Abstract
Anaplastic thyroid cancer (ATC) is a highly lethal disease. First-line therapy for a patient diagnosed with this disease includes surgical resection or chemoradiation. Due to variable treatment and its rarity, there is a paucity of prospective and/or randomized controlled literature studying the initial therapy for patients diagnosed with ATC. To understand which therapy is more appropriate in terms of survival and quality of life, we evaluated the available literature and our own institutional experience with the management of ATC for recommendations regarding this topic. This chapter provides a summary of the pertaining literature and offers recommendations based on these sources for first-line management of a patient with newly diagnosed ATC.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Anaplastic thyroid carcinoma (ATC) is a rare but highly lethal disease. It comprises 1.7% of all newly diagnosed thyroid cancers each year in the United States [1]. In a review article looking at 1771 patients with ATC who were reported in clinical studies, 64% were women, the median survival was 5 months and the 1-year survival was 20% [1]. It typically occurs in the sixth or seventh decade of life. Although there is much scientific research into disease understanding and treatment of ATC, the median overall survival has not changed in recent decades. ATC is poorly differentiated in that it does not produce thyroglobulin, cannot transport iodine and it lacks genetic changes associated with follicular-origin thyroid cancers [2]. Thus, radioactive iodine as an adjuvant therapy is not considered for ATC as it is for the majority of thyroid cancers. In most cases, the prognosis is dismal even with the most aggressive treatment. Clinically, ATC presents most commonly with a rapidly growing neck mass in a short period of time, sometimes days to weeks. Histopathological confirmation is usually obtained by fine needle aspiration but often an open or core needle biopsy is required for diagnosis.
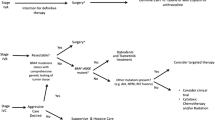
The first-line therapy for a newly diagnosed patient with ATC is controversial. Given the near certain lethality from the disease soon after diagnosis, clinicians have a small window of time to prescribe treatment expeditiously that may improve survival and quality of life. As far as published data and recommendations on management of ATC, the rarity of the tumor forces researchers to utilize multi-institutional data often spanning over several decades during which treatment and dose, and approach has changed. The decision to operate versus chemoradiation therapy at the onset hinges on several patient factors: location(s) and spread of the cancer, proximity and danger to adjacent vital structures, the imminent life-threating concerns from synchronous metastases, comorbidities, and the wishes of the patient. The chapter includes a review of the American Thyroid Association (ATA) guidelines on ATC and published literature to arrive at the most evidence-based approach to first-line therapy (Table 12.1). Overall survival of patients with ATC patients and clinical trials have only lead to small differences in median survival (generally at most 2 months). Depending on an individual patient, the possibility of improving median survival by a few weeks or months may be considered significant or futile [3]. The published clinical trials on ATC have exemplified the challenges for trial design in this very rare and quickly lethal disease. Most trials have been small and often have been terminated early due to poor accrual or inadequate treatment response to justify risks [4].
Early Multidisciplinary Assessment
An early multidisciplinary discussion is integral to the evaluation, workup and treatment of a patient with ATC. Histopathologic diagnosis is necessary. Usually this diagnosis can be made with fine needle aspiration cytology. Infrequently, a surgical incisional biopsy will need to be done to secure the diagnosis. Once the diagnosis is confirmed, an expeditious discussion among primary physician, endocrinologist, medical oncologist, radiation oncologist, surgeon, and palliative care specialist is due in order to form an acceptable mutually agreed upon plan in the patient’s best interests. Decisions regarding first-line therapy should be made based on available literature, initial staging, presence of synchronous metastases, comorbidities, and patient’s wishes.
