Abstract
Hepatic encephalopathy (HE) is a metabolically induced neuropsychiatric syndrome that is not always reversible that can result in significant morbidity and mortality. The incidence is unknown, but it is estimated that most individuals with cirrhosis develop some degree of HE, and advanced age is a risk factor. Cognitive, behavioral, and motor dysfunction are the characteristic features of HE, although the pattern and severity differ among grades. Neuropsychologists are most likely to encounter HE in the context of liver transplant evaluations. The current chapter reviews the classification and pathogenesis of HE and diagnosis and treatment considerations and also provides clinical cases as examples and reviews practical issues that will arise for neuropsychologists involved in the care of patients with HE.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Minimal hepatic encephalopathy
- Portosystemic encephalopathy
- End-stage liver disease
- Chronic liver disease
- Cirrhosis
- Neuropsychological
- Neuroimaging
Hepatic encephalopathy (HE), also referred to as portosystemic encephalopathy (PSE), is a metabolically induced, usually reversible neuropsychiatric syndrome resulting from failure of the liver to perform its detoxifying function. HE is usually associated with acute or chronic liver dysfunction but can also be due to portosystemic shunts that divert portal blood into circulation before removal of toxins by the liver. In its mildest form, HE manifests as subtle cognitive or motor difficulties that may not be detectable upon clinical exam alone. HE is one of the most serious complications of liver dysfunction and is a feature of fulminant hepatic failure. In its most severe form, HE results in coma and death. Between one-third and one-half of hospitalizations of patients with cirrhosis are due to HE, and the frequency of hospitalization for HE has doubled over the past decade, with average hospital stays between 5 and 7 days [1, 2]. HE is a marker of poor prognosis [3], resulting in death in over 75% of patients within 3 years of their first episode [4]. In patients with acute liver failure, prognosis is even grimmer, with only about half surviving hospitalization [5]. Although rare, acute liver failure is the most frequent indication for emergency liver transplantation in most countries [6].
Classification and Grading of HE
In an attempt to provide consistency within the literature, scientific study, and treatment of HE, the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) convened in 2014 to create a standardized Practice Guideline [7]. This classification system is based on etiology, severity of symptoms, time course, and whether the episode is precipitated by known or unknown factors. Each area should be addressed and rated at each encounter.
Underlying Etiology
The type of HE is based on underlying liver dysfunction. Type A is associated with acute liver dysfunction, type B with portosystemic bypass in the absence of liver disease, and type C with liver cirrhosis, which is the most common (see Table 41.1).
Continuum of Severity
Severity of HE is graded on a scale from minimal to 4, where minimal represents a normal clinical examination and 4 is coma. Minimal and grade 1 are also known as “covert” HE, and grades 2–4 are considered “overt” HE. The most widely used method of grading HE is the West Haven criteria (WHC) [8, 9], which is determined by clinical examination and based on the subjective evaluation of the clinician (see Table 41.2). This method has been criticized for lack of sensitivity to detect subtle brain dysfunction [10]. For this reason, neuropsychological or neurophysiological measures are recommended to identify covert HE [11, 12]. Identifying covert HE is essential so that symptoms can be monitored and treatment be initiated given that covert or minimal hepatic encephalopathy has a negative effect on quality of life and ability to maintain functioning [13,14,15,16,17,18,19,20]. In general, basic activities of daily living are preserved, while activities that involve divided attention, visuospatial abilities, and motors skills, such as driving, are often impaired [21].
It is important to remember that although specific criteria have been determined to be characteristic of each grade, clear distinctions between grades sometimes cannot be made, and patients may fluctuate from grade to grade within minutes or hours, further clouding the clinical picture. According to Bajaj and colleagues, once the patient exhibits disorientation to time and asterixis, the patient is considered to have moved down the continuum from covert to overt HE [22].
Time Course
Elucidating the clinical course of HE can facilitate identification of the underlying etiology so that correction of the precipitating event can be accomplished as quickly as possible. A firm grasp of the history and timing of episodes of HE allows the clinician to develop an effective treatment plan and set appropriate expectations for family members and caregivers. Table 41.3 displays the possible time courses of HE and the most common underlying factors associated with each [23].
Precipitating or Spontaneous Factors
Quick evaluation and confirmation of precipitating factors that contribute to the onset of HE will hasten treatment and improve the possibility of reversal. If there are no significant precipitating factors found, the possibility of progression of the underlying liver disease must be considered. The most common precipitating factors of HE are presented in Table 41.4.
Epidemiology
Unfortunately, an accurate incidence of HE in the population is difficult to ascertain. Estimations of the occurrence of HE are based on the incidence of cirrhosis. Cirrhosis is a result of damage to the liver causing scar tissue and interfering with the liver’s ability to function properly. Cirrhosis can be caused by alcohol use, chronic viral hepatitis, and steatohepatitis, also known as nonalcoholic fatty liver disease (NAFLD). NAFLD is related to obesity and metabolic syndrome and is estimated to become the single most common indication for liver transplantation [24].
According to the National Health and Nutrition Examination Survey (NHANES), 0.27% of the US population is estimated to have cirrhosis. 26.4% of this population has a 2-year mortality rate [25]. It is believed that minimal HE occurs in up to 80% of cirrhotic patients at some point in time in their disease process [15]. Overt HE is estimated to have an incidence rate of up to 45% of cirrhotic patients. For patients who have undergone a TIPS (transjugular intrahepatic portosystemic shunt), up to 50% are at risk for overt HE [2]. Complications of cirrhosis such as minimal HE, infections, variceal bleeding, and ascites increase the likelihood of overt HE in the first 5 years of diagnosis [26]. There is suspicion that diabetes and hepatitis C virus infection may also contribute to this risk [27,28,29,30,31]. Persons who have an episode of overt HE are likely to have another episode of HE within the following year; individuals with recurrent overt HE have a 40% cumulative risk of developing HE in the next 6 months [32].
For patients who have undergone a TIPS, up to 50% are at risk for overt HE and death [2]. In 2002 the Model for End-Stage Liver Disease (MELD) score replaced the Child-Pugh score for assessing transplantation need. This formula, which was updated in 2016 [33], is currently used to prioritize patients for liver transplantation by the United Network for Organ Sharing and Eurotransplant. Scores range from 6 to 40, with higher scores conferring a higher mortality risk (see Table 41.5). There are easily accessible apps and online calculators to obtain a patient’s MELD score.
Pathogenesis
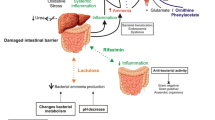
The exact mechanisms underlying HE are complex and still largely unknown, but ammonia neurotoxicity plays a major role [34,35,36,37]. A primary reason ammonia may build up in the blood stream is disruption of the urea cycle. Urea is a nitrogen-containing waste product of protein metabolism. When protein is metabolized, deamination (breakdown) of amino acids produces ammonia. In addition to protein metabolism, intestinal bacteria produce ammonia that is then absorbed into the portal system, the major source of blood flow to the liver. A healthy liver would quickly convert ammonia into urea, which would then be excreted primarily by the kidneys. In the presence of liver dysfunction, ammonia is synthesized more slowly into urea or not at all, allowing ammonia to accumulate in the blood stream. Healthy muscle tissue metabolizes ammonia in this manner, but individuals with cirrhosis are impaired due to muscle wasting, physician recommendations for low-protein “liver failure” diets, and an increased catabolic state (i.e., when the body is breaking down tissue). Certain medications (e.g., benzodiazepines) sensitize the central nervous system (CNS) to ammonia, even at normal levels. Natural benzodiazepines may also be important since a benzodiazepine antagonist (e.g., flumazenil) briefly improves the clinical course of some patients who were not administered pharmaceutical doses of benzodiazepines [38].
