Abstract
Nonalcoholic steatohepatitis (NASH) is becoming the leading cause of chronic liver disease and a major health issue owing to its close association with the worldwide epidemics of obesity and diabetes [1]. A significant proportion of patients can experience disease progression with the occurrence of cirrhosis, hepatocellular carcinoma and end-stage liver disease [2]. This results in an increase in the overall and liver-related mortality [3, 4]. Patients at risk of disease progression need to be identified as not all individuals with metabolic risk factors will experience disease progression [5]. Prognostic markers have mostly been derived from histological studies and found that the degree of inflammation is the strongest and independent predictor for fibrosis progression [6]. Thus, therapies that could reduce liver inflammation would be the most meaningful option to control this disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Nonalcoholic steatohepatitis (NASH) is becoming the leading cause of chronic liver disease and a major health issue owing to its close association with the worldwide epidemics of obesity and diabetes [1]. A significant proportion of patients can experience disease progression with the occurrence of cirrhosis, hepatocellular carcinoma and end-stage liver disease [2]. This results in an increase in the overall and liver-related mortality [3, 4]. Patients at risk of disease progression need to be identified as not all individuals with metabolic risk factors will experience disease progression [5]. Prognostic markers have mostly been derived from histological studies and found that the degree of inflammation is the strongest and independent predictor for fibrosis progression [6]. Thus, therapies that could reduce liver inflammation would be the most meaningful option to control this disease.
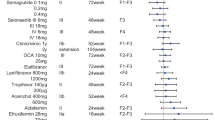
While simple to recommend, diet and lifestyle measures as a first-line therapy for nonalcoholic steatohepatitis (NASH) are hardly a model of successful therapy as most clinicians can testify. They can be complex to implement, hard to sustain, and of limited efficacy in advanced stages of the disease. The need for specific pharmacotherapy is now acknowledged by practitioners, the pharmaceutical industry, and regulators and is largely expected by patients. The result is a clear move away from products developed second-hand for NASH (such as pioglitazone or metformin) or from generic, non-specific hepatoprotectors (such as pentoxifillin, ursodeoxycholic acid, or antioxidants) toward molecules developed and tested specifically for NASH that aim to correct one or several of the pathways of liver injury in this disease. The two most advanced molecules, obeticholic acid (OCA) and elafibranor, have shown encouraging data on improving hepatic histology. Both compounds appear to clear NASH, with OCA improving liver fibrosis and elafibranor improving the glycemic and lipid profile. Cenicriviroc is also being tested as an antifibrotic drug in NASH.
17.1 What Are the Relevant Pharmacological Targets?
Our current understanding of the pathophysiology of NASH is that excessive fat accumulation coexisting with overweight, particularly when localized to visceral adipose tissue, promotes insulin resistance. Uninhibited lipolysis, a consequence of insulin resistance increases delivery of free fatty acids to the liver [7]. In addition, hyperinsulinemia and the subsequent increase in serum glucose will enhance a maladaptive hepatic lipogenic response and inhibit lipid disposal through beta-oxidation [8]. The resulting increase of intrahepatic flux of numerous lipid species promotes liver damage through multiple lipocytotoxic pathways: oxidative stress, mitochondrial dysfunction, apoptosis, free cholesterol toxicity, and endoplasmic reticulum stress [9]. The resulting cell injury and accompanying inflammation (part of which is modulated by cross-talk with the inflamed adipose tissue) sets the stage, in the long run, for liver fibrosis to occur.
This brief description suggests that the relevant mechanisms of action for NASH drugs could be: (1) weight loss agents; (2) insulin sensitizers; (3) antidiabetic drugs with antihyperglycemic properties; (4) hepatoprotectants with broad anti-inflammatory properties; and (5) antifibrotic drugs. These drugs can therefore be classified into two broad categories: drugs that improve the underlying metabolic conditions that promoted the emergence of NASH; and hepatoprotectants that specifically target the mechanisms of hepatic cell injury. As some pathways can be involved in both hepatic inflammation and insulin resistance, some drugs might belong to both categories. Alternatively, combination therapy with molecules that act on distinct metabolic and hepatoprotective pathways could also be envisioned. Depending on how vast the NASH drug pipeline will be, tailored therapy for particular patients could thus become a reality in the near future.
17.2 Where Do We Stand with Pharmacological Therapies?
An ideal drug candidate for NASH should reduce hepatic inflammation and liver cell injury, should correct the underlying insulin resistance, and should have antifibrotic effects. However, primarily “anti-NASH” drugs that have no direct antifibrotic effect could, theoretically, result in a subsequent reduction of fibrosis if a sustained resolution of NASH is achieved. Conversely, purely antifibrotic drugs with no anti-NASH activity and no interference with insulin resistance will leave the triggers for fibrogenesis intact. Therefore, even if an antifibrotic is effective, efforts to curb the underlying pro-fibrotic condition must be considered [10]. We will here review some of the novel anti-NASH agents that are now in late stages of drug development. Many other agents are in preclinical phases of development or in early human studies and will not be reviewed here. These agents that target fibrotic pathways, hepatic lipogenesis, endothelial adhesion molecules, apoptosis, miRNA, endotoxin, nuclear receptors among others are part of a very diverse and rich pipeline for NASH.
17.3 Available Agents with Limited Testing
17.3.1 Insulin-Sensitizing and Antidiabetic Agents
Metformin. Metformin is a safe and inexpensive compound that acts as an insulin-sensitizing agent by reducing hepatic glucose production and increasing peripheral insulin utilization. It reduces body weight. The efficacy in NASH is not proven. An open-label study showed histological improvement (reduction in a histological index) [11], but this was not confirmed in other open-label [12, 13] or randomized trials [14] and a meta-analysis [15]. It is possible that higher weight loss in some patients could explain histological improvement. The anti-steatogenic effect of metformin is weak and is consistent with its inability to restore serum adiponectin levels [16]. Metformin is not recommended for the treatment of NASH. Recent studies however have suggested an association between metformin use and reduced risk of hepatocellular carcinoma [17]. Other studies, in diabetic patients with NASH cirrhosis, have shown that continued treatment with metformin is associated with less episodes of cirrhosis decompensation [18]. Similarly, in a monocentric cohort of diabetics with NASH and advanced fibrosis or cirrhosis, metformin was associated with increased transplant-free survival and reduced risk of HCC [19].