Prognostic Factors
Prognostic factors for better suitability and improved outcomes to undergo aggressive treatment include younger age, smaller tumor size, and lack of distant metastases. Existing data suggests a better prognosis for patients less than 70 years old of age. Others have found improved survival outcomes even for those under the age of 60 years [5, 6]. Tumor size less than 5 cm have been associated with a relatively better prognosis [5, 7, 8]. The presence of distant metastases, local tumor extension, rapid tumor growth and poor performance status have all been noted to be poor prognostic factors in a published series from 2005 [9]. In the treatment of ATC, multimodality therapy can be of benefit, however institution of all three modalities, radiation, chemotherapy, and surgery are not always feasible. These prognostic factors should be considered in deciding whether to offer an aggressive tri-modality treatment, but which may have increased complications and significant side effects. Available treatments for ATC are very burdensome for the patient and patients with a reduced performance status or poor prognostic factors may be considered for less aggressive treatment or palliation. Ito et al. demonstrated that patients with a clinically resectable tumor and who underwent multimodal therapy with surgery, chemotherapy and radiation treatment had the longest survival of 13.7 months [10] in comparison to patients who underwent uni or bi-modal treatments.
Determine Resectability
Thyroid ultrasound provides a rapid, non-invasive evaluation of neck mass and regional lymph nodes. Computed tomography (CT) imaging with iodinated contrast can provide important locally invasive structural information and will help determine extent of disease and resectability. If the patient has symptoms or imaging features suggestive of invasion into the recurrent laryngeal nerve, trachea, or esophagus, laryngoscopy for upper airway and larynx, bronchoscopy for trachea, and esophagoscopy for esophagus are warranted as part of the preoperative staging.
Initial resectability requires a detailed evaluation with neck imaging and depends on the presence or absence of local invasion to adjacent structures. Figure 12.1 is a coronal image of a patient with tumor extension into the superior vena cava. This tumor was deemed unresectable due to local invasion into superior vena cava as well into the larynx. Although a decision to resect such aggressive tumors also varies from surgeon to surgeon, it should primarily be dictated by the ability to obtain a R0 or R1 resection with minimal morbidity in the safest way possible.
Metastases with ATC are common. In a series of 41 autopsy cases of ATC, 91% had metastases. The most common sites of metastases were the lungs (78%), intrathoracic lymph nodes (58%), neck lymph nodes (51%), pleura (29%), adrenal glands (24%), liver (20%), brain (18%), heart (18%), and retroperitoneal lymph nodes (18%) [11]. Moreover, up to 50% of patients have distant metastases at presentation [2, 12]. Dedicated imaging of the rest of the body can identify synchronous metastases that may alter first line management. For instance, an impending neurologic crisis either from a growing brain metastasis or a vertebral metastasis compromising the spinal cord, would constitute sufficient cause for delaying primary thyroid surgery until after emergent neurologic care is administered. Similarly, life-threatening pulmonary hemorrhage from metastatic lung disease may demand priority over neck operation [13, 14]. However, the presence of non-life-threating distant metastases is not a strict contraindication for neck resection. In fact, the workup of distant metastases with biopsy and imaging should not delay the treatment of the primary neck disease often warrants immediate attention [14]. Therefore, in a patient with suspected distant metastases and locally resectable disease in the neck, should be offered a neck resection as first line therapy to obtain local tumor control and prevent airway obstruction. Because ATC frequently invades adjacent organs and tissues, the potential for complete tumor extirpation is not always possible at the time of presentation. Nevertheless, impending airway compromise from primary neck disease will be priority in most cases of ATC.
