Abstract
In the current chapter, the various strategies for biofuel production focusing on rice straw have been discussed. The basic aim is to address the technical applications in enhancing biofuel production using lignocellulosic biomass. The overall price can be minimized using lignocellulosic biomass fractionation at biological platform, where lignin separation and also conversion of biomass into biofuel production is possible at a single platform. The inclusion of chemical pretreatment methods often produces toxic components thus inhibiting the cell to grow during further conversion, thus results in low productivity. In such case, microbial consortia may be a good option. Understanding of bioprocessing steps can lead to the development of sustainable technology for pilot-scale economical productions, to meet the current demand for biofuel.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In the current scenario, the principal requirements of human being are food feed and fuel. Regarding fossil fuel its going to deplete very soon in coming years. Biofuel is the only alternative to rapidly depleting fossil fuel, because of its sustainability, efficiency, and economics when used for blending thus helps in reducing overall cost. A variety of biofuels exist today of which bioethanol always being in great demand and thus high production is required.
According to a report of 2014, around 74% of biofuel ethanol was produced, while biodiesel is produced in second highest amount (Gupta and Verma 2015). In 2016, the growth rate of global biofuel production was 2.6%, while in current scenario biofuel demand is increasing at 6.5% per annum, while petroleum reserve is decreasing day by day. Of all global productions, India is producing only 1% of biofuel, while consumption rate is high up to 3.1%. Consequently, options are available where many biomasses can be converted into ethanol, biodiesel (Mofijur et al. 2015), and other gaseous or liquid biofuels. There are various advantages of using biofuel such as (1) high energy (2) CO2 mitigation (3) renewable (4) eco-friendly (5) and can be produced from nonedible biomass.
According to a report of European Union, the target of ethanol production has been set to 8 billion liters up to 2020. Japan has a target of 6 billion liters of ethanol up to 2030. India has a target of 4 billion gallon tons (Energetica India Report 2009). The current costs of biochemical cellulosic ethanol are estimated to be between US$4.03 and $5.60 per US gallon of annual capacity (Binod et al. 2010). The current cost of ethanol today is $1.22 per gallon while it may reduce further up to $70 per gallon (http://www.biofuelsdigest.com/bdigest/2017/05/18/ethanol-and-biodiesel-dropping-below-the-production-cost-of-fossil-fuels/).
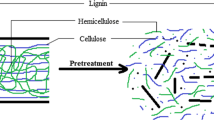
The utilization of lignocellulosic biomass includes delignification, hydrolysis, and saccharification before final conversion into ethanol. Complex bonding of lignocellulosic plant structure makes it a recalcitrant owing to the presence of lignin, hemicellulose, and other materials with cellulosic content. Table 1 shows cellulosic composition in various biomasses. Pretreatment is one of the mandatory requirements, in order to get rid of lignin from lignocellulosic biomass. Therefore, delignification is one of the costly and challenging procedures and need technical improvement for pilot-scale applications (Bhatt 2014; Phitsuwan et al. 2013).
In this context, biorefineries concept seems practicable, where every fraction of biomass is processed into value-added product. In the current chapter, we will discuss issues of bioprocessing steps in biofuel ethanol production with a special focus on rice straw which is the globally largest available biomass (Abedinifar et al. 2009).
-
Sustainable Biofuel Production: Indian Scenario
As per as the report of NUiCONE proceedings, India has now changed strategies to enhance its biofuel production beyond 1%. Therefore, around 85% increase in biofuel production has been observed since 2009. Also, the Government of India has now changed the blending mandate from 5 to 20% in 2017. Therefore, sustainable technology is required for the enhancing current production of 52.32–83.58 Mt (around >26%). In 2008, the US participation was around 51%, Brazil was 36%. EU’s contribution was only 4% to ethanol production, while in 2011, US contribution was around 46%, Brazil was 22%, while EU was 17% see Fig. 1.
The United States is the world’s largest producer of ethanol, and has produced nearly 15 billion gallons in 2015 alone. Together, the U.S. and Brazil produce 85% of the world’s ethanol. The vast majority of U.S. ethanol is produced from corn, while Brazil primarily uses sugar.
2 Biofuel Classifications
Based on the types of biomass, biomass can be classified as mentioned in Table 2 and various feedstock used in biofuel production has also been mentioned. Out of which corn and molasses are used mainly for biofuel ethanol production. Biodiesel production is another strategy to cope up with demand in the current scenario, which uses various agricultural and nonagricultural waste as a source. Ethanol production has been mentioned in billions of gallons of different biomasses.
First generation (1st G) biofuel includes sugar crops (sugar beet, sugarcane), edible crops (corn, sorghum), oilseed crops (soybean, canola), and animal fats. Second generation (2nd G) biofuel includes all types of cellulosic biomass, nonfood crops, and waste biomass, while third (3rd G) generation biofuel is based on the use of algae and municipal solid wastes (Singh et al. 2011).
There are various generations of biofuel such as first, second, third, and fourth generation biofuel. First generation biofuel includes all kind of edible feedstock which can be hydrolyzed by simple steam and further by enzymatic conversion into ethanol. Second generation biofuel is mostly based on the use of lignocellulosic biomass for biofuel production. These are less competitive to edible crops and are mostly available as agriculture, municipal, or industrial waste (Araújo et al. 2017). This biomass is rich in cellulose and hemicellulose which can be converted into ethanol after several bioprocessing steps. Recent research shows that production of bioethanol from these wastes is little expansive due to (1) feedstock cost, (2) feedstock harvesting, (3) feedstock densification, (4) feedstock pretreatment, (5) by-product separations (6) environmental and health impact. Biobutanol is another fuel which is obtained using the same feedstock but with different microbes (Araújo et al. 2017). Third-generation biofuel is obtained as bio-oil using algae as feedstock while fourth-generation biofuel is obtained as bio-solar fuel and electro-fuel as shown in Table 2.
Economically sustainable lignocellulosic-based ethanol production depends on
-
(1)
Suitable substrate
-
(2)
Suitable pretreatment applications without inhibitors productions
-
(3)
Suitable biocatalyst
-
(4)
Robust yeast cell bioethanol conversion (e) proper detoxification.
The order of cost of production is 3G > 2G > 1G. The ethanol production cost of molasses-based feedstock varies between 0.78 and 0.97 US$/L. However, production of ethanol at pilot scale is still in process of demonstration scale from the 3G feedstock. The cheapest ethanol production is in Brazil, where a combination of readily available resources and cheap labor makes prices of about $0.20 per liter possible.
3 Lignocellulosic Ethanol: Production Technology
There are various modes of ethanol production using rice straw such as SSF, SHF, sequential hydrolysis and fermentation process, SSMSF, SESF (Abedinifar et al. 2009; Karimi et al. 2006; Shinozaki and Kitamoto 2011; Ko et al. 2009). Each technique has its own merits and demerits but the basic idea is to utilize the pentose sugar present in hemicellulose. Thus, much novel work has been reported such as the use of lactic acid bacteria which has the capability to produce lactic acid during fermentation and helpful in the insolubilization of cellulose that is able to ferment glucose and (Kim et al. 2010). Thus, it was an integration of enzymatic hydrolysis and fermentation in one step called separate hydrolysis and fermentation. Ethanol production by Mucor indicus and Rhizopus oryzae from rice straw was successful in bypassing the end product inhibition (Abedinifar et al. 2009).
The bioconversion of cellulosic materials includes the formation of soluble sugars from cellulose in paper/agricultural residues and depends on the coordinated action of individual components such as β-exoglucanase, β-endoglucanase, β-glucosidase of cellulase enzyme.
3.1 Ethanol from Lignocellulosic Biomass
There are various biomasses used for lignocellulosic-based ethanol production such as rice and paddy straw because, water hyacinth corn stover. Predominantly, bio-ethanol is derived from stover and switchgrass in the U.S., while sugarcane bagasse is used mainly in Brazil and India, and rice husk and straw from China and India (Khoo 2015).