Thiazolidinediones (glitazones) are PPAR gamma agonists, which are potent insulin-sensitizing agents and marketed for treatment of type 2 diabetes. They promote adipocyte differentiation into small, insulin sensitive adipocytes. With long-term treatment, fat storage is redirected from illegitimate storage sites, such as the liver and muscle, toward the adipose tissue, which alleviates hepatic and muscle insulin resistance and reduces lipotoxicity. Glitazones also increase adiponectin levels, an anti-steatogenic and anti-inflammatory cytokine, which is reduced in NAFLD. Glitazones are the best studied pharmacological class in NASH. Several open-label and controlled studies are available with pioglitazone or rosiglitazone, as well as one pediatric trial with pioglitazone. Unfortunately these trials are heterogeneous for daily doses, duration of therapy, and included population (diabetics or non-diabetics) [20]. The largest trial so far is a NASH CRN-sponsored trial comparing pioglitazone at a low dose of 30 mg/day vs. vitamin E (400 IU/day) vs. placebo for 2 years in patients without full-blown diabetes [21]. Although the primary endpoint (histological improvement, defined as a reduction in the NAS without worsening of fibrosis) was not formally met, pioglitazone improved all individual histological features (except for fibrosis) and in particular achieved clearance of steatohepatitis—currently considered the optimal end point in NASH trials [22, 23]. Importantly, when the analysis was limited to patients with well-defined steatohepatitis upon inclusion, pioglitazone reached the primary endpoint with an even more stringent than usual p-value of 0.025. Thus the PIVENS trial [21] should not be seen as a negative trial for pioglitazone but rather an underpowered trial displaying a strong trend toward histological improvement for this drug. The histological benefit occurred together with ALT improvement and partial correction of insulin resistance [21]. In particular, a short-term 6-month treatment with pioglitazone improves adipose tissue insulin sensitivity, which correlates with hepatic histological improvement [24]. Similar results were reported in two other randomized trials of 6 months and 1 year duration [25, 26]. For reasons still unclear, rosiglitazone failed to show histological benefit in the hallmark histological lesions of steatohepatitis, even though there was a significant reduction in steatosis and a biochemical (ALT) and metabolic (HOMA) response [27]. Most of these effects were obtained in the first year of therapy, and prolonged therapy for up to 3 years did not result in further improvement [28].
The enthusiasm for glitazones as a treatment for NASH is seriously dampened by the side effects of these drugs [20]. First and most immediate is weight gain, which is due to adipose tissue buildup and is not always reversible upon discontinuation. Bone fractures in women have been reported with both glitazones and seem to be due to an increased rate of bone loss. Congestive heart failure is a rare complication, yet it warranted a black box warning for both glitazones. Recently, the demonstration of an increased risk of bladder cancer with pioglitazone justified its market withdrawal in some European countries. Finally, an increased risk of cardiovascular events, especially myocardial infarction with rosiglitazone, has been hotly debated and the magnitude of the risk is still uncertain [20]. Nonetheless, rosiglitazone has received a black box warning in the USA and has been withdrawn from the market in many European countries.
17.3.2 Antioxidants
Vitamin E (Vit E). Vit E (alpha tocopherol) is a naturally occurring antioxidant that inhibits TGF-beta, prevents hepatic stellate cell activation, and improves liver necrosis and fibrosis in animal models. The PIVENS study showed that a 2-year treatment with Vit E at 800 IU/day in adult patients significantly reverses steatohepatitis and improves all histological features of NASH (except fibrosis) compared to placebo [21]. Interestingly, this beneficial effect of Vit E was not associated with an improvement in insulin sensitivity. The TONIC trial confirmed the histological efficacy of Vit E 800 IU/day in a pediatric population: after 2 years of treatment Vit E cleared NASH and improved ballooning more often than placebo [29]. Of note, there was no effect on steatosis or inflammation, and, despite prolonged therapy, still no effect on fibrosis. Also, these histological endpoints were only secondary endpoints. The reduction in ALT, which was the primary endpoint, was not achieved by Vit E, as the trend was not statistically significant [29]. A smaller 2-year pediatric Italian trial did not show any histological or biochemical efficacy of the combination of VitE (600 mg/day) and Vit C vs. placebo [30]. In this trial, however, an intense diet and lifestyle intervention program was successfully implemented in both groups and resulted in similar weight loss and improvement in insulin resistance, thus blurring the differences between the antioxidant and control arm.
Pending confirmation by other investigators, the histological results of the two NASH CRN-sponsored trials on Vit E [21, 29] seem encouraging. Nonetheless the controversy around the long-term safety of Vit E supplements dictates restraint in generalizing recommendations of use for Vit E: several meta-analyses suggest increased mortality in patients taking Vit E supplements [31, 32]; one meta-analysis showed a 20% increase in the risk of hemorrhagic stroke [33]; and another large trial suggested an increase in the risk of prostate cancer in men older than 50 years [34].
17.4 Agents in Development
17.4.1 FXR Agonists and Obeticholic Acid
Recent discoveries have identified bile acids as key regulators of liver and metabolic homeostasis. Their action is mediated through nuclear hormone receptors such as the farnesoid X receptor (FXR) and TGR5 [35]. FXR activation results primarily in a reduction of bile acid synthesis from cholesterol by altering expression of a host of genes but mainly by downregulating CYP7A1 [36]. This limits the size of the circulating bile acid pool and promotes choleresis thus protecting against the toxic accumulation of bile acids. Obeticholic acid (OCA), a first-in-class FXR agonist, is a synthetic bile acid with picomolar agonistic activity on FXR [36]. The bile acid effects have translated into clinical efficacy in patients with primary biliary cirrhosis [37] with a reduction in phosphatase alkaline, a biochemical surrogate for clinical events in the natural history of the disease [38]. Based on these results, it is expected that OCA will be approved for this indication. FXR activation also has a wide range of metabolic effects: inhibition of hepatic neoglucogenesis and hepatic glucose production, reduction of lipogenesis and enhancement of beta-oxidation, improvement in peripheral insulin sensitivity [39]. Interestingly, FXR activation has also anti-inflammatory actions [40] with resultant protection against liver inflammation and fibrosis in experimental models of NASH [41, 42].
A small randomized trial in type 2 diabetic patients with NAFLD showed an improvement in hepatic and muscle insulin sensitivity as measured by the euglycemic clamp, a modest but dose-related weight loss, and a reduction in ALT levels [43]. This study provided the proof of principle of an improvement of insulin sensitivity and possibly NAFLD in humans. It was followed by a much larger trial that tested the oral administration of 25 mg OCA QD vs. placebo over 72 weeks of therapy in non-cirrhotic NASH patients [44]. The therapeutic phase of the FLINT trial was stopped early, partly because a preplanned interim analysis showed improved histology in more patients on OCA than on placebo (45% vs. 21%). The primary endpoint was a two-point reduction in the composite Nonalcoholic Fatty Liver Disease Score (NAS) without worsening of fibrosis. However, beyond this composite end point, OCA was able to significantly improve all histological lesions constitutive of NASH including liver fibrosis. Although the trial was not designed for fibrotic endpoints, there was a significant reduction in the fibrosis score (one stage) in 35% of OCA-treated patients vs. 19% in the placebo arm. The reduction in fibrosis was observed regardless of the baseline fibrosis stage. The study included patients at high risk of progression (half of the participants had type 2 diabetes) and “non-responders” to vitamin E (20%). The primary endpoint was reached in secondary analyses of all subgroups of patients. There was a trend in favor of a higher rate of resolution of NASH in the OCA group (22% vs. 13% in the placebo group) which became significant (19% vs. 8%, p < 0.05) in a subgroup analysis restricted to patients with well-characterized NASH at baseline. As far as safety and tolerability, two issues emerged: pruritus and an increase in LDL cholesterol. Pruritus occurred in 23% of OCA-treated patients vs. 6% in the placebo group, but discontinuation was very rare (only one patient). It is however a concern as the NASH population is overwhelmingly asymptomatic. Further studies will test whether lower doses of OCA reduce the incidence of pruritus. An increase in LDL cholesterol occurred early on therapy, plateaued with continued therapy then reversed once the drug was discontinued. Post hoc analyses showed that statins, when initiated during the trial, were able to mitigate the excursion in LDL. Future studies are needed to better characterize alterations in lipid profile and to determine if this results in an increase in cardiovascular risk, if any. Interestingly, in animal models of atherosclerosis, FXR agonists reduce atherosclerosis and vascular cholesterol load and inflammation. A large phase 3 trial [REGENERATE trial (NCT02548351)] comparing three groups (OCA 10 mg QD vs. OCA 25 mg QD vs. placebo) is ongoing in non-cirrhotic NASH patients. The 18-month preliminary analysis [45] of 931 patients (ITT population) confirmed that 25 mg OCA QD induces a fibrosis reduction of at least 1 stage with no worsening of NASH (23.1% vs. 11.9%, p = 0.0002). Pruritus was confirmed as the main adverse event in up to 51% in OCA 25 mg QD group, with the highest incidence in the first 3 months and leading to 9% of discontinuation as per protocol requirement. Currently a large 2-year study in cirrhotic patients is ongoing (REVERSE trial). There is rationale for a benefit of OCA in cirrhosis: in rodents, OCA reduces bacterial translocation by increasing the expression of intestinal tight junction proteins which resulted in a normalization of the endotoxin-TLR4 signaling [46, 47]. Also, OCA can reduce the intrahepatic vascular resistance and improving endothelial vasorelaxation by restoring hepatic e-NOS activity [48]. This suggests beneficial effects on portal hypertension which together with reduced risk of infections due to reduced bacterial translocation could result into clinical benefit in cirrhotic patients.
The main side effects observed with OCA has led to the development of second generation, non-bile acid FXR agonists with the hope of a reduced incidence of pruritus and lipid changes. Early trials are ongoing with several compounds such as tropifexor, cilofexor, and others. Data on efficacy are not yet available.
17.4.2 PPAR Alpha/Delta Agonists and Elafibranor
Another innovative insulin sensitizer is elafibranor, a dual PPARα/δ agonist. PPARs (α, β, and γ) are fatty acid-activated nuclear receptors that have a wide range of physiological actions. PPARδ activation emerged as a potent metabolic regulator that induces hepatic fatty acid β-oxidation, inhibits hepatic lipogenesis [49], reduces hepatic glucose production, and improves hepatic inflammation [50, 51]. PPARα is a major regulator of fatty acid disposal through mitochondrial beta-oxidation, but has also anti-inflammatory actions as it inhibits inflammatory genes induced by NF-kB and acute phase response genes induced by IL6 [52]. Combining these two modes of action can thus improve many of the pathways of injury involved in NASH. Animal data confirmed that elafibranor has hepatoprotective effects in dietary models of NASH or fibrosis with a reduction in steatosis, hepatic inflammation, and pro-inflammatory genes [53]. Importantly, this compound exhibited antifibrotic properties in fibrosis models that were independent of metabolic and insulin resistance abnormalities [53], thereby suggesting a universal antifibrotic potency in rodents. Elafibranor is a PPAR modulator with preferential activity on PPARα and additional activity on PPARδ, but no PPARγ actions [54]. It undergoes extensive enterohepatic cycling and is liver-targeted with little or no muscle action [55]. Human studies performed in abdominally obese, insulin-resistant patients, with or without diabetes, have shown that elafibranor improves hepatic and peripheral insulin sensitivity, dyslipidemia, inflammatory markers, and liver function tests [54, 56].
The results of a large, international, phase IIb trial, the GOLDEN505 trial have been reported [57]. In this randomized trial, 274 NASH patients received elafibranor 80 mg/day, 120 mg/day, or placebo for 1 year. While the lower 80 mg dose did not improve histology, the higher dose was more effective than placebo at inducing NASH resolution without fibrosis worsening. The optimal definition for this histological outcome is still under debate, but these positive results were obtained with a modified, more stringent definition that is consensually emerging. There was no effect on fibrosis (1-year trial duration only) although patients who cleared steatohepatitis (responders) had an improvement in fibrosis after 1 year of therapy, while nonresponders did not. Importantly, improvement in the activity score (i.e. reduction in hepatocyte ballooning and inflammation) was correlated with reduction in fibrosis [58]. This validates the concept that resolution of NASH will be followed by a reversal of fibrosis, a cornerstone of the current surrogate endpoints used in drug development. As anticipated from earlier phase 2 trials, elafibranor improved lipid parameters, glucose homeostasis and insulin sensitivity as well as systemic inflammatory markers. Remarkably, the cardio-metabolic improvement was achieved on top of standard of practice management of the comorbidities in these patients with metabolic syndrome. The drug was well tolerated although a few patients had an increase in creatinine, which was reversible after discontinuation of treatment. The increase was less than that observed with fibrates and, similarly to fibrates, it is not expected to be associated with renal insufficiency. A phase 3 trial, RESOLVE-IT, evaluating the efficacy and safety of elafibranor 120 mg once daily for 72 weeks, has been stopped because the trial did not meet the predefined primary endpoint of NASH resolution without worsening of fibrosis in the ITT population of 1,070 patients at interim analysis. The response rate in the 717 patients enrolled on study drug was 19.2% for patients who received elafibranor 120 mg compared to 14.7% for patients in the placebo arm.
17.4.3 Chemokines and Cenicriviroc
Chemokines are chemotactic cytokines specialized in leukocyte recruitment at sites of tissue injury, inflammation, and fibrosis. Chemokines and their receptors form a complex network of redundant ligand-complex binding as one receptor may bind different chemokines, but their overall effect is the promotion of local inflammatory and fibrotic response [59]. CCL2 (a.k.a. monocyte chemoattractant protein, MCP1) and CCL5 (RANTES) are particularly involved in liver and adipose tissue inflammation and hepatic fibrosis [60,61,62]. Cenicriviroc (CVC) is a selective inhibitor of CCR2 and CCR5 with nanomolar potency. It was developed initially as an anti-HIV agent as CVC blocks the use of CCR5 as a co-receptor for entry into host cells by HIV. CVC blocks the binding of MCP-1 to CCR2 and of RANTES, macrophage inflammatory protein-1α (MIP-1α) and MIP-β to CCR5. There is a strong rationale for the use of CVC in NASH. CVC decreases recruitment, migration, and infiltration of pro-inflammatory monocytes to the site of liver injury mainly via CCR2 antagonism, thereby having the potential to reduce chronic liver inflammation. CVC also disrupts co-receptor and cytokine signaling pathways or “cross-talk” of intrahepatic immune cells within the inflamed liver via CCR2 and CCR5 antagonism, resulting in decreased Kupffer cell and hepatic stellate cell activation and migration, and therefore reduced fibrogenesis. CVC demonstrated significant antifibrotic effects in diet-induced (mouse model of NASH with streptozotocin and high-fat diet [63]) and chemically induced (rat thioacetamide [TAA] [64]) models of liver fibrosis, as well as in a model of kidney fibrosis. It also reduced lobular inflammation and hepatocyte ballooning in the dietary NASH model. Studies in up to 48 weeks in HIV-infected individuals did not show any safety concern.
A large, randomized phase 2b trial in NASH, the CENTAUR trial has been conducted in patients with fibrotic NASH or NASH at high risk of progression [65]. This trial tests CVC vs. placebo over a 2-year period. The primary analysis was performed after 1 year of therapy [66]. Entering year 2, half of the patients in the placebo arm were switched to the active arm and exploratory results at year 2 were reported. At neither time points, cenicriviroc improved the activity score or resolved NASH. However, there was a doubling of the rate of a one stage or more fibrosis regression at year 1 vs. placebo. During the second year of therapy, there was no additional antifibrotic effect, but exploratory analyses on a small number of patients suggest a higher durability of the antifibrotic effect. A registrational, phase 3 trial of Cenicriviroc is ongoing (the AURORA study).
17.4.4 Fatty Acid-Bile Acid Conjugates and Aramchol
Aramchol is a first-in-class, novel synthetic small molecule produced by conjugating two natural components, a fatty acid, arachidic acid, and a bile acid, cholic acid linked by a stable amide bond. It was initially synthesized to treat gallstones as the saturated fatty acid has cholesterol-solubilizing properties and the bile acid enabled secretion into the bile and entry into the enterohepatic circulation [67]. However, empirical observations of animals fed a high-fat, lithogenic diet documented a strong reduction in liver fat that occurred much earlier than did gallstone dissolution [68]. The anti-steatogenic mechanism is probably related to the inhibition of stearoyl CoA desaturase1 (SCD1) activity well documented in human liver [69]. This results in decreased synthesis of mono-unsaturated fatty acids and of triglyceride stores. Moreover, aramchol activates cholesterol efflux by stimulating the ABCA1 transporter, a universal cholesterol efflux pump [70] which can explain the anti-atherogenic effects in some animal models [69]. Since liver-specific SCD1 inhibition in rodents reversed hepatic insulin resistance and reduced neoglucogenesis [71] several SCD1 inhibitors were tested as a treatment of diet-induced metabolic complications. However, systemic inhibition of SCD1 resulted in severe skin and eye side effects, and most of them have been discontinued [72]. Aramchol does not induce these side effects possibly because of the liver targeting or the partial and not complete inhibition of SCD1. A small phase 2a study performed in patients with biopsy documented NAFLD tested two doses of aramchol vs. placebo over a 3-month period and did not raise any significant safety concern [73]. The higher, 300 mg daily, dose resulted in significant reduction in liver fat as measured by magnetic resonance spectroscopy (MRS). There was also a trend toward an increase in serum adiponectin and an improvement in flow-mediated dilation [73], an early marker of endothelial dysfunction in patients with NASH [74].
A large international phase 2b trial is ongoing in patients with histologically documented NASH, high liver fat content measured by MRS and several features of the metabolic syndrome (NCT 02279524). This trial of 1-year duration tests still higher doses of daily aramchol, 400 and 600 mg. The results, reported in abstract form, have shown a reduction in liver fat by magnetic resonance spectroscopy and also histologically a higher rate of NASH resolution in the high-dose aramchol arm than in placebo with a numerically higher rate of fibrosis regression which was not statistically significant. A registrational phase 3 trial is planned for testing the histological efficacy on a much larger scale.
17.4.5 Acetyl-Coenzyme A Carboxylase Inhibitors
Hepatic fat is mainly derived from free fatty acids that are released from the adipose tissue while de novo lipogenesis (DNL), the formation of new fatty acids from excess carbohydrates and amino acids, contributes for only about 5% of liver fat content [75]. However, in the setting of NAFLD, DNL is increased 2–3 times. The cytosolic enzyme acetyl-CoA carboxylase (ACC1) converts acetyl-coenzyme A (CoA) to malonyl-CoA which is a key substrate for fatty acid synthesis. This is the rate-limiting step in DNL, and it is 2–3 times greater in the setting of NASH. A second isoform of ACC (ACC2) is located in the mitochondria and is known to inhibit carnitine palmitoyltransferase I, the carrier protein of fatty acids into mitochondria for ß-oxidation, resulting in the oxidation of free fatty acids. Therefore, ACC inhibition both limits the production of fatty acids and promotes their breakdown.