Operative Resection as First Line Therapy
In patients with newly diagnosed ATC who are deemed resectable, the goal is a gross tumor resection with R0 or R1 margins. Several single center retrospective studies suggest that either an R0 or R1 resection correlates with an improved disease-free and overall survival with or without adjunct therapy. One study of 120 patients examined the utility of restricted radical surgery with the intent to clear as much tumor without removal of vital organs such as the esophagus, larynx, and trachea [15]. Overall survival was poor with median survival time of 3.1 months regardless of approach, nevertheless the patients who had a R0 resection had a 15% 5-year survival whereas no patient survived at 5 years who underwent a R1 or R2 resection [15]. In another study of 33 patients, those patients treated with a potentially curative resection had a median survival of 43 months versus 3 months [16]. The operation should be a total or near-total thyroidectomy. In those with extrathyroidal extension, a more aggressive resection is warranted if gross disease can be safely cleared [10, 17]. In a study of 75 patients that looked at multimodal treatment, the patients that had better locoregional control were those that underwent R0/R1 resection with and without chemoradiation. The survival benefit was minimal but there were three patients who survived for more than 5 years and all of them underwent gross tumor resection [18]. Another retrospective study looking at 40 consecutive patients with ATC evaluated surgical resection followed by chemoradiation and found that patients with less invasive primary tumors that were resected initially and had adjuvant therapy had a survival benefit compared to those with more aggressive and less resectable disease (9.6 months versus 4.0 months) [10].
Patients with locoregional invasion should be offered a resection if gross tumor resection can be achieved with minimal morbidity [14]. Since ATC can spread via direct contiguous invasion and through the lymphatics, adjacent anatomical structures and applicable surgical planes in relation to the tumor must be carefully evaluated. More than 80% of patients with ATC present with primary tumors that have already invaded into surrounding structures including the trachea, esophagus, and carotid artery [19, 20]. Moreover, nearly 40% of patient with ATC taken for resection required extended resections [15]. Operative debulking with gross positive margins should never be a goal as the rate of local recurrence are very high. In a retrospective study with 67 patients, 6-month, 1 year, and 3-year survival rates were reported as 92%, 92%, and 83%, respectively after complete resection. This was much diminished to 53%, 35%, and 0% if the patient underwent debulking surgery [8].
Airway Management
Frequently airway obstruction can be the initial presentation or can develop during the treatment of ATC. Often times, mortality from these aggressive tumors results from airway issues. There is a paucity of literature regarding airway management in ATC.
Historically, prophylactic tracheostomy was employed in many cases of ATC for prevention of airway compromise or suffocation. Current guidelines do not recommend for a routine and elective tracheostomy either with oncological operation or as a prophylactic procedure alone [14]. Tracheostomy is often fraught with inherent issues such as excessive secretions, wound infections and potential local complications including bleeding and tumor overgrowth or tube dislodgement. Furthermore, placement of a tracheostomy usually necessitates an inpatient hospital or nursing facility stay and can delay future treatments. Holting et al. in their experience of 170 patients with ATC showed that tracheostomy resulted in delayed radiation treatments and this was mainly due to tracheostomy related complications [21, 22]. Moreover, tracheostomy resulted in a reduction in median survival from 5 months to 2 months. From a quality or life standpoint, many patients with tracheostomy will lose their voice permanently as decannulation or downsizing with the use of speech valve can rarely be achieved. A potential for future tracheostomy in unstable and unintubatable patients can also lead to a decision to perform prophylactic tracheostomy in a small minority of patients.
A rapidly enlarging central compartment mass, unilateral or bilateral vocal cord paralysis, direct tracheal invasion, bleeding into the trachea, or upper airway edema are the usual causes of airway obstruction [23]. Hoarseness, stridor or dyspnea could indicate impending airway obstruction [24]. Every patient with suspected ATC should undergo evaluation of the vocal cords. The best way to evaluate the vocal cords is with fiberoptic laryngoscopy; however, mirror examination may also be acceptable. Fiberoptic laryngoscopy will also help to assess the opposite vocal cord, mobility of the vocal cords, any endolaryngeal pathology and can also identify any extension of disease in the subglottic or upper tracheal regions [14].
