Abstract
Physical activity is a key determinant of metabolic control and is commonly recommended for people with non-alcoholic fatty liver disease (NAFLD), usually alongside weight loss and dietary change. Physical activity and exercise have both been shown to improve liver health in NAFLD and should be included as part of the clinical care of all patients, regardless of where they sit on the NAFLD disease spectrum [1]. Reducing or breaking up sedentary time should also be a key therapeutic target with these patients.
Physical activity and exercise confer benefits, independent of weight loss, and are useful for those patients struggling to lose weight and are a key tool in weight loss maintenance. Both aerobic exercise and resistance training effectively reduce liver fat. The choice of training should be tailored based on patients’ preferences to be maintained in the long term and should be used as an adjunct to dietary change.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Physical activity
- Exercise
- Sedentary behaviour
- Physical inactivity
- Aerobic
- Resistance
- High-intensity interval training
- Exercise prescription
16.1 Introduction
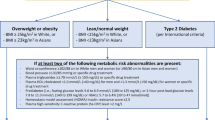
Even though physical activity and exercise are recommended as part of treatment for NAFLD [1, 2], there have been no large-scale studies with adequate statistical power to guide health practitioners in prescribing exercise programmes or for generating physical activity guidelines for the management of these patients. Evidence for the benefit of physical activity comes from prospective studies showing that individuals who maintain a physically active lifestyle are less likely to develop insulin resistance (IR), impaired glucose tolerance, or type 2 diabetes [3,4,5,6]. Physical activity levels have been shown to be lower in people with NAFLD than their “healthy” counterparts [7,8,9,10], and links have been made between low cardiorespiratory fitness and NAFLD severity [11, 12].
Being physically inactivity is not just a lack of physical activity, but rather a distinct behaviour in itself, often called “sedentary behaviour.” This is becoming a growing problem in the general population [13], and low levels of physical activity are compounded by an increase in physical inactivity. Sedentary behaviour, including activities such as sitting, is reported to be higher in people predisposed to the metabolic syndrome, excessive adiposity, and type 2 diabetes [14,15,16,17] and has been shown to be higher in NAFLD [10]. Consequently, increases in sedentary time could play a potential role in the development of or predisposition towards NAFLD, independent of physical activity/exercise and needs to be considered when introducing lifestyle interventions.
16.2 Physical Activity, Exercise and Metabolic Health
Although much attention has historically been given to the role of nutrition in the management of obesity and NAFLD, emerging evidence suggests that energy expenditure also plays an integral role in adequate metabolic control. Our everyday lives consist of activities which, without us paying conscious effort, have a profound impact upon our health and well-being. Activities related to energy expenditure can typically be broken into four distinct categories throughout the day: (1) sedentary behaviour or inactivity, (2) physical activity, (3) exercise and (4) sleep.
Sedentary behaviour is not simply a lack of physical activity but is a cluster of individual behaviours where sitting or lying is the dominant mode of posture, and energy expenditure is very low. The definition of being sedentary or physically inactive is controversial. Some groups define inactivity as expending less than 1.5 kcal/kg/day in leisure physical activities (National Population Health Survey of Canada: www.hc-sc.gc.ca/fn-an/surveill/nutrition/population/index-eng.php), while the UK National Obesity Forum indicates that 3000–6000 steps/day is sedentary or inactive (www.national obesityforum.org.uk). In the US National Health Interview Survey, adults were classified as sedentary if they did not report any sessions of light to moderate or vigorous leisure-time physical activity of at least 10 min a day (www.cdc.gov/nchs/nhis). Sedentary behaviours are multi-faceted and might include behaviours at work or school, at home, during transport and in leisure time. Typically, key sedentary behaviours include screen time (TV viewing, computer use), motorised transport and sitting.
Physical activity is defined as “any bodily movement produced by contraction of skeletal muscles and resulting in energy expenditure above the basal level” [18] and constitutes many of the activities carried out as part of the daily routine. The term “physical activity” should not be confused with “exercise.” Exercise is a subcategory of physical activity in which planned, structured, and repetitive bodily movements are performed to maintain or improve physical fitness. Physical activity includes exercise as well as other activities which involve bodily movement and are done as part of playing, working, active transportation, house chores and recreational activities.
Physical activity can be defined in terms of its metabolic equivalent (MET) level, a physiological measure expressing the energy cost of the task. It is defined as the ratio of metabolic rate (and therefore the rate of energy consumption) during a specific physical activity to a reference metabolic rate, set by convention to 3.5 mL·O2·kg−1·min−1 or equivalently 1 kcal·kg−1 h−1 or 4.184 kJ·kg−1 h−1 [19]. One MET is considered as the resting metabolic rate (RMR) measured during quiet sitting. Activities of less than 3 METs are classed as “light” (e.g. desk work, watching television, slow walking), 3–6 METs as “moderate” (e.g. walking at 3–4 mph, cycling less than 10 mph), and over 6 METs as “vigorous” (e.g. running, circuit training).
With sleep playing an important role in physiological and cognitive well-being, alongside the large proportion of our lives which is spent asleep, it is not surprising that variations in sleep, whether duration or pattern, influence metabolic and mental health. Cross-sectional and prospective cohorts reveal that self-reported sleep duration of less than 7 h is associated with an excess risk of cardiovascular disease (up to 33%), type 2 diabetes and all-cause mortality [20, 21].
16.3 Sedentary Behaviour and Metabolic Control
Sedentary behaviour, also referred to as physical inactivity, holds strong epidemiological, physiological and molecular relationships with the development of over 30 long-term conditions [22]. Subtle changes in sedentary behaviour may contribute to obesity and metabolic disorders, potentially as much as lack of moderate–vigorous physical activity. Both TV sitting (a reliable marker of overall sedentary behaviour) and physical activity are associated with cardio-metabolic health when viewed separately [23, 24] or together [25]. Beyond cardio-metabolic health, 3+ h of daily sitting is linked to all-cause mortality (RR 1.30; 95% CI, 1.06–1.56) [23]. Sedentary behaviour, including activities such as sitting, is reported to be higher in people predisposed to the metabolic syndrome, excessive adiposity and type 2 diabetes [26]. In addition, prospective studies show that a change in TV viewing over 5 years was associated with waist circumference and clustered cardio-metabolic risk score, independent of physical activity [27]. Even if adults meet the public health guideline for leisure-time physical activity, they may have a high risk of becoming overweight or developing metabolic disorders if they spend a large amount of time in sedentary behaviours during the rest of the day [28].
Increasing sedentary behaviour is becoming a growing problem in the general population [13], and low levels of physical activity are compounded by an increase in physical inactivity. One of the seminal studies linking everyday physical inactivity with adverse health showed that people with jobs that involve a lot of sitting (e.g. bus drivers) had double the incidence of cardiovascular disease as those whose jobs include more standing and walking activities (e.g. bus conductors) [29]. The most direct effect of sitting still is that the work performed by the large skeletal muscles in the legs, back and trunk required for upright movement decreases. Sitting for prolonged periods also causes the loss of opportunity for cumulative energy expenditure resulting from the thousands of intermittent muscular contractions throughout the day [30]. Sedentary behaviours involving sitting or lying down are characterised by a low MET value of less than 2, and lower mean daily MET levels are related adversely to metabolic biomarkers and to poorer health outcomes [28]. A recent study by Hallsworth et al. [10] found average daily MET levels were significantly lower in patients with NAFLD when compared to healthy controls.
The majority of the general population are unaware of the potential insidious dangers of sitting too much or the possible benefits of at least maintaining daily low-intensity intermittent non-exercise activity throughout the day. Often, these non-exercise activities occur subconsciously. Energy expenditure of “standing workers” (e.g. shop assistants) was approximately 1400 kcal/day, for work involving some manual labour around 2300 kcal/day, whereas seated workers burned only around 700 kcal/day. More than 90% of the calories burned during all forms of physical activity were due to this pattern of standing and non-exercise ambulatory movements [30]. The frequency and cumulative duration of non-exercise activity throughout the day is extremely high. People perform intermittent bouts of non-exercise activity throughout most of the day, 7 days/week, 365 days/year. In contrast, the frequency of exercise is more limited, generally to less than 150 min/week. Given the broader opportunities and implications for daily low-intensity activity, it is possible that maintaining this level of activity has greater implications for health and well-being than moderate–vigorous physical activity for those who do not prefer more structured exercise.
Classically, there are three components of human daily energy expenditure (Fig. 16.1): basal metabolic rate (BMR), the thermic effect of food and activity thermogenesis. BMR is the energy required for the core bodily functions and is measured at complete rest while fasted. It accounts for about 60% of daily energy expenditure in a sedentary person. Nearly all of its variability is accounted for by body size, or more precisely lean body mass, with bigger and/or leaner people having a higher BMR. The thermic effect of food is the energy expended in response to a meal and is that associated with digestion, absorption and fuel storage. This accounts for about 10% of daily energy needs and does not vary greatly between people. The remaining component activity thermogenesis can be subdivided into exercise and non-exercise activity thermogenesis (NEAT) which incorporates general, everyday activity. NEAT is the most variable component of human expenditure, and may be the easiest to manipulate for health benefits. NEAT varies between two people of similar size by 2000 kcal/day because of people’s different occupations and leisure-time activities [31]. Occupations that involve physical labour, such as farming, confer higher NEAT values than those that involve more sedentary work. Variability in leisure activities also affects NEAT—those people that choose to sit in the evening watching the television exhibit lower NEAT than those that are out walking the dog. Obesity is associated with low NEAT; obese individuals stand and ambulate for 2.5 h/day less than lean sedentary controls [16]. If we can attempt to address this, either at an individual level by encouraging the person to move more, or at an environmental/societal level by ensuring there are more opportunities to stand/walk throughout the day, then we may have a positive impact on obesity levels and metabolic control.
Components of total daily energy expenditure [31]
The links between sedentary behaviour and metabolic health extend beyond the total amount of time spent inactive. Healy et al. [15] report that more interruptions in sedentary time were associated with a decrease in metabolic risk factors. This suggests that it is not only the amount of sedentary time that is important but also the manner in which it is accumulated. As sedentary time comprises a large proportion of waking hours (over 50% for most people—[30]), small changes regarding the interruption of this with regular, short breaks of light-intensity activity could be incorporated across numerous settings and workplaces, increasing NEAT, resulting in beneficial metabolic effects [31]. Regular participation in moderate–vigorous intensity exercise should still be promoted as the predominant physical activity message. However, encouraging a reduction in sedentary time through increasing light-intensity day-to-day activity may be another important public health message for reducing obesity and overall metabolic risk [15, 31]. Encouraging our patients with NAFLD to have regular breaks from sitting throughout the day, especially if they hold a sedentary job, will enhance their daily NEAT levels and increase their calorie expenditure. This is an important therapeutic message to relay to our patients with NAFLD regardless of their disease severity.
Researchers hypothesise that signals harming the body during high levels of physical inactivity are different from those that boost health above normal after exercising regularly [32, 33]. Lipoprotein lipase (LPL) is the first protein directly interacting with and regulating lipoproteins to be studied at the cellular level during physical inactivity. Physical inactivity has a powerful effect on suppressing LPL activity in skeletal muscle, the rate-limiting enzyme for the hydrolysis of triglyceride-rich lipoproteins [34]. Local contractile activity and/or inactivity is the major physiological variable regulating LPL function within the skeletal muscle, and a localised reduction in contractile activity is a potent physiological factor reducing LPL activity. Low LPL function has been linked with blunted triglyceride uptake in skeletal muscle and reduced plasma HDL cholesterol levels.
Increased skeletal muscle LPL has been reported following short-term exercise training [32]. LPL activity was measured in six muscles after intensive training for 2 weeks. Exercise increased LPL activity 2- to 2.5-fold in the least oxidative regions of the leg muscle (fast-twitch white fibres), whereas the most oxidative (slow-twitch red fibres) postural leg muscles that already had high LPL due to non-exercise activity did not display any further increase in LPL after training [30]. LPL activity is generally much greater in the red oxidative muscle types than in the white glycolytic muscles. By removing the normally high level of postural support by oxidative muscles, this abolished the difference of LPL activity between muscle fibre types. This suggests that the difference in LPL activity between fibre types is primarily due to the level of recruitment in normal daily activity [33] and thus, local changes in metabolism during even light–moderate contractions are the most important physiological stimulus for LPL regulation in skeletal muscle.
There is a growing body of evidence reporting that the majority of people at risk of developing the metabolic syndrome, obesity, NAFLD and type 2 diabetes spend excessive amounts of time inactive and have low levels of NEAT [10, 14,15,16,17]. These results are real and applicable to our everyday lives, with one study reporting that with every 1 h increase of television viewing per day that there was a 26% increase in the prevalence of metabolic syndrome [14]. The magnitude of the negative effect of television watching was about the same as the positive health benefit derived from the 30 min of extra physical activity/exercise recommended to improve health. Given the balance between the negative health consequences of physical inactivity and the modest positive effects of exercise in comparison, it is important to identify both activity and sedentary behaviour in developing clinically meaningful interventions.
16.4 Sedentary Behaviour and NAFLD
Increases in sedentary time could play a potential role in the development of NAFLD and, in turn, provide a potential avenue for therapy. Current physical inactivity physiology would suggest that a reduction in LPL activity, as a result of fewer cumulative muscle contractions throughout the day, could predispose to NAFLD through the resultant circulatory hyperlipidaemia. An increase in circulating fatty acids, with fewer being hydrolysed as lipoproteins, will lead to an increased delivery of circulating fatty acids to the liver and hence predisposition to or progression of NAFLD. Increasing circulating fatty acids also exacerbates IR [35] and hyperinsulinaemia which could subsequently increase de novo lipogenesis within the liver. A high level of sedentary behaviour reduces NEAT energy expenditure increasing the risk of a person becoming overweight/obese which is linked to NAFLD predisposition.
Targeting a reversal of sedentary behaviour may provide an additional therapeutic avenue to complement physical activity and exercise guidelines. Decreasing overall sedentary time and increasing breaks throughout the day could be a useful therapeutic message to relay to people with NAFLD, and may be perceived as being more achievable by patients initially than increasing physical activity levels. Any means of increasing NEAT, whether it be at work or during leisure time, may exert positive metabolic benefits. There is limited but promising evidence from prospective cohort studies that identify sedentary behaviour as an independent risk factor for NAFLD [36].
16.5 Physical Activity and Metabolic Control
General health guidelines promote at least 150 min/week of moderate–vigorous leisure-time physical activity or 10,000 steps per day for the primary prevention of cardiovascular disease and decreasing the risks for metabolic diseases [37,38,39]. However, the majority of people in the general population do not follow this prescription for enough moderate–vigorous exercise, and this may be contributing to the rising numbers of people being affected by obesity, type 2 diabetes and NAFLD.
Evidence for the benefit of physical activity comes from studies showing that individuals who exercise and maintain a physically active lifestyle are less likely to develop IR, impaired glucose tolerance or type 2 diabetes [3,4,5,6]. Physical activity appears to result in insulin-receptor upregulation in muscle tissue increasing delivery of glucose and insulin to the muscles, and translocation of GLUT4 to the muscle cell membrane, enhancing non-insulin-dependent glucose uptake [8, 40, 41]. Exercise also has a beneficial effect on NEFA metabolism by enhancing whole-body lipid oxidation [42, 43] and favourably affects overall lipid profile [40, 44], reducing the risk of cardiovascular disease. Physical activity, including exercise, has been shown to improve mitochondrial number and density in skeletal muscle [45]. This results in an increase in oxidative capacity which enhances fat oxidation. Physical activity offers an insulin-independent way of aiding glucose homeostasis in the face of IR and promotes fat oxidation, thus reducing hyperlipidaemia, all of which is key in the prevention and management of metabolic disorders including NAFLD.
16.6 Physical Activity and NAFLD
Physical activity levels are reported to be lower in people with NAFLD than their “healthy” counterparts. A cross-sectional study of Japanese men showed that the prevalence of NAFLD was inversely related to the frequency of self-reported exercise [7]. Those people that exercised for more than 30 min/day on at least 3 days/week were half as likely to have NAFLD as their sedentary counterparts, despite a similar BMI. In a subsequent cross-sectional report, these observations were expanded to state that people without fatty liver engaged in nearly three times more resistance activity than people with NAFLD [8]. Among the NAFLD group, those that engaged in physical activity of any kind or duration had lower fasting serum insulin levels and a lower rate of abdominal obesity even though they had a similar BMI to their inactive counterparts. However, in both of these studies, physical activity levels were obtained from self-reported, non-validated, physical activity questionnaires developed for the purpose of the research, rather than being objectively measured. Perseghin et al. (2007) demonstrated that a higher level of habitual physical activity is associated with a lower level of liver fat and suggested that this relationship may be due to the effect of exercise per se (n = 191) [9]. Again, this study relied upon self-reporting of physical activity levels rather than using an objective measure, but did use a questionnaire validated for use in the general population. A recent study that used a multi-sensor array to measure activity levels in NAFLD revealed that people with NAFLD spent more time physically inactive and achieved lower levels of physical activity than their healthy counterparts on a day-to-day basis [10]. People with NAFLD not only carried out a lower average level of physical activity but also undertook less moderate and vigorous activity than people without NAFLD. The lower levels of these higher intensity activities may have implications as the intensity of the activity may also play a key role in improving metabolic control. Increasing physical activity levels in people with NAFLD is likely to be of benefit, not only to liver health, but overall metabolic profile, and should be encouraged in a bid to prevent NAFLD progression, the development of type 2 diabetes or cardiovascular disease.