Firsocostat (GS-0976) is a liver-targeted, inhibitor of ACC1 and ACC2 in development for the treatment of NASH expected to decrease DNL and increase mitochondrial β-oxidation. In preclinical and animal models, ACC blockade decreased hepatic steatosis, inflammation, and insulin resistance [76]. In a phase 2 trial of 126 non-cirrhotic NASH patients treated for 12 weeks [77] firsocostat reduced liver fat more than placebo: 48% of NAFLD patients receiving 20 mg of GS-0976 had a relative reduction of ≥30% from baseline in MRI-PDFF versus 15% of patients receiving placebo. A notable side effect was hypertriglyceridemia (>500 mg/dL) observed in 14–18% of patients on active drug, predominantly in those with pre-existing triglyceride levels higher than 250 mg/dL; it was asymptomatic and reversible upon treatment with fibrates. Based on these encouraging results trials testing combined therapy of firsocostat with selonsertib and non-biliary FXR agonists are currently underway.
17.4.6 Incretin Mimetics and Liraglutide
Among existing therapies for type 2 diabetes, incretin mimetics which are glucagon-like peptide-1 receptor (GLP-1R) agonists hold promise for the treatment of NASH. GLP-1, a peptide product of the L cells of the small intestine and proximal colon, stimulates insulin secretion from the β cells and inhibits glucagon secretion from the α cells in a glucose-dependent manner [78]. GLP-1 also enhances satiety and delays gastric emptying [78]. However, because of their short half-life due to rapid degradation by specific enzymes (such as dipeptidyl peptidase, DPP-IV), native GLP-1 cannot be used as a pharmacological agent. GLP-1R agonists have a much longer half-life than natural GLP-1 allowing either a daily or a once-weekly administration [79]. There seems to be some controversy over the presence of receptors for GLP-1 in hepatocytes and stellate cells. Some studies have shown the presence of a cognate receptor for GLP-1 on human hepatocytes [80]; signaling through these receptors improves hepatic insulin sensitivity [81] by inducing phosphorylation of key signaling pathways [80]. GLP-1 R binding in hepatocytes results in an induction of PPARα and γ expression, which increases disposal of hepatocyte fatty acids by beta-oxidation and lipid export [81, 82]. In vivo studies have confirmed an anti-steatogenic effect of exendin in mice [81, 83]. Several potentially beneficial effects have been demonstrated in humans by metabolic studies including the euglycemic clamp: patients with NAFLD had decreased de novo lipogenesis, decreased adipose tissue lipolysis, and reduced hepatic glucose production upon administration of 1.8 mg liraglutide daily [84]. Moreover, because it induces weight loss, liraglutide at the dose of 3 mg/day [85] is now approved for treatment of obesity or overweight with comorbidities. Some of the effects of GLP1 R agonists seem to be mediated independent of weight loss: there are human data showing that an acute administration of exenatide improves hepatic and adipose tissue insulin resistance before any changes in weight occur [86]. Other GLP1-R agonists are approved for glycemic control in diabetic patients.
Data from large registration trials have shown that diabetic patients treated with liraglutide improved ALT levels and possibly steatosis, measured by CT-scan imaging [87]. Taken together all the above data form a compelling rationale for testing liraglutide in patients with NASH. A British study randomized 52 NASH patients and analyzed 23 of them treated with liraglutide, 1.8 mg/day, and 22 with placebo, in a randomized controlled trial of a 1-year duration [88]. Patients treated with liraglutide experienced more often reversal of NASH (39% vs. 9%, p < 0.02) and less often progression of fibrosis. There was no significant effect on lobular inflammation and ALT and only a marginally significant effect on hepatocyte ballooning, an indication of the very small sample size of this trial. Hence, these results, although encouraging, especially in the light of the preclinical data and the weight loss effect, clearly need further confirmation before any recommendations can be made.
17.4.7 Antifibrotic Agents: Simtuzumab, Galectin-3 Inhibitor, and Caspase Inhibitors
Since the overall objective when treating NASH patients is to reduce the progression to cirrhosis, it would be important to have antifibrotic drugs directly blocking the fibrogenic process. There are very few well-conducted trials of antifibrotic agents and those that are available are negative [89,90,91,92]. Lysil oxidase and lysil oxidase-like (LOXL) are a family of enzymes expressed and secreted by fibrogenic cells and that catalyze oxidative deamination of lysyl and hydroxylysine residues in collagen precursors and elastin [93]. This results in covalent cross-linking of the extracellular matrix, a phenomenon that is believed to greatly contribute to the deposition and stabilization of the hepatic scar [94]. LOXL2, a member of the LOXL family, is upregulated in hepatocytes and its expression is correlated with collagen deposition in various hepatic fibrotic diseases [95] including steatohepatitis in humans [96]. LOXL2 regulates fibroblast activation, TGF-β signaling, and latent TGF-β activation [96]. Experimental studies have shown that inhibition of LOXL2 with an inhibitory monoclonal antibody results in a reduction in liver and lung fibrosis [96]. Simtuzumab is a humanized monoclonal IgG4 antibody with a long half-life of 10–20 days and that can be administered either IV or subcutaneously. Unfortunately two large trials in patients with NASH and bridging fibrosis and in cirrhotic NASH failed to demonstrate any antifibrotic potency on the histological stage of fibrosis or on the hepatic venous pressure gradient (HVPG) [97].
Galectins are a family of proteins that bind to galactose residues present on glycoproteins from extracellular matrix components (collagens, laminin, fibronectin, integrins, elastin) and also on cell surface proteins such as CD4, CD8, or TGF-beta receptors [98]. Galectin-3, a member of the galectin family expressed at high levels on macrophages, regulates multiple cellular processes including cell adhesion and migration, immune cell function, and inflammation [99]. It is upregulated in hepatic human fibrosis and promotes fibrosis in vitro and in vivo [100]. GR-MD-02, a complex polysaccharide polymer (a galactoarabino-rahmnogalacturonan) is a pharmacological inhibitor of galectin-3 that reduces liver fibrosis and portal hypertension in a thioacetamide model of fibrosis/cirrhosis [101]. The antifibrotic effects were confirmed in a dietary NASH model in diabetic mice where GR-MD-02 prevented accumulation of collagen and reduced stellate cell activation [102]. Remarkably the drug also improved hepatocyte ballooning and lobular inflammation and reduced fat accumulation; these anti-NASH effects are probably related to a reduction in iNOS, a marker of inflammation, and in CD-36 expressing pro-inflammatory macrophages [102]. A phase I dose-ranging study has shown good safety and tolerability in humans receiving this compound intravenously (NCT01899859). A larger, phase 2a study in NASH patients with cirrhosis and portal hypertension testing intravenous infusions of GR-MD-02 every 2 weeks for 1 year failed to show an overall benefit on HVPG.