When pathologic ammonia is allowed to reach the brain, astrocytes provide the primary means to eliminate it through the synthesis of glutamine [37]. Glutamine is produced by adding one molecule of ammonia to glutamate, an amino acid present in over 90% of neurons, where it acts as an excitatory neurotransmitter. As glutamine accumulates, its osmotic effect causes the astrocyte to take in water, resulting in brain edema and increased intracranial pressure (ICP). Thus, HE is hypothesized to occur when astrocytes are unable to maintain osmotic equilibrium in response to the ammonia-induced increase in glutamine. On autopsy, astrocytes of patients with chronic liver disease show morphologic features characteristic of Alzheimer type II astrocytosis (e.g., pale, enlarged, and frequently paired nuclei, prominent nucleole, proliferation of cytoplasmic organelles) [37].
Another by-product of the ammonia-induced increase in glutamine that may contribute to the pathogenesis of HE is oxidative stress [39,40,41], which results when reactive oxygen species (ROS) such as free radicals and peroxides cannot be removed efficiently, causing significant damage to cell structures and even cell death. Ammonia has been shown to generate ROS when added to astrocyte cultures [42, 43], and glutamine increases free radical production [44]. Ammonia also induces oxidative and nitrosative stress in mitochondria after being carried in and released by glutamine [45,46,47].
Other neurotransmitter systems also are affected by ammonia both directly and indirectly through alteration of transmitter synthesis and recirculation [37, 48]. Altered serotonergic and dopaminergic transmission has been described [49,50,51], as has activation of glutamatergic NMDA receptors and modulation of γ-aminobutyric acid (GABA) receptors by elevated levels of neurosteroids and endogenous benzodiazepines [45, 52]. Overstimulation of excitatory NMDA receptors by ammonia has been shown to induce neuromodulation, neurodegeneration, and neuronal apoptosis [53].
Inflammatory mediators, such as pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1, and IL-6, whether produced in the brain as a result of edema and/or ICP or in the periphery in response to infection also have been implicated in the pathogenesis of HE [40, 41, 54]. This hypothesis is supported by a more rapid progression to severe HE in the presence of infection in patients with acute liver failure [55, 56], as well as astrocyte swelling induced by cytokine exposure in cell cultures [57].
Clinical Presentation
Cognitive, behavioral, and motor dysfunction are the characteristic features of HE, although the pattern and severity differ among grades. Patients with overt HE display changes in mental status over the course of hours or days consistent with the diagnostic criteria for delirium detailed in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [58]. Overt HE can develop spontaneously but is often precipitated by electrolyte imbalances, increased nitrogen load, medications, infection, or a host of other factors (see Table 41.3). Once HE and any precipitating factors are identified and treated, patients usually return to baseline functioning within a few days (i.e., episodic HE). In cases of persistent HE, which is less common, the patient’s mental status continues to fluctuate for more than 4 weeks without returning to baseline, and this is an indication for liver transplantation [59].
The most severe grade of HE, grade 4, is the easiest to recognize, as patients are usually in a coma. Although patients may respond to pain, there often is no response to voice or gentle physical prodding and no spontaneous speech. Patients may open their eyes, but this is not done on command or in conjunction with any purposeful behavior. Decerebrate or decorticate posturing may be seen, even without sternal pressure [60], and may be a sign of raised ICP. Increased ICP is associated with poor outcome, including high rates of mortality, if not controlled [61].
Hallmarks of grade 3 are somnolence and confusion, including disorientation to place [62]. Patients in grade 3 are difficult to rouse and keep awake and may not orient to the clinician. Once awakened, they have trouble paying attention and participating in conversation. They may act strangely and laugh inappropriately, display paranoia, or become easily agitated. Motor findings may include clonus (i.e., rapid involuntary muscle contraction and relaxation after forced extension or stretching), Babinski’s sign (i.e., toes splay out instead of curve inward when sole of foot is rubbed with a blunt instrument), or nystagmus (i.e., rapid involuntary eye movements that are usually side to side but can be up and down).
In grade 2, patients are often lethargic but easy to arouse and engage in conversation. Their movements and thinking are slow. Their speech tends to be slow and monotonous and also may be soft and dysarthric. They typically are aware of their location (i.e., setting and city) but usually are not oriented to time (i.e., month or day of the week). Although most can obey simple commands and recognize common objects, they typically cannot perform simple addition and subtraction and have trouble remembering recent events. Cranial nerves are usually intact, but patients in grade 2 may display either decreased or increased tone or deep tendon reflexes, reduced speed or clumsiness of rapid alternating movements, ataxia, tremor, or asterixis (i.e., “flapping” of the wrist when arms are held straight out with wrists flexed and fingers outstretched and widely separated). Patients too lethargic to lift their arms can be instructed to grasp the examiner’s hands or extend the tongue since sustained movement in patients with asterixis oscillates between tense and relaxed (i.e., never constant) [63]. They may have fetor hepaticus, a uniquely pungent, sweet odor of the breath.
Patients in grade 1 HE are usually alert and typically oriented to place and generally to time. They may sometimes appear lethargic, but they more often report that they are tired, and their sleep–wake cycle is off. They may be sleeping more than usual or have reversal in their sleep–wake cycle, so they sleep more during the day and need medication to sleep at night. These patients often can perform simple arithmetic but have trouble with multiplication or division. Handwriting may be small and difficult to read. Similar to patients in grade 2, memory for recent events is impaired. Motor abnormalities are similar to those displayed by patients in grade 2, as well, although dysarthria, tremor, and hyperreflexia are the most common in grade 1 [62, 64]. It is important to remember, however, that motor abnormalities in overt HE can be transient and do not always align with a particular grade of HE. The possible exception to this is asterixis, which, when present, is usually an indicator of grade 2 [59].
As noted above, patients with minimal HE usually display no obvious abnormalities on clinical exam. However, they sometimes exhibit subtle motor dysfunction, with motor akinesia (i.e., difficulty initiating motor movements), tremor, and rigidity being most common [65]. They or their family members may complain of cognitive problems; disturbances in sleep, appetite, and sexuality; and reduced efficiency in performing work and home activities. The ability to perform basic activities of daily living, such as bathing and dressing, is often not affected. Cognitive testing displays a frontal–subcortical pattern of deficits, with impairments most often seen in psychomotor speed, attention/concentration, visuospatial/constructional skills, and executive functions [66,67,68]. Poor performances on measures of learning and memory may be found but usually are secondary to attentional and visuospatial/perceptual difficulties rather than deficits in memory per se [69, 70]. Intellectual functioning and language abilities typically are preserved.
Differential Diagnosis
Because the symptoms of HE are not specific, it should be considered only in patients with known or suspected liver disease or other portosystemic shunts. The clinician must additionally rule out other causes of mental status change with neurological symptoms, including intracranial bleeding, metabolic abnormalities, ischemic brain disease, CNS infection or neoplasm, Wilson’s disease, substance-induced delirium, and postictal state (see Table 41.6). Seizures and focal neurological signs, such as hemiparesis and hemiplegia, are uncommon [71] and may suggest another etiology. If HE does not resolve within 72 h of treatment, another cause of encephalopathy or unresolved precipitating factor should be considered.
Treatment
The 2014 EASL/AASLD Practice Guidelines for treatment of overt HE, type C, recommend a four-pronged approach. The first step is to identify the person with altered consciousness and begin supportive therapies. The second step involves ruling out other neurological diseases that may account for the altered mental status. Identifying any known precipitating factors that were found on the diagnostic work-up is the third step. The fourth step is to start known treatments for the precipitating event.
Given the primary role of ammonia neurotoxicity in the pathogenesis of HE, management strategies focus on reduction or elimination of ammonia, in addition to treatment of precipitating factors, when identified [72, 73]. The most commonly administered treatment for HE is lactulose, which is a nonabsorbable disaccharide that remains undigested until it reaches the colon. It reduces plasma ammonia levels by inhibiting ammonia production of bacteria and increasing fecal nitrogen excretion. It is usually administered orally, but in the more severe grades of HE or in patients with ascites (i.e., fluid retention in the abdominal cavity) or peritonitis (i.e., inflammation of visceral or abdominal lining), administration via retention enema is preferred [59, 63].
In spite of its long-standing and widespread use, the efficacy of lactulose has been questioned [74], and patients are often noncompliant due to unpleasant side effects, such as increased intestinal gas, abdominal distention and cramping, and diarrhea [59, 75]. On the other hand, Sharma and colleagues report that there is not enough evidence to not recommend nonabsorbable disaccharides for the treatment of HE despite inconsistent study outcomes [75]. Nonresponse to lactulose has been shown to be predicted by high MELD scores, high white cell count, low blood sodium, low mean arterial pressure, and hepatocellular carcinoma [76].