16.7 Exercise and NAFLD
Exercise is one of the cornerstones of NAFLD and NASH management although the evidence underpinning this is still in its infancy compared to other conditions (type 2 diabetes for example). This is likely the product of studies combining diet and exercise interventions until recently. Indeed, in 2012 two independent systematic reviews could only identify a maximum of six studies that had undertaken randomised control trials to explore the effects of exercise on liver fat in people with NAFLD [46, 47]. A more recent systematic review in 2017 was able to identify 24 exercise studies, showing the rapid increase in work in this area [48]. These reviews reveal that exercise, without weight loss, produced a 20–30% relative reduction in intrahepatic lipid.
Different forms of exercise (aerobic, resistance/strength training or high-intensity intermittent training) appear to have similar effects on liver fat [46,47,48,49]. More vigorous aerobic exercise does not hold additional benefit for liver fat compared with moderate aerobic exercise [50, 51]. However, it should be noted that all exercise trials are still small and have a wide range of variability in terms of their protocol intensities. The studies to date have been relatively short, lasting in the main between 8 and 12 weeks. Longer-term studies are starting to be published and reveal that if patients continue to exercise for 12 months, the benefits remain [52]. However, if patients do not continue to exercise, the benefits are lost [53]. Moreover, further studies should take into account genetic background of the patients and its influence on response to physical activity. Indeed, PNPLA3 seems to influence response to lifestyle intervention. Patients bearing unfavourable genotype GG did respond better than patients with genotype CC or CG [54].
The mechanisms underlying the change in liver fat following exercise in NAFLD reflect changes in energy balance, circulatory lipids and insulin sensitivity. Much of the early work in exercise in NAFLD has been debated as exercise was accompanied by either dietary changes or diet-induced weight loss leaving the question of whether there is an exercise-only effect. More recent, better-controlled studies are able to not only demonstrate that there is an exercise-only effect on liver fat but also begin to explore the underlying mechanisms. Exercise has little effect on hepatic insulin sensitivity, but does improve peripheral insulin sensitivity [55] producing a net improvement in insulin action and as a consequence, reducing hepatic de novo lipogenesis. It should be noted that the direct benefits of exercise on glycaemic control are significant, but modest even in people with impaired glucose control [5]. However, tracer studies also show that exercise has a direct effect on lipid flux, with an increase in VLDL clearance contributing to the reduction in liver fat with exercise [56], so not all of the changes in liver fat are attributable to insulin sensitivity alone.
Exercise alone, in the absence of any change in body weight or composition, may enhance insulin sensitivity and glucose homeostasis. Exercise, or muscle contraction per se, provides an insulin-independent way of stimulating glucose uptake from the circulation into skeletal muscle. As the muscle contracts, GLUT4 transporters translocate to the muscle cell wall increasing the capacity for glucose uptake [8]. A larger mass of skeletal muscle, as a consequence of exercise, increases overall glucose storage capacity. Exercise also enhances fatty acid metabolism by enhancing whole-body lipid oxidation [42, 43]. Thus, in people who are IR or have type 2 diabetes, exercise provides a way of improving glycaemic control.
In patients with type 2 diabetes, skeletal muscle mitochondria are reduced in size, and there is reduced activity of the electron transport chain [57]. Mitochondria are normally adaptable organelles and in skeletal muscle in healthy individuals there is considerable plasticity in terms of mitochondrial content, allowing the muscle to adapt to match energy demands of physical activity [45]. Endurance training increases fat oxidation during submaximal exercise. Mild or moderate-intensity exercise (25–65% of VO2max) is associated with a five- to tenfold increase in fat oxidation above resting amounts because of increased energy requirements of muscle and enhanced fatty acid availability [58]. Several factors contribute to this adaptive response: increased density of the mitochondria in the skeletal muscles, which increases the capacity for fat oxidation; a proliferation of capillaries within skeletal muscle, which enhances fatty acid delivery to muscle; an increase in carnitine transferase, which facilitates fatty acid transport across the mitochondrial membrane, and an increase in fatty acid binding proteins, which regulate myocyte fatty acid transport [58, 59]. In people with type 2 diabetes, mitochondria were found to increase both in size and density after a 4-month lifestyle intervention of daily moderate–intensity exercise with moderate weight loss [45]. Increased fatty acid oxidation during endurance exercise permits sustained physical activity and delays the onset of glycogen depletion and hypoglycaemia.
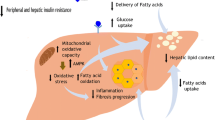
Although not the liver itself, there is an important reduction in visceral adipose tissue with exercise. Visceral fat has been directly linked with liver inflammation and fibrosis, independent of IR and hepatic steatosis [60]. The precise mechanism of how visceral fat applies its detrimental effects on liver metabolism, fibrotic and inflammatory consequences remains unclear although influx of fatty acids and synthesis of cytokines and adipokines have been shown to promote liver fat accumulation, IR and inflammation [60]. There is much that is not known in the field of exercise and NAFLD, including the effect of exercise on inflammation (a key mediator in the progression of NAFLD), effect on gut microbiota and appetite for a start. However, given that people with NAFLD are at nearly double the risk of developing cardiovascular disease than those without [61], the beneficial effects of exercise on cardiovascular function [62] should be explored further. Indeed, it is possible that the major benefits for exercise in NAFLD are not in the liver, but in improving cardiovascular function. A schematic representation of the mediators of response to exercise in NAFLD can be seen in Fig. 16.2.
16.8 Aerobic Exercise and NAFLD
Aerobic exercise, sometimes referred to as cardio or cardiovascular exercise, is any activity that uses large muscle groups and can be maintained continuously over a period of time. It is generally rhythmic in nature and is a type of exercise that overloads the heart and lungs and causes them to work harder than at rest [63]. Multiple studies have highlighted the benefits of aerobic exercise, in NAFLD independent of weight loss [46,47,48]. The protocols used in these studies largely follow the guidelines for physical activity prescription in the general population of 150 mins moderate-to-vigorous intensity exercise per week [37, 38] and utilise a combination of static cycling, walking/jogging and circuit-based exercise. For a large proportion of patients with NAFLD, these exercise levels may be too high a target to be aiming for initially as their baseline levels are significantly lower than this [10]. This is not surprising as figures from the Health Survey for England show that only 67% of men and 55% of women in the general population meet theses exercise targets. One barrier to exercise people often site is lack of time. High-intensity intermittent training (HIIT) is a relatively new method of exercising. HIIT consists of exercise divided into high-intensity bouts interspersed with recovery periods and can provide comparable or greater benefits to cardiorespiratory fitness than continuous moderate–intensity exercise of longer duration [64]. Studies have found that some volunteers prefer HIIT to continuous exercise routines as it is less time consuming [65, 66]. HIIT has also been shown to improve liver fat and cardiac function in patients with NAFLD [49] and is another option to offer patients in the clinical setting. It is worth noting that more vigorous aerobic exercise does not hold additional benefit for liver fat compared with moderate aerobic exercise [50, 51]—the majority of patients with NAFLD would benefit from a combined exercise approach, which targets not only liver health but also type 2 diabetes and CVD risk. Ultimately, exercise prescription for our patients with NAFLD should be individualised to promote adoption and long-term adherence to the exercise regimen and should take into consideration patients’ other comorbidities, their baseline capabilities and personal preferences [67].
16.9 Resistance Exercise and NAFLD
Resistance exercise, often known as strength or weight training, works the muscles against a load. Resistance exercise provides an alternative to aerobic exercise; it improves muscular strength, muscle mass and metabolic control, safely and effectively, in vulnerable populations independent of weight loss [68]. It places less of a demand on the cardiorespiratory system and may therefore be accessible to more patients [69] thus proving a particularly useful tool in the management of our NAFLD patients with multiple comorbidities.
Evidence that resistance exercise can improve body composition is increasing, and it is now recommended by the American College of Sports Medicine and the American Heart Association as an integral component to any exercise programme [70, 71]. A meta-analysis comparing aerobic training with weight training concluded that weight training resulted in greater increases in fat-free mass [72]. An increase in muscle mass may improve insulin sensitivity by increasing the available glucose storage area, thereby reducing the amount of insulin required to maintain a normal glucose tolerance. An increased muscle mass may also improve fat oxidation due to an increase in the number of mitochondria.
Resistance exercise has been shown to decrease respiratory exchange ratio (RER) after exercise, indicating elevated fat oxidation [70]. This reduction in RER has been reported to last hours after a single bout of resistance exercise [71, 73]. This represents a shift towards greater fat relative to carbohydrate oxidation during the post-exercise period. Enhanced fat oxidation, observed as an acute response to resistance exercise, is due to glucose sparing for the purpose of glycogen replenishment, thus resulting in fatty acids being the primary substrate for energy provision after resistance exercise.
Strenuous resistance exercise could be beneficial in weight control, not only because of the direct caloric cost of the activity and the residual elevation of the post-exercise VO2 but also because of the greater post-exercise fat oxidation. Energy expenditure has been found to be elevated for as long as 38 h after an acute bout of heavy resistance exercise [74]. Results suggest that the energy required to recover from resistance training may be of significant use to a weight control/loss programme. For the first 24-h period following exercise, metabolism was increased by 21% and over a further 24 h by 19%. These differences could equate to 404 kcal and 369 kcal increases per day, respectively, for average build individuals [74].
-
Recommendations for exercise prescription in NAFLD [67]
-
Aerobic (e.g. jogging, cycling):
-
150–300 min/week of moderate-to-vigorous intensity (50–70% VO2peak) ≥3 days/week
-
-
Resistance (strength training):
-
2–3 sets of 8–12 repetitions (70–85% 1RM) 2–3 days/week
-
-
For weight maintenance: ↑ volume of exercise
-
For improvement in cardiorespiratory fitness and glycaemic control: ↑ intensity of exercise
16.10 Diet, Sedentary Behaviour, Physical Activity and Exercise
Although exercise has a significant and clinically meaningful effect on liver lipid (20–30% relative reduction), its effects are modest in comparison to weight reduction which can produce >80% reduction in liver fat [46]. This is important as, clinically, supporting people to manage their weight through diet approaches will produce greater changes in liver fat than exercise alone. However, completely disassociating exercise and diet may not be beneficial as data suggests that cardiorespiratory fitness is a determinant of response to dietary intervention in NAFLD, with those with a greater cardiorespiratory fitness having a greater response to dietary intervention [75]. This creates a difficult paradox where those with the lowest cardiorespiratory fitness, who will find exercise most difficult, also have the lowest response to diet-induced lifestyle interventions. Additionally, high levels of physical activity (i.e. 200–300 min/week) are crucial for weight loss maintenance [76], and since physical activity has an independent effect in NAFLD treatment, it provides another treatment option for those who have difficulties in weight loss.
16.11 Physical Activity Measurement
In order to utilise physical activity/exercise as a treatment strategy in the management of NAFLD, we need a means to accurately measure levels of sedentary behaviour, physical activity and exercise. Sensitive and specific tools are required to best characterise the habitual patterns of activity in our patients and to monitor the effectiveness of lifestyle interventions. These tools may also assist clinicians in providing accurate feedback to the patient as to their current activity levels, and enable individual activity targets to be set, monitored and worked towards as part of the patient’s treatment package. Several different methodologies exist for the measurement and assessment of physical activity and energy expenditure (EE). These methodologies range from expensive and objective laboratory measures such as doubly labelled water to subjective measures such as self-reported physical activity questionnaires. All of these tools have benefits and limitations, and their appropriate use depends on multiple factors, especially the context in which they are being used. The most clinically useful measures are discussed below:
Physical activity questionnaires: There are a large number of self-recall physical activity questionnaires. The most frequently used are the Baecke and IPAQ. Self-reported physical activity is valid [77,78,79] and useful in understanding broad differences in physical activity in large cross-sectional studies. However, these techniques are not sensitive to monitor changes in activity patterns or allow accurate determination of energy expenditure (EE) and are subject to recall error [80]. They can be useful to use on an individual patient basis to gain an estimate of current activity levels thus allowing the clinician to open the conversation about changing activity habits, but are not sensitive enough to detect small changes made through lifestyle interventions.
Heart rate monitors: Heart rate monitors are routinely used to measure physical activity in both research and recreation, with an increase in heart rate used as a surrogate marker for an increase in physical exertion. However, heart rate monitors are only accurate in measuring moderate–vigorous activities, as in lower intensity activities, confounding factors, such as stress, emotions, illness and caffeine intake, have a significant impact on results [81]. Heart rate monitors may therefore be deemed an inappropriate technique, when used in isolation, for measuring day-to-day activity which is generally of low–moderate intensity. They also do not provide information about the type of activity or activity patterns across the day/week.
Pedometers: Pedometers are simple devices, which use up and down motions as estimates of steps. Pedometers provide a low-cost means of crudely measuring physical activity. The major drawback to this method is that pedometers measure footfalls, and thus any activity undertaken which does not involve ambulation (e.g. weight lifting, biking, swimming) is inaccurately recorded. Pedometers also fail to capture intensity, frequency or duration of activity. In most cases, pedometers prove accurate in counting steps; however, they are much less accurate in predicting EE, with error rates of ±30% [82].
Accelerometry: An accelerometer is an electromechanical device that will measure acceleration forces. Basic, uniaxial accelerometers measure acceleration of the body or body parts in one plane and take into account the speed, direction and duration of movements and convert these to movement counts to allow for estimation of EE. Biaxial or triaxial accelerometers provide information about movement in multiple directions, and show a better relationship to physical activity EE than uniaxial units [83]. All accelerometers are subject to motion artefacts, and cannot distinguish movement from activities such as driving a car, from actual “physical” activity. Error rate for accelerometry ranges from 14 to 30% against laboratory measures [84, 85] with uniaxial units prone to the greatest recording error due to their relative insensitivity to whole-body movement.
Multi-sensor array: Multi-sensor systems, or multi-sensor arrays, combine measures such as heart rate, accelerometry and body temperature to provide an overall more accurate picture of physical activity patterns. Multi-sensor arrays utilise pattern detection algorithms (typically determined by the respective manufacturer) to combine physiological signals detected from the different sensors to first identify the wearer’s context, and then apply an appropriate formula to estimate EE from the sensor values [86]. These monitors are generally easy and comfortable to use and have an average error rate of 8–10% when compared to laboratory measures [86, 87].
16.12 Summary
In the absence of approved pharmacotherapies for NAFLD, lifestyle change remains the cornerstone of clinical care [88]. Structured exercise produces significant, but modest, improvements in liver lipid [46]. Evidence-based guidelines for sedentary behaviour and physical activity are lacking in NAFLD. General guidelines for physical activity of 150 min of moderate exercise per week or 10,000 steps per day are good rules of thumb, based on guidelines for the primary prevention of cardiovascular disease [39]. However, the current literature cannot inform us how much sitting is too much, we just know that it is better to sit less than to sit more. Furthermore, it is better to have more breaks in sedentary behaviour than less [89]. Targeting a reversal of sedentary behaviour may also provide an additional therapeutic avenue to complement physical activity and exercise as therapies for NAFLD, but has not been tested yet. There remains a significant lack of large-scale studies exploring physical activity and exercise in NAFLD, with and without dietary change/pharmacotherapy, limiting the generation of guidelines specific for NAFLD. Despite the relative infancy of evidence, the available data suggests that physical activity and exercise provide useful tehraputic tools for the prevention and management of NAFLD and NASH and should be supported.
References
European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL–EASD–EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402.
Chalasani N, Younossi ZM, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23.
Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, et al. Exercise and type 2 diabetes. The American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33:e147–67.
Thomas D, Elliott E, Naughton G. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;(3):CD002968.
Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care. 2006;29:2518–27.
Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–27.
Hsieh SD, Yoshinaga H, Muto T, Sakurai Y. Regular physical activity and coronary risk factors in Japanese men. Circulation. 1998;97:661–5.
Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Zvibel I, Goldiner I, Blendis L, et al. Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology. 2008;48:1791–8.
Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A, Belloni E, et al. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care. 2007;30:683–8.
Hallsworth K, Thoma C, Moore S, Ploetz T, Anstee QM, Taylor R, Day CP, et al. Non-alcoholic fatty liver disease is associated with higher levels of objectively measured sedentary behaviour and lower levels of physical activity than matched healthy controls. Frontline Gastroenterol. 2015;6:44–51.
Krasnoff JB, Painter PL, Wallace JP, Bass NM, Merriman RB. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2008;47:1158–66.
Church TS, Kuk JL, Ross R, Priest EL, Biltoff E, Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023–30.
Blair SN. Physical inactivity: the biggest public health problem of the 21st century. Br J Sports Med. 2009;43:1–2.
Dunstan D, Salmon J, Owen N, Armstrong T, Zimmet P, Welborn T, Cameron A, et al. Associations of TV viewing and physical activity with the metabolic syndrome in Australian adults. Diabetologia. 2005;48:2254–61.
Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, Owen N. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian diabetes, obesity and lifestyle study (AusDiab). Diabetes Care. 2008;31:369–71.
Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–6.
Dunstan DW, Salmon J, Owen N, Armstrong T, Zimmet PZ, Welborn TA, Cameron AJ, et al. Physical activity and television viewing in relation to risk of undiagnosed abnormal glucose metabolism in adults. Diabetes Care. 2004;27:2603–9.
Wittink H, Engelbert R, Takken T. The dangers of inactivity; exercise and inactivity physiology for the manual therapist. Man Ther. 2011;16:209–16.
Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–516.
Tamakoshi A, Ohno Y, JACC Study Group. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004;27:51–4.
Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9.
Levine JA. Sick of sitting. Diabetologia. 2015;58:1751–8.
Grontved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305:2448–55.
Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care. 2002;25:1612–8.
Chu AH, Moy FM. Joint association of sitting time and physical activity with metabolic risk factors among middle-aged Malays in a developing country: a cross-sectional study. PLoS One. 2013;8:e61723.
Dunstan DW, Salmon J, Healy GN, Shaw JE, Jolley D, Zimmet PZ, Owen N, et al. Association of television viewing with fasting and 2-h postchallenge plasma glucose levels in adults without diagnosed diabetes. Diabetes Care. 2007;30:516–22.
Wijndaele K, Healy GN, Dunstan DW, Barnett AG, Salmon J, Shaw JE, Zimmet PZ, et al. Increased cardiometabolic risk is associated with increased TV viewing time. Med Sci Sports Exerc. 2010;42:1511–8.
Sugiyama T, Healy GN, Dunstan D, Salmon J, Owen N. Joint associations of multiple leisure-time sedentary behaviours and physical activity with obesity in Australian adults. Int J Behav Nutr Phys Act. 2008;5:35.
Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work. Lancet. 1953;265:1053–7.
Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655–67.
Levine JA. Nonexercise activity thermogenesis—liberating the life-force. J Intern Med. 2007;262:273–87.
Hamilton M, Etienne J, McClure W, Pavey B, Holloway A. Role of local contractile activity and muscle fibre type on LPL regulation during exercise. Am J Phys. 1998;275:1016–22.
Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol. 2003;551:673–82.
Zderic TW, Hamilton MT. Physical inactivity amplifies the sensitivity of skeletal muscle to the lipid-induced downregulation of lipoprotein lipase activity. J Appl Physiol. 2006;100:249–57.
Taylor R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia. 2008;51:1781–9.
Ryu S, Chang Y, Jung HS, Yun KE, Kwon MJ, Choi Y, Kim CW, et al. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J Hepatol. 2015;63:1229–37.
ACSM. American College of Sports Medicine position stand. Appropriate physical activity intervention for weight loss and weight regain for adults. Med Sci Sports Exerc. 2009;41:459–71.
Department of Health. UK physical activity guidelines. In: Department of Health; 2011.
Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388–91.
Agosti V, Graziano S, Artiaco L, Sorrentino G. Biological mechanisms of stroke prevention by physical activity in type 2 diabetes. Acta Neurol Scand. 2009;119:213–23.
Hayashi T, Wojtaszewski JFP, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. Am J Phys. 1997;273:E1039–51.
Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, Day CP, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60:1278–83.
Trenell MI, Hollingsworth KG, Lim EL, Taylor R. Increased daily walking improves lipid oxidation without changes in mitochondrial function in type 2 diabetes. Diabetes Care. 2008;31:1644–9.
Kadoglou NPE, Iliadis F, Sailer N, Athanasiadou Z, Vitta I, Kapelouzou A, Karayannacos PE, et al. Exercise training ameliorates the effects of rosiglitazone on traditional and novel cardiovascular risk factors in patients with type 2 diabetes mellitus. Metabolism. 2010;59:599–607.
Toledo FGS, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56:2142–7.
Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255–66.
Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157–66.
Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, Takano Y, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66:142–52.
Hallsworth K, Thoma C, Hollingsworth KG, Cassidy S, Anstee QM, Day CP, Trenell MI. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci. 2015;129:1097–105.
Keating SE, Hackett DA, Parker HM, O’Connor HT, Gerofi JA, Sainsbury A, Baker MK, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63:174–82.
Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS, Chen Z, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: a randomized clinical trial. JAMA Intern Med. 2016;176:1074–82.
Zhang HJ, Pan LL, Ma ZM, Chen Z, Huang ZF, Sun Q, Lu Y, et al. Long-term effect of exercise on improving fatty liver and cardiovascular risk factors in obese adults: a 1-year follow-up study. Diabetes Obes Metab. 2017;19:284–9.
Pugh CJ, Sprung VS, Jones H, Richardson P, Shojaee-Moradie F, Umpleby AM, Green DJ, et al. Exercise-induced improvements in liver fat and endothelial function are not sustained 12 months following cessation of exercise supervision in nonalcoholic fatty liver disease. Int J Obes. 2016;40:1927–30.
Shen J, Wong GL, Chan HL, Chan RS, Chan HY, Chu WC, Cheung BH, et al. PNPLA3 gene polymorphism and response to lifestyle modification in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2015;30:139–46.
Cuthbertson DJ, Shojaee-Moradie F, Sprung VS, Jones H, Pugh CJ, Richardson P, Kemp GJ, et al. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin Sci (Lond). 2016;130:93–104.
Shojaee-Moradie F, Cuthbertson DJ, Barrett M, Jackson NC, Herring R, Thomas EL, Bell J, et al. Exercise training reduces liver fat and increases rates of VLDL clearance but not VLDL production in NAFLD. J Clin Endocrinol Metab. 2016;101:4219–28.
Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–71.
Horowitz JF, Klein S. Lipid metabolism during endurance exercise. Am J Clin Nutr. 2000;72:558S–63.
Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52:2191–7.
van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, London R, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449–57.
Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73.
Hallsworth K, Thoma C, Hollingsworth KG, Cassidy S, Anstee QM, Day CP, Trenell MI. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci (Lond). 2015;129:1097–105.
ACSM. ACSM’s resource manual for guidelines for exercise testing and prescription. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2006.
Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590:1077–84.
Guiraud T, Nigam A, Juneau M, Meyer P, Gayda M, Bosquet L. Acute responses to high-intensity intermittent exercise in CHD patients. Med Sci Sports Exerc. 2011;43:211–7.
Coquart JB, Lemaire C, Dubart AE, Luttembacher DP, Douillard C, Garcin M. Intermittent versus continuous exercise: effects of perceptually lower exercise in obese women. Med Sci Sports Exerc. 2008;40:1546–53.
Keating SE, George J, Johnson NA. The benefits of exercise for patients with non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2015;9:1247–50.
Larose J, Sigal RJ, Boule NG, Wells GA, Prud’Homme D, Fortier MS, Reid RD, et al. Effect of exercise training on physical fitness in type II diabetes mellitus. Med Sci Sports Exerc. 2010;42:1439–47.
Gordon BA, Benson AC, Bird SR, Fraser SF. Resistance training improves metabolic health in type 2 diabetes: a systematic review. Diabetes Res Clin Pract. 2009;83:157–75.
Ormsbee MJ, Choi MD, Medlin JK, Geyer GH, Trantham LH, Dubis GS, Hickner RC. Regulation of fat metabolism during resistance exercise in sedentary lean and obese men. J Appl Physiol. 2009;106:1529–37.
Ormsbee MJ, Thyfault JP, Johnson EA, Kraus RM, Choi MD, Hickner RC. Fat metabolism and acute resistance exercise in trained men. J Appl Physiol. 2007;102:1767–72.
Ballor D, Keesey R. A meta-analysis of the factors affecting exercise-induced changes in body mass, fat mass and fat-free mass in males and females. Int J Obes. 1991;15:717–26.
Melby C, Scholl C, Edwards G, Bullough R. Effect of acute resistance exercise on postexercise energy expenditure and resting metabolic rate. J Appl Physiol. 1993;75:1847–53.
Schuenke M, Mikat R, McBride J. Effect of an acute period of resistance exercise on excess post-exercise oxygen consumption: implications for body mass management. Eur J Appl Physiol. 2002;86:411–7.
Kantartzis K, Thamer C, Peter A, Machann J, Schick F, Schraml C, Konigsrainer A, et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. 2009;58:1281–8.
Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023.
Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Activity Health. 2009;6:790–804.
Hagströmer M, Oja P, Sjöström M. The international physical activity questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9:755–62.
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95.
Warren JM, Ekelund U, Besson H, Mezzani A, Geladas N, Vanhees L. Assessment of physical activity— a review of methodologies with reference to epidemiological research: a report of the exercise physiology section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2010;17:127–39.
Crouter SE, Albright C, Bassett DRJ. Accuracy of polar S410 heart rate monitor to estimate energy cost of exercise. Med Sci Sports Exerc. 2004;36:1433–9.
Crouter SE, Schneider PL, Karabulut M, Bassett DRJ. Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Med Sci Sports Exerc. 2003;35:1455–60.
Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity. 2007;15:2371–9.
Fehling PC, Smith DL, Warner SE, Dalsky GP. Comparison of accelerometers with oxygen consumption in older adults during exercise. Med Sci Sports Exerc. 1999;31:171–5.
Chen KY, Acra SA, Majchrzak K, Donahue CL, Baker L, Clemens L, Sun M, et al. Predicting energy expenditure of physical activity using hip- and wrist-worn accelerometers. Diabetes Technol Therap. 2003;5:1023–33.
Welk GJ, Blair SN, Wood K, Jones S, Thompson RW. A comparative evaluation of three accelerometry-based physical activity monitors. Med Sci Sports Exerc. 2000;32:S489–97.
St-Onge M, Mignault D, Allison DB, Rabasa-Lhoret R. Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am J Clin Nutr. 2007;85:742–9.
Harrison SA, Day CP. Benefits of lifestyle modification in NAFLD. Gut. 2007;56:1760–9.
Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, Owen N. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31:661–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hallsworth, K., Trenell, M. (2020). Physical Activity in NAFLD: What and How Much?. In: Bugianesi, E. (eds) Non-Alcoholic Fatty Liver Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-95828-6_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-95828-6_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95827-9
Online ISBN: 978-3-319-95828-6
eBook Packages: MedicineMedicine (R0)