Abstract
Obesity—namely visceral obesity—is definitely the core of the metabolic syndrome, having insulin resistance as the central metabolic defect. Body fat accumulation and insulin resistance mutually feed each other, and progressive lipotoxicity, i.e., accumulation of fat in adipose tissue stores, finally involves several organs, including the liver. The primary defect may be genetic in origin, or promoted by epigenetic modifications, or, more commonly, by unhealthy lifestyles (both unhealthy diet and poor physical activity), progressively leading to obesity. Nonalcoholic fatty liver disease is one of such conditions, possibly further impairing insulin sensitivity and favoring other comorbidities. The presence of “lean NAFLD” does not confute this theory: it must be regarded as a condition with a much more genetic imprinting in subjects who long maintain an adequate lifestyle. However, in most cases, favored by the accumulation of body fat and reduced physical activity that are common along the years, also these subjects are prone to develop visceral obesity. They might be considered “metabolically unhealthy normal weight” subjects, as opposed to the “metabolically healthy obese” individuals. In most cases, a variable drive of genes and lifestyle is probably the basis for differences that tend to produce obesity and metabolic diseases, including NAFLD, in the course of life.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Obesity, Lipotoxicity, and the Metabolic Syndrome
1.1.1 Pathophysiology
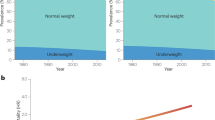
Obesity, i.e., accumulation of body fat, stems from positive energy balance, independently of the absolute amount of calorie intake and energy expenditure via physical activity. According to Unger hypothesis [1], adipocytes were specifically developed to protect organs and tissues during periods of overnutrition, also providing reserve for periods of undernutrition. To satisfy these needs, the adipocytes turned into a versatile endocrine gland, able to regulate food intake via leptin, acting on hypothalamus. A second hormone, adiponectin, counterbalances the action of leptin and is reduced in obesity [2]. Mutation in leptin and leptin receptors genes and changes in leptin and adiponectin levels might regulate fat accumulation, but unhealthy lifestyle probably remains the most relevant factor responsible for increased total body fat. Under these conditions, fatty acid recirculation may exceed the anti-steatotic potential of adipose tissue, and lipotoxic disease develops, characterized by fatty infiltration of non-adipose organs and tissues, including the liver. The secretion of pro-inflammatory cytokines and pro-oxidant substances by adipose tissue favors insulin resistance on glucose and lipid metabolism, leading to a cluster of metabolic changes grouped to define the metabolic syndrome (MetSyn). The definition of MetSyn changed in the course of the years; obesity per se has never been considered a mandatory feature, but waist circumference (a surrogate marker of visceral obesity) was always included and the cutoffs, also related to gender and ethnic differences, were progressively reduced to include cases classified in the overweight range by body mass index (BMI). Alberti and Zimmet first proposed enlarged waist circumference as mandatory feature [3], and the proposal was followed by the International Diabetes Federation [4] and is now widely accepted. In a pivotal study based on statistical analysis of factors associated with the so-called insulin-resistance syndrome, Maison et al. identified BMI and waist-to-hip ratio (a surrogate marker of visceral obesity) as the core components of MetSyn, supporting recent classifications [5]. However, many more metabolic alterations stem from insulin resistance, which have never been included in the definition (Fig. 1.1). The sequence of events starting from liver fat accumulation (steatosis) to hepatic necroinflammation with/without fibrosis (steatohepatitis) to cirrhosis, when unrelated to alcohol abuse, constitutes another nominated but not elected component of MetSyn (nonalcoholic fatty liver disease—NAFLD). NAFLD is one of the most prevalent liver diseases worldwide, occurring in all countries and all ethnic groups [6], largely associated with obesity and other components of MetSyn [7, 8]. Fatty liver may also occur in normal-weight individuals (approx. 10–15% of total cases) [9], but it is much more prevalent in overweight/obese people and also in these cases liver fat positively correlates with insulin resistance [10, 11]: accordingly, nonalcoholic NAFLD and its progressive states (nonalcoholic steatohepatitis—NASH—and NASH-cirrhosis) may be considered the hepatic manifestation of MetSyn [12]. The association of NAFLD with MetSyn is so strict that several critical editorials have suggested that a new name should be given to NAFLD, to better highlight its pathogenic role [13,14,15]. This would achieve two main goals: (a) to identify the etiology in a positive way, avoiding the negative definition of “nonalcoholic”; (b) to consider the metabolic involvement as a possible comorbid condition of other liver diseases (namely alcoholic or viral liver disease). A proposal has recently been made to rename NAFLD as MAFLD (metabolic associated fatty liver disease), and it has immediately gained a wide consensus [16].