Concerning airway management, a patient’s desire and wishes should be taken into consideration in decision-making. A diagnosis of ATC does not necessitate a tracheostomy upfront if the patient is clinically stable from a respiratory standpoint. Medical management with steroids and nebulized epinephrine in conjunction with comfort care measures can be employed. In situations of respiratory distress, securing the airway is the priority. Additionally, management of acute airway issues in the operating room has been advised [23]. Awake intubation in a semirecumbent position, preferably using a flexible laryngoscope should be attempted. The trachea could be almost impossible to reach especially in the presence of a bulky anterior mass requiring the use of a long tracheostomy tube. For long segment tracheal compression, use of airway stents or endotracheal tube via cricothyroidotomy tube have been also described. Figure 12.2 demonstrates a coronal CT image of a patient who had a long tracheostomy tube placed via a cricothyroidotomy for impending airway compromise.
The presence of airway compromise in ATC is almost universally associated with a dismal prognosis. Prophylactic tracheostomy in a patient with a biopsy-proven ATC is not indicated. In contrast to most acute airway situations, a clear and informed discussion about the prognosis and goals of care should be considered prior to any airway interventions as these could be the source of much morbidity in a clinical entity with dismal prognosis.
Medical Management as First-Line Therapy
Two subgroups of patients are candidates for medical therapy as first-line therapy. If a patient who has biopsy-proven ATC is deemed to have a tumor that would not allow for a safe resection, neoadjuvant radiotherapy and/or chemotherapy should be administered. Also, in the case where systemic disease is present upon presentation and the neck disease is confined and does not need immediate resection, initial chemoradiation is indicated. If a patient is truly unresectable from a safety and morbidity standpoint, chemoradiation is the preferred initial management. Patients with ATC who present with locoregionally confined with borderline unresectable disease should consider radiotherapy with or without systemic therapy as these patients may become operable candidates [14] (Fig. 12.3).
Radiotherapy
Radiation therapy is delivered with either definitive or palliative intent. Definitive radiotherapy refers to high-dose radiation treatment with the intention to provide long-term local control. Palliative radiotherapy, on the other hand, refers to lower dose treatment in order to alleviate symptoms locally albeit for a shorter duration [14]. In the setting of ATC, external beam radiotherapy (EBRT) is the primary modality of radiation therapy utilized. Intensity-modulated radiotherapy (IMRT) is a type EBRT that allows for radiation to be more conformal to the tumor while sparing adjacent normal tissues. Radiation dose is prescribed in Gray (Gy). Once daily radiation is called standard fractionation. Hyperfractionation is radiation therapy given more than once a day. Hyperfractionated accelerated radiation therapy allows the same total dose to be given in a shorter time frame. This is utilized in ATC with the intent of combatting the rapid repopulation of the tumor.
Dose Benefit
Radiation has been found to prolong survival in ATC, typically by a few months. It is often delivered in conjunction with chemotherapy, but even radiation as a single modality prolongs survival although for a short duration. Radiation dose is a significant prognostic factor. Survival benefit was found in a single institution retrospective review where patients were treated to either a curative dose (>40 Gy) versus a palliative dose of 40 Gy or less [25]. There was a statistically significant improvement in survival for those patients receiving greater than 40 Gy [25] of radiation. Dose is also a significant prognostic factor. Improvements in survival with increasing dose were found to be at 40 Gy in several studies [18, 25, 26]. A higher dose (60 Gy) in some studies demonstrated improvements in survival [26, 27]. In the modern era, higher doses can be delivered with better target coverage and decreased toxicity to normal structures such as the spinal cord utilizing IMRT [28, 29].