Therefore, alternative treatments for HE are a topic of intense study [77]. Nonabsorbable antibiotics, such as neomycin, vancomycin, and rifaximin, have been suggested with the goal of reducing bacteria-producing ammonia in the gut. While the efficacy of neomycin and vancomycin has not been well established, rifaximin has been found to be equivalent or superior to placebo, other antibiotics, and nonabsorbable disaccharides for both lowering ammonia and improving cognitive functioning [78]. The combination of rifaximin and lactulose has been found to reduce mortality when compared to lactulose and placebo [79].
L-ornithine L-aspartate (LOLA) is the salt of two natural amino acids (i.e., ornithine and aspartate) and is another treatment option occasionally used outside of the United States. LOLA is believed to reduce ammonia levels by converting ammonia to urea and glutamine [80]. LOLA delivered via IV has shown to lower plasma ammonia rates and improve performance on psychometric testing [81].
Due to the lack of effective treatments for HE, prevention is the goal [12, 63], particularly given evidence of increased severity of cognitive impairment with each additional episode of overt HE [63]. Along with diligent management of underlying liver disease and its complications, close monitoring of dietary protein intake is recommended in patients with a history of HE, as large amounts of protein can increase plasma ammonia levels and possibly precipitate HE, while too little protein correlates with mortality and development of complications [82, 83]. Up to 75% of patients with HE are found to be malnourished due to lack of protein [84]. Adequate protein intake is essential to improve nutrition to avoid loss of muscle mass and lower the risk of accelerated fasting metabolism. Malnutrition itself is a risk factor for HE in cirrhotic patients [85]. Patients with cirrhosis should be assessed for sarcopenia and nutritional status (AASLD Practice Guideline).
Probiotics are currently being studied for those recovering from HE and in prevention of recurrence of HE. It is hypothesized that gut dysbiosis may contribute to inflammatory processes that potentiate brain edema and neuro-inflammation associated with HE [86]. While there is some evidence that probiotics reduce plasma ammonia levels and are comparable to lactulose for secondary prophylaxis of [87], other studies show no effect on mortality, recovery from HE, or quality of life [88]. Due to mixed evidence and wide variability in the content of probiotics, they are not currently recommended for treatment of HE [89].
Findings on dietary supplementation with branched-chain amino acids have been mixed, with some studies showing positive effects on cognitive functioning [90, 91], particularly in patients with persistent HE [92], and prolonged event-free survival [85], and others showing no effect at all [93]. Gluud, Borre, Cordoba, Marchesini, et al. (2013) performed a meta-analysis on eight studies that evaluated treatments of HE comparing lactulose, rifaximin, and BCAAs [94, 95]. They concluded that BCAAs improve presentation of symptoms of both minimal and overt HE but have no effect on survival per se.
Liver transplantation is indicated for patients with recurrent episodic or persisting HE due to increased mortality rates [35], with extracorporeal albumin dialysis serving as a potential bridge to liver transplantation [96, 97].
Clinical Evaluation
Although the core manifestations of HE have been recognized and agreed upon for years, a “gold standard” for the diagnosis of HE remains elusive. Definition and classification of even the basic behavioral and motor alterations need further refinement to distinguish among grades of HE, particularly the less severe grades. Therefore, diagnosis must be based on multiple approaches, including clinical examination, laboratory findings, neuroimaging, neurophysiological measures, and neuropsychological assessment.
Clinical Examination
The clinical interview and physical and neurological exams are the mainstays for assessing HE. The clinician must ensure a history of known or suspected liver disease or the presence of a portosystemic shunt and exclude other potential causes of encephalopathy. Early identification of HE is crucial as delays in diagnosis may result in death. A thorough review of possible precipitating factors also is critical so that appropriate treatment can be initiated promptly. For inpatients with HE, examination of mental status should be performed at least 2–3 times a day [98].
In determining grade of HE, the WHC (Table 41.2) can be employed quickly and easily and provides a useful “ballpark” of the patient’s clinical status [11]. In more severe grades of HE, the Glasgow Coma Scale (GCS) [99] may be a useful adjunct, supplying additional information about ocular and motor responses and thus allowing for wider separation among patients in grades 3 and 4 [62]. In less severe grades, and particularly in minimal HE, neurocognitive tests and neurophysiological measures are recommended [12].
Because some of the items in the WHC are not operationally defined and do not correspond well to the progression of HE, Ortiz and colleagues [91, 100] developed the Clinical Hepatic Encephalopathy Staging Scale (CHESS). The CHESS consists of nine manifestations of HE that can be easily recognized and categorized into dichotomous groups (see Table 41.7) and was designed to provide a means to monitor the severity of HE. The CHESS provides a score from 0 (low) to 9 (high), which reflects the severity of HE, not the grade. Factor analysis supported two factors corresponding to “mild” and “severe” HE, which is consistent with recent proposals to classify HE into more clinically meaningful categories of “low-” (grades 1 and 2) or “high-grade” (grades 3 and 4) rather than trying to make fine-grained differentiations among grades. Like the WHC, the CHESS should be augmented with the GCS for more severe HE and with neurocognitive and/or neurophysiological measures for less severe grades.
A modified version of the WHC, the Hepatic Encephalopathy Scoring Algorithm (HESA), was developed by Hassanein and colleagues in an attempt to improve its objectivity and sensitivity [64]. The HESA combines the clinical exam with neuropsychological tests to determine HE grade, relying heavily on subjective clinical evaluation in the more severe grades where neuropsychological testing is not possible and more heavily on objective testing in the less severe grades where dysfunction may not be as evident on clinical exam (see Table 41.8). Initial findings confirm increased sensitivity and accuracy of the HESA compared to the WHC in grading HE [64].
Laboratory Findings
Blood ammonia levels are often elevated in patients with overt HE but do not always correlate with HE grade [101, 102]. However, significantly elevated blood ammonia levels (>150–200 μmol/l) in a comatose patient without a history of recent seizures are strongly suggestive of HE [59]. It is important to perform the assay within 30 min of drawing blood, or levels may be artificially inflated [103].
Neuroimaging
The primary role of neuroimaging in evaluation of HE is to rule out other possible etiologies of neurobehavioral changes [104] and to establish the presence of cerebral edema, particularly in acute liver failure. Because clinical symptoms of increased ICP (e.g., hypertension, bradycardia) may not be present, ICP monitoring devices may be helpful to identify cerebral edema early and prevent herniation until liver transplantation can be performed [105]. Typical neuroimaging findings in HE include hyperintensities in the globus pallidus on magnetic resonance imaging (MRI) T1-weighted images (see Fig. 41.1), elevated glutamine/glutamate peaks and decreased myoinositol and choline signals on proton magnetic resonance spectroscopy (1H MRS), and white matter abnormalities on MRI fast fluid-attenuated inversion recovery sequences (FLAIR) and diffusion-weighted images (DWI) [106]. In cirrhotic patients with minimal HE, T2 hyperintensities along the corticospinal tract (see Fig. 41.2) are suggestive of mild edema [107, 108] and have been found to relate to abnormalities in central motor pathways that resolve (as do some cognitive difficulties) after liver transplantation [109]. In patients with HE due to portosystemic shunt and no liver disease, MRI can be especially helpful as dietary manganese that is not cleared by the liver accumulates in the basal ganglia and is detected as hyperintensities on T1-weighted images when exam may have found mild Parkinson-like movement changes only [103]. Qi, Zhang, and Zhong et al. [110] used fMRI and found that there is disrupted influence between the globus pallidus and the anterior cingulate cortex, which affects both cognitive and emotional processing [110]. This study confirmed previous investigations indicating decreased functional connection between the globus pallidus and the cuneus. They also reported an increase in connectivity from the pallidum to the precuneus that may indicate a compensatory mechanism in play and decreased input from the globus pallidus to the right inferior temporal gyrus and left superior temporal gyrus that may explain visual deficits.
Hyperintensities in the globus pallidus secondary to hepatic encephalopathy. Transverse T1-weighted MR images of the brain in a patient with chronic liver failure and parkinsonism. Observe the bilateral and symmetric high T1 signal-intensity change involving the globus pallidus and the anterior midbrain
Hyperintensities in the corticospinal tract secondary to hepatic encephalopathy. (a) Transverse T2-weighted fast FLAIR images obtained in a patient with liver cirrhosis during an episode of hepatic encephalopathy. Observe the symmetric areas of increased signal intensity along the corticospinal tract in both cerebral hemispheres. (b) This signal-intensity abnormality almost completely reverses on a follow-up study obtained few months later, when the patient showed no signs of overt hepatic encephalopathy
Neurophysiological Measures
Advantages of neurophysiological measures are that they are not influenced by demographic variables, such as gender, education, or cultural background, and they are easy to administer by staff without extensive training. Electroencephalogram (EEG) has been used to diagnose HE since the 1950s [111]. However, because findings are not specific to HE, EEG and other neurophysiological measures are most useful in the comatose patient [112], when the diagnosis is uncertain (i.e., focal neurological signs or seizure activity is present or the patient has “normal” mental status) or when evidence of worsening HE is needed [113]. The most common EEG findings in HE are slowed mean dominant frequencies, and in minimal HE, you may see relatively slowed activity within the δ (delta) and θ (theta) frequency bands [114]. In patients with minimal HE, changes in EEG have been shown to be predictive of developing overt HE and thus may have prognostic utility [115]. EEG has been criticized for use in detecting HE because it measures cortical rather than subcortical activity, which is where most of the pathology in HE is hypothesized to exist.
Other neurophysiological measures that have been used to identify HE include evoked potentials (EPs) and critical flicker frequency (CFF). EPs, the latency between presentation and detection of a stimulus, may be slightly delayed in patients with minimal HE, shown most often using P300 oddball paradigms [116,117,118,119], but findings are not specific and often confounded by alcohol use or diabetes, which also delay EPs, and are frequently found in patients with cirrhosis [120]. In CFF, the patient is asked to press a button when a steady light has changed into a flicker and when a flickering light has become a steady, fused light. Patients with minimal and lower grades of HE have shown reduced ability to detect the light flickering or fusing [76, 121, 122]. A recent meta-analysis of the diagnostic accuracy of the CFF revealed sensitivity of 61% (95% CI, 55–67) and specificity of 79% (95% CI 75–83) [123].
Neuropsychological Assessment
Neuropsychologists are most likely to encounter HE in the context of liver transplant evaluations. Pretransplant evaluations usually are conducted on an outpatient basis, but occasionally they must be performed while the patient is hospitalized and awaiting transplantation. Of course, the possibility of HE, particularly minimal HE, must always be considered in patients with cirrhosis, regardless of reason for referral or inpatient versus outpatient status. Neuropsychologists also are called upon to assess for HE in the context of clinical trials for management of HE and when insertion of TIPS for management of portal hypertension is planned [124]. TIPS for management of portal hypertension is planned. Onset or worsening of HE is common after placement of TIPS, occurring in 35–55% of patients within the first year [125]. Baseline assessment and subsequent monitoring are important for identifying and treating HE before it escalates and the patient’s status becomes critical, particularly in the first 3 months, since 90% of post-TIPS HE occurs in this time frame [125, 126]. The level of neuropsychological assessment will depend on the severity of HE, with more comprehensive testing reserved for those with covert HE. It often is difficult for patients with grades 2 and 3 to participate reliably for more than 10–15 min. Fatigue is also frequently a factor, even in patients with no or minimal HE, so full-day evaluations are not routinely employed.
There is evidence that cognitive impairment may remain after the treatment and resolution of overt HE. Bajaj and colleagues examined 226 cirrhotic patients and found that patients with a history of overt HE were more likely to have persistent cognitive problems and patients with further episodes of overt HE displayed deficits in multiple areas of cognition [63]. Given growing evidence of cumulative effects of recurrent overt HE on neuropsychological functioning, the role neuropsychologists plays in educating patients and families about the effects of neuropsychological impairments on daily functioning cannot be understated.
Clinical Interview
Changes in cognitive and motor functioning secondary to minimal HE are often subtle and result in cognitive inefficiencies rather than frank impairment but still significantly affect daily functioning, including ability to work and drive. With regard to driving, patients with minimal HE report more traffic violations and motor vehicle accidents than those without cognitive dysfunction [14, 15, 127]. Common cognitive complaints include trouble paying attention, concentrating, remembering, and completing tasks. Aphasia, significant memory problems such as repeating stories or forgetting recent events even when reminded, and lateralized motor problems (i.e., weakness or motor abnormality on one side only) are uncommon and usually indicate another etiology. Patients often have difficulty pinpointing when the symptoms began but usually indicate that they are not worsening significantly over time. Report of gradual cognitive decline over time in the absence of recurrent episodic HE is suggestive of possible neurodegenerative disease process, psychological factors, or medical conditions other than minimal HE contributing to cognitive complaints.
Additional complaints often include fatigue and changes in appetite, sleep, energy, and activity levels. Patients with minimal HE report reduced HRQOL, such as limited social interactions and recreational pastimes and difficulties managing home and work duties [13, 16, 17, 128]. Although the patient may endorse affective symptoms, it is important to establish that these changes do not occur in conjunction with increasingly depressed or anxious mood.
As with any patient referred for neuropsychological assessment, ruling out other possible causes of cognitive impairment, including stroke, seizure disorder, traumatic brain injury, or other neuromedical condition, is necessary. Gathering information about psychiatric and substance use histories, academic and social functioning, and family medical history also is important for differential diagnosis. Information from a collateral source is helpful when assessing patients with minimal HE due to the possibility of poor insight and/or awareness [127] and essential when assessing patients with overt HE who often cannot report reliably.
Test Selection
Selection of measures will depend on the setting (inpatient vs. outpatient), severity of HE, and reason for evaluation (e.g., pretransplant, monitoring of HE in clinical trials, or following TIPS). In the case of pretransplant outpatient evaluations, most patients are either unimpaired or have minimal HE, so comprehensive neuropsychological evaluation is appropriate. Assessment of current intellectual or estimated premorbid functioning, language, visuospatial/constructional skills, attention and processing speed, executive functioning, learning and memory, emotional status, and HRQOL is recommended. Because one of the purposes of the pretransplant evaluation is to rule out neurodegenerative diseases, such as Alzheimer’s disease, it is important to include tests that can distinguish cortical from subcortical patterns of deficits. A couple of studies have found support for the utility of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [129] in pretransplant evaluations [67, 130], with one study confirming the expected subcortical pattern of deficits using the Randolph Cortical–Subcortical Deviation Score detailed in the RBANS manual [67]. When pretransplant evaluations must be conducted on an inpatient basis and the patient can tolerate more detailed assessment (i.e., is at grade 2 HE or less), the RBANS may be a good choice since it taps multiple cognitive domains, can be administered in less than 30 min, and is easy to transport.
With regard to emotional status, brief self-report measures rather than longer measures of psychopathology (e.g., Minnesota Multiphasic Personality Inventory-2) [131] are used to minimize fatigue. Of course, if there are concerns about significant psychopathology, particularly in the context of pretransplant evaluation, the use of a more comprehensive measure of psychological functioning may be warranted. For HRQOL, the Medical Outcomes Study Short Form (SF-36) [132] is commonly used and enables comparisons to other chronic diseases, but disease-specific measures also are available, including the Chronic Liver Disease Questionnaire [133], the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK)—Quality Assessment [134], and the Liver Disease Quality of Life Instrument [135]. Recently, a measure of HRQOL for use specifically with minimal HE patients showed promising initial validity [136]. Table 41.9 displays a sample outpatient pretransplant battery and suggested modifications for inpatient status.
When monitoring HE in the course of clinical trials, you want to select measures that can be completed by patients with more severe HE but also are sensitive enough to detect subtle changes in cognition in the less severe grades. This was one of the goals of the HESA, which allows one to measure changes in HE severity across all grades and is now required in Federal Drug Administration (FDA)-sponsored studies [64]. Although more validation of the HESA is needed, particularly in the lower grades, it is a viable option for clinical trials, as the neuropsychological measures administered are well known and widely used with modifications to ensure feasibility of administration and scoring in the inpatient setting while maintaining sensitivity for detecting impairment.
When the goal is to identify the presence of minimal HE outside the context of pretransplant evaluation or clinical trials, such as when conducting evaluations pre- and post-TIPS insertion or for monitoring risk of developing overt HE during clinic visits, a comprehensive battery may not be necessary or appropriate. The consensus statement generated by the 1998 working group mentioned previously [12] recommended at least two of the following four measures be used to assess for minimal HE: Parts A and B of the Trail Making Test (TMT) [138] (also known as the Number Connection Test), block design test, and digit symbol test. Also recommended was the Psychometric Hepatic Encephalopathy Score (PHES) [70], which has been validated in several languages across several countries, including Germany, Italy, and Spain [146]. The PHES is a composite score based on demographic-adjustedz scores from Parts A and B of the TMT, digit symbol, line tracing, and serial dotting. Scores ≤ −4 are considered to reflect minimal HE.
The PHES, along with the RBANS, also was recommended recently by a group of experts convened by the International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) for use in patients at risk for developing minimal HE [147]. One limitation of the PHES for use in the United States is that line tracing and serial dotting have not yet been normed in the United States. A limitation of the RBANS is that it has not been systematically studied as a method for detecting or monitoring HE [148]. Computerized cognitive measures are another method beginning to be used, with the inhibitory control task (ICT), a computerized variant of the continuous performance test, showing good initial validity [14, 149, 150], including ability to predict future car crashes and traffic violations [127].
Case Example: Characterization of Overt and Minimal HE [133]
Following is a case example of a 46-year-old non-Hispanic White man with end-stage liver disease (ESLD) secondary to hepatitis C virus and alcoholic hepatitis. Mr. J graduated from high school and worked primarily as a machinist until he became disabled from ESLD. He was being followed in a hepatology clinic at a university hospital and agreed to participate in a research study examining quality of life in persons with chronic liver disease. As part of this research protocol, a brief neurocognitive battery consisting of a modified version of the Rey Complex Figure Test (RCF) [151], Digit Cancellation (DC) [151], Trail Making Test (TMT), and the written version of the Symbol Digit Modalities Test (SDMT) [152] was administered during a routine clinic visit. Mr. J completed this battery on three occasions: once during an episode of overt HE judged to be grade 1, once during minimal HE, and once 5 months post-transplant. His raw scores on these measures at each of the three time points are presented in Table 41.10.
Cognitive performance on all measures during Mr. J’s episode of overt HE was more than three standard deviations below the normative mean, and he evidenced a mild tremor while performing tasks. He exhibited significant difficulty copying this version of the RCF, which was modified to be more simplistic than the original figure. Even after having viewed the figure three times, his learning score (i.e., raw score = 5) revealed that he did not encode much additional information beyond that encoded on the initial (copy) trial (i.e., raw score = 3.5). Moreover, he forgot half of the details of the figure after a 20-min delay. On a measure of selective attention, Digit Cancellation, he required a long time to complete the task and made a significant number of errors (both omission and commission). He was able to complete the TMT, albeit very slowly, and he made several cognitive-switching errors on Part B. On the SDMT, he performed very slowly and made a few errors. His cognitive and motor findings during this episode of overt HE are typical of those seen in patients with grade 1 HE [153].
A couple of months later, after his episode of overt HE had resolved, Mr. J’s performance on this brief battery was significantly improved. His action tremor was gone, and his test scores were essentially within normal limits, except for SDMT, which was approximately 1.5 standard deviations below the normative mean. Five months post-transplant, Mr. J exhibited continued improvement, particularly on measures relying on executive function (i.e., RCF learning, TMT Part B, and SDMT). Although some of these improvements may have been due to practice effects, others were too significant to be attributed to practice effects alone. The contrast between test performances during minimal HE and post-transplant suggests that although Mr. J generally performed within normal limits on all but one task (i.e., SDMT) pretransplant, he was still performing below his baseline. The pattern of findings also is consistent with the literature showing compromised frontal–subcortical circuits.
Case Example: Overt HE in Post-TIPS and Continued ETOH Use
Identifying information in the following case example was altered to protect the patient’s privacy. Mr. H is a 36-year-old, divorced, Caucasian man with 13 years of formal education and a significant history of heavy drinking. He had been admitted to the hospital for HE after an accidental overdose of Tylenol and was referred for neuropsychological evaluation to characterize neurocognitive functioning, provide treatment recommendations, and educate family members about his behavior and prognosis.
Past medical history was noteworthy for hospitalization 13 months earlier for HE associated with recent heavy drinking. Mr. H’s hospital course was complicated by pneumonia and acute respiratory failure, requiring intubation. Liver biopsy revealed cirrhosis, and he had portal hypertension, which was treated with TIPS. Mr. H recovered and was discharged. Although he was independent with his activities of daily living, he did not return to his premorbid level of functioning and remained unemployed post-discharge. Mr. H was being followed by cardiology for alcoholic cardiomyopathy.
Upon admission for the current episode of HE, toxicology screens were negative for substances including alcohol. He had an elevated ammonia level (78 umol/L) and a MELD score of 39. Neuroimaging revealed mild dilation of the ventricles and sulci, compatible with generalized cerebral volume loss, and abnormal T1 hyperintensities in the bilateral basal ganglia, which the radiologist interpreted as consistent with a history of elevated manganese levels (See Figs. 41.3 and 41.4). As noted earlier, the inability of the liver to clear manganese from the diet often manifests as T1 hyperintensities, particularly in the context of portosystemic shunt, suggesting that the patient’s current episode of HE may have been a complication of TIPS [154]. Mr. H was disoriented and agitated and was treated with lactulose and rifaximin. After approximately 2½ weeks, Mr. H’s medical status had stabilized, including normalization of ammonia levels, and he was transferred to a locked psychiatric unit. While his neurocognitive abilities had improved, staff reported that he continued to hallucinate and become confused during the evening hours.
At the time of the clinical interview, Mr. H had been in the locked psychiatric unit for 9 days. He was alert and oriented and ambulated independently. He had significant yellowing of his sclera. His speech was fluent but tangential and nonsensical at times and noteworthy for word-finding problems and confabulatory responses. He was an inconsistent historian and had difficulty relaying the sequencing of his medical history. Of note, Mr. H denied having had alcohol since his hospital admission for HE the previous year; however, his mother indicated that she had found empty liquor bottles in the house and that his friends had told her that he had resumed drinking. He reportedly has gotten lost while driving and had unexplained scrapes on his car. There was no history of previous neuropsychological testing.
During neurocognitive testing 3 days later, he appeared motivated to perform well, which was confirmed by performance validity measures (see Table 41.11). Mr. H’s performance on the Brief Cognitive Status Exam, a cognitive screening measure, was within normal limits, although he struggled with the inhibition task and made multiple commission errors. Intellectual test results ranged from borderline to average. His ability to define words was a significant weakness, and difficulties with naming and category fluency were apparent although phonemic fluency was intact. He also struggled with duplicating designs using blocks and tended to copy designs in a sloppy fashion, with decreasing accuracy noted when the precepts became complex. His free recall of previously copied figures after a delay was impaired, but he was able to accurately identify all of the previously copied designs in a recognition format. Memory for verbal information was intact. On measures of timed visual scanning, sequencing, attention, and inhibition, Mr. H exhibited difficulties, performing in the impaired range after correcting for age and education.
Mr. H represents a complex case in terms of differential diagnosis for the etiology of his neurocognitive dysfunction. He had two documented episodes of overt HE requiring hospitalization in the 14 months prior to neuropsychological evaluation, but the contribution of continued alcohol use cannot be ruled out entirely in spite of negative toxicology results at the time of his most recent hospitalization. Neuropsychological testing indicated impairment in areas of attention, language, managing complex information, visuospatial abilities, and visual memory. While this pattern of dysfunction is generally consistent with findings associated with minimal HE, it is also generally consistent with findings associated with recent alcohol detoxification [155]. As Mr. H was tested within 1 month of onset of HE, continued improvement is expected as long as he remains abstinent from alcohol. Long-term follow-up, along with reliable verification of alcohol-free status, is needed in order to establish the etiology and stability of his neurocognitive dysfunction.