3.1.1 Corn Stover
Ethanol production from corn stover (Saha et al. 2013) was studied by many workers. Corn stover is rich in cellulose, i.e., 37% while having less lignin comparatively. Hydrothermal pretreatment combined with enzymatic saccharification leads to conversion of 72% glucose (Saha et al. 2013) while ethanol yield was 0.49 g/g biomass. Corncob was reported to produce more furfural as compared to corn stover, which is a toxic component released after pretreatment and should be an inhibitor for the growth of microbes involved in hydrolysis and saccharification. Therefore, corn stover is suitable for ethanol production (Kadam and McMillan 2003). Microbial pretreatment of corn stover with Ceriporiopsis subvermispora was attempted with enzymatic hydrolysis by some workers, which resulted in 66% production of glucose in 35 days (Wan and Li 2010). In another work, steam pretreatment was given in SSF mode which resulted in 70% ethanol with 10% insoluble solid biomass with yeast concentration 5 g/l. In SSF mode, alkaline pretreatment of corn stover coupled with fungal treatment with Phanerochaete chrysosporium or Gloeophyllum trabeum and fermentation with Saccharomyces cerevisiae or Escherichia coli K011 resulted in 3.09. g/100. g stover at day 4 (Vincent et al. 2014). In an attempt to resolve the problem of released toxic during pretreatments such as furfural and other inhibitory compounds, corn stover were hydrolyzed and hemicellulose fraction was treated with by roto-evaporation and lime neutralization resulted in removal of more than 50% furfural and acetic acid, which was volatile and resulted in enhanced ethanol production of 31.1 g/L and the corresponding ethanol yield on fermentable sugars of 0.406 g/g were obtained within 72 h in batch fermentation of the detoxified hydrolysate with immobilized cells (Zhao and Xia 2010). In another report, corn stover was pretreated with dilute H3PO4 (0.0–2.0%, v/v) or 1% acid combined with biological treatments with Escherichia coli strain FBR and condition were optimized by response surface methodology resulted in 85% glucose yield. In a similar effort to produce butanol using ABE technology, simultaneous saccharification, fermentation, and recovery (SSFR) were attempted which resulted in hydrolysis of 97% sugar from corn stover (Qureshi et al. 2014).
3.1.2 Rice Straw
Rice straw burning was recently in the news in Punjab at a large level, and as a proof images were released as shown in Fig 2. As an estimate, around 2.5 tons of rice straw has been burnt per acre of land due to the season of next crop within 20 days. In Punjab, around 11 MT (million tons) of rice is grown which produces around 21 MT of rice straw alone which resulted in the release of >70% CO2 7% CO, and 0.66% CH4 along with 2.09% N2O (Binod et al. 2010). Besides warning by the government and court, farmers are burning this useful biomass due to the lack of appropriate technology. One such attempt has been done to convert them into pellets which later can be used into fuel generations by using techniques of pyrolysis. The need is to develop farmer-friendly technology, which can be used in one step to yield glucose and thus ethanol.
Source RFA renewable fuel associations https://www.afdc.energy.gov/data/10331
4 Bioprocessing of Lignocellulosic Biomass
Removal of recalcitrant lignin requires various complex chemical or biochemical processing to get rid of cellulose and hemicellulose, which is the main carbohydrate to be used in fermentation and sachharification.
Different studies show that composition of biomass depends on various biotic and abiotic factors, ecosystems and time of harvesting. Variation of lignin content varies from 3 to 20%, cellulose content from 17 to 14%, which is the most suitable for ethanol production. After separation of cellulosic biomass, enzymatic hydrolysis and fermentation lead to the conversion of ethanol production. Owing to environmental issue combination of treatment is preferred to make biochemical process feasible in one step. A recent review of Sindhu et al (2016) shows that combined pretreatment is more efficient in delignification as compared to a single chemical pretreatment process (Sindhu et al. 2016). Mood et al. (2013) show that alkali pretreatment alone can be used with other pretreatment processes even with enzymatic pretreatment (Mood et al. 2013), which is ineffective and time consuming, therefore, Mishima et al (2008) show that enzymatic efficiency can be improved by using at least 20 chemical pretreatment methods (Mishima et al. 2008; Klein et al. 2016). According to Singh et al. (2015), lignocellulosic biomass pretreatment is challenging and need further research for making it cost-effective (Singh et al. 2015). As per our own research Bhatt (2014), alkali pretreatment leads to less solubilization of cellulose and hemicellulose than acid pretreatment (Bhatt 2014). To improve enzymatic treatment, other strategies have been worked out by some workers such as the use of ionic liquids, or use of microwave-assisted technology. In such a case, the main objective was to reduce the solubilization of cellulose and hemicellulose while maximizing the removal of Lignin. In this regard, the experiment of Klein et al. (2016) demonstrated that microwave-assisted chemical pretreatment is more effective for enzymatic-based lignin removal (De Bhowmick et al. 2017; Klein et al. 2016). The main aim is to save the environment by using fewer chemicals during pretreatment.
Therefore, fermentable sugar obtained after enzymatic hydrolysis is more effective for ethanol production. A similar work of Xia et al. (2013) shows that up to 94.6% sugar can be derived by using acid pretreatment (Kapoor et al. 2017; Xia et al. 2013).
Ethanol production from rice straw uses either solid-state fermentation (SSF) or simultaneous saccharification and fermentation (SSF) or by separate enzymatic hydrolysis and fermentation (SHF) (Singh et al. 2016). SHF may be a better option as compared to SSF mode (Akhtar et al. 2017). In some experiments, microwave pretreatment was combined with alkali pretreatment, and SSF mode was more useful as compared to SHF mode (Swain and Krishnan 2015).
4.1 Bioprocessing of Rice Straw
Rice straw is most abundantly grown in India and is suitable for ethanol production due to abundant cellulose and hemicellulose fractions present which can be hydrolyzed instantly for the production of ethanol but its bioprocessing is challenging due to the presence of ash and silica which interfere with microbial fermentation (Belal 2013).
Since rice straw can be promising and also a sustainable feedstock, therefore, extensive research has been done to resolve a technical issue related to rice straw biomass conversion a disadvantage linked with this biomass.
Bioprocessing of rice straw can be divided into four main parts (1) pretreatment which includes chemical, physical, and biological or combined, (2) mode of saccharification and fermentation for ethanol productions. Therefore, the choice of suitable pretreatment technology is a deciding factor where a large fraction of cellulose and hemicellulose can be available for hydrolysis and enzymatic saccharification. In one report, pretreatment of various lignocellulosic biomass including rice straw with Trichoderma reesei resulted in enhanced hydrolysis of rice straw comparatively (Singh et al. 2016) (Fig. 3).

Source https://mediawiki.middlebury.edu/wiki/OpenSourceLearning/Biofuels, http://biofuel.org.uk/types-of-biofuels.html
Bioprocessing of ethanol production from lignocellulosic biomass.
4.1.1 Pretreatment of Rice Straw
The main objective of pretreatment is to reduce crystallinity and degree of polymerization of rice straw so that potential carbon can be unlocked such as cellulose, hemicellulose, and other components such as lignin. In this regard steaming, milling, irradiation, and temperature along with pressure are applied which is useful in reducing crystallinity (Nguyen et al. 2010; Poornejad et al. 2013; Chang et al. 2016, 2017). The purpose of milling is to reduce the surface area of rice stalk and making available of rice husk biomass for biofuel production. Since powdered form of rice straw is fine and can be converted into animal feedstock but due to the presence of large amount of silica, RICE straw cannot even be converted into animal feed since presence of silica can tear the jaw and can give wounds in animals, therefore getting rid of silica is not an easy task.
The only drawback of rice straw is the presence of around 75% silica while its advantages are its availability in large amounts in the world. Presence of Silica makes bioprocessing of rice straw tough, thus, it is even composting is difficult. Presence of silica acts as a barrier in the protection of leaves and is present over leaves and also as a layer of plant part thus, its hydrolysis is also very difficult. Only a few reports are available around the world for technology which can make rice straw free of silica.
Some workers had attempted some new methodologies to get rid of this silica from rice husk (Araújo et al. 2017). Its composting is also very difficult owing to the presence of silica and lignin. Also, treatment with ionic liquids is helpful for complete lignin removal. Various ionic liquids have experimented till date (Sindhu et al. 2017). Some workers reported that in presence of SDS, ionic liquid is able to release lignin up to 46% by increasing the temperature up to 100 °C Lau et al. (2015) along with tetrabutyl-phosphonium hydroxide resulted in the removal of silica before lignin removal (Lau et al. 2015). One more recent work shows that silica can be removed up to 91% with combined pretreatment of organosolv and with sodium carbonate (Khaleghian et al. 2017).
4.1.2 Mechanical Pretreatment
Milling is beneficial in the reduction of particle size which is advantageous in increasing surface/volume ratio of straw which makes chemical or biological treatment accessible. Milling includes chopping, or grinding or pressing.
4.1.3 Ultrasonic Pretreatment
Ultrasound releases approximately 10–100 kJ/mol, which is sufficient for decreasing the crystallinity by destroying the microfibril structure of fiber cellulose up to 78.4–66.3%, and mean particle size reduced up to 0.4 mm after sonication (Bussemaker and Zhang 2013). Generally, 40 kHz is employed at an industrial scale of 40 kHz, about 0.025 W/mL, 25 °C, at 30 min, (Luo et al. 2014; Chuetor et al. 2015). With fungal treatment at 28 °C the net glucose obtained was around 38% with rice straw. Mostly acidic pretreatment or alkali pretreatment is done. Some workers reported that upon mixed treatment of 0.5 M NaOH and with 60% ethanol around 100% removal of lignin was obtained when treated on wheat straw (Sun et al. 2016). Irradiation is helpful in breaking the strong bond which is almost impossible to break by any other mode but certainly, they are helpful with other methods such as chemical or heating.
Strong cavitational effect of ultrasound mixed with suitable solvent is helpful in reducing the load of lignin complexed with cellulose or hemicellulose. Many reports show the combined effect of ultrasound and alkaline pretreatment or hydrothermal treatment is very effective in other lignocellulosic biomasses such as bagasse and rice straw (Wu et al. 2017). Since the technology is costly so, not recommended for industrial applications.
4.1.4 Microwave Treatment
Some reports show the use of microwave along with alkali pretreatment and shows to increase enzymatic digestibility of rice straw (Singh et al. 2014) and microwave pretreatment with organic solvent (acetic acid and propionic acid) further reported to increase enzymatic digestibility of rice straw as a result glucose yield was obtained up to 80% (Gong et al. 2010).
4.1.5 Chemical Pretreatment
Rice straw and rice stalk have been pretreated with many methods such as aqueous ammonia (Araújo et al. 2017), dilute acid (Lee et al. 2015), sodium carbonate and fungus Mucor hiemalis, (Khaleghian et al. 2015), alkaline pulping and steam explosion pretreatment (Ibrahim et al. 2011), calcium capturing by carbonation (Park et al. 2010), microwave alkali pretreatment (Singh et al. 2011), dilute sulfuric acid and sulpho-methylation (Zhu et al. 2015), using a cocktail of hydrolytic and oxidizing enzyme (Dhiman et al. 2015), organic acid treatment (Amnuaycheewa et al. 2016), biological pretreatment (Bak et al. 2009; Salvachúa et al. 2011; Okamoto et al. 2011; Arora et al. 2016; Karimi et al. 2006; Bak et al. 2010; Das et al. 2013).
Though many pretreatment technologies are available as discussed previously but every technology has its own advantages and disadvantages, for example, acid treatment has the benefit that it can help in the dissolution of lignin from cellulose before hydrolysis (Mishima et al. 2008; Khaleghian et al. 2017). Though it needs a large quantity of acids which is not environmentally friendly but dilute acid has advantages that it can protect the conversion of hemicellulose into xylan and other inhibitory compounds such as furfural which acts as an inhibitor for the action of cellulase and other microbial enzymes. Acid pretreatment sometimes require high temperature up to 180 °C and use of organic acid is helpful in increasing cellulose depolymerization, for example, pretreatment of the rice straw with 75% (v/v) aqueous ethanol and 1% w/w H2SO4 at 150 °C for 60 min resulted in the production of total sugar concentration up to 31. g/L (Amiri et al. 2014). Many workers have got good results with the use of concentrated phosphoric acid during pretreatments of rice straw (Moradi et al. 2013) and reported that now enzymes are more accessible and thus enhance enzymatic loading reported (Amiri et al. 2014). Further, lowering of acid concentration of acid H2SO4 (0.25%v/v), HCl, H3PO4, and oxalic acid and NaOH (0.25w/v) helps in more release of glucose yield up to 84–91% with very low concentration of furan from rice straw. Pretreated rice straw with acid hydrolysate technology uses the term PRSAH, where rice straw is treated with 1% acid and 1% alkali is beneficial in increasing cellulose content from 38 to 50% during enzymatic treatment with glucose yield 0.58 g/g from PRASH (Chen et al. 2014).
Though dilute acid pretreatment is useful in the rapid hydrolysis of hemicellulose and also in the release of cellulose providing a path for good enzyme accessibility, but they also reported to have many disadvantages such as formation of various inhibitors (Wi et al. 2013; Balan 2014; De Bhowmick et al. 2017). Acid treatment converts glucose into HMF, while xylose into furfural, along with acetic acid and formic acid; or may convert lignin into derived phenolics, oligomers, and re-polymerized furans or pseudo-lignin (Jönsson and Martín 2016). Some authors agree that before acid treatment there should be alkali pretreatment, which helps in good release of hemicellulose.
4.1.6 Alkaline Pretreatment
Use of alkali pretreatment is for breaking lignin from recalcitrant cellulose and hemicellulose. Alkali is useful in breaking the ester bond present in lignin and hemicellulose. A little supply of high temperature is helpful in breaking the ether bond. Thus, the overall effort is very high solubilization of hemicellulose and lignin. Aqueous ammonia and alkaline peroxides are helpful in overall increase impact in promoting solubilization (Cabrera et al. 2014). Alkaline peroxide pretreatment is mostly helpful in reducing the temperature requirement for enhanced saccharification by enzymatic hydrolysis at 30 °C with reducing sugar up to 92% using rice hull. A comparison of inhibitors released during acid or alkali pretreatment shows that most of the inhibitors such as formic and acetic acids and phenolic compounds, while 5-hydroxymethylfurfural (HMF) and furfural are released during acid pretreatment and while during alkali pretreatment inhibitors were not released (Bolado-Rodríguez et al. 2016). An increase of temperature, reduction in time of incubation of enzyme was observed for 60% lignin removal along with an increase in crystalline index from 40 to 52, and in SSF mode there was 98% of conversion yield of ethanol using rice straw (Phitsuwan et al. 2017). In another work, the use of sodium carbonate at mild conditions followed by fermentation using Zygomycetes fungus Mucor hiemalis (Khaleghian et al. 2015) results in 90% removal of silica from rice straw while 65% increased enzymatic hydrolysis in SSF mode at 100 °C.
A novel pretreatment study was conducted by Silva et al. (2013), where the objective was to detoxify the inhibitors produced during acid pretreatment by the use of Ozonation in alkaline medium (pH8) in the presence of H2O2 and ethanol production was done by using Pichia stipius yeast (Silva et al. 2013).