Hyperfractionation
However, even though there is a dose response and survival benefit with radiotherapy upfront, survival remains incredibly low. Many institutions have tried to improve survival by altering fractionation. The rationale for the use of hyperfractionation in ATC is due to its rapid doubling time. With hyperfractionation accelerated treatment, the total prescribed dose is delivered over a shorter treatment time. There are several issues with this method, one is patient convenience as the fractions are typically separated by 6 h to decrease toxicity and this involves the patient coming to the radiation center either twice a day or staying for over 6 h at the center. In addition, hyperfractionation typically causes increased acute toxicity such as dysphagia, esophagitis, erythema, and desquamation, but not late side effects [30, 31]. Hyperfractionation has been measured in multiple trials and there is evidence for improved local control, although not always statistically significant, over standard fractionation [7, 25]. Wang et al. treated their radical radiation patients to 60 Gy. Their patients were treated without chemotherapy and a comparison was performed between standard fractionation given in the earlier period of the review (14 patients) vs. twice daily fractionation given in the latter period of the study (9 patients). Standard fractionation was delivered once daily to a total dose of 60 Gy in 30 fractions (6 week course of treatment) or twice daily radiation, most commonly 60 Gy in 40 fractions of 1.5 Gy each (taking 4 weeks to achieve desired dosing) [25]. It was found that there was a trend toward improved overall survival to 13.6 months for patients receiving hyperfractionation vs 10.3 months for standard fractionation, but this difference was not statistically significant (p = 0.3). An important caveat here is that hyperfractionation was only offered to patients with good performance status of Eastern Cooperative Oncology Group (ECOG) grade 2 or better [25].
Hyperfractionated accelerated treatment has resulted in improved local control, but not survival. Other studies have looked to further accelerate the radiation treatment, although at times with severe toxicity. One protocol treated with 1 Gy four times daily, with each fraction separated by 3 h and had a 6% rate of radiation myelitis [32]. Other regimens include increasing acceleration by increase in the per fraction dose, as employed by three consecutive protocols run at Lund Hospital [33]. One protocol treated all patients with 46 Gy of radiation. The protocol initially called for treating with 1 Gy twice daily. The second protocol increased the dose per fraction to 1.3 Gy and the third protocol to 1.6 Gy per fraction. Thus each protocol accelerated the time to completion over the previous one. Acceleration showed the benefit of improved local control but did not improve overall survival [33]. The first two protocols had a treatment break after 30 Gy for surgery, but the third protocol was completed without a break. The final radiation protocol had 100% local control, but the shortest survival at 2 months [33]. Given that overall survival has not improved significantly, some institutions have gone back to the standard fractionation [27, 30].
Many patients with ATC are found to be unresectable and can be offered definitive radiation treatment including hyperfractionation if they have good performance status [34, 35]. In one small series of five patients, survival was 13 months with radiotherapy and chemotherapy [34]. Additionally, radiation therapy could also convert an inoperable tumor to an operable one. Besic et al. in 2001 retrospectively divided the eligible patients treated in his institution into 26 patients who received upfront surgery versus those who were treated with upfront chemoradiation (53 patients) [35]. The patients with upfront chemoradiation had a worse prognosis when compared to upfront surgery group likely due to more aggressive tumors, extension outside of the thyroid capsule, and lymph node metastases. There was no difference in overall survival at 1 year between those two groups, 21% chemotherapy and/or radiotherapy first and 25% for surgery first [35]. Of those 53 patients, 12 became operable. The best overall survival of 50% at 1 year was found to be in those 12 patients who first underwent chemoradiation , followed by surgery [35].
Combined Treatment Modalities
Surgery in addition to radiation improves outcomes. A multivariate analysis of the SEER database found that the combination of surgical resection and radiotherapy decreased the cause-specific mortality and was statistically significant [5]. For those patients who can undergo upfront surgery followed by radiotherapy, they may have a better outcome. In another study, there were three patients who had gross tumor resection upfront followed by radiotherapy and survived more than 2 years. Among patients who did not have surgery and only received radiotherapy, none lived greater than 2 years [36]. Another study also found improved survival in those who had a potentially curable resection followed by radiation and chemotherapy had a median survival of 43 months versus 3 months for those who only had a palliative surgery [16].
In patients undergoing surgery first, several studies have found a local control benefit to those who underwent hyperfractionated radiation as opposed to daily treatment [7, 37]. Although not statistically significant, there were no local recurrences for those receiving hyperfractionated radiation [7]. For patients who underwent an R0 or R1 resection, complete locoregional control was 89% in a previously mentioned study [18].