Clinical Pearls
-
HE is associated with impaired abilities to perform complex tasks (e.g., driving), reduced HRQOL, and poor outcome, including death.
-
Severity of HE is usually graded on a scale from minimal to 4 (coma), and sometimes distinctions among grades are difficult to determine due to fluctuations in a patient’s status or limitations in the methods available for grading HE.
-
Overt HE typically requires hospitalization and quick identification and treatment of precipitating events to prevent continued deterioration and death.
-
Blood ammonia levels may not correspond to clinical severity of HE and have little clinical significance if serially followed.
-
Minimal HE is present in 50–80% of cirrhotic patients and usually undetected unless tested with neuropsychological or neurophysiological measures.
-
Although HE should be high on the list of diagnostic possibilities in delirious patients with cirrhosis, other causes of mental status change, such as alcohol withdrawal, occult gastrointestinal bleed, infection, and dehydration, must be ruled out since they are also common in patients with cirrhosis.
-
In patients with worsening of HE but no clear precipitating factor, check for noncompliance with lactulose or other HE treatments since patients sometimes are not compliant due to unpleasant drug side effects or poor memory.
-
In patients with minimal HE, a frontal–subcortical pattern of deficits and cognitive inefficiencies is a characteristic; aphasia, significant forgetting such as that seen in Alzheimer’s disease, and lateralized deficits suggest another etiology.
-
Traffic violations and motor vehicle accidents are more common in cirrhotic patients with minimal HE than those without, so careful inquiry about driving is needed, and physician recommendation for the patient to stop driving may be advised.
-
Gut dysbiosis is an emerging area of research and has demonstrated a relationship with HE-related cognitive impairment.
-
NAFLD is estimated to become the main reason for liver transplant.
-
Neuropsychological evaluation can aid in decisionmaking for priority placement for liver transplant.
References
Leevy CB, Phillips JA. Hospitalizations during the use of Rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci. 2007;52(3):737–41.
Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25:3–9.
Stewart CA, et al. Hepatic encephalopathy as a predictor of survival in patients with end-stage liver disease. Liver Transpl. 2007;13(10):1366–71.
Bustamante J, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30(5):890–5.
Bernuau J, Durand F. Early prediction of encephalopathy in hospitalized patients with severe acute liver disease: the narrow window of opportunity for transplant-free survival. J Hepatol. 2009;51(6):977–80.
Bernal W, et al. Acute liver failure. Lancet. 2010;376(9736):190–201.
Vilstrup H, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the liver. Hepatology. 2014;60(2):715–35.
Atterbury CE, Maddrey WC, Conn HO. Neomycin-sorbitol and lactulose in the treatment of acute portal-systemic encephalopathy. A controlled, double-blind clinical trial. Am J Dig Dis. 1978;23(5):398–406.
Conn HO, et al. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology. 1977;72(4 Pt 1):573–83.
Citro V, et al. Mental status impairment in patients with west haven grade zero hepatic encephalopathy: the role of HCV infection. J Gastroenterol. 2007;42(1):79–82.
Dhiman RK, et al. Minimal hepatic encephalopathy: consensus statement of a working party of the Indian National Association for study of the liver. J Gastroenterol Hepatol. 2010;25(6):1029–41.
Ferenci P. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th world congresses of gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716–21.
Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci. 2003;48(8):1622–6.
Bajaj JS, et al. Minimal hepatic encephalopathy: a vehicle for accidents and traffic violations. Am J Gastroenterol. 2007;102(9):1903–9.
Bajaj JS, et al. Navigation skill impairment: another dimension of the driving difficulties in minimal hepatic encephalopathy. Hepatology. 2008;47(2):596–604.
Groeneweg M, et al. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology. 1998;28(1):45–9.
Prasad S, et al. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45(3):549–59.
Schomerus H, et al. Latent portasystemic encephalopathy. Dig Dis Sci. 1981;26(7):622–30.
Watanabe A, et al. Evaluation of neuropsychological function in patients with liver cirrhosis with special reference to their driving ability. Metab Brain Dis. 1995;10(3):239–48.
Wein C, et al. Minimal hepatic encephalopathy impairs fitness to drive. Hepatology. 2004;39(3):739–45.
Montgomery JY, Bajaj JS. Advances in the evaluation and management of minimal hepatic encephalopathy. Curr Gastroenterol Rep. 2011;13(1):26–33.
Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50(6):2014–21.
Strauss E, da Costa MF. The importance of bacterial infections as precipitating factors of chronic hepatic encephalopathy in cirrhosis. Hepato-Gastroenterology. 1998;45(21):900–4.
Marchesini G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–23.
Scaglione S, et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49(8):690–6.
D’Amico G, et al. Survival and prognostic indicators in compensated and decompensated cirrhosis. Dig Dis Sci. 1986;31(5):468–75.
Bustamante J, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30(5):890–5.
Benvegnu L, et al. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53(5):744–9.
Gentilini P, et al. Long course and prognostic factors of virus-induced cirrhosis of the liver. Am J Gastroenterol. 1997;92(1):66–72.
Hartmann IJ, et al. The prognostic significance of subclinical hepatic encephalopathy. Am J Gastroenterol. 2000;95(8):2029–34.
Watson H, et al. Satavaptan treatment for ascites in patients with cirrhosis: a meta-analysis of effect on hepatic encephalopathy development. Metab Brain Dis. 2013;28(2):301–5.
Agrawal A, et al. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am J Gastroenterol. 2012;107(7):1043–50.
Kalra A, Wedd JP, Biggins SW. Changing prioritization for transplantation: MELD-Na, hepatocellular carcinoma exceptions, and more. Curr Opin Organ Transplant. 2016;21(2):120–6.
Bjerring PN, et al. The brain in acute liver failure. A tortuous path from hyperammonemia to cerebral edema. Metab Brain Dis. 2009;24(1):5–14.
Córdoba J, Mínguez B. Hepatic encephalopathy. Semin Liver Dis. 2008;28(1):070–80.
Shawcross D, Jalan R. The pathophysiologic basis of hepatic encephalopathy: central role for ammonia and inflammation. Cell Mol Life Sci. 2005;62(19–20):2295–304.
Butterworth RF. Effects of hyperammonaemia on brain function. J Inherit Metab Dis. 1998;21(S1):6–20.
Barbaro G, et al. Flumazenil for hepatic encephalopathy grade III and IVa in patients with cirrhosis: an italian multicenter double-blind, placebo-controlled, cross-over study. Hepatology. 1998;28(2):374–8.
Butterworth RF. Thiamine deficiency-related brain dysfunction in chronic liver failure. Metab Brain Dis. 2009;24(1):189–96.
Haussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut. 2008;57(8):1156–65.
Seyan AS. Changing face of hepatic encephalopathy: role of inflammation and oxidative stress. World J Gastroenterol. 2010;16(27):3347–57.
Kosenko E, et al. Sources of oxygen radicals in brain in acute ammonia intoxication in vivo. Brain Res. 2003;981(1–2):193–200.
Master S, Gottstein J, Blei AT. Cerebral blood flow and the development of ammonia-induced brain edema in rats after portacaval anastomosis. Hepatology. 1999;30(4):876–80.
Norenberg M. Oxidative and nitrosative stress in ammonia neurotoxicity. Hepatology. 2003;37(2):245–8.
Albrecht J, Norenberg MD. Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology. 2006;44(4):788–94.
Bjerring PN, et al. Cerebral glutamine concentration and lactate–pyruvate ratio in patients with acute liver failure. Neurocrit Care. 2008;9(1):3–7.
Rama Rao KV, et al. Role of oxidative stress in the ammonia-induced mitochondrial permeability transition in cultured astrocytes. Neurochem Int. 2005;47(1-2):31–8.