Although hepatic steatosis (pure fatty liver, without necroinflammation and fibrosis: i.e., nonalcoholic fatty liver—NAFL) is regarded as a benign stage, it may also progress to NASH in a subgroup of patients, and progression is difficult to forecast [17]. Visceral obesity is probably the main risk factor for NAFLD progression and inappropriate storage of triglycerides in adipocytes and higher concentrations of free fatty acids may add to increased hepatic lipid storage, insulin resistance, and progressive liver damage [18].
This is the general background linking whole-body fat (obesity) to hepatic fat accumulation (NAFLD), where four issues remain unsolved: (a) do obesity and NAFLD stem from a similar genetic background and similar lifestyles?; (b) do they coexist by simple association or is there a cause/effect relationship and, in this case, which comes first?; (c) have they a similar outcome and similar treatment?; and finally (d) does the existence of “lean NAFLD” challenge the pivotal role of adipose tissue accumulation?
In the following sections we will address these questions, in order to answer the title question.
1.1.1.1 Do Obesity and NAFLD Stem from Similar Lifestyles and a Similar Genetic Background?
The relationship between NAFLD and obesity is largely driven by similar pathogenic factors. Obesity is a complex disease, occurring from both genetic and lifestyle promoters (Fig. 1.2).
Representation of the effects of genes, epigenetics, and lifestyles in the course of the years on the relative risk of NAFLD, NAFLD progression, and associated diseases. The relative importance of genes and epigenetic modifications is particularly high in infancy, whereas the importance of unhealthy lifestyles (both unhealthy diet and scarce physical activity) leading to obesity and NAFLD grows along the years
1.1.1.1.1 Dietary Factors
The present epidemic of obesity is largely dependent on excessive calorie intake and sedentary lifestyles, and at any stage of life obesity and NAFLD remain systematically associated (Fig. 1.2); similarly, there is considerable interest on calorie intake and dietary components in the development of NAFLD. In the presence of unhealthy lifestyles and behavioral factors, leading to enlarged adipose tissue and insulin resistance (IR), both lipolysis and de novo lipogenesis are expected to increase the risk of hepatic lipid depots, in association with high calorie (either high-fat or high-carbohydrate) diets [19]. Conflicting results have been reported on the dietary composition of patients with NALFD. In general, calorie intake did not differ between NAFLD and control subjects [20, 21], but macronutrient composition may differ. Studies using food frequency questionnaires (FFQs) reported a higher-than-normal habitual dietary fat intake [22], but in other cohorts higher carbohydrate intake and no differences in dietary fats were reported [21]. Notably, different dietary fats have different effects on liver fat: diets rich in monounsaturated fatty acids (MUFAs) fat or n-6 polyunsaturated fatty acids (n-6 PUFAs) tend to reduce liver fat [23], whereas a high intake of saturated FAs increases liver fat more than a similar amount of n-6 PUFA [24].
Fructose-rich foods are the prototype of an unhealthy diet [25]. Fructose is largely metabolized in the liver, and fuels de novo lipogenesis, favoring steatosis [26]. Fructose is used to enrich sweetened beverages and processed foods, and its consumption is associated with a higher risk of obesity, as well as NAFLD detected by ultrasonography or magnetic resonance imaging [27, 28]. The deleterious effect of fructose might be specifically related to industrial fructose from processed foods and beverages, with limited effect of fruit fructose, when consumed with the several healthy nutrients also present in fruit, sharing antioxidant properties. This would explain the dichotomy between the risk associated with fructose and the beneficial effects of the Mediterranean diet, suggested to reduce the risk of NAFLD and NAFLD progression [29].
Also physical activity regulates triglyceride turnover and, indirectly, liver fat. Physical activity is also intimately associated with obesity, but its association with liver fat is independent of weight gain/weight loss. Any type (aerobic vs. resistance) [30], volume (time spent in exercise), and intensity (from low- to moderate- to high-intensity) of physical activity, including leisure time and non-exercise activity, are important to decrease liver fat accumulation, compared with the time spent sedentarily, an additional risk factor for both obesity and NAFLD [31, 32].
1.1.1.1.2 Genetic Predisposition
A lot of data support a primary role of genetic factors shared between obesity and NAFLD (Fig. 1.2). Several genes have been reported to favor whole-body fat accumulation, although they do not completely account for obesity but should always be considered as cofactors interacting with unhealthy lifestyles [33]. Adiponutrin (PNPLA3) is an adipocyte protein with both lipolytic and lipogenic properties, regulated by insulin [34]. Gene polymorphism of the wild-type allele has been consistently associated with obesity, and in 2008, Romeo et al., in a genome-wide analysis of a large population of differing ethnic origin, identified a PNPLA3 allele strongly associated with both increased hepatic fat levels and hepatic inflammation. Notably, subjects homozygotes for the genetic variant had a much higher hepatic fat content and susceptibility to NAFLD [35]. The variant promotes hepatic injury, independently of insulin resistance and BMI [36], is also associated with higher risk of disease progression to advanced fibrosis and cirrhosis [37, 38], and confers a higher susceptibility to hepatocellular carcinoma [39].
Kozlitina et al. identified another variant in TM6SF2 rs58542926, a gene on chromosome 19, also associated with hepatic lipid accumulation [40], and also this variant was shown to increase the risk of disease progression [41, 42]. These data led the European Association for the Study of the Liver (EASL), together with the sister Associations of Diabetes and Obesity, to discuss the opportunity to include these two variants in a comprehensive assessment of the risk for disease progression in their joint NAFLD clinical practice guidelines [43]. Other variants may also modulate the risk (MBOAT7, GCKR, and MERTK), and one variant (a protein-truncating HSD17B13 variant) appears to be associated with a reduced NAFLD risk [44], opening a new frontier to disease prevention, via identification of subjects at higher risk.
According to Barker’s hypothesis of fetal and infant origin of adult disease [45], epigenetic should also be considered. Epigenetic modifications are stable changes in the expression of DNA promoted by environmental risk factors in parents or in the intrauterine environment. The risk of NAFLD is not only associated with high BMI at birth [46] but also with low birth weight for gestational age [47]. Whether this reflects a more rapid catch-up following intrauterine retardation or a profound alteration in metabolic processes remains to be determined. The close relation with insulin resistance supports epigenetic regulation as the main driver [48]. In a comprehensive analysis of genetic predisposition, present and childhood demographic, metabolic and lifestyle variables, also including birth weight, the importance of in utero epigenetic modifications was extensively demonstrated [46].
As long as genetics remains a non-modifiable risk factor, lifestyle modifications, including diet and physical activity, targeting visceral adiposity remain the standard of care for patients with NAFLD and MetSyn. The health-care systems and hepatology communities need to implement measures aimed at reducing their causes; in the area of NAFLD, child and adult obesity are a priority to reduce the burden of liver disease [49].
1.1.1.2 Do Obesity and NAFLD Coexist by Simple Association or Is There a Cause/Effect Relationship and, in this Case, Which Comes First?
Both childhood NAFLD and adult NAFLD are definitely more common in children and adults with obesity, respectively; long-term obesity might thus favor NAFLD progression to more severe stages, including liver failure and hepatocellular carcinoma, but the initial sequence of events remains difficult to determine. In a seminal study, Suzuki et al. tested the temporal occurrence of the various features of MetSyn in a cohort of subjects undergoing repeated screening in a Japanese workplace, free of any insulin resistance-related conditions, where elevated aminotransferase was assumed as surrogate biomarkers for NAFLD [50]. According to their analysis, weight gain and hypertriglyceridemia preceded NAFLD, whereas hypertension and altered glucose metabolism occurred later. Notably, weight gain and weight loss were consistently associated with altered and normal liver enzymes, respectively. These data were confirmed in a different cohort where incident fatty liver at ultrasounds was associated with the risk of incident hypertension [51] as well as incident diabetes [52].
Also epidemiological data and modeling studies support these findings. In Italy, the prevalence of NAFLD increases systematically along with obesity rates, with a time lag of approximately 5 years [8], and it is a significant risk factor for the future development of type 2 diabetes [53]. These data have been reproduced in different countries and different ethnic groups, and suggest that the future burden of NASH-cirrhosis might be extremely challenging for health-care systems [54].
A few long-term cohort studies are also available to support the role of weight gain on NAFLD and its long-term consequences. In apparently healthy individuals with no history of alcohol abuse, weight gain, and weight loss were associated with NAFLD incidence and remission, respectively, in a 7-year follow-up [55], and in a large cohort of normal-weight Korean individuals also a modest 2-kg weight gain was associated with the development of ultrasonographically detected fatty liver in a 5-year follow-up [56]. In a cohort of 44,248 Swedish men (18–20 years) enrolled into military service in their teens between 1969 and 1970, the risk of severe liver disease (i.e., diagnosis of decompensated liver disease, cirrhosis, or liver-related death) was associated with BMI and overweight in a follow-up of nearly 40 years [57]. The longer is the obesity status, the higher is the risk of NAFLD and its long-term consequences. Suomela et al. identified enlarged waist circumference, high body mass index (BMI), and sedentary lifestyles among the main drivers of future NAFLD, measured by ultrasonography in middle-aged adults [46]. In a Danish study of 285,884 schoolboys and girls, followed for over 30 years, the risk of primary liver cancer was increased by 20–30% in the presence of overweight/obesity at ages 7–13 [58].
By contrast, weight loss induced by lifestyle changes is significantly associated with improved liver function in cirrhosis. In the presence of obesity, an intensive program coupling hypocaloric diet with supervised physical activity significantly reduced measures of portal hypertension by an extent dependent on weight loss in subjects with cirrhosis (24% with NAFLD) [59].
1.1.1.3 Have Obesity and NAFLD a Similar Clinical Outcome and Similar Treatment?
The burden of obesity per se on cardiovascular risk, chronic kidney disease (CKD), and cancer is well-known, and significantly impacts on life expectancy. Cardiovascular disease at age 60 reduces life expectancy by 6–10 years, but when coupled with metabolic diseases (cardiometabolic multimorbidity) life expectancy is reduced by 15 years [60]. The Emerging Risk Factors Collaboration group recently reported the effects of cardiometabolic multimorbidity, defined by the simultaneous coexistence of more than one conditions among type 2 diabetes, coronary heart disease and stroke, in adults who were overweight and obese compared with subjects with healthy weight. In over 120,000 adults, stratified according to BMI and without risk factors at baseline, and a mean follow-up of 10.7 years, the risk of developing cardiometabolic multimorbidity doubled in overweight individuals (odds ratio [OR] 2.0, 95% CI 1.7–2.4), and further increased to 4.5 (3.5–5.8) in type 1 obesity, and to 14.5 (10.1–21.0) in subjects with obesity class II–III. The association was maintained irrespective of gender, socio-economic status, age, and lifestyles [61]. This study highlights the importance of obesity, when coupled with other metabolic diseases, i.e., of MetSyn in deadly outcomes.
The association of obesity with CKD is also well demonstrated. Hsu et al. identified obesity as a risk factor for end-stage kidney disease in 2006 [62] and again metabolic multimorbidity significantly increases the risk. In subjects with and without MetSyn, both overweight and obesity more than double the risk of CKD [63], but CKD is also a correlate of cardiovascular morbidity, further increasing the burden of disease [64].
The most intriguing association of obesity is the risk of cancer, largely ignored by patients and scarcely perceived by health professionals. The most impressive data came from the seminal study of Calle et al. [65], in a prospective study of more than 900,000 adults, free of cancer at enrollment. During a follow-up of 16 years, the risk of death from cancer (any site) was increased by more than 50% in individuals with obesity, with particular risks for specific sites (including the liver). These data have been repeatedly confirmed in different settings and different ethnic groups [66]; notably, long-term weight loss induced by bariatric surgery not only increases life expectancy, but initial data are accumulating on its role in reducing the risk of incident cancer [67]. Of note, the cancer risk associated with obesity might be directly driven by liver fat [68], with NAFLD as the main predictor of future extrahepatic cancer also in obese individuals [69].
How much do the same factors dictate the outcome of NAFLD? Although NAFLD may progress to NASH-cirrhosis and end-stage liver disease remains a dreadful outcome, the majority of cases have a cardiovascular outcome. In a long-term follow-up study of a NAFLD cohort, Ekstedt et al. found an increased risk of cardiovascular death [70], although fatty liver was unable to predict cardiovascular death in subjects with established coronary artery disease [71]. Similarly, NAFLD patients are at higher risk of hepatocellular cancer [72], also in the absence of cirrhosis. In an ultrasonography-defined NAFLD cohort followed by regular check-ups for over 7 years, the cancer incidence rate was significantly increased (hazard ratio [HR] 1.32; 95% confidence interval [CI] 1.17–1.49) [73]. After adjustment for demographic and metabolic factors, three cancers were significantly associated with NAFLD: hepatocellular carcinoma (HR 16.7; 95% CI 2.1–133.8), colorectal cancer in males and breast cancer in females, i.e., cancers significantly associated with obesity, independently of fatty liver.
Weight loss is the standard treatment of both obesity and NAFLD. These issues will be dealt with in another chapter; suffice here to say that weight loss, both achieved by lifestyle changes or by bariatric surgery simultaneously decreases the burden of both obesity and NAFLD and NAFLD progression to more advanced stages of the disease.
1.1.1.4 Does the Existence of “Lean NAFLD” Challenge the Pivotal Role of Adipose Tissue Accumulation?
Since the original identification of NAFLD as a specific condition associated with MetSyn, it became apparent that a variable proportion of cases was not associated with obesity. These cases, identified as “lean NAFLD,” account for 10–15% of total NAFLD individuals in different cohorts, depending on age, gender, and particularly on the clinical setting [9]. In most cases they are by no means lean, but have a limited amount of body fat, fulfill the criteria for normal weight or overweight, but frequently have an excess of visceral fat (visceral obesity) [6]. They might represent a variant of the so-called “metabolically obese, normal weight” phenotype [74], described in at least 5% of the population in Western countries. This subgroup, lying on the opposite end of “metabolically healthy obese” population along a spectrum dictated by genes, diet, physical activity, and inflammation [74], comprises individuals who are non-obese, frequently sedentary, and who have impaired insulin sensitivity, increased cardiovascular risk and increased liver lipid levels as the consequence of a decreased capacity of fat-storing cells [75]. When compared with individuals with overweight or obese NAFLD, these subjects are usually younger, are nonetheless insulin resistant, and have higher plasma triglyceride levels, possible expression of a more severe alteration in lipid metabolism [76], but variable and sometimes more severe degree of necroinflammation and fibrosis [77]. In their most advanced stages, they are frequently identified as “cryptogenic cirrhosis”, which has produced some debate in the interpretation of diagnostic tests and on the identification of NASH-cirrhosis and cryptogenic cirrhosis as different entities [78, 79].
The histologic and clinical outcome of lean NAFLD has attracted a lot of attention. The largest series of “lean NAFLD” comes from studies carried out in Eastern countries, which are at higher risk of insulin resistance for minimal visceral adiposity, as demonstrated by the specific cutoffs of waist circumference for MetSyn dictated by International agencies [4]. In a systematic review with meta-analysis, Sookoian et al. compared the histological outcomes of lean NAFLD series (n = 493 individuals) with overweight/obese NAFLD individuals [80]. Contrary to initial findings, the authors concluded that lean NAFLD is characterized by less severe histological features as compared to overweight/obese NAFLD [80]. Also disease progression has never been clearly defined. In the study of Fracanzani et al., the risk of cardiovascular-related events was not systematically different between lean and overweight/obese NAFLD during a follow-up of 49 months, but the numbers of deaths and events were too small to derive solid conclusions [77]. On the contrary, in a large multicenter analysis published only in abstract form, the death rate of lean NAFLD was reported to be higher compared to the event rate in non-lean individuals [81]. This occurred despite lean NAFLD being characterized by less severe disease, a low number of comorbidities and lower levels of liver enzymes.
In conclusion, lean NAFLD remains a scarcely defined condition, which partly blurs the relation between obesity and NAFLD. However, it might indeed represent the end of a large spectrum where different genetic and lifestyle factors interact to determine liver disease incidence and progression.
1.2 Conclusions
The accumulation of fat droplets in the hepatic parenchyma is driven by factors synergistically acting to increase triglyceride flow to the liver (diet and metabolic factors, endotoxemia from gut microbiota, genetic factors). They are shared between obesity and NAFLD, as are the levels of adipokynes, both leptin and adiponectin, that are putative mediators of lipotoxicity [2].
A large body of evidence supports the concept that NAFLD rarely dissociates from obesity, and in these cases visceral fat accumulation is nonetheless present, also accounting for NAFLD progression to fibrosis and cirrhosis, as well as to T2DM and other metabolic abnormalities. The best evidence comes from intervention studies, showing that body weight loss, whatever the strategy used to reduce obesity (lifestyle changes, low-calorie diet, physical activity, bariatric surgery), remains the most effective way to reduce the incidence and prevalence of NAFLD in selected cohorts and in the general population, its progression to cirrhosis, and liver disease-related morbidity and progression also in the presence of cirrhosis [59]. An expert report, recently released from the European Association for the Study of the Liver focusing on the burden of liver disease in Europe (HEPAHEALTH project), concludes that tackling obesity is the only way to reduce the burden of NAFLD, by combining health policies with food interventions at population level [49]. We need to develop new strategies to counsel, motivate, educate toward healthier lifestyles the high number of individuals at risk of advanced liver disease all over the world [49]. Web-based programs are at the forefront [82], and should be exploited considering the difficulties faced by Liver units in preparing adequate educational programs. However, their effectiveness is limited if not integrated with face-to-face visits and contacts with specialists trained in motivational interviewing, considering the scarce motivation from patients’ side [83]. Interventions aimed at curbing the NAFLD epidemics are urgently needed not only to reduce the burden on National Health Systems but also to decrease the environmental impact and the costs of the Western dietary model [84]. The Mediterranean diet is qualifying as a dietary pattern able to reduce the risk of obesity, NAFLD, and associated cardiovascular risk, also favoring a sustainable healthy eating behavior [85].
References
Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–36.
Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–69.
Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–62.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5.
Maison P, Byrne CD, Hales CN, Day NE, Wareham NJ. Do different dimensions of the metabolic syndrome change together over time? Evidence supporting obesity as the central feature. Diabetes Care. 2001;24:1758–63.
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20.
Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23.
Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47:181–90.
Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore). 2012;91:319–27.
Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–5.
Rosso C, Mezzabotta L, Gaggini M, Salomone F, Gambino R, Marengo A, et al. Peripheral insulin resistance predicts liver damage in nondiabetic subjects with nonalcoholic fatty liver disease. Hepatology. 2016;63:107–16.
Alizadeh S, Ahmadi M, Ghorbani Nejad B, Djazayeri A, Shab-Bidar S. Metabolic syndrome and its components are associated with increased chronic kidney disease risk: evidence from a meta-analysis on 11,109,003 participants from 66 studies. Int J Clin Pract. 2018;72:e13201.
Bellentani S, Tiribelli C. Is it time to change NAFLD and NASH nomenclature? Lancet Gastroenterol Hepatol. 2017;2:547–8.
Dufour JF. Time to abandon NASH? Hepatology. 2016;63:9–10.
Eslam M, Sanyal AJ, George J. Toward more accurate nomenclature for fatty liver diseases. Gastroenterology. 2019;157:590–3.
Eslam M, Sanyal AJ, George J, International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999–2014. e1991.
Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–54, e641–649; quiz e639–640.
Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14:72–81.
Mazzotti A, Caletti MT, Sasdelli AS, Brodosi L, Marchesini G. Pathophysiology of nonalcoholic fatty liver disease: lifestyle-gut-gene interaction. Dig Dis. 2016;34(Suppl 1):3–10.
Cortez-Pinto H, Jesus L, Barros H, Lopes C, Moura MC, Camilo ME. How different is the dietary pattern in non-alcoholic steatohepatitis patients? Clin Nutr. 2006;25:816–23.
Solga S, Alkhuraishe AR, Clark JM, Torbenson M, Greenwald A, Diehl AM, et al. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004;49:1578–83.
Green CJ, Hodson L. The influence of dietary fat on liver fat accumulation. Nutrients. 2014;6:5018–33.
Utzschneider KM, Bayer-Carter JL, Arbuckle MD, Tidwell JM, Richards TL, Craft S. Beneficial effect of a weight-stable, low-fat/low-saturated fat/low-glycaemic index diet to reduce liver fat in older subjects. Br J Nutr. 2013;109:1096–104.
Rosqvist F, Iggman D, Kullberg J, Cedernaes J, Johansson HE, Larsson A, et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63:2356–68.
Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–71.
Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68:1063–75.
Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Blendis L, Halpern Z, et al. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol. 2007;47:711–7.
Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95:283–9.
Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: evidence and plausible mechanisms. Liver Int. 2017;37:936–49.
Hannukainen JC, Nuutila P, Borra R, Kaprio J, Kujala UM, Janatuinen T, et al. Increased physical activity decreases hepatic free fatty acid uptake: a study in human monozygotic twins. J Physiol. 2007;578:347–58.
Ryu S, Chang Y, Jung HS, Yun KE, Kwon MJ, Choi Y, et al. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J Hepatol. 2015;63:1229–37.
Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31:661–6.
Valenti LVC, Baselli GA. Genetics of nonalcoholic fatty liver disease: a 2018 update. Curr Pharm Des. 2018;24:4566–73.
Johansson LE, Hoffstedt J, Parikh H, Carlsson E, Wabitsch M, Bondeson AG, et al. Variation in the adiponutrin gene influences its expression and associates with obesity. Diabetes. 2006;55:826–33.
Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5.
Romeo S, Sentinelli F, Dash S, Yeo GS, Savage DB, Leonetti F, et al. Morbid obesity exposes the association between PNPLA3 I148M (rs738409) and indices of hepatic injury in individuals of European descent. Int J Obes. 2010;34:190–4.
Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–94.
Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, Dongiovanni P, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209–17.
Liu YL, Patman GL, Leathart JB, Piguet AC, Burt AD, Dufour JF, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75–81.
Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjaerg-Hansen A, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–6.
Liu YL, Reeves HL, Burt AD, Tiniakos D, McPherson S, Leathart JB, et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309.
Sookoian S, Castano GO, Scian R, Mallardi P, Fernandez Gianotti T, Burgueno AL, et al. Genetic variation in transmembrane 6 superfamily member 2 and the risk of nonalcoholic fatty liver disease and histological disease severity. Hepatology. 2015;61:515–25.
European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402.
Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med. 2018;378:1096–106.
Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111.
Suomela E, Oikonen M, Pitkanen N, Ahola-Olli A, Virtanen J, Parkkola R, et al. Childhood predictors of adult fatty liver. The Cardiovascular Risk in Young Finns Study. J Hepatol. 2016;65:784–90.
Nobili V, Marcellini M, Marchesini G, Vanni E, Manco M, Villani A, et al. Intrauterine growth retardation, insulin resistance, and nonalcoholic fatty liver disease in children. Diabetes Care. 2007;30:2638–40.
Dudley KJ, Sloboda DM, Connor KL, Beltrand J, Vickers MH. Offspring of mothers fed a high fat diet display hepatic cell cycle inhibition and associated changes in gene expression and DNA methylation. PLoS One. 2011;6:e21662.
Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69:718.
Suzuki A, Angulo P, Lymp J, St Sauver J, Muto A, Okada T, et al. Chronological development of elevated aminotransferases in a nonalcoholic population. Hepatology. 2005;41:64–71.
Sung KC, Wild SH, Byrne CD. Development of new fatty liver, or resolution of existing fatty liver, over five years of follow-up, and risk of incident hypertension. J Hepatol. 2014;60:1040–5.
Sung KC, Wild SH, Byrne CD. Resolution of fatty liver and risk of incident diabetes. J Clin Endocrinol Metab. 2013;98:3637–43.
Targher G, Marchesini G, Byrne CD. Risk of type 2 diabetes in patients with non-alcoholic fatty liver disease: causal association or epiphenomenon? Diabetes Metab. 2016;42:142–56.
Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896–904.
Zelber-Sagi S, Lotan R, Shlomai A, Webb M, Harrari G, Buch A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol. 2012;56:1145–51.
Chang Y, Ryu S, Sung E, Woo HY, Cho SI, Yoo SH, et al. Weight gain within the normal weight range predicts ultrasonographically detected fatty liver in healthy Korean men. Gut. 2009;58:1419–25.
Hagstrom H, Stal P, Hultcrantz R, Hemmingsson T, Andreasson A. Overweight in late adolescence predicts development of severe liver disease later in life: a 39 years follow-up study. J Hepatol. 2016;65:363–8.
Berentzen TL, Gamborg M, Holst C, Sorensen TI, Baker JL. Body mass index in childhood and adult risk of primary liver cancer. J Hepatol. 2014;60:325–30.
Berzigotti A, Albillos A, Villanueva C, Genesca J, Ardevol A, Augustin S, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology. 2017;65:1293–305.
Emerging Risk Factors Collaboration, Di Angelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314:52–60.
Kivimaki M, Kuosma E, Ferrie JE, Luukkonen R, Nyberg ST, Alfredsson L, et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health. 2017;2:e277–85.
Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–8.
Cao X, Zhou J, Yuan H, Wu L, Chen Z. Chronic kidney disease among overweight and obesity with and without metabolic syndrome in an urban Chinese cohort. BMC Nephrol. 2015;16:85.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305.
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78.
Sjostrom L, Gummesson A, Sjostrom CD, Narbro K, Peltonen M, Wedel H, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–62.
Allen AM, Hicks SB, Mara KC, Larson JJ, Therneau TM. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity—A longitudinal cohort study. J Hepatol. 2019;71:1229–36.
Marchesini G, Petroni ML, Cortez-Pinto H. Adipose-tissue-associated cancer risk: is it the fat around the liver, or the fat inside the liver? J Hepatol. 2019;71:1073–5.
Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73.
Wong VW, Wong GL, Yip GW, Lo AO, Limquiaco J, Chu WC, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60:1721–7.
Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–40.
Kim GA, Lee HC, Choe J, Kim MJ, Lee MJ, Chang HS, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol. 2018;68:140–6.
Navarro E, Funtikova AN, Fito M, Schroder H. Can metabolically healthy obesity be explained by diet, genetics, and inflammation? Mol Nutr Food Res. 2015;59:75–93.
Gomez-Ambrosi J, Catalan V, Rodriguez A, Andrada P, Ramirez B, Ibanez P, et al. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care. 2014;37:2813–21.
Vos B, Moreno C, Nagy N, Fery F, Cnop M, Vereerstraeten P, et al. Lean non-alcoholic fatty liver disease (Lean-NAFLD): a major cause of cryptogenic liver disease. Acta Gastroenterol Belg. 2011;74:389–94.
Fracanzani AL, Valenti L, Bugianesi E, Vanni E, Grieco A, Miele L, et al. Risk of nonalcoholic steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease and low visceral adiposity. J Hepatol. 2011;54:1244–9.
Caldwell S, Marchesini G. Cryptogenic vs. NASH-cirrhosis: the rose exists well before its name. J Hepatol. 2018;68:391–2.
Thuluvath PJ, Kantsevoy S, Thuluvath AJ, Savva Y. Is cryptogenic cirrhosis different from NASH cirrhosis? J Hepatol. 2018;68:519–25.
Sookoian S, Pirola CJ. Systematic review with meta-analysis: the significance of histological disease severity in lean patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2018;47:16–25.
Dela Cruz AC, Bugianesi E, George J, Day CP, Liaquat H, Charatcharoenwitthaya P, et al. Characteristics and long-term prognosis of lean patients with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:S-909.
Mazzotti A, Caletti MT, Brodosi L, Di Domizio S, Forchielli ML, Petta S, et al. An internet-based approach for lifestyle changes in patients with NAFLD: two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69:1155–63.
Centis E, Moscatiello S, Bugianesi E, Bellentani S, Fracanzani AL, Calugi S, et al. Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J Hepatol. 2013;58:771–7.
Pieniak Z, Zakowska-Biemans S, Kostyra E, Raats M. Sustainable healthy eating behaviour of young adults: towards a novel methodological approach. BMC Public Health. 2016;16:577.
Germani A, Vitiello V, Giusti AM, Pinto A, Donini LM, del Balzo V. Environmental and economic sustainability of the Mediterranean diet. Int J Food Sci Nutr. 2014;65:1008–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Brodosi, L., Barbanti, F.A., Petroni, M.L., Marchignoli, F., Marchesini, G. (2020). Obesity and NAFLD: Same Problem?. In: Bugianesi, E. (eds) Non-Alcoholic Fatty Liver Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-95828-6_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-95828-6_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95827-9
Online ISBN: 978-3-319-95828-6
eBook Packages: MedicineMedicine (R0)