K, et al. Nonalcoholic fatty liver disease and serum lipoproteins: the multi-ethnic study of atherosclerosis. Atherosclerosis. 2013;227(2):429–36.
Nass KJ, van den Berg EH, Faber KN, Schreuder T, Blokzijl H, Dullaart RPF. High prevalence of apolipoprotein B dyslipoproteinemias in non-alcoholic fatty liver disease: the lifelines cohort study. Metab Clin Exp. 2017;72:37–46.
Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49(4):755–65.
Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274(42):30028–32.
Koo SH, Dutcher AK, Towle HC. Glucose and insulin function through two distinct transcription factors to stimulate expression of lipogenic enzyme genes in liver. J Biol Chem. 2001;276(12):9437–45.
Alkhouri N, Tamimi TA, Yerian L, Lopez R, Zein NN, Feldstein AE. The inflamed liver and atherosclerosis: a link between histologic severity of nonalcoholic fatty liver disease and increased cardiovascular risk. Dig Dis Sci. 2010;55(9):2644–50.
Sun ZL, Xie QY, Guo GL, Ma K, Huang YY. Serum fetuin-A levels in patients with cardiovascular disease: a meta-analysis. Biomed Res Int. 2014;2014:691540.
Reynolds JL, Skepper JN, McNair R, Kasama T, Gupta K, Weissberg PL, et al. Multifunctional roles for serum protein fetuin-A in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol. 2005;16(10):2920–30.
Marechal C, Schlieper G, Nguyen P, Kruger T, Coche E, Robert A, et al. Serum fetuin-A levels are associated with vascular calcifications and predict cardiovascular events in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6(5):974–85.
Sato M, Kamada Y, Takeda Y, Kida S, Ohara Y, Fujii H, et al. Fetuin-A negatively correlates with liver and vascular fibrosis in nonalcoholic fatty liver disease subjects. Liver Int. 2015;35(3):925–35.
Celebi G, Genc H, Gurel H, Sertoglu E, Kara M, Tapan S, et al. The relationship of circulating fetuin-A with liver histology and biomarkers of systemic inflammation in nondiabetic subjects with nonalcoholic fatty liver disease. Saudi J Gastroenterol. 2015;21(3):139–45.
Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG, et al. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008;118(24):2555–62.
Kahraman A, Sowa JP, Schlattjan M, Sydor S, Pronadl M, Wree A, et al. Fetuin-A mRNA expression is elevated in NASH compared with NAFL patients. Clin Sci. 2013;125(8):391–400.
Jin L, Lin Z, Xu A. Fibroblast growth factor 21 protects against atherosclerosis via fine-tuning the multiorgan crosstalk. Diabetes Metab J. 2016;40(1):22–31.
Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, et al. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56(5):1387–94.
Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17(5):779–89.
Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y, et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation. 2015;131(21):1861–71.
Shen Y, Ma X, Zhou J, Pan X, Hao Y, Zhou M, et al. Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovasc Diabetol. 2013;12:124.
Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH, et al. Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2013;33(10):2454–9.
Choi HY, Hwang SY, Lee CH, Hong HC, Yang SJ, Yoo HJ, et al. Increased selenoprotein p levels in subjects with visceral obesity and nonalcoholic fatty liver disease. Diabetes Metab J. 2013;37(1):63–71.
Misu H, Takamura T, Takayama H, Hayashi H, Matsuzawa-Nagata N, Kurita S, et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010;12(5):483–95.
Yang SJ, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: implications for insulin resistance, inflammation, and atherosclerosis. J Clin Endocrinol Metab. 2011;96(8):E1325–9.
Tofler GH, Massaro J, O’Donnell CJ, Wilson PWF, Vasan RS, Sutherland PA, et al. Plasminogen activator inhibitor and the risk of cardiovascular disease: the Framingham Heart Study. Thromb Res. 2016;140:30–5.
Kotronen A, Joutsi-Korhonen L, Sevastianova K, Bergholm R, Hakkarainen A, Pietilainen KH, et al. Increased coagulation factor VIII, IX, XI and XII activities in non-alcoholic fatty liver disease. Liver Int. 2011;31(2):176–83.
Tripodi A, Fracanzani AL, Primignani M, Chantarangkul V, Clerici M, Mannucci PM, et al. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;61(1):148–54.
Verrijken A, Francque S, Mertens I, Prawitt J, Caron S, Hubens G, et al. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2014;59(1):121–9.
Alessi MC, Juhan-Vague I. PAI-1 and the metabolic syndrome: links, causes, and consequences. Arterioscler Thromb Vasc Biol. 2006;26(10):2200–7.
Targher G, Bertolini L, Scala L, Zenari L, Lippi G, Franchini M, et al. Plasma PAI-1 levels are increased in patients with nonalcoholic steatohepatitis. Diabetes Care. 2007;30(5):e31–2.
Chang ML, Hsu CM, Tseng JH, Tsou YK, Chen SC, Shiau SS, et al. Plasminogen activator inhibitor-1 is independently associated with non-alcoholic fatty liver disease whereas leptin and adiponectin vary between genders. J Gastroenterol Hepatol. 2015;30(2):329–36.
Vaughan DE. PAI-1 and atherothrombosis. J Thromb Haemost. 2005;3(8):1879–83.
Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther. 2010;28(5):e72–91.
Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342(24):1792–801.
Lauridsen BK, Stender S, Kristensen TS, Kofoed KF, Kober L, Nordestgaard BG, et al. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: Mendelian randomization and meta-analysis of 279 013 individuals. Eur Heart J. 2018;39(5):385–93.
Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901–10.
Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–64.
Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377(21):2063–72.
Mantovani A, Rigolon R, Pichiri I, Bonapace S, Morani G, Zoppini G, et al. Nonalcoholic fatty liver disease is associated with an increased risk of heart block in hospitalized patients with type 2 diabetes mellitus. PLoS One. 2017;12(10):e0185459.
Mantovani A, Rigamonti A, Bonapace S, Bolzan B, Pernigo M, Morani G, et al. Nonalcoholic fatty liver disease is associated with ventricular arrhythmias in patients with type 2 diabetes referred for clinically indicated 24-hour Holter monitoring. Diabetes Care. 2016;39(8):1416–23.
Wild SH, Walker JJ, Morling JR, McAllister DA, Colhoun HM, Farran B, et al. Cardiovascular disease, cancer, and mortality among people with type 2 diabetes and alcoholic or nonalcoholic fatty liver disease hospital admission. Diabetes Care. 2018;41(2):341–7.
Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol. 2018;14(2):99–114.
Lombardi R, Onali S, Thorburn D, Davidson BR, Gurusamy KS, Tsochatzis E. Pharmacological interventions for non-alcohol related fatty liver disease (NAFLD): an attempted network meta-analysis. Cochrane Database Syst Rev. 2017;3:CD011640.
Sanyal AJ, Neuschwander-Tetri BA, Tonascia J. End points must be clinically meaningful for drug development in nonalcoholic fatty liver disease. Gastroenterology. 2016;150(1):11–3.
European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402.
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–609.
Glen J, Floros L, Day C, Pryke R. Non-alcoholic fatty liver disease (NAFLD): summary of NICE guidance. BMJ. 2016;354:i4428.
Marchesini G, Petta S, Dalle Grave R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: pathophysiology, evidence, and practice. Hepatology (Baltimore, Md). 2016;63(6):2032–43.
Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: evidence and plausible mechanisms. Liver Int. 2017;37(7):936–49.
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–81.
Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136(3):e1–e23.
Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59(1):138–43.
Bozzetto L, Prinster A, Annuzzi G, Costagliola L, Mangione A, Vitelli A, et al. Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care. 2012;35(7):1429–35.
Properzi C, O’Sullivan TA, Sherriff JL, Ching HL, Jeffrey GP, Buckley RF, et al. Ad libitum Mediterranean and low fat diets both significantly reduce hepatic steatosis: a randomized controlled trial. Hepatology (Baltimore, Md). 2018;68(5):1741–54.
Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–78.e5; quiz e14–5.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–71.
Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305–15.
Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–85.
Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–307.
Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35(1):66–75.
DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364(12):1104–15.
Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone clinical trial in macroVascular events): a randomised controlled trial. Lancet. 2005;366(9493):1279–89.
Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–31.
Morgan CL, Inzucchi SE, Puelles J, Jenkins-Jones S, Currie CJ. Impact of treatment with pioglitazone on stroke outcomes: a real-world database analysis. Diabetes Obes Metab. 2018;20(9):2140–7.
Yaghi S, Furie KL, Viscoli CM, Kamel H, Gorman M, Dearborn J, et al. Pioglitazone prevents stroke in patients with a recent transient ischemic attack or ischemic stroke: a planned secondary analysis of the IRIS trial (insulin resistance intervention after stroke). Circulation. 2018;137(5):455–63.
Tanaka N, Aoyama T, Kimura S, Gonzalez FJ. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol Ther. 2017;179:142–57.
Luo W, Xu Q, Wang Q, Wu H, Hua J. Effect of modulation of PPAR-gamma activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci Rep. 2017;7:44612.
Tsukamoto H, She H, Hazra S, Cheng J, Miyahara T. Anti-adipogenic regulation underlies hepatic stellate cell transdifferentiation. J Gastroenterol Hepatol. 2006;21(Suppl 3):S102–5.
Byndloss MX, Olsan EE, Rivera-Chavez F, Tiffany CR, Cevallos SA, Lokken KL, et al. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science (New York, NY). 2017;357(6351):570–5.
Tomas J, Mulet C, Saffarian A, Cavin JB, Ducroc R, Regnault B, et al. High-fat diet modifies the PPAR-gamma pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc Natl Acad Sci U S A. 2016;113(40):E5934–e43.
Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem. 1997;272(8):5128–32.
Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science (New York, NY). 1996;274(5295):2100–3.
Floyd ZE, Stephens JM. Controlling a master switch of adipocyte development and insulin sensitivity: covalent modifications of PPARgamma. Biochim Biophys Acta. 2012;1822(7):1090–5.
Kraakman MJ, Liu Q, Postigo-Fernandez J, Ji R, Kon N, Larrea D, et al. PPARgamma deacetylation dissociates thiazolidinedione’s metabolic benefits from its adverse effects. J Clin Invest. 2018;128(6):2600–12.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the study of liver diseases. Hepatology (Baltimore, Md). 2018;67(1):328–57.
Goldenstein H, Levy NS, Lipener YT, Levy AP. Patient selection and vitamin E treatment in diabetes mellitus. Expert Rev Cardiovasc Ther. 2013;11(3):319–26.
Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the physicians’ health study II randomized controlled trial. JAMA. 2008;300(18):2123–33.
Sesso HD, Christen WG, Bubes V, Smith JP, MacFadyen J, Schvartz M, et al. Multivitamins in the prevention of cardiovascular disease in men: the physicians’ health study II randomized controlled trial. JAMA. 2012;308(17):1751–60.
Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37–46.
Magan-Fernandez A, Rizzo M, Montalto G, Marchesini G. Statins in liver disease: not only prevention of cardiovascular events. Expert Rev Gastroenterol Hepatol. 2018;12(8):743–4.
Browning JD. Statins and hepatic steatosis: perspectives from the Dallas Heart Study. Hepatology (Baltimore, Md). 2006;44(2):466–71.
Eslami L, Merat S, Malekzadeh R, Nasseri-Moghaddam S, Aramin H. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;(12):CD008623.
Kim RG, Loomba R, Prokop LJ, Singh S. Statin use and risk of cirrhosis and related complications in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2017;15(10):1521–30.e8.
Byrne CD, Patel J, Scorletti E, Targher G. Tests for diagnosing and monitoring non-alcoholic fatty liver disease in adults. BMJ. 2018;362:k2734.
Acknowledgements
ES and CDB are supported in part by the Southampton NIHR Biomedical Research Centre.
Conflict of Interests
All authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Scorletti, E., Byrne, C.D. (2020). NAFLD and Cardiovascular and Cardiac Disease: Clinical Implications. In: Bugianesi, E. (eds) Non-Alcoholic Fatty Liver Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-95828-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-95828-6_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95827-9
Online ISBN: 978-3-319-95828-6
eBook Packages: MedicineMedicine (R0)