Butterworth RF. Hepatic encephalopathy: a neuropsychiatric disorder involving multiple neurotransmitter systems. Curr Opin Neurol. 2000;13(6):721–7.
Lozeva V, et al. Increased brain serotonin turnover correlates with the degree of shunting and hyperammonemia in rats following variable portal vein stenosis. J Hepatol. 2004;40(5):742–8.
Lozeva-Thomas V. Serotonin brain circuits with a focus on hepatic encephalopathy. Metab Brain Dis. 2004;19(3-4):413–20.
Pidoplichko VI, Dani JA. Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage. Proc Natl Acad Sci. 2006;103(30):11376–80.
Ahboucha S, Butterworth RF. Pathophysiology of hepatic encephalopathy: a new look at GABA from the molecular standpoint. Metab Brain Dis. 2004;19(3-4):331–43.
Albrecht J, Bender AS, MD N. Potassium-stimulated GABA release is a chloride-dependent but sodium- and calcium-independent process in cultured astrocytes. Acta Neurobiol Exp (Wars). 1998;58(3):169–75.
Odeh M. Pathogenesis of hepatic encephalopathy: the tumour necrosis factor-? Theory. Eur J Clin Investig. 2007;37(4):291–304.
Rolando N. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32(4 Pt 1):734–9.
Vaquero J, et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology. 2003;125(3):755–64.
Haussinger D, Schliess F. Astrocyte swelling and protein tyrosine nitration in hepatic encephalopathy. Neurochem Int. 2005;47(1–2):64–70.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. (5th ed.) Arlington, Virginia: American Psychiatric Association; 2013.
Munoz SJ. Hepatic encephalopathy. Med Clin N Am. 2008;92(4):795–812.
Wehbe E, et al. Reversible hepatic decerebration: a case report and review of the literature. Eur J Gastroenterol Hepatol. 2010;22(6):759–60.
Mukherjee KK, Chhabra R, Khosla VK. Raised intracranial pressure in hepatic encephalopathy. Indian J Gastroenterol. 2003;22(S2):62–5.
Hassanein T, et al. Performance of the hepatic encephalopathy scoring algorithm in a clinical trial of patients with cirrhosis and severe hepatic encephalopathy. Am J Gastroenterol. 2009;104(6):1392–400.
Bajaj JS, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138(7):2332–40.
Hassanein TI, Hilsabeck RC, Perry W. Introduction to the hepatic encephalopathy scoring algorithm (HESA). Dig Dis Sci. 2008;53(2):529–38.
Jover R, et al. Minimal hepatic encephalopathy and extrapyramidal signs in patients with cirrhosis. Am J Gastroenterol. 2003;98(7):1599–604.
McCrea M, et al. Neuropsychological characterization and detection of subclinical hepatic encephalopathy. Arch Neurol. 1996;53(8):758–63.
Mooney S, et al. Utility of the repeatable battery for the assessment of neuropsychological status (RBANS) in patients with end-stage liver disease awaiting liver transplant. Arch Clin Neuropsychol. 2007;22(2):175–86.
Weissenborn K, et al. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34(5):768–73.
Ortiz M, et al. Neuropsychological abnormalities in cirrhosis include learning impairment. J Hepatol. 2006;44(1):104–10.
Weissenborn K, et al. Memory function in early hepatic encephalopathy. J Hepatol. 2003;39(3):320–5.
Cadranel J-F, et al. Focal neurological signs in hepatic encephalopathy in cirrhotic patients: an underestimated entity? Am J Gastroenterol. 2001;96(2):515–8.
Morgan MY, et al. The treatment of hepatic encephalopathy. Metab Brain Dis. 2007;22(3–4):389–405.
Phongsamran PV, et al. Pharmacotherapy for hepatic encephalopathy. Drugs. 2010;70(9):1131–48.
Als-Nielsen B. Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ. 2004; https://doi.org/10.1136/bmj.38048.506134.EE. (published 30 March 2004).
Sharma P, Sharma BC. Management of overt hepatic encephalopathy. J Clin Exp Hepatol. 2015;5(Suppl 1):S82–7.
Sharma P, et al. Critical flicker frequency: diagnostic tool for minimal hepatic encephalopathy. J Hepatol. 2007;47(1):67–73.
Mullen KD, Amodio P, Morgan MY. Therapeutic studies in hepatic encephalopathy. Metab Brain Dis. 2007;22(3–4):407–23.
Patidar KR, Bajaj JS. Covert and overt hepatic encephalopathy: diagnosis and management. Clin Gastroenterol Hepatol. 2015;13(12):2048–61.
Sharma BC, et al. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108(9):1458–63.
Sharma P, Sharma BC. Management patterns of hepatic encephalopathy: a Nationwide survey in India. J Clin Exp Hepatol. 2015;5(3):199–203.
Kircheis G, et al. Therapeutic efficacy of L-ornithine-L-aspartate infusions in patients with cirrhosis and hepatic encephalopathy: results of a placebo-controlled, double-blind study. Hepatology. 1997;25(6):1351–60.
Kondrup J, Müller MJ. Energy and protein requirements of patients with chronic liver disease. J Hepatol. 1997;27(1):239–47.
Merli M, Riggio O. Dietary and nutritional indications in hepatic encephalopathy. Metab Brain Dis. 2009;24(1):211–21.
Montano-Loza AJ, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10(2):166–73, 173 e1.
Amodio P, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58(1):325–36.
Ahluwalia V, et al. Impaired gut-liver-brain Axis in patients with cirrhosis. Sci Rep. 2016;6:26800.
Agrawal NK, Sharma B. Prevalence of osteoporosis in otherwise healthy Indian males aged 50 years and above. Arch Osteoporos. 2013;8:116.
McGee RG, et al. Probiotics for patients with hepatic encephalopathy. Cochrane Database Syst Rev. 2011;9(11):CD008716.
Flamm SL. Hot topics in primary care: diagnosis of cirrhosis and evaluation of hepatic encephalopathy: common errors and their significance for the PCP. J Fam Pract. 2017;66(4 Suppl):S34–9.
Egberts EH, et al. Branched chain amino acids in the treatment of latent portosystemic encephalopathy. Gastroenterology. 1985;88(4):887–95.
Plauth M, et al. Long-term treatment of latent portosystemic encephalopathy with branched-chain amino acids. J Hepatol. 1993;17(3):308–14.
Marchesini G, et al. Long-term oral branched-chain amino acid treatment in chronic hepatic encephalopathy. J Hepatol. 1990;11(1):92–101.
Schulz GJ, Campos ACL, JCU C. The role of nutrition in hepatic encephalopathy. Curr Opin Clin Nutr Metab Care. 2008;11:275–80.
Gluud LL, et al. Oral branched-chain amino acids have a beneficial effect on manifestations of hepatic encephalopathy in a systematic review with meta-analyses of randomized controlled trials. J Nutr. 2013;143(8):1263–8.
Gluud LL, et al. Lactulose, rifaximin or branched chain amino acids for hepatic encephalopathy: what is the evidence? Metab Brain Dis. 2013;28(2):221–5.
Stadlbauer V, Wright GAK, Jalan R. Role of artificial liver support in hepatic encephalopathy. Metab Brain Dis. 2009;24(1):15–26.
Stange J. Liver support by extracorporeal blood purification: a clinical observation. Liver Transpl. 2000;6(5):603–13.
Bajaj JS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther. 2010;31(5):537–47.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. Lancet. 1974;2(7872):81–4.
Ortiz M, et al. Development of a clinical hepatic encephalopathy staging scale. Aliment Pharmacol Ther. 2007;26(6):859–67.
Bass NM. Review article: the current pharmacological therapies for hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25(S1):23–31.
Sotil EU, et al. Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transpl. 2009;15(2):184–92.
Eroglu Y, Byrne WJ. Hepatic encephalopathy. Emerg Med Clin North Am. 2009;27(3):401–14.
Mullen KD. Review of the final report of the 1998 working party on definition, nomenclature and diagnosis of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25(S1):11–6.
Jalan R. Pathophysiological basis of therapy of raised intracranial pressure in acute liver failure. Neurochem Int. 2005;47(1–2):78–83.
Rovira A, Alonso J, Cordoba J. MR imaging findings in hepatic encephalopathy. Am J Neuroradiol. 2008;29(9):1612–21.
Córdoba J, et al. The development of low-grade cerebral edema in cirrhosis is supported by the evolution of 1H-magnetic resonance abnormalities after liver transplantation. J Hepatol. 2001;35(5):598–604.
Rovira A, et al. Normalization of T2 signal abnormalities in hemispheric white matter with liver transplant. Neurology. 2002;59(3):335–41.
Córdoba J, et al. T2 hyperintensity along the cortico-spinal tract in cirrhosis relates to functional abnormalities. Hepatology. 2003;38(4):1026–33.
Qi R, et al. Altered effective connectivity network of the basal ganglia in low-grade hepatic encephalopathy: a resting-state fMRI study with granger causality analysis. PLoS One. 2013;8(1):e53677.
Foley JM, Watson CW, RD A. Significance of the electroencephalographic changes in hepatic coma. Trans Am Neurol Assoc. 1950;51:161–5.
Guerit J-M, et al. Neurophysiological investigations of hepatic encephalopathy: ISHEN practice guidelines. Liver Int. 2009;29(6):789–96.
Guérit JM, et al. Consensus on the use of neurophysiological tests in the intensive care unit (ICU): electroencephalogram (EEG), evoked potentials (EP), and electroneuromyography (ENMG). Neurophysiol Clin/Clin Neurophysiol. 2009;39(2):71–83.
Amodio P, et al. Spectral versus visual EEG analysis in mild hepatic encephalopathy. Clin Neurophysiol. 1999;110(8):1334–44.
Saxena N, et al. Electrophysiological and neuropsychological tests for the diagnosis of subclinical hepatic encephalopathy and prediction of overt encephalopathy. Liver. 2002;22(3):190–7.
Kügler CFA, et al. Visual event-related P300 potentials in early portosystemic encephalopathy. Gastroenterology. 1992;103(1):302–10.
Kügler CFA, et al. Dynamics of cognitive brain dysfunction in patients with cirrhotic liver disease: an event-related P300 potential perspective. Electroencephalogr Clin Neurophysiol. 1994;91(1):33–41.
Saxena N, et al. Auditory P300 event-related potentials and number connection test for evaluation of subclinical hepatic encephalopathy in patients with cirrhosis of the liver: a follow-up study. J Gastroenterol Hepatol. 2001;16(3):322–7.
Weissenborn K, et al. Neurophysiological assessment of early hepatic encephalopathy. Electroencephalogr Clin Neurophysiol. 1990;75(4):289–95.
Amodio P, et al. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis. 2004;19(3-4):253–67.
Kircheis G. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology. 2002;35(2):357–66.
Romero-Gómez M, et al. Value of the critical flicker frequency in patients with minimal hepatic encephalopathy. Hepatology. 2007;45(4):879–85.
Torlot FJ, McPhail MJ, Taylor-Robinson SD. Meta-analysis: the diagnostic accuracy of critical flicker frequency in minimal hepatic encephalopathy. Aliment Pharmacol Ther. 2013;37(5):527–36.
O’Carroll RE. Neuropsychological aspects of liver disease and its treatment. Neurochem Res. 2008;33(4):683–90.
Colombato L. The role of Transjugular intrahepatic portosystemic shunt (TIPS) in the Management of Portal Hypertension. J Clin Gastroenterol. 2007;41(Supplement 3):S344–51.
Montagnese S, et al. Hepatic encephalopathy: you should only comment on what you have actually measured. J Gastroenterol. 2010;45(3):342–3.
Bajaj JS, et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology. 2009;50(4):1175–83.
Schomerus H, Hamster W. Quality of life in cirrhotics with minimal hepatic encephalopathy. Metab Brain Dis. 2001;16(1-2):37–41.
Randolph C. Repeatable battery for the assessment of neuropsychological status. San Antonio: The Psychological Corporation; 1998.
Sorrell JH, et al. Cognitive impairment in people diagnosed with end-stage liver disease evaluated for liver transplantation. Psychiatry Clin Neurosci. 2006;60(2):174–81.
Butcher JN, et al. Minnesota multiphasic personality inventory-2. Minnepolis: University of Minnesota Press; 2001.
Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83.
Younossi ZM, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45(2):295–300.
Ray Kim W. Reliability and validity of the NIDDK-QA instrument in the assessment of quality of life in ambulatory patients with Cholestatic liver disease. Hepatology. 2000;32(5):924–9.
Gralnek IM, et al. Development and evaluation of the liver disease quality of life instrument in persons with advanced, chronic liver disease-the LDQOL 1.0. Am J Gastroenterol. 2000;95(12):3552–65.
Zhou Y-q, et al. Development and evaluation of the quality of life instrument in chronic liver disease patients with minimal hepatic encephalopathy. J Gastroenterol Hepatol. 2009;24(3):408–15.
Wechsler D. Wechsler Test of Adult Reading. San Antonio: The Psychological Corporation; 2001.
Hartlage L. The Halstead-Reitan neuropsychology test battery: theory and clinical interpretation second edition by Ralph M. Reitan, PhD & Deborah Wolfson, PhD, Tucson, Az: Neuropsychology Press, 1993. Arch Clin Neuropsychol. 1994;9(3):289–90.
Golden C, Freshwater SM. Stroop Color and Word Test: Revised examiner’s manual. Wood Dale: Stoelting Co.; 2002.
Kaplan E, Goodglass H, Weintraub S. Boston naming test. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2001.
Benton AL. Development of a multilingual aphasia battery. J Neurol Sci. 1969;9(1):39–48.
Kongs SK, et al. Wisconsin Card Sorting Test - 64-Card Version. Odessa: Psychological Assessment Resources; 2000.
Matthews CG, Klove H. Instruction manual for the adult neuropsychology test battery. Madison: University of Wisconsin Medical School; 1964.
Beck AT, Steer RA, Brown G. Beck depression inventory. 2nd ed. San Antonio: The Psychological Corporation; 1996.
Beck AT, et al. Beck anxiety inventory. San Antonio: The Psychological Corporation; 1988.
Amodio P, et al. Detection of minimal hepatic encephalopathy: normalization and optimization of the psychometric hepatic encephalopathy score. A neuropsychological and quantified EEG study. J Hepatol. 2008;49(3):346–53.
Randolph C, et al. Neuropsychological assessment of hepatic encephalopathy: ISHEN practice guidelines. Liver Int. 2009;29(5):629–35.
Bajaj JS. Current and future diagnosis of hepatic encephalopathy. Metab Brain Dis. 2010;25(1):107–10.
Amodio P, et al. Improving the inhibitory control task to detect minimal hepatic encephalopathy. Gastroenterology. 2010;139(2):510–518.e1-2.
Bajaj JS, et al. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology. 2008;135(5):1591–1600.e1.
Franklin GM, et al. Correlation of neuropsychological and MRI findings in chronic/progressive multiple sclerosis. Neurology. 1988;38(12):1826–9.
Smith A. Symbol digit modalities test. Los Angeles: Western Psychological Services; 1973.
Mattarozzi K, et al. Distinguishing between clinical and minimal hepatic encephalopathy on the basis of specific cognitive impairment. Metab Brain Dis. 2005;20(3):243–9.
Ripamonti R, et al. Transjugular intrahepatic portosystemic shunt-related complications and practical solutions. Semin Intervent Radiol. 2006;23(2):165–76.
Oscar-Berman M, et al. Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. Handb Clin Neurol. 2014;125:183–210.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Musgrave, H., Hilsabeck, R.C. (2019). Hepatic Encephalopathy. In: Ravdin, L.D., Katzen, H.L. (eds) Handbook on the Neuropsychology of Aging and Dementia. Clinical Handbooks in Neuropsychology. Springer, Cham. https://doi.org/10.1007/978-3-319-93497-6_41
Download citation
DOI: https://doi.org/10.1007/978-3-319-93497-6_41
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93496-9
Online ISBN: 978-3-319-93497-6
eBook Packages: Behavioral Science and PsychologyBehavioral Science and Psychology (R0)