In some cases, chemotherapy is administered concurrently with radiation to act as a radiation sensitizer by making its the anti-tumor properties more effective for local control [14]. Concurrent chemotherapy is now added to most radiation regimens. However, some data suggests that there is no impact on survival [8, 26]. Conversely, there are studies to support improvement in local response [31], local control [3], progression-free survival [27], and overall survival [9, 38]. Regardless, given the high rate of distant metastases and dismal prognosis, a search for better systemic treatment continues.
Systemic Therapy
There are no standard chemotherapy guidelines for ATC and, therefore, there is no clear data on the most appropriate timing of systemic therapy. The importance of performing comprehensive pre-operative staging to address tumor invasiveness to find out whether patients could benefit from neoadjuvant therapy is unfortunately challenged by this tumor’s rapid doubling time which often require expeditious therapeutic intervention. Systemic chemotherapy and radiotherapy have been used therapeutically together in the neoadjuvant setting to downstage locally unresectable tumor to enable complete resection. Furthermore, chemoradiation is also used in the adjuvant setting to control locoregional disease and distant metastases, and in the palliative setting [2].
Neoadjuvant Therapy
There have been studies suggesting that neoadjuvant chemoradiation should be considered if disease at presentation is considered unresectable. Besic et al. compared outcomes for a primary surgery group with a primary chemotherapy ± radiation group (16 out of 18 patients had radiotherapy) and found there was no survival difference between the two. Notably, the neoadjuvant therapy group was older, had larger and more rapidly growing disease not confined to the thyroid and had more frequent lymph node metastases [35]. In mouse model studies of ATC, administration of a selective BRAF V600E inhibitor PLX4720 for 1 week in mice with unresectable disease enabled thyroidectomy to be performed and modestly affected lifespan. However, tumor growth resumed after this agent was stopped [39].
Cytotoxic Agents
Doxorubicin is the only FDA-approved drug for systemic therapy that may be used to treat ATC, and, while it has achieved modest effects against advanced ATC , it is often used in multi-modal fashion [2]. Since no single cytotoxic agent or combination of agents has demonstrated a very significant survival advantage especially in stage IVC ATC, systemic chemotherapy is recommended through clinical trials [2]. A randomized trial of patients comparing doxorubicin plus cisplatin (n = 18) to doxorubicin alone (n = 21) concluded with 3 complete responses and 3 partial responses in the combination therapy group (18%) versus 1 partial response in the single agent group (5%) [1]. The sequence of therapy was variable (adjuvant vs. induction) but overall response rate was 50% in this small case series [40]. A phase II trial with paclitaxel demonstrated a longer median survival of 32 weeks compared to only 7 weeks in non-responders [3].
Targeted Therapies
The invasive nature of ATC is in part a result of the accumulation of activated/mutated oncogenes and defective tumor suppressor genes along with the high percentage of patients who have distant metastases at diagnosis [41]. Smallridge et al. reported the prevalence of the following mutations: p53 (55%), RAS (22%), BRAF (26%), PIK3CA (17%) and PTEN (12%) [42]. Liu et al. used polymerase chain reaction (PCR) to analyze the role of the MAPK and PI3K pathway in ATC and found tyrosine kinase receptors like epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR) and vascular endothelial growth factor (VEGFR) are frequently amplified in ATC [43]. Furthermore, mutations in the tumor suppressor gene TP53 which controls cell proliferation and apoptosis have been reported in 50–80% cases [44]. Identifying and targeting specific mutations in ATC has shown promising results in pre-clinical mouse model studies [45]. However, translating what is understood about the molecular pathogenesis of ATC from pre-clinical laboratories to clinical trials has not resulted in large survival benefits in this highly lethal tumor.
Several trials targeting tyrosine kinase inhibitors have demonstrated very limited efficacy. Sorafenib, a BRAF/VEGFR inhibitor is being used in to treat advanced radioiodine refractory thyroