Abstract
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of liver pathology, ranging from simple hepatic steatosis to more advanced forms of liver damage, up to cirrhosis. It has been described an important increase in the prevalence of NAFLD, likely related to the dramatic rise in the incidence of obesity during the past three decades. Despite an increase in public awareness, overweight/obesity and related conditions, such as NAFLD, remain underdiagnosed and underestimated.
The literature of last decades contains several advances not only to further elucidate the genetic/pathogenetic mechanisms that lead to pediatric NAFLD but also allows to make many steps forward both on the diagnosis and therapy of children and adolescents with NAFLD.
The aim of this chapter is to provide an overview of the actual knowledge in the field of pediatric NAFLD, with particular attention to the novel discoveries in terms of diagnosis and therapy and its future implications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
The epidemic of obesity has resulted in a parallel incremental burden of nonalcoholic fatty liver disease (NAFLD) worldwide. Also, in children and adolescents, NAFLD represents the most common cause of chronic liver disease in industrialized countries [1]. NAFLD in children is now considered a metabolic condition, which is strongly associated with the other metabolic features, such as hypertension and insulin resistance, increasing the risk of developing type 2 diabetes mellitus, metabolic syndrome, and cardiovascular disease at a young age [2, 3].
The exact prevalence of pediatric NAFLD is actually unknown, but available data describe a prevalence ranging from 3 to 12% in the general pediatric population, with peaks of 70% in obese children. In Western countries, the prevalence of NAFLD is estimated to be around 20–46%, while in Asian children the prevalence is lower, about 5–18%. Moreover, in Asia and Pacific Islands, significant differences are reported between urban and rural populations with a prevalence of 16–32% in urban areas versus a prevalence of 9% in rural populations. Obesity-related NAFLD was reported in 77% of obese/overweight Chinese children. In Australia, the prevalence of pediatric NAFLD was estimated to be approximately 10% in the total population and 27.6% among overweight and obese children [4]. These ethnic differences may be related to genetic, environmental, or sociocultural factors as well as differences in body composition, insulin sensitivity, and adipocytokine profile.
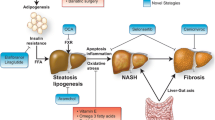
As it is note, NAFLD is characterized by accumulation of fat in the hepatocytes (>5%) in the absence of other causes of liver steatosis, such as Wilson’s disease, deficiency of alfa-1-antitripsin, celiac disease, autoimmune hepatitis, HCV infection, metabolic disorders, and alcohol or drug consumption. The simple hepatic steatosis is usually a benign condition, but in some cases, it may progress to more advanced forms of liver injury, characterized by the presence of inflammation and various degrees of fibrosis [nonalcoholic steatohepatitis (NASH)] up to cirrhosis, predisposing to liver failure and/or hepatocellular carcinoma (HCC) [2].
The natural history of pediatric NAFLD remains to be fully understood, considering the paucity of data available at medium/long-term and the complex interplay between liver involvement and other obesity-related metabolic impairments [5]. Several studies reported that children with NAFLD observed in a tertiary center have a significantly shorter survival compared to the general population of the same age and sex, and that, in some cases, NAFLD in children may progress to cirrhosis and end-stage liver disease [5].
In this chapter, the clinical, diagnostic, and therapeutic strategies of NAFLD currently known will be discussed, with particular interest to novel future scenarios in diagnosis and therapeutic approach to pediatric NAFLD.
11.2 Pathogenesis
In the last two decades, several advances have been made in the knowledge of pathogenesis of NAFLD. The two-hit hypothesis has been exceeded by the multi-hit theory, in which several agents have been identified as contemporary actors in the onset and progression of liver damage. Also in this theory, the starting point is represented by hepatic steatosis, which can be the result of several possible factors, such as dietary habits, environmental and genetic factors, insulin resistance, obesity with adipocyte proliferation, and changes in the composition of intestinal microbiota [6]. Subsequent noxae, such as pro-inflammatory cytokines, oxidative stress, circulating endotoxins, and activation of hepatic stellate cells, contribute to the progression of liver damage.
Adipose tissue is a metabolically active endocrine organ that causes the release of pro-inflammatory cytokines, such as TNF-α and IL-6, whereas beneficial adipokines are suppressed. This situation leads to the development of peripheral insulin resistance and hyperinsulinemia and increased fatty acid delivery to the hepatocyte. The disruption of normal insulin signaling in the hepatocyte and increased abundance of fatty acids leads to disordered lipid metabolism, characterized by the over-activation of de novo lipogenesis (DNL) transcriptional factors, causing more fatty acid and glucose products to be shunted into these lypogenetic pathways [7].
The role of intestinal microbiota has been recently considered within this metabolic dysregulation. A bad “obesogenic” diet (rich in fats and lipids) and increase of intestinal bacteria products (i.e., endotoxins, proteins, metabolites, lipopolysaccharides (LPS)) with the subsequent activation of the toll-like receptor (TLR) pathway, may act as promoter of inflammation and progression of hepatic steatosis to NASH and fibrosis. This process seems also be aggravated by the increased intestinal permeability that has been demonstrated in subjects with liver disease, where the gut seems to go through a tight junction disruption process that could be reversed by changes in the microbiota composition.
These discoveries in the pathogenetic mechanisms are particularly relevant because of the possible therapeutic implications of prebiotics/probiotics and dietetic supplements in the treatment of NAFLD/NASH [8, 9].
11.3 Diagnosis
NAFLD is generally asymptomatic and the diagnosis is frequently made following the incidental discovery of hypertransaminasemia and/or hepatic steatosis. Hypertransaminasemia with a mild elevation of liver enzymes is a common finding in pediatric NAFLD, even if it is clearly demonstrated that aminotransferases serum levels may be in the normal range in several cases, independently from severity of liver damage [10]. However, serum ALT and AST concentration remains a cheap test for initial evaluation of NAFLD, even if its sensitivity is unsatisfactory. To make diagnosis of NAFLD, it is important to exclude other possible causes of hepatic steatosis and therefore an adequate screening panel should be done in all patients. Moreover, considering the strict association between NAFLD and metabolic syndrome, evaluation of metabolic parameters such as lipid profile, glyco-insulinemic status, uric acid concentrations, and blood pressure measurements are strongly recommended in patients with NAFLD [11].
A recent position paper by the ESPGHAN Hepatology Committee has clarified the diagnostic approach to NAFLD in childhood. NAFLD is more frequent in children aged more than 10 years and is usually present with overweight/obesity. As previously stated, the diagnosis of NAFLD needs the recognition of fatty liver and the exclusion of other causes of liver steatosis [2].
Liver biopsy remains the current gold standard for the diagnosis of NAFLD, and it is the only way to distinguish between NASH and simple steatosis, and to determine the presence and characteristics of fibrosis and the severity of liver damage. However, since liver biopsy is an invasive procedure, its use should be limited to selected patients, as stated by ESPGHAN Hepatology Committee [2].
In the last two decades, several biomarkers have been tested as diagnostic tools for NAFLD/NASH, but till now none of the markers evaluated is completely satisfactory in the diagnostic work-up. The major limitation of all these tests is the incapacity to estimate the severity of disease and the presence of fibrosis []. Higher levels of C-reactive protein and pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-6, have been associated with progression of damage from NAFLD to NASH, but these markers lack specificity for NAFLD.
Recently cytokeratin-18, a marker of hepatocyte apoptosis, fragment levels, and the cathepsin-D (a lysosomal protease) were identified as reliable markers of NASH. In fact, it has been demonstrated that the cathepsin-D have a high diagnostic value to distinguish pediatric patients with hepatic inflammation from children with steatosis, while the cytokeratin-18 correlates significantly with hepatic fibrosis and with NAFLD severity [12, 13].
Another new marker of liver disease is the cathepsin D (CATD). The CATD is a proteolytic enzyme contained in the lysosomes of eukaryotic cells. The CATD are involved in autolysis processes, causing cleavage of the protein cellular constituents into peptides and amino acids. They also have the function of eliminating the extracellular proteins, such as the bacterial residues and the products of the antigen–antibody reaction [14]. A pediatric study of 2015 has shown that the plasma levels of CATD levels were significantly lower in patients with liver inflammation than those with only steatosis [15]. Moreover, the levels of CATD have been progressively reduced and negatively correlated with severity of liver inflammation, steatosis, hepatocellular ballooning, and the score of hepatic steatosis nonalcoholic activities. In addition, the levels of CATD are better correlated with the progression of nonalcoholic hepatic steatosis pediatric compared to ALT and CK-18 [14]. CATD showed a high diagnostic accuracy, with AUROC = 0.94, for the differentiation between hepatic steatosis and inflammation, and has reached nearly the highest precision (AUROC = 0.99) after addition of CK-18 [15]. The observation that plasma CATD correlated with the development of NASH and regression is promising for NASH diagnosis, but especially for the liver inflammation, which proceeds the onset of fibrosis.
Recently, genome-wide association studies (GWAS) have identified single-nucleotide polymorphisms (SNPs) of genes implicated in NAFLD/NASH pathogenesis capable to influence liver damage and fibrosis progression in adult and pediatric patients. Firstly, the PNPLA3, also known as adiponutrin, is a member of the patatin-like phospholipase family. The rs738409 C>G single-nucleotide polymorphism, encoding the Ile148Met variant protein of PNPLA3, is described as genetic determinant of hepatic steatosis. Several studies have established a strong link between PNPLA3 and the development of NAFLD [16]. PNPLA3 is associated with an increased risk of advanced fibrosis among patients with a variety of liver diseases and is an independent risk factor for hepatocellular carcinoma (HCC) among patients with NASH [17]. Besides PNPLA3, combined GWAS datasets have identified other SNPs associated with liver fat content and other aspects of the NAFLD phenotype, involving genes implicated in insulin resistance, oxidative stress, and fibrogenesis. For diagnosis and risk stratification of NAFLD, genetic screening tests are now available; these tests are easy to perform and have a low cost and can assess the risk of the subject of developing severe forms of NAFLD. Currently, a simple oral swab that searches for mutations in a combination of four genes (KLF6, PNPLA3, SOD2, and LPIN1), each of which is related to NAFLD, is able to estimate the risk of development of severe form of hepatopathy [18, 19].
The most used technique in the diagnostic work-up of NAFLD is abdominal ultrasound. It is widely used because it is simple, economic, and widely available. Unfortunately, US examination diagnose liver steatosis, only if fat is present in 30% of hepatocytes, and it do not detect minor degree of steatosic infiltration (<30%). Overall, the sensitivity of ultrasound in NAFLD ranges from 60 to 94%, with specificity from 84 to 100% [20]. The main limitation of liver ultrasound is represented by the inability in distinguishing between NAFLD and NASH due to its incapacity to detect liver fibrosis. This limitation is partially exceeded by transient elastography, an ultrasonographic technique based on the evaluation of tissue elasticity through ultrasound by measuring the propagation of specific elastic waves (shear waves, S-waves) emitted by the probe. However, abdominal obesity may reduce its utility in patients with NASH, and the detection accuracy of this method increases with worsening grades of fibrosis. For these reasons, it is not standardized in pediatric patients, and large studies are required to define normal values and the accuracy of transient elastography in children [21, 22].
Acoustic force impulse imaging radiation (ARFI) is an integrated US elastography method in US conventional machines in which a region of interest in the liver is mechanically excited with an acoustic pulse that induce localized tissue displacement, which translates in wave propagation. The wave propagation velocity correlates with liver stiffness and fibrosis. In a meta-analysis in patients with NAFLD (n = 77), the ARFI imaging had AUROC of 0.86 for the diagnosis of fibrosis [22].
Shear-wave elastography (SWE) are new methods that evaluate elasticity of the tissues using conventional USA. They allow the operator to select a specific area of the liver parenchyma, avoiding inclusion of focal lesions or large blood vessels and overcome some limitations of transient elastography. Acoustic imaging radiation impulse force showed good results in the differentiation stages of fibrosis in adults, especially in adult patients with chronic viral hepatitis. A recent study tested the SWE in 68 children with biopsy-proven NASH (37 males, age 8–17 years), demonstrating that SWE showed a high correlation with hepatic fibrosis (rho = 0.84, p < 0.001). The diagnostic accuracy in the determination of the presence of any grade of fibrosis showed an AUC of 0.92 (cutoff = 5.1 kPa), whereas the presence of significant fibrosis could be established with an AUC of 0.97 (cutoff = 6.7 kPa). These results are in line with a previous study of those that evaluated the accuracy of transient elastography in another pediatric NASH population [23].
Computed tomography (CT) and magnetic resonance imaging (MRI) are more sensitive and specific than ultrasound in determining liver steatosis, but these techniques have some limitations in pediatric setting. Firstly, both are expensive and not always widely available in pediatric setting. Moreover, CT implies an unjustified radiation exposure and MRI in several cases needs of sedation of pediatric patients. In addition, neither MRI nor CT are able to stage the disease and cannot distinguish between NAFLD and NASH, given that they could not detect hepatic fibrosis [2].
11.4 Treatment
Considering the actual knowledge on natural history of NAFLD/NASH in adults and children and health burden of metabolic comorbidities generally associated to NAFLD, the effective treatment of these conditions is now become a public health problem, mainly in children. The final goals of ideal treatments are to reverse hepatic histological damage, reducing long-term hepatic and extrahepatic complications and improving patient’s quality of life and life expectation. Today, lifestyle intervention, based on hypocaloric diet and regular physical exercise, continues to be the basilar therapeutic approach, even if its results are often unsatisfactory, especially for the difficult compliance. Unfortunately, none of the drugs until now tested in children have proven to be completely adequate in the treatment of liver disease and therefore novel possible targets or pharmacological associations direct against the novel pathogenetic mechanisms are now being evaluated.
11.4.1 Actual Therapeutic Approaches
Gradual weight loss, due to behavioral modifications based on balanced diet and regular physical exercise, is still considered the first therapeutical approach to fatty liver in children. The available data demonstrated in several adults and pediatrics reports the effectiveness of this approach in terms of hepatic and extrahepatic insulin sensitivity, inflammation, and oxidative stress.
The main problem of this therapeutic strategy in pediatric NAFLD is represented by the difficulty in maintaining compliance of children and their families with the proposed programs, with disappointing results. Some reports described an inacceptable success rates as low as 10% after 2 years of intervention [24]. No clear indications are established for diet in pediatric patients with NAFLD, but a balanced diet containing low fat meats and fish, vegetables, legumes, and fruits with reduction in sugar-sweetened beverages, saturated fat, starches, and salt is generally recommended [25]. Moreover, in the last decade several data have demonstrated the toxic effect of high fructose intake on metabolic status and liver damage; therefore, a significant reduction of daily fructose intake is now strongly recommended [26].
As for pharmaceutical armamentarium, till now none of the available drugs has been revealed totally satisfactory per sé in the treatment of NAFLD. Starting from the new information gradually emerging about pathogenetic mechanisms of NAFLD, several molecules have been proposed, without however conclusive recommendation. The main limitations of many available studies are the frequent small sample size and the heterogeneity of diagnostic tools used to detect NASH and the different outcome measures. Only few randomized controlled clinical trials (RCTs) have been made or are ongoing in pediatric population.
-
Insulin-sensitizing agents. Based on the strict interplay between insulin resistance and metabolic and liver damage typical of NAFLD/NASH, insulin sensitizer have been the first drug evaluated in children. Metformin activates the 5′adenosine monophosphate (AMP)-activated protein kinase (AMPK) pathway, which increases lipid and glucose catabolism. Recently, the large clinical TONIC trial, which enrolled 173 patients, reported that metformin (500 mg twice daily) caused only minor reductions in serum transaminase levels and had no significant effect on liver histology [27].
-
Antioxidants. In the pathogenetic mechanism of NAFLD, the reactive oxygen species, generated by fatty acids oxidation, cause direct cellular damage and activate pro-inflammatory cytokines. The main antioxidant tested in children has been vitamin E (alpha-tocopherol), a fat-soluble vitamin. Initial small studies showing some efficacy of vitamin E in reducing transaminases levels were not confirmed in the TONIC trial, in which vitamin E was no better than placebo in attaining the primary end point, that is, a sustained decrease in ALT levels. Likewise, there was only a limited effect on hepatocellular ballooning and NAFLD activity score [27].
-
Dietetic supplementations. Polyunsaturated fatty acids (PUFA) are long-chain fatty acids, distinguished in two groups: omega-3 PUFA, which are synthesized from α-linolenic acid, and omega-6 PUFA, which derive from linoleic acid. These acids are also defined “essential” fatty acids because they cannot be synthesized de novo by cells of human body and therefore should be taken in adequate amount with the diet. The omega-3 PUFA are important regulators of hepatic gene transcription, with anti-inflammatory, insulin sensitizing, and anti-steatotic effects [28]. On the contrary, the metabolites derived from omega-6 PUFA pathways induce pro-inflammatory and pro-thrombogenic activation.
As it is well-known, the actual Western diets are characterized by a higher consumption of omega-6 fatty acid respect to omega-3 fatty acid, with a consequent imbalance between omega-6/omega-3. Several data have demonstrated, in animal and human studies, beneficial effects of omega-3 supplementation in hepatic and metabolic features of NAFLD. Particularly, Nobili and coworkers have described in well-conducted double-blind randomized controlled trial that oral administration of docosahexaenoic acid (DHA) in pediatric NAFLD causes at 6 and 24 months an amelioration of transaminases and triglycerides concentrations, insulin sensitivity index, and hepatic steatosis [21].
Based on the new advances in NAFLD pathogenesis, mainly following the development of “gut–liver axis theory,” probiotics have been proposed as a possible strategy for treatment of liver disease, such as NAFLD. As previously stated, poor diet and slowed intestinal transit, frequent in obese patients, may induce small intestinal bacterial overgrowth (SIBO) and thereby increase the release of endotoxins [mainly gut-derived lipopolysaccharides (LPS)] and tumor necrosis factor (TNF)-α. These inflammatory mediators easily cross the intestinal barrier, which is more permeable in patients with NAFLD, and promote the progression of NAFLD to NASH with a profibrogenic phenotype [29]. VSL#3, a mixture of eight probiotic strains (Streptococcus salivarius subsp. thermophilus, Bifidobacterium [B. breve, B. infantis, B. longum], Lactobacillus acidophilus, L. plantarum, L. casei, and L. delbrueckii subsp. bulgaricus) has been tested in animal models and human studies that include children. These studies show a beneficial effect on the intestinal barrier, reducing inflammation and permeability as well as liver damage, as measured by hepatic steatosis and aminotransferase levels [30]. These results, in association with optimal safety and tolerability, make probiotics a promising therapeutic tool in pediatric NAFLD. However, to confirm these results, further larger randomized studies are still needed.
11.4.2 Novel Therapies
Bile Acid. Bile acids are synthesized in hepatocytes from cholesterol through enzymatic pathways and then conjugated with glycine or taurine before secretion into bile and release into the small intestine. In the small intestine, conjugated bile acids are not only involved in lipid absorption and transport but have also been increasingly recognized to function as nuclear receptor binders and to have a role in function of microbiota. Bacteria within the intestine can also chemically modify bile acids and thereby alter the composition of the bile acids [31]. Besides the classic role as detergents to facilitate fat absorption, bile acids have also been recognized as important cell signaling molecules regulating lipid and carbohydrate metabolism and inflammatory response. These molecular functions are mediated through their binding and activation of the nuclear hormone receptor, farnesoid X receptor (FXR), and the G-protein-coupled cell surface receptor TGR5. Intestinal FXR activity upregulates endocrine FGF19 expression, which inhibits hepatic bile acid synthesis via CYP7A1 signaling [32]. Recently, it was showed that hepatic FXR protein content and plasma FGF19 concentrations in children and adolescents with NASH were decreased compared to levels in children with “simple” fatty liver. Hepatic FXR protein level was positively correlated with serum FGF-19 concentrations, and both FXR and FGF19 concentration were inversely and independently associated with NASH [18].
When activated, FXR migrates into the cell nucleus and modulates the transcription of specific genes involved in the regulation of inflammation and glucose and lipid metabolism. Numerous studies, mostly based on animal models, have shown that the use of FXR agonists (e.g., obeticholic acid) could improve hepatic steatosis and steatohepatitis [33].
Recently, ursodeoxycholic acid (UDCA) has been supposed to be involved in several other mechanisms, such as glutathione synthesis and activation of glucocorticoid receptor, contributing to the antioxidants anti-inflammatory pathways. Despite these effects, administration of UDCA does not result in concrete benefits in treating NAFLD [34]. In contrast, the FLINT study, conducted in an adult population with NASH, showed that treatment with obeticholic acid (6-ethylchenodeoxycholic acid), FXR agonist, was effective in about half of the treated patients. Obeticholic acid improved the biochemical and histological characteristics of NASH compared to placebo. All histological parameters of NASH (steatosis, hepatocellular swelling, and lobular inflammation) and fibrosis have improved. The improvement of fibrosis, although limited, demonstrated that this therapy could be useful in preventing progression to cirrhosis [35]. In pediatric age, this bile acid has not yet been tested.
Finally, Liraglutide is an analog of glucagone-like peptide 1 (GLP-1), a gut-derived incretin hormone that induces weight loss and insulin sensitivity. In 2016, the LEAN study, conducted on 52 adult patients with NASH, showed that liraglutide led to histological resolution of steatohepatitis. The treatment was safe and well tolerated by the patients, but today no information is available about liraglutide in pediatric setting [36].
Many other trials must be done to understand the therapeutic role of these molecules in NAFLD, mainly in pediatric population.
Bariatric surgery. Bariatric surgery and non-surgical obesity treatments based on minimally invasive intragastric balloons are emerging as therapeutic alternatives that should be carefully considered in obese children with NAFLD, mostly in patients with numerous, unsuccessful weight loss attempts [37]. In 2015, a position paper of ESPGHAN has established eligibility criteria for bariatric surgery in pediatric patients: selected obese patients with BMI >40 kg/m2 and severe comorbidities (including NASH with advanced fibrosis) or with BMI more than 50 kg/m2 and mild comorbidities (including NASH) [38].
In a recent pediatric trial, laparoscopic sleeve gastrectomy, a restrictive intervention consisting in the removal of the gastric fundus, has proved to be more effective than lifestyle approach, even when combined with intragastric devices, in reducing NASH and fibrosis in obese patients after 1 year of treatment and in improving dyslipidemia, sleep apnea, and hypertension [39].
A novel technique used in children is the adjustable gastric banding (AGB). The banding operation is characterized by an externally compressive device on the upper portion of the stomach, which can be inflated or deflated with a subcutaneous port, permitting adjustment of the degree of gastric compression to limit stomach distention and food intake. The main benefit of this technique is to be completely reversible, with discrete efficacy pales in comparison with other bariatric operations. The weight loss response with AGB is highly variable, and prospective studies in adults show a body weight loss of about 20%. Currently the trials are underway in adolescent populations [40].
Bariatric surgery should not be considered as a first-line therapy and a careful evaluation of the patient, considering emotional, psychological, and clinical features, should be performed before performing surgery. Moreover, further studies are needed in order to evaluate long-term efficacy and safety of these procedures (Fig. 11.1).
11.5 Conclusion
NAFLD is often perceived by the patients as a “minor” disease compared to other liver conditions, but recent studies stated that fibrotic potential of NAFLD is as severe as that of chronic hepatitis C, with an average interval time of transition from NASH to cirrhosis estimated around 8–10 years [41]. Prevalence data have decreed NAFLD as the most widespread liver disease in Western countries. 30–40% and 3–5% of adult US population is affected by NAFLD and NASH, respectively: this reflects the risk for millions of people to develop, over the years, end-stage liver disease potentially requiring liver transplantation (LT). Over the past 25 years, in the USA, the number of LTs performed for NASH cirrhosis has doubled from 5.5 to 11% of all reported LTs, and to date NAFLD is the third cause of LT preceded only by alcoholic liver disease and hepatitis C virus. Considering the prevalence trend of NAFLD, the delay of diagnosis due to the absence of non-invasive diagnostic tool, the absence of effective treatments, NASH cirrhosis is expected to become the main indication for LT by 2030 [42].
Because of its natural history, LT for NASH cirrhosis is a rare occurrence in the pediatric context, in fact the average age of transplantation is around 58.5 ± 8 years old [43]. However, some reports of severe hepatic disease and HCC being to be described now also in adolescents and young adults. Moreover, it is important to consider that the worldwide prevalence of NAFLD in children is a worrying phenomenon because this disease is closely associated with the development of both cirrhosis and cardiometabolic syndrome in adulthood. Therefore, the identification of early disease markers and prompt therapeutic approach represents important objectives of the research programs in this field in the next decades.
References
Nobili V, Svegliati-Baroni G, Alisi A, Miele L, Valenti L, Vajro P. A 360-degree overview of paediatric NAFLD: recent insights. J Hepatol. 2013;58:1218–29.
Vajro P, Lenta S, Socha P, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012;54:700–13.
Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–10.
Hadžić N, Baumann U, McKiernan P, McLin V, Nobili V. Long-term challenges and perspectives of pre-adolescent liver disease. Lancet Gastroenterol Hepatol. 2017;2(6):435–45.
Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, et al. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58:1538–44.
Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43(8):617–49.
Mann JP, Raponi M, Nobili V. Clinical implications of understanding the association between oxidative stress and pediatric NAFLD. Expert Rev Gastroenterol Hepatol. 2017;11(4):371–82.
Doulberis M, Kotronis G, Gialamprinou D, et al. Non-alcoholic fatty liver disease: an update with special focus on the role of gut microbiota. Metabolism. 2017;71:182–97.
Del Chierico F, Nobili V, Vernocchi P, et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. 2017;65(2):451–64.
Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–8.
Manco M, Marcellini M, Devito R, Comparcola D, Sartorelli MR, Nobili V. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int J Obes. 2008;32:381–7.
Jazwinski AB, Thompson AJ, Clark PJ, Naggie S, Tillmann HL, Patel K. Elevated serum CK18 levels in chronic hepatitis C patients are associated with advanced fibrosis but not steatosis. J Viral Hepat. 2012;19(4):278–82.
Vos MB, et al. Cytokeratin 18, a marker of cell death, is increased in children with suspected nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2008;47(4):481–5.
Bateman AC, Hübscher SG. Cytokeratin expression as an aid to diagnosis in medical liver biopsies. Histopathology. 2010;56(4):415–25.
Walenbergh SM, et al. Plasma cathepsin D levels: a novel tool to predict pediatric hepatic inflammation. Am J Gastroenterol. 2015;110(3):462–70.
Pingitore P, Romeo S. The role of PNPLA3 in health and disease. Biochim Biophys Acta. 2019;1864(6):900–6. pii: S1388-1981(18)30145-8
Mangge H, Baumgartner BG, Zelzer S, Pruller F, Schnedl WJ, Reininghaus EZ, Haybaeck J, Lackner C, Stauber R, Aigner E, et al. Patatin-like phospholipase 3 (rs738409) gene polymorphism is associated with increased liver enzymes in obese adolescents and metabolic syndrome in all ages. Aliment Pharmacol Ther. 2015;42:99–105.
Carpino G, Pastori D, Baratta F, et al. PNPLA3 variant and portal/periportal histological pattern in patients with biopsy-proven non-alcoholic fatty liver disease: a possible role for oxidative stress. Sci Rep. 2017;7(1):15756.
Nobili V, Donati B, Panera N, et al. A 4-polymorphism risk score predicts steatohepatitis in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2014;58(5):632–6.
Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–9.
Nobili V, Monti L, Alisi A, Lo Zupone C, Pietrobattista A, Tomà P. Transient elastography for assessment of fibrosis in paediatric liver disease. Pediatr Radiol. 2011;41(10):1232–8.
Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–74.
Garcovich M, et al. Liver stiffness in pediatric patients with fatty liver disease: diagnostic accuracy and reproducibility of shear-wave elastography. Radiology. 2016;13:161002.
Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–78.e5.
Africa JA, Newton KP, Schwimmer JB. Lifestyle interventions including nutrition, exercise, and supplements for nonalcoholic fatty liver disease in children. Dig Dis Sci. 2016;61:1375–86.
Softic S, Cohen DE, Kahn CR. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig Dis Sci. 2016;61(5):1282–93.
Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–68.
Della Corte C, Carpino G, De Vito R, De Stefanis C, Alisi A, Cianfarani S, Overi D, Mosca A, Stronati L, Cucchiara S, Raponi M, Gaudio E, Byrne CD, Nobili V. Docosahexanoic acid plus vitamin D treatment improves features of NAFLD in children with serum vitamin D deficiency: results from a single centre trial. PLoS One. 2016;11(12):e0168216.
Miele L, Marrone G, Lauritano C, et al. Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr Pharm Des. 2013;19:5314–24.
Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39(11):1276–85.
Molinaro A, Wahlström A, Marschall HU. Role of bile acids in metabolic control. Trends Endocrinol Metab. 2018;29(1):31–41. pii: S1043-2760(17)30151-0.
Nobili V, Alisi A, Mosca A, et al. Hepatic farnesoid X receptor protein level and circulating fibroblast growth factor 19 concentration in children with NAFLD. Liver Int. 2018;38(2):342–9.
Sepe V, Distrutti E, Fiorucci S, Zampella A. Farnesoid X receptor modulators 2014-present: a patent review. Expert Opin Ther Pat. 2018;28(5):351–64.
Steinacher D, Claudel T, Trauner M. Therapeutic mechanisms of bile acids and nor-ursodeoxycholic acid in non-alcoholic fatty liver disease. Dig Dis. 2017;35(3):282–7.
Neuschwander-Tetri BA, Loomba R, Sanyal AJ, NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–65.
Eguchi Y, Kitajima Y, Hyogo H, Japan Study Group for NAFLD (JSG-NAFLD). Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J). Hepatol Res. 2015;45(3):269–78.
Nobili V, Della Corte C, Liccardo D, Mosca A, Caccamo R, Morino GS, Alterio A, De Peppo F. Obalon intragastric balloon in the treatment of paediatric obesity: a pilot study. Pediatr Obes. 2015;10(5):e1–4.
Nobili V, Vajro P, Dezsofi A, et al. Indications and limitations of bariatric intervention in severely obese children and adolescents with and without nonalcoholic steatohepatitis: ESPGHAN Hepatology Committee position statement. J Pediatr Gastroenterol Nutr. 2015;60:550–61.
Manco M, Mosca A, De Peppo F, et al. The benefit of sleeve gastrectomy in obese adolescents on nonalcoholic steatohepatitis and hepatic fibrosis. J Pediatr. 2017;180:31–37.e2.
Albaugh VL, Abumrad NN. Surgical treatment of obesity. F1000Res. 2018;7:F1000 Faculty Rev-617. https://doi.org/10.12688/f1000research.13515.1. eCollection 2018.
Fiorucci S, Biagioli M, Distrutti E. Future trends in the treatment of non-alcoholic steatohepatitis. Pharmacol Res. 2018;134:289–98.
Alkhouri N, Hanouneh IA, Zein NN, et al. Liver transplantation for nonalcoholic steatohepatitis in young patients. Transpl Int. 2016;29(4):418–24.
Pais R, Barritt AS, Calmus Y, et al. NAFLD and liver transplantation: current burden and expected challenges. J Hepatol. 2016;65(6):1245–57.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Della Corte, C., Mosca, A., Pietrobattista, A., Basso, M.S., Nobili, V. (2020). NAFLD in Children: Implication for the Future. In: Bugianesi, E. (eds) Non-Alcoholic Fatty Liver Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-95828-6_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-95828-6_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95827-9
Online ISBN: 978-3-319-95828-6
eBook Packages: MedicineMedicine (R0)