Caspases are a family of 11 intracellular cysteine proteases mediating apoptosis and regulating inflammatory and immune responses to dying cells. They produce hemodynamically active, pro-inflammatory microparticles that cause intrahepatic inflammation, vasoconstriction, and extrahepatic splanchnic vasodilation. Excessive hepatocyte apoptosis has been described in patients with NASH and considered a drug target as it induces inflammation and fibrosis. Emricasan (IDN-6556), an oral pan-caspase inhibitor, decreases hepatic apoptosis, inflammation, and fibrosis in animal models of acute hepatitis and chronic models of nonalcoholic steatohepatitis (NASH) [103, 104]. In a multicenter randomized study, 86 patients with cirrhosis Child-Pugh class A or B and MELD scores 11–18 received Emricasan 25 mg BID or placebo for 3 months [105]. Emricasan treatment improved MELD and Child-Pugh scores in patients with high MELD (≥15) due to improvements in INR and total bilirubin. In another study including 23 compensated cirrhotics with portal hypertension (HVPG above 5 mmHg), Emricasan was administered for 28 days and induced a significant decrease in HVPG in patients with severe PH (HVPG ≥12 mmHg) at baseline [106]. Larger studies will be needed to better characterize the safety profile of Emricasan and the potential clinical benefit.
17.5 Conclusion
Drug development for NASH has accelerated strongly over the past few years. Earlier studies such as the PIVENS trial have provided the proof of principle that histological improvement and even NASH resolution is possible with drugs such as insulin sensitizers (glitazones) or antioxidants (vitamin E) [21]. Retrospective studies have documented the prognostic significance of histological lesions in NAFLD [107, 108], suggesting that these lesions could be acceptable surrogates of disease control on therapy. Tools for a precise histological description and classification have been refined from the NASH CRN classification [109] to the FLIP/SAF algorithm [110]. Major advances also occurred in the regulatory field. Both the European and the American drug agencies now agree that NASH is a valid indication for therapy and as such, it can follow a regulatory path for drug approval. Trial outcomes with clinical and regulatory value have been defined and are currently being used in several large trials of new drugs in NASH [10]. What remains to be done is the discovery and validation of biomarkers that would help diagnose patients at risk of advanced or progressive NASH and also monitor disease progression. Renewed and sustained efforts for drug discovery and dedication from physicians to recruit and complete clinical trials will be key to providing patients with NASH with safe and effective drugs in the near future.
References
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23.
Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85.
Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73.
Soderberg C, Stal P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcranz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602.
Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–84.
Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371–9.
Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–25. e6
Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of NASH: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–88.
Peverill W, Powell LW, Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci. 2014;15:8591–638.
Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015;61:1392–405.
Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, Modi A, Nagabhyru P, Sumner AE, Liang TJ, Hoofnagle JH. Clinical trial: pilot study of metformin for the treatment of nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2008;29:172–82.
Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–90.
Nair S, Diehl AM, Wiseman M, Farr GH Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–8.
Haukeland JW, Konopski Z, Eggesbo HB, von Volkmann HL, Raschpichler G, Bjoro K, Haaland T, Loberg EM, Birkeland K. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853–60.
Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104.
Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169–76.
Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, Lin JH, Wu CY. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2012;62:606–15.
Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, Roberts LR, Chaiteerakij R. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology. 2014;60:2008–16.
Vilar-Gomez E, Vuppalanchi R, Desai A, Gawrieh S, Ghabril M, Saxena R, Cummings OW, Chalasani N. Long-term metformin use may improve clinical outcomes in diabetic patients with non-alcoholic steatohepatitis and bridging fibrosis or compensated cirrhosis. Aliment Pharmacol Ther. 2019;50(3):317–28.
Ratziu V, Caldwell S, Neuschwander-Tetri BA. Therapeutic trials in nonalcoholic steatohepatitis: insulin sensitizers and related methodological issues. Hepatology. 2010;52:2206–15.
Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85.
Ratziu V, Bellentani S, Cortez-Pinto H, Day CP, Marchesini G. A position paper on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–84.
Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–53.
Gastaldelli A, Harrison SA, Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S, Cusi K. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology. 2009;50:1087–93.
Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–307.
Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–84.
Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E, Grimaldi A, Poynard T. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled fatty liver improvement with rosiglitazone therapy (FLIRT) trial. Gastroenterology. 2008;135:100–10.
Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, Hartmann-Heurtier A, Bruckert E, Poynard T. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445–53.
Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, Tonascia J, Unalp A, Clark JM, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–68.
Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR, Piemonte F, Marcellini M, Angulo P. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology. 2008;48:119–28.
Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–57.
Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176.
Schurks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702.
Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL Jr, Baker LH. Vitamin E and the risk of prostate cancer: the selenium and vitamin E cancer prevention trial (SELECT). JAMA. 2011;306:1549–56.
Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11:55–67.
Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov Today. 2012;17:988–97.
Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, Kowdley KV, Vincent C, Bodhenheimer HC Jr, Pares A, Trauner M, Marschall HU, Adorini L, Sciacca C, Beecher-Jones T, Castelloe E, Bohm O, Shapiro D. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751–61. e8
Lammers WJ, van Buuren HR, Hirschfield GM, Janssen HL, Invernizzi P, Mason AL, Ponsioen CY, Floreani A, Corpechot C, Mayo MJ, Battezzati PM, Pares A, Nevens F, Burroughs AK, Kowdley KV, Trivedi PJ, Kumagi T, Cheung A, Lleo A, Imam MH, Boonstra K, Cazzagon N, Franceschet I, Poupon R, Caballeria L, Pieri G, Kanwar PS, Lindor KD, Hansen BE. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338–49.e5; quiz e15.
Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–9.
Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology. 2008;48:1632–43.
Zhang S, Wang J, Liu Q, Harnish DC. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J Hepatol. 2009;51:380–8.
Verbeke L, Mannaerts I, Schierwagen R, Govaere O, Klein S, Vander Elst I, Windmolders P, Farre R, Wenes M, Mazzone M, Nevens F, van Grunsven LA, Trebicka J, Laleman W. FXR agonist obeticholic acid reduces hepatic inflammation and fibrosis in a rat model of toxic cirrhosis. Sci Rep. 2016;6:33453.
Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574.
Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65.
Younossi Z. Positive results from REGENERATE: a phase 3 international, randomized, placebo-controlled study evaluating obeticholic acid treatment for NASH. ILC 2019: J Hepatol. 2019.
Ubeda M, Lario M, Munoz L, Borrero MJ, Rodriguez-Serrano M, Sanchez-Diaz AM, del Campo R, Lledo L, Pastor O, Garcia-Bermejo L, Diaz D, Alvarez-Mon M, Albillos A. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J Hepatol. 2016;64:1049–57.
Verbeke L, Farre R, Verbinnen B, Covens K, Vanuytsel T, Verhaegen J, Komuta M, Roskams T, Chatterjee S, Annaert P, Vander Elst I, Windmolders P, Trebicka J, Nevens F, Laleman W. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am J Pathol. 2015;185:409–19.
Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, Elst IV, Windmolders P, Vanuytsel T, Nevens F, Laleman W. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286–98.
Qin X, Xie X, Fan Y, Tian J, Guan Y, Wang X, Zhu Y, Wang N. Peroxisome proliferator-activated receptor-delta induces insulin-induced gene-1 and suppresses hepatic lipogenesis in obese diabetic mice. Hepatology. 2008;48:432–41.
Barish GD, Narkar VA, Evans RM. PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116:590–7.
Bojic LA, Huff MW. Peroxisome proliferator-activated receptor delta: a multifaceted metabolic player. Curr Opin Lipidol. 2013;24:171–7.
Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62:720–33.
Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, Baron M, Lucas A, Tailleux A, Hum DW, Ratziu V, Cariou B, Hanf R. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–52.
Cariou B, Zair Y, Staels B, Bruckert E. Effects of the new dual PPAR alpha/delta agonist GFT505 on lipid and glucose homeostasis in abdominally obese patients with combined dyslipidemia or impaired glucose metabolism. Diabetes Care. 2011;34:2008–14.
Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, Baron M, Lucas A, Tailleux A, Hum DW, Ratziu V, Cariou B, Hanf R. Hepato-protective effects of the dual PPARalpha/delta agonist GFT505 in rodent models of NAFLD/NASH. Hepatology. 2013;58(6):1941–52.
Cariou B, Hanf R, Lambert-Porcheron S, Zair Y, Sauvinet V, Noel B, Flet L, Vidal H, Staels B, Laville M. Dual peroxisome proliferator-activated receptor alpha/delta agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care. 2013;36:2923–30.
Ratziu V, Harrison S, Franque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J. Elafibranor, an agonist of the peroxisome proliferator-activated receptor alpha and delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–59.
Ratziu V, Francque S, Harrison SH, Anstee QM, Bedossa P, Hum DW, Megnien S, Hanf R, Staels B, Sanyal A. Improvement in NASH histological activity highly correlates with fibrosis regression. Hepatology. 2016;64:LB21.
Berres ML, Koenen RR, Rueland A, Zaldivar MM, Heinrichs D, Sahin H, Schmitz P, Streetz KL, Berg T, Gassler N, Weiskirchen R, Proudfoot A, Weber C, Trautwein C, Wasmuth HE. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J Clin Invest. 2010;120:4129–40.
Zimmermann HW, Tacke F. Modification of chemokine pathways and immune cell infiltration as a novel therapeutic approach in liver inflammation and fibrosis. Inflamm Allergy Drug Targets. 2011;10:509–36.
Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–21.
Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, Huss S, Klussmann S, Eulberg D, Luedde T, Trautwein C, Tacke F. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61:416–26.
Lefebvre E, Hashiguchi T, Jenkins H, Nabhan A, Yoneyama H, Friedman SL, Wolfgang GH. Anti-fibrotic and anti-inflammatory activity of the dual CCR2 and CCR5 antagonist cenicriviroc in a mouse model of NASH. Hepatology. 2013;58:221A–2A.
Hong F, Chou H, Friedman SL. Significant anti-fibrotic activity of cenicriviroc, a dual CCR2/CCR5 antagonist, in a rat model of thioacetamide-induced liver fibrosis and cirrhosis. Hepatology. 2013;58:S1.
Friedman SL, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L, Ratziu V. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials. 2016;47:356–65.
Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A, Simon K, Fischer L, Melchor-Khan L, Vest J, Wiens BL, Vig P, Seyedkazemi S, Goodman Z, Wong VW, Loomba R, Tacke F, Sanyal A, Lefebvre E. A randomized, placebo-controlled trial of Cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67(5):1754–67.
Gilat T, Somjen GJ, Mazur Y, Leikin-Frenkel A, Rosenberg R, Halpern Z, Konikoff F. Fatty acid bile acid conjugates (FABACs)—new molecules for the prevention of cholesterol crystallisation in bile. Gut. 2001;48:75–9.
Gilat T, Leikin-Frenkel A, Goldiner I, Juhel C, Lafont H, Gobbi D, Konikoff FM. Prevention of diet-induced fatty liver in experimental animals by the oral administration of a fatty acid bile acid conjugate (FABAC). Hepatology. 2003;38:436–42.
Leikin-Frenkel A, Gonen A, Shaish A, Goldiner I, Leikin-Gobbi D, Konikoff FM, Harats D, Gilat T. Fatty acid bile acid conjugate inhibits hepatic stearoyl coenzyme A desaturase and is non-atherogenic. Arch Med Res. 2010;41:397–404.
Goldiner I, van der Velde AE, Vandenberghe KE, van Wijland MA, Halpern Z, Gilat T, Konikoff FM, Veldman RJ, Groen AK. ABCA1-dependent but apoA-I-independent cholesterol efflux mediated by fatty acid-bile acid conjugates (FABACs). Biochem J. 2006;396:529–36.
Gutierrez-Juarez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006;116:1686–95.
Powell DA. An overview of patented small molecule stearoyl coenzyme-A desaturase inhibitors (2009–2013). Expert Opin Ther Pat. 2014;24:155–75.
Safadi R, Konikoff FM, Mahamid M, Zelber-Sagi S, Halpern M, Gilat T, Oren R. The fatty acid-bile acid conjugate aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12:2085–2091.e1.
Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–80.
Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–88.
Harriman G, Greenwood J, Bhat S, Huang X, Wang R, Paul D, Tong L, Saha AK, Westlin WF, Kapeller R, Harwood HJ Jr. Acetyl-CoA carboxylase inhibition by ND-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proc Natl Acad Sci U S A. 2016;113:E1796–805.
Loomba R, Kayali Z, Noureddin M, Ruane P, Lawitz EJ, Bennett M, Wang L, Harting E, Tarrant JM, McColgan BJ, Chung C, Ray AS, Subramanian GM, Myers RP, Middleton MS, Lai M, Charlton M, Harrison SA. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155:1463–1473.e6.
Mells JE, Anania FA. The role of gastrointestinal hormones in hepatic lipid metabolism. Semin Liver Dis. 2013;33:343–57.
Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34(Suppl 2):S279–84.
Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, Anania FA. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584–92.
Mells JE, Fu PP, Sharma S, Olson D, Cheng L, Handy JA, Saxena NK, Sorescu D, Anania FA. Glp-1 analog, liraglutide, ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a Western diet. Am J Physiol Gastrointest Liver Physiol. 2012;302:G225–35.
Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, Faraci G, Pacetti D, Vivarelli M, Nicolini D, Garelli P, Casini A, Manco M, Mingrone G, Risaliti A, Frega GN, Benedetti A, Gastaldelli A. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285–97.
Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173–81.
Armstrong MJ, Hull D, Guo K, Barton D, Hazlehurst JM, Gathercole LL, Nasiri M, Yu J, Gough SC, Newsome PN, Tomlinson JW. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol. 2015;64:399–408.
Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DCW, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JPH. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22.
Gastaldelli A, Gaggini M, Daniele G, Ciociaro D, Cersosimo E, Tripathy D, Triplitt C, Fox P, Musi N, DeFronzo R, Iozzo P. Exenatide improves both hepatic and adipose tissue insulin resistance: a dynamic positron emission tomography study. Hepatology. 2016;64:2028–37.
Armstrong MJ, Houlihan DD, Rowe IA, Clausen WH, Elbrond B, Gough SC, Tomlinson JW, Newsome PN. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: individual patient data meta-analysis of the LEAD program. Aliment Pharmacol Ther. 2013;37:234–42.
Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hubscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–90.
Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, Lee WM, Lok AS, Bonkovsky HL, Morgan TR, Ghany MG, Morishima C, Snow KK, Dienstag JL. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429–41.
McHutchison J, Goodman Z, Patel K, Makhlouf H, Rodriguez-Torres M, Shiffman M, Rockey D, Husa P, Chuang WL, Levine R, Jonas M, Theodore D, Brigandi R, Webster A, Schultz M, Watson H, Stancil B, Gardner S. Farglitazar lacks antifibrotic activity in patients with chronic hepatitis C infection. Gastroenterology. 2010;138:1365–73, 1373.e1–2.
Pockros PJ, Jeffers L, Afdhal N, Goodman ZD, Nelson D, Gish RG, Reddy KR, Reindollar R, Rodriguez-Torres M, Sullivan S, Blatt LM, Faris-Young S. Final results of a double-blind, placebo-controlled trial of the antifibrotic efficacy of interferon-gamma1b in chronic hepatitis C patients with advanced fibrosis or cirrhosis. Hepatology. 2007;45:569–78.
Poynard T, Bruix J, Schiff ER, Diago M, Berg T, Moreno-Otero R, Lyra AC, Carrilho F, Griffel LH, Boparai N, Jiang R, Burroughs M, Brass CA, Albrecht JK. Improved inflammatory activity with peginterferon alfa-2b maintenance therapy in non-cirrhotic prior non-responders: a randomized study. J Hepatol. 2013;58:452–9.
Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–72.
Kagan HM. Lysyl oxidase: mechanism, regulation and relationship to liver fibrosis. Pathol Res Pract. 1994;190:910–9.
Vadasz Z, Kessler O, Akiri G, Gengrinovitch S, Kagan HM, Baruch Y, Izhak OB, Neufeld G. Abnormal deposition of collagen around hepatocytes in Wilson’s disease is associated with hepatocyte specific expression of lysyl oxidase and lysyl oxidase like protein-2. J Hepatol. 2005;43:499–507.
Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, Neufeld G, Van Vlasselaer P, Smith V. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–17.
Sanyal A, Abdelmalek M, Diehl AM, Caldwell SH, Shiffman ML, Ghalib R, Lawitz E, Rockey D, Schall RA, Jia C, McColgan BJ, Myers RP, Subramanian GM, McHutchison J, Ratziu V, Afdhal N, Goodman Z, Harrison SH, Bosch J. Efficacy and safety of simtuzumab for the treatment of nonalcoholic steatohepatitis with bridging fibrosis or cirrhosis: results of two phase 2b, dose-ranging, randomized, placebo-controlled trials. J Hepatol. 2017;46:PS094.
Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17.
Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160–71.
Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci U S A. 2006;103:5060–5.
Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, Friedman SL. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One. 2013;8:e75361.
Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One. 2013;8:e83481.
Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191–6.
Barreyro FJ, Holod S, Finocchietto PV, Camino AM, Aquino JB, Avagnina A, Carreras MC, Poderoso JJ, Gores GJ. The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver Int. 2015;35:953–66.
Frenette CT, Morelli G, Shiffman ML, Frederick RT, Rubin RA, Fallon MB, Cheng JT, Cave M, Khaderi SA, Massoud O, Pyrsopoulos N, Park JS, Robinson JM, Yamashita M, Spada AP, Chan JL, Hagerty DT. Emricasan improves liver function in patients with cirrhosis and high model for end-stage liver disease scores compared with placebo. Clin Gastroenterol Hepatol. 2019;17:774–783.e4.
Garcia-Tsao G, Fuchs M, Shiffman M, Borg BB, Pyrsopoulos N, Shetty K, Gallegos-Orozco JF, Reddy KR, Feyssa E, Chan JL, Yamashita M, Robinson JM, Spada AP, Hagerty DT, Bosch J. Emricasan (IDN-6556) lowers portal pressure in patients with compensated cirrhosis and severe portal hypertension. Hepatology. 2019;69:717–28.
Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver fibrosis, but no other histologic features, associates with long-term outcomes of patients with Nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–97.
Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, Goodman Z. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874–82.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21.
Bedossa P. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565–75.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Stern, C., Ratziu, V. (2020). Pharmacological Options for NASH. In: Bugianesi, E. (eds) Non-Alcoholic Fatty Liver Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-95828-6_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-95828-6_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95827-9
Online ISBN: 978-3-319-95828-6
eBook Packages: MedicineMedicine (R0)