4.1.7 Biological Pretreatment of Rice Straw
The biological/enzymatic application has less been in use for ethanol production at industrial scale. There are many challenges which need attention. Pretreatment is done usually to separate unwanted lignin complex with cellulose and hemicellulose and another problem with rice straw is the presence of silica (15%). Silica separation has not been attempted using any of biological pretreatment methods. Silica and lignin reduce the overall activity of microbes. Microbial growth ceases due to release of inhibitors by lignin, cellulose, or hemicellulose degradation at high acidic pretreatment and high temperature. Laccase is one of the microbes which is known to degrade and solubilize lignin from lignocellulosic biomass. Method for separation of silica was suggested by Ludueña et al. (2011) in which overnight soaking with KOH was suggested in 1:12 ratio (Ludueña et al. 2011).
Laccase is copper-containing oxidative enzyme, which is produced by fungi from class Basidiomycetes, ascomycetes, and deuteromycetes in solid-state fermentation mode.
Under optimal conditions of environmental factor, microbes start producing laccase, lignin peroxidase, and phenoloxidase. Some wood-rotting fungi called as white rot fungus and some mushrooms such as Pleurotus are also known to produce such enzymes. Wheat and rice straw have been used in the past to produce enzymes such as laccase and cellulase (Lee et al. 2012; Nakanishi et al. 2012; Jin and Ning 2013; Parenti et al. 2013; Rastogi et al. 2016; Postemsky et al. 2017).
At pilot scale, there are only a few reports for biological pretreatment-based ethanol production. There are many reports of application of biological pretreatments (Bak et al. 2010; Toquero and Bolado 2014; Mustafa et al. 2017). Mostly fungus is used for combined pretreatment since treatment alone is not useful (Bak et al. 2009). Several Basidiomycetes species such as Ceriporiopsis subvermispora, Phanerochaete chrysosporium, Pleurotus ostreatus, Phlebia subserialis, and Pichia guilliermondii can grow on different lignocellulosic biomass have been evaluated for their delignification efficiencies (Kumar et al. 2009). Now, it has been realized that use of microbial consortia (mix of bacteria and fungus) is more helpful in value-added product formation after pretreatment (Shen et al. 2018; Toquero and Bolado 2014). Generally, biological pretreatment with laccase secretion leads to lignin dissolution but also loss of cellulose leads to overall decrease in ethanol production. To cope up with this problem, use of NaCl is the suggested and the addition of which can control the growth of cells, this technique is called as inhibitor-mediated-intensified biological pretreatment technology IMBP (Kumar et al. 2017). Kogo et al. (2017) have used Trichoderma reesei and Humicola insolens for simultaneous enzyme production and hydrolysis (Kogo et al. 2017). Trichoderma and Humicola are best to known produce cellulase enzyme, which shows an increased effect in alkaline pretreatment.
4.1.8 Microbes for Pentose Utilizations
Pentose utilization is a major issue in the conversion of lignocellulosic biomass into ethanol. In the past, many microbes were genetically modified to utilize pentose released from rice straw hydrolysate, for example, genetically engineered strain Corynebacteriu glutamicum wild type was modified
4.1.9 Application of Microbial Consortia
Microbial consortia are important in the current scenario for biological pretreatment since a group of microbe is more effective as compared to the single microbe. The synergistic action of microbes results in improving enzyme activity thus rapid action is expected. This can solve the most problematic part of lignocellulosic digestion, which is lignin degradation and its degradation takes a number of days. Microbial degradation of lignin using microbial consortia has been described in detail by many researchers around the world but still less adopted by the industries due to various challenges (Ding et al. 2016; Jia et al. 2016; de Lima Brossi et al. 2016). Laccase (EC 1.10.3.2) is known as lignin degrader enzyme (multicopper blue oxidase) that couples the four electron reduction of oxygen with the oxidation of a broad range of organic substrates, including phenols, polyphenols, anilines, and even certain inorganic compounds by a one-electron transfer mechanism (Margot et al. 2013).
Consortia are interactive groupings of microorganisms ranging from defined species communities to undefined, multispecies aggregations. Further, several aspects of applied microbial consortia have been reviewed. Some workers have discussed the advantages of using consortia and the difficulty in achieving selective biofuel production. Microbial consortia have been demonstrated because of their enhanced characteristics over monoculture approaches in the conversion of cellulose and other sugar mixtures to alcohol (Xing et al. 2012). Wan and Li (2010) worked on microbial delignification of corn stover by Ceriporiopsis subvermispora for improving cellulose digestibility. MnP and laccase were detected during the degradation of corn stover by C. subvermispora. For major hydrolytic enzymes, xylanase was the only enzyme detected which resulted in 39% lignin degradation. Overall glucose yield was about 72% after the enzymatic hydrolysis in 18 days (Wan and Li 2010).
Zuroff and Curtis (2012) reviewed on developing symbiotic consortia for lignocellulosic biofuel production. The author concluded that the designing consortia with an understanding of cooperative microbial energetics could allow the development of efficient biofuel production processes using existing natural or genetically modified/selected organisms and could pave the way for future bioprospecting and genetic engineering. Engineering microbial consortia to produce biofuel involves short-circuiting the catabolic cascade to accumulate the biofuel of interest. The energetic, metabolic, and physiological conditions that allow this to occur are the key process in design considerations for a biofuel production platform. Natural and engineered interactions appear to be promising methods for community control and regulation. In the meantime, ample lignin degrading, cellulolytic, and fuel-producing organisms have been characterized and are available to explore the potential of symbiotic relationships for biofuel production. Decreased rates and the relatively low value of biofuels suggest the need for a new paradigm of low-cost bioprocessing technology. Organism, consortia, and bioprocess design must advance hand-in-hand with technical and economic feasibility in order to make lignocellulosic biofuels a reality (Zuroff and Curtis 2012).
5 Bioreactor and Optimizations Conditions
Bioreactor design at pilot-scale production of ethanol using a variety of cellulosic biomass operates in various modes such as batch (Gusakov et al. 1985), airlift (Zheng et al. 2005), packed bed reactor (Canabarro et al. 2017), batch tube reactor for biomass hydrolysis (Reactor and Engineering 2001), rotating fibrous bed reactor (Lan et al. 2013) for ethanol production. There is a continuous research for improvement process parameters in order to improve the cost of production and accordingly conditions are adjusted. As depicted in Fig. 4, there are four main types of processing commonly operating for industrial ethanol production. (1) Separate Hydrolysis and Fermentation (SHF); (2) Simultaneous Saccharification and Fermentation (SSF); (3) Simultaneous Saccharification and Co-fermentation (SSCF); (4) Consolidated Bioprocessing (CBP).
Source Modified from (Devarapalli and Atiyeh 2015) abbreviation used SHF = Separate Hydrolysis and Fermentation; SSF = Simultaneous Sachharification and Fermentation; SSCF = Simultaneous Sachharification and Co-fermentation; CBP = Consolidated Bioprocessing
5.1 Separate Hydrolysis and Fermentation (SHF)
A very good review has been presented by Nguyen et al. (2017) in which pros and cons of SHF has been discussed. SHF process includes enzyme production, hydrolysis, hexose and pentose utilization separately, which has benefits that it can reduce the end product inhibitions but on the other hand reduction in the yield of ethanol has been reported. It may be due to low enzyme loading, culture in stress condition that perform lower production. It also has been reported that in SSF mode carbon starvation takes place before glucose consumptions. Temperature beyond 37 °C lowers ethanol production while at 37 °C ethanol production was around 80 g/l. Also, because of high temperature, low cell viability was observed.
There is a separate reactor, thus hexose and pentose utilization occurs separately and proceeds at optimum conditions. Many factors related to the production of enzymes and fermentation can be set to optimum in separate hydrolysis and fermentation. The main limitation is the inhibition of enzyme production because of glucose and cellobiose, therefore during hydrolysis overall efficiency is reduced.
5.2 Solid-State Fermentation (SSF)
Solid-state fermentation is another condition for production of ethanol from cellulose, where first, enzyme production is done and then enzyme is mixed with cellulosic biomass for hydrolysis. SSF condition requires mixing of microbes to enhance the biomass fermentation simultaneously in order to relieve the product inhibition by the sugar produced during fermentation (Swain and Krishnan 2015; Bak et al. 2010). The main challenges encountered during production is providing an optimal condition for microbes to produce enzymes cellulase.
Therefore, another strategy was adapted as SSCF (see Fig. 4), where both hexose and pentose have been co-fermented by using mostly genetically modified S. cerevisiae and Zymomonas mobilis. While in CBP, both hydrolysis and fermentation are performed in a single step by using single microbes thus the high efficiency of ethanol production has been achieved.
Ethanol from rice straw hydrolysate using Pichia stipitis can be increased twice and also results in reduced inhibitors production (furfural and 5-hydroxymethyl furfural) by using ammonia. In addition xylose fermentation could result in more ethanol production (Lin et al. 2012). Bak et al. (2010) demonstrated that fungal pretreatment of rice straw (Phanerochaete chrysosporium) along with manganese peroxidase could result in 62.7% ethanol yield for 96 h using rice straw in SSF mode (Bak et al. 2010). Swain and Krishnan (2015) demonstrated that xylitol from rice straw improved much after aqueous ammonia pretreatment, where sequential fermentation technique was applied to improve lignin digestibility in repeated batch fermentation using Candida tropicalis. Thus adapting two-stage batch fermentation could result in 98% ethanol production (Swain and Krishnan 2015). Zahed et al. (2016) applied mixed mode of treatment such as batch and continuous co-fermentation. Hence, the focus was on to hydrolyze substrate rice straw into maximum sugar 81% and to reduce furfural by 505 xylitol yield was 68% in continuous mode dilution rate was 0.03 l per hours (Zahed et al. 2016).
6 Future Scope
In summary, we can conclude that the future of biofuel lies in the economical production of ethanol using rice straw using microbial consortia-based technology and adapting suitable bioreactor condition platform so that maximum utilization of biomass can be done in an efficient way. As per the report, EU has set a target of 10% bioethanol production but also has to reduce CO2 emission by 6% against the current emission of more than 30% (Union 2009). Therefore, for sustainable production of ethanol using largest available biomass on earth, rice straw requires adapting continuous bioreactor condition along with batch fermentation which may be helpful in minimizing inhibitor formation after acid pretreatment under co-fermentation conditions (Zahed et al. 2016). As per data available, bioprocessing of 1 ton of rice straw yields around 239–253 L ethanol with 292 kg CO2 eq/ton straw (Soam et al. 2016).
The author reported that 1% increase in enzyme during hydrolysis increases ethanol yield to 2.9%. The author also reported that reducing chemical pretreatment is essential since it accounts for release of 30% CO2 while biological pretreatment also account for the release of CO2 emissions which can be reduced by recycling of enzymes at various stages of hydrolysis of biomass. Against dilute acid treatment, steam explosion technique is a more viable option for large-scale biomass pretreatment. Another benefit of evaluation of techno-economical aspect is reducing rice straw biomass burning, improving socioeconomical aspects of farmers and thus helpful in setting favorable policy as per Indian scenario concerns.
References
Abedinifar S, Karimi K, Khanahmadi M, Taherzadeh MJ (2009) Ethanol production by Mucor indicus and Rhizopus oryzae from rice straw by separate hydrolysis and fermentation. Biomass Bioenerg 33(5):828–833. https://doi.org/10.1016/j.biombioe.2009.01.003
Akhtar N, Goyal D, Goyal A (2017) Characterization of microwave-alkali-acid pre-treated rice straw for optimization of ethanol production via simultaneous saccharification and fermentation (SSF). Energy Convers Manag 141:133–144. https://doi.org/10.1016/j.enconman.2016.06.081
Amiri H, Karimi K, Zilouei H (2014) Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Biores Technol 152:450–456. https://doi.org/10.1016/j.biortech.2013.11.038
Amnuaycheewa P, Hengaroonprasan R, Rattanaporn K, Kirdponpattara S, Cheenkachorn K, Sriariyanun M (2016) Enhancing enzymatic hydrolysis and biogas production from rice straw by pretreatment with organic acids. Ind Crops Prod 87:247–254. https://doi.org/10.1016/j.indcrop.2016.04.069
Araújo K, Mahajan D, Kerr R, da Silva M (2017) Global biofuels at the crossroads: an overview of technical, policy, and investment complexities in the sustainability of biofuel development. Agriculture 7(4):32
Arora A, Priya S, Sharma P, Sharma S, Nain L (2016) Evaluating biological pretreatment as a feasible methodology for ethanol production from paddy straw. Biocatal Agric Biotechnol 8:66–72. https://doi.org/10.1016/j.bcab.2016.08.006
Bak JS, Ko JK, Choi IG, Park YC, Seo JH, Kim KH (2009) Fungal pretreatment of lignocellulose by Phanerochaete chrysosporium to produce ethanol from rice straw. Biotechnol Bioeng 104:471–482. https://doi.org/10.1002/bit.22423
Bak JS, Kim MD, Choi IG, Kim KH (2010) Biological Pretreatment of Rice Straw by Fermenting with Dichomitus squalens. New Biotechnol 27:424–434. https://doi.org/10.1016/j.nbt.2010.02.021
Balan Venkatesh (2014) Current challenges in commercially producing biofuels from lignocellulosic biomass. Int Sch Res Not 2014(May):e463074. https://doi.org/10.1155/2014/463074
Belal EB (2013) Bioethanol production from rice straw residues. Braz J Microbiol [Publication of the Brazilian Society for Microbiology] 44(1):225–234. https://doi.org/10.1590/S1517-83822013000100033
Bhatt SM (2014) Lignocellulosic feedstock conversion, inhibitor detoxification and cellulosic hydrolysis—a review. Biofuels 5(6):633–649. https://doi.org/10.1080/17597269.2014.1003702
Binod P, Sindhu R, Singhania RR, Vikram S, Devi L, Nagalakshmi S, Kurien N, Sukumaran RK, Pandey A (2010) Bioethanol production from rice straw: an overview. Biores Technol 101:4767–4774. https://doi.org/10.1016/j.biortech.2009.10.079
Bolado-Rodríguez S, Toquero C, Martín-Juárez J, Travaini R, García-Encina PA (2016) Effect of thermal, acid, alkaline and alkaline-peroxide pretreatments on the biochemical methane potential and kinetics of the anaerobic digestion of wheat straw and sugarcane bagasse. Biores Technol 201:182–190. https://doi.org/10.1016/j.biortech.2015.11.047
Bussemaker MJ, Zhang D (2013) Effect of ultrasound on lignocellulosic biomass as a pretreatment for biorefinery and biofuel applications. Ind Eng Chem Res 52 (10):3563–3580
Cabrera E, Muñoz MJ, Martín R, Caro I, Curbelo C, Díaz AB (2014) Alkaline and alkaline peroxide pretreatments at mild temperature to enhance enzymatic hydrolysis of rice hulls and straw. Biores Technol 167:1–7. https://doi.org/10.1016/j.biortech.2014.05.103
Canabarro NI, Alessio C, Foletto EL, Kuhn RC, Priamo WL, Mazutti MA (2017) Ethanol production by solid-state saccharification and fermentation in a packed-bed bioreactor. Renew Energy 102:9–14. https://doi.org/10.1016/j.renene.2016.10.026
Chang KL, Chen XM, Han YJ, Wang XQ, Potprommanee L, Ning XA, Liu JY et al (2016) Synergistic effects of surfactant-assisted ionic liquid pretreatment rice straw. Biores Technol 214:371–375. https://doi.org/10.1016/j.biortech.2016.04.113
Chang KL, Chen XM, Wang XQ, Han YJ, Potprommanee L, Liu JY, Liao YL, Ning XA, Sun SY, Huang Q (2017) Impact of surfactant type for ionic liquid pretreatment on enhancing delignification of rice straw. Biores Technol 227:388–392. https://doi.org/10.1016/j.biortech.2016.11.085
Chen WH, Chen YC, Lin JG (2014) Study of chemical pretreatment and enzymatic saccharification for producing fermentable sugars from rice straw. Bioprocess Biosyst Eng 37(7):1337–1344. https://doi.org/10.1007/s00449-013-1106-0
Chuetor S, Luque R, Barron C, Solhy A, Rouau X, Barakat A (2015) Innovative combined dry fractionation technologies for rice straw valorization to biofuels. Green Chem 17(2):926–936. https://doi.org/10.1039/C4GC01718H
Das A, Paul T, Jana A, Halder SK, Ghosh K, Maity C, Mohapatra PKD, Pati BR, Mondal KC (2013) Bioconversion of rice straw to sugar using multizyme complex of fungal origin and subsequent production of bioethanol by mixed fermentation of Saccharomyces cerevisiae MTCC 173 and Zymomonas mobilis MTCC 2428. Ind Crops Prod 46:217–225. https://doi.org/10.1016/j.indcrop.2013.02.003
De Bhowmick G, Sarmah AK, Sen R (2017) Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Biores Technol
de Lima Brossi MJ, Jiménez DJ, Cortes-Tolalpa L, van Elsas JD (2016) Soil-derived microbial consortia enriched with different plant biomass reveal distinct players acting in lignocellulose degradation. Microbial Ecol 71(3):616–627. https://doi.org/10.1007/s00248-015-0683-7
de Souza AP, Leite DCC, Pattathil S, Hahn MG, Buckeridge MS (2013) Composition and structure of sugarcane cell wall polysaccharides: implications for second-generation bioethanol production. BioEnergy Res 6(2):564–579
Devarapalli M, Atiyeh HK (2015) A review of conversion processes for bioethanol production with a focus on syngas fermentation. Biofuel Res J 2(3):268–280
Dhiman SS, Haw JR, Kalyani D, Kalia VC, Kang YC, Lee JK (2015) Simultaneous pretreatment and saccharification: green technology for enhanced sugar yields from biomass using a fungal consortium. Biores Technol 179:50–57. https://doi.org/10.1016/j.biortech.2014.11.059
Ding MZ, Song H, Wang EX, Liu Y, Yuan YJ (2016) Design and Construction of Synthetic Microbial Consortia in China. Synthetic and Systems Biotechnology 1(4):230–235. https://doi.org/10.1016/j.synbio.2016.08.004
Gong G, Liu D, Huang Y (2010) Microwave-assisted organic acid pretreatment for enzymatic hydrolysis of rice straw. Biosyst Eng 107(2):67–73. https://doi.org/10.1016/j.biosystemseng.2010.05.012
Gupta A, Verma JP (2015) Sustainable bio-ethanol production from agro-residues: a review. Renew Sustain Energy Rev. https://doi.org/10.1016/j.rser.2014.08.032
Gusakov AV, Sinitsyn AP, Klyosov AA (1985) Kinetics of the enzymatic hydrolysis of cellulose: 1. A mathematical model for a batch reactor process. Enzyme Microbial Technol 7(7):346–352. https://doi.org/10.1016/0141-0229(85)90114-0
Ibrahim MM, El-Zawawy WK, Abdel-Fattah YR, Soliman NA, Agblevor FA (2011) Comparison of alkaline pulping with steam explosion for glucose production from rice straw. Carbohyd Polym 83(2):720–726. https://doi.org/10.1016/j.carbpol.2010.08.046
Imman S, Arnthong J, Burapatana V, Champreda V, Laosiripojana N (2015) Fractionation of rice straw by a single-step solvothermal process: effects of solvents, acid promoters, and microwave treatment. Renew Energy 83:663–673
Jia X, Liu C, Song H, Ding M, Du J, Ma Q, Yuan Y (2016) Design, analysis and application of synthetic microbial consortia. Synth Syst Biotechnol 1(2):109–117. https://doi.org/10.1016/j.synbio.2016.02.001
Jin X, Ning Y (2013) Laccase production optimization by response surface methodology with Aspergillus fumigatus AF1 in unique inexpensive medium and decolorization of different dyes with the crude enzyme or fungal pellets. J Hazard Mater 262:870–877. https://doi.org/10.1016/j.jhazmat.2013.09.024
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Biores Technol 199:103–112
Kadam KL, McMillan JD (2003) Availability of corn stover as a sustainable feedstock for bioethanol production. Biores Technol. https://doi.org/10.1016/S0960-8524(02)00269-9
Kapoor M, Soam S, Agrawal R, Gupta RP, Tuli DK, Kumar R (2017) Pilot scale dilute acid pretreatment of rice straw and fermentable sugar recovery at high solid loadings. Biores Technol 224:688–693. https://doi.org/10.1016/j.biortech.2016.11.032
Karimi K, Emtiazi G, Taherzadeh MJ (2006) Ethanol production from dilute-acid pretreated rice straw by simultaneous saccharification and fermentation with Mucor indicus, Rhizopus oryzae, and Saccharomyces cerevisiae. Enzyme Microbial Technol 40(1):138–144. https://doi.org/10.1016/j.enzmictec.2005.10.046
Khaleghian H, Karimi K, Behzad T (2015) Ethanol production from rice straw by sodium carbonate pretreatment and Mucor hiemalis fermentation. Ind Crops Prod 76:1079–1085. https://doi.org/10.1016/j.indcrop.2015.08.008
Khaleghian H, Molaverdi M, Karimi K (2017) Silica removal from rice straw to improve its hydrolysis and ethanol production. Ind Eng Chem Res 56(35):9793–9798. https://doi.org/10.1021/acs.iecr.7b02830
Khoo HH (2015) Review of bio-conversion pathways of lignocellulose-to-ethanol: sustainability assessment based on land footprint projections. Renew Sustain Energy Rev. https://doi.org/10.1016/j.rser.2015.02.027
Kim JH, Block DE, Shoemaker SP, Mills DA (2010) Conversion of rice straw to bio-based chemicals: an integrated process using lactobacillus brevis. Appl Microbiol Biotechnol 86(5):1375–1385
Klein M, Griess O, Pulidindi IN, Perkas N, Gedanken A (2016) Bioethanol production from Ficus religiosa leaves using microwave irradiation. J Environ Manag 177:20–25
Ko JK, Bak JS, Jung MW, Lee HJ, Choi IG, Kim TH, Kim KH (2009) Ethanol Production from rice straw using optimized aqueous-ammonia soaking pretreatment and simultaneous saccharification and fermentation processes. Biores Technol 100(19):4374–4380. https://doi.org/10.1016/j.biortech.2009.04.026
Kogo T, Yoshida Y, Koganei K, Matsumoto H, Watanabe T, Ogihara J, Kasumi T (2017) Production of rice straw hydrolysis enzymes by the fungi Trichoderma reesei and Humicola insolens using rice straw as a carbon source. Biores Technol 233:67–73
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729
Kumar MN, Ravikumar R, Thenmozhi S, Sankar MK (2017) Development of natural cellulase inhibitor mediated intensified biological pretreatment technology using Pleurotus florida for maximum recovery of cellulose from paddy straw under solid state condition. Biores Technol 244:353–361
Lan TQ, Wei D, Yang ST, Liu X (2013) Enhanced cellulase production by Trichoderma viride in a rotating fibrous bed bioreactor. Biores Technol 133:175–182. https://doi.org/10.1016/j.biortech.2013.01.088
Lau BBY, Luis ET, Hossain MM, Hart WES, Cencia-Lay B, Black JJ, To TQ, Aldous L (2015) Facile, room-temperature pre-treatment of rice husks with tetrabutylphosphonium hydroxide: enhanced enzymatic and acid hydrolysis yields. Biores Technol 197:252–259
Lee KM, Kalyani D, Tiwari MK, Kim TS, Dhiman SS, Lee JK, Kim IW (2012) Enhanced enzymatic hydrolysis of rice straw by removal of phenolic compounds using a novel laccase from Yeast Yarrowia lipolytica. Biores Technol 123:636–645. https://doi.org/10.1016/j.biortech.2012.07.066
Lee C, Zheng Y, VanderGheynst JS (2015) Effects of pretreatment conditions and post-pretreatment washing on ethanol production from dilute acid pretreated rice straw. Biosys Eng 137:36–42. https://doi.org/10.1016/j.biosystemseng.2015.07.001
Lin TH, Huang CF, Guo GL, Hwang WS, Huang SL (2012) Pilot-scale ethanol production from rice straw hydrolysates using xylose-fermenting Pichia stipitis. Biores Technol 116:314–319. https://doi.org/10.1016/j.biortech.2012.03.089
López-Linares JC, Ballesteros I, Tourán J, Cara C, Castro E, Ballesteros M, Romero I (2015) Optimization of uncatalyzed steam explosion pretreatment of rapeseed straw for biofuel production. Biores Technol 190:97–105
Ludueña L, Fasce D, Alvarez VA, Stefani PM (2011) Nanocellulose from rice husk following alkaline treatment to remove silica. BioResources 6(2):1440–1453
Luo J, Fang Z, Smith RL (2014) Ultrasound-enhanced conversion of biomass to biofuels. Prog Energy Combust Sci. https://doi.org/10.1016/j.pecs.2013.11.001
Margot J, Bennati-Granier C, Maillard J, Blánquez P, Barry DA, Holliger C (2013) Bacterial versus fungal laccase: potential for micropollutant degradation. AMB Express 3:1–30. https://doi.org/10.1186/2191-0855-3-63
Mishima D, Kuniki M, Sei K, Soda S, Ike M, Fujita M (2008) Ethanol production from candidate energy crops: water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes L.). Biores Technol 99:2495–2500. https://doi.org/10.1016/j.biortech.2007.04.056
Mofijur M, Masjuki HH, Kalam MA, Ashrafur Rahman SM, Mahmudul HM (2015) Energy Scenario and biofuel policies and targets in ASEAN countries. Renew Sustain Energy Rev. https://doi.org/10.1016/j.rser.2015.02.020
Mood SH, Golfeshan AH, Tabatabaei M, Jouzani GS, Najafi GH, Gholami M, Ardjmand M (2013) Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew Sustain Energy Rev 27:77–93. https://doi.org/10.1016/j.rser.2013.06.033
Moradi F, Amiri H, Soleimanian-Zad S, Ehsani MR, Karimi K (2013) Improvement of acetone, butanol and ethanol production from rice straw by acid and alkaline pretreatments. Fuel 112:8–13. https://doi.org/10.1016/j.fuel.2013.05.011
Mustafa AM, Poulsen TG, Xia Y, Sheng K (2017) Combinations of fungal and milling pretreatments for enhancing rice straw biogas production during solid-state anaerobic digestion. Biores Technol 224:174–182. https://doi.org/10.1016/j.biortech.2016.11.028
Nakanishi A, Bae JG, Fukai K, Tokumoto N, Kuroda K, Ogawa J, Nakatani M, Shimizu S, Ueda M (2012) Effect of pretreatment of hydrothermally processed rice straw with laccase-displaying yeast on ethanol fermentation. Appl Microbiol Biotechnol 94(4):939–948. https://doi.org/10.1007/s00253-012-3876-8
Nguyen TAD, Kim KR, Han SJ, Cho HY, Kim JW, Park SM, Park JC, Sim SJ (2010) Pretreatment of rice straw with ammonia and ionic liquid for lignocellulose conversion to fermentable sugars. Biores Technol 101:7432–7438. https://doi.org/10.1016/j.biortech.2010.04.053
Nguyen TY, Cai CM, Kumar R, Wyman CE (2017) Overcoming factors limiting high-solids fermentation of lignocellulosic biomass to ethanol. In: Proceedings of the National Academy of Sciences, 201704652
Okamoto K, Nitta Y, Maekawa N, Yanase H (2011) Direct ethanol production from starch, wheat bran and rice straw by the white rot fungus Trametes hirsuta. Enzyme Microbial Technol 48(3):273–277. https://doi.org/10.1016/j.enzmictec.2010.12.001
Parenti A, Muguerza E, Iroz AR, Omarini A, Conde E, Alfaro M, Castanera R, Santoyo F, Ramírez L, Pisabarro AG (2013) Induction of laccase activity in the white rot fungus Pleurotus ostreatus using water polluted with wheat straw extracts. Biores Technol 133:142–149. https://doi.org/10.1016/j.biortech.2013.01.072
Park JY, Shiroma R, Al-Haq MI, Zhang Y, Ike M, Arai-Sanoh Y, Ida A, Kondo M, Tokuyasu K (2010) A novel lime pretreatment for subsequent bioethanol production from rice straw—calcium capturing by carbonation (CaCCO) process. Biores Technol 101(17):6805–6811. https://doi.org/10.1016/j.biortech.2010.03.098
Phitsuwan P, Sakka K, Ratanakhanokchai K (2013) Improvement of lignocellulosic biomass in planta: a review of feedstocks, biomass recalcitrance, and strategic manipulation of ideal plants designed for ethanol production and processability. Biomass Bioenerg 58:390–405
Phitsuwan P, Permsriburasuk C, Baramee S, Teeravivattanakit T, Ratanakhanokchai K (2017) Structural analysis of alkaline pretreated rice straw for ethanol production. Int J Polym Sci. https://doi.org/10.1155/2017/4876969
Poornejad N, Karimi K, Behzad T (2013) Improvement of saccharification and ethanol production from rice straw by NMMO and [BMIM][OAc] pretreatments. Ind Crops Prod 41(1):408–413. https://doi.org/10.1016/j.indcrop.2012.04.059
Postemsky PD, Bidegain MA, González-Matute R, Figlas ND, Cubitto MA (2017) Pilot-scale bioconversion of rice and sunflower agro-residues into medicinal mushrooms and laccase enzymes through solid-state fermentation with Ganoderma lucidum. Biores Technol 231:85–93. https://doi.org/10.1016/j.biortech.2017.01.064
Qureshi N, Singh V, Liu S, Ezeji TC, Saha BC, Cotta MA (2014) Process integration for simultaneous saccharification, fermentation, and recovery (SSFR): production of butanol from corn stover using Clostridium beijerinckii P260. Biores Technol 154:222–228. https://doi.org/10.1016/j.biortech.2013.11.080
Rastogi S, Soni Raman, Kaur J, Soni SK (2016) Unravelling the capability of Pyrenophora phaeocomes S-1 for the production of ligno-hemicellulolytic enzyme cocktail and simultaneous bio-delignification of rice straw for enhanced enzymatic saccharification. Biores Technol 222:458–469. https://doi.org/10.1016/j.biortech.2016.10.012
Reactor, Tube, and Biochemical Engineering (2001) In design of a batch tube reactor for biomass hydrolysis 91(1):377–386
Saha BC, Yoshida T, Cotta MA, Sonomoto K (2013) Hydrothermal pretreatment and enzymatic saccharification of corn stover for efficient ethanol production. Ind Crops Prod 44:367–372. https://doi.org/10.1016/j.indcrop.2012.11.025
Saha BC, Qureshi N, Kennedy GJ, Cotta MA (2016) Biological pretreatment of corn stover with white-rot fungus for improved enzymatic hydrolysis. Int Biodeterior Biodegradation 109:29–35
Salvachúa D, Prieto A, López-Abelairas M, Lu-Chau T, Martínez ÁT, Martínez MJ (2011) Fungal pretreatment: an alternative in second-generation ethanol from wheat straw. Biores Technol 102:7500–7506. https://doi.org/10.1016/j.biortech.2011.05.027
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy. https://doi.org/10.1016/j.renene.2011.06.045
Shen F, Li H, Wu X, Wang Y, Zhang Q (2018) Effect of organic loading rate on anaerobic co-digestion of rice straw and pig manure with or without biological pretreatment. Biores Technol 250:155–162
Shinozaki Y, Kitamoto HK (2011) Ethanol production from ensiled rice straw and whole-crop silage by the simultaneous enzymatic saccharification and fermentation process. J Biosci Bioeng 111(3):320–325. https://doi.org/10.1016/j.jbiosc.2010.11.003
Silva JPA, Carneiro LM, Roberto IC (2013) Treatment of rice straw hemicellulosic hydrolysates with advanced oxidative processes: a new and promising detoxification method to improve the bioconversion process. Biotechnol Biofuels 6(1). https://doi.org/10.1186/1754-6834-6-23
Sindhu R, Kuttiraja M, Binod P, Janu KU, Sukumaran RK, Pandey A (2011) Dilute acid pretreatment and enzymatic saccharification of sugarcane tops for bioethanol production. Biores Technol 102:10915–10921. https://doi.org/10.1016/j.biortech.2011.09.066
Sindhu R, Binod P, Pandey A (2016) Biological pretreatment of lignocellulosic biomass–an overview. Biores Technol 199:76–82
Sindhu R, Binod P, Mathew AK, Abraham A, Gnansounou E, Ummalyma SB, Thomas L, Pandey A (2017) Development of a novel ultrasound-assisted alkali pretreatment strategy for the production of bioethanol and xylanases from chili post harvest residue. Biores Technol
Singh A, Tuteja S, Singh N, Bishnoi NR (2011) Enhanced saccharification of rice straw and hull by microwave-alkali pretreatment and lignocellulolytic enzyme production. Biores Technol 102(2):1773–1782. https://doi.org/10.1016/j.biortech.2010.08.113
Singh R, Tiwari S, Srivastava M, Shukla A (2014) Microwave assisted alkali pretreatment of rice straw for enhancing enzymatic digestibility. J Energy 2014:1–7. https://doi.org/10.1155/2014/483813
Singh J, Suhag M, Dhaka A (2015) Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: a review. Carbohyd Polym 117:624–631
Singh R, Srivastava M, Shukla A (2016) Environmental sustainability of bioethanol production from rice straw in india: a review. Renew Sustain Energy Rev. https://doi.org/10.1016/j.rser.2015.10.005
Soam S, Kapoor M, Kumar R, Borjesson P, Gupta RP, Tuli DK (2016) Global warming potential and energy analysis of second generation ethanol production from rice straw in India. Appl Energy 184:353–364. https://doi.org/10.1016/j.apenergy.2016.10.034
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Biores Technol 199:49–58
Swain MR, Krishnan C (2015) Improved conversion of rice straw to ethanol and xylitol by combination of moderate temperature ammonia pretreatment and sequential fermentation using Candida tropicalis. Ind Crops Prod 77:1039–1046. https://doi.org/10.1016/j.indcrop.2015.10.013
Toquero C, Bolado S (2014) Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Biores Technol 157:68–76. https://doi.org/10.1016/j.biortech.2014.01.090
Umagiliyage AL, Choudhary R, Liang Y, Haddock J, Watson DG (2015) Laboratory scale optimization of alkali pretreatment for improving enzymatic hydrolysis of sweet sorghum bagasse. Ind Crops Prod 74:977–986
Union, Europäische (2009) Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing directives 2001/77/EC and 2003/30/EC. Official J European Union 5:2009
Valdez-Vazquez I, Pérez-Rangel M, Tapia A, Buitrón G, Molina C, Hernández G, Amaya-Delgado L (2015) Hydrogen and butanol production from native wheat straw by synthetic microbial consortia integrated by species of Enterococcus and Clostridium. Fuel 159:214–222
Vincent M, Pometto AL, Van Leeuwen JH (2014) Ethanol production via simultaneous saccharification and fermentation of sodium hydroxide treated corn stover using Phanerochaete chrysosporium and Gloeophyllum trabeum. Biores Technol 158:1–6. https://doi.org/10.1016/j.biortech.2014.01.083
Wan C, Li Y (2010) Microbial pretreatment of corn stover with Ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol production. Biores Technol 101(16):6398–6403
Wi SG, Choi IS, Kim KH, Kim HM, Bae HJ (2013) Bioethanol production from rice straw by popping pretreatment. Biotechnol Biofuels 6(1). https://doi.org/10.1186/1754-6834-6-166
Wu J, Zhang X, Wan J, Ma F, Tang Y, Zhang X (2011) Production of fiberboard using corn stalk pretreated with white-rot fungus Trametes hirsute by hot pressing without adhesive. Biores Technol 102(24):11258–11261
Wu H, Dai X, Zhou SL, Gan YY, Xiong ZY, Qin YH, Ma J, Yang L, Wu ZK, Wang TL (2017) Ultrasound-assisted alkaline pretreatment for enhancing the enzymatic hydrolysis of rice straw by using the heat energy dissipated from ultrasonication. Biores Technol 241:70–74
Xia A, Cheng J, Song W, Yu C, Zhou J, Cen K (2013) Enhancing enzymatic saccharification of water hyacinth through microwave heating with dilute acid pretreatment for biomass energy utilization. Energy 61:158–166
Xing MN, Zhang XZ, Huang H (2012) Application of metagenomic techniques in mining enzymes from microbial communities for biofuel synthesis. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2012.01.021
Zahed O, Jouzani GS, Abbasalizadeh S, Khodaiyan F, Tabatabaei M (2016) Continuous co-production of ethanol and xylitol from rice straw hydrolysate in a membrane bioreactor. Folia Microbiol 61(3):179–189
Zhao J, Xia L (2010) Ethanol production from corn stover hemicellulosic hydrolysate using immobilized recombinant yeast cells. Biochem Eng J 49:28–32. https://doi.org/10.1016/j.bej.2009.11.007
Zheng YG, Chen XL, Wang Z (2005) Microbial biomass production from rice straw hydrolysate in airlift bioreactors. J Biotechnol 118(4):413–420. https://doi.org/10.1016/j.jbiotec.2005.04.022
Zhu S, Huang W, Huang W, Wang K, Chen Q, Wu Y (2015) Pretreatment of rice straw for ethanol production by a two-step process using dilute sulfuric acid and sulfomethylation reagent. Appl Energy 154:190–196. https://doi.org/10.1016/j.apenergy.2015.05.008
Zuroff TR, Curtis WR (2012) Developing symbiotic consortia for lignocellulosic biofuel production. Appl Microbiol Biotechnol 93(4):1423–1435
http://www.energetica-india.net/articles/renewable-energy (2009)
http://nuicone.org/site/common/proceedings/Chemical/oral/CH_21.pdf
Acknowledgements
The author expresses thanks to all faculties of CoD of Natural Science Department, Director R&D department, and special thanks for VC, SBBSU, Director Dr. Vijay Dhir and all those who are involved directly or indirectly in supporting me during manuscript preparations.
Conflict of Interest
The author declares no conflict of interest with any agency.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Bhatt, S.M., Bal, J.S. (2019). Bioprocessing Perspective in Biorefineries. In: Srivastava, N., Srivastava, M., Mishra, P., Upadhyay, S., Ramteke, P., Gupta, V. (eds) Sustainable Approaches for Biofuels Production Technologies. Biofuel and Biorefinery Technologies, vol 7. Springer, Cham. https://doi.org/10.1007/978-3-319-94797-6_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-94797-6_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-94796-9
Online ISBN: 978-3-319-94797-6
eBook Packages: EnergyEnergy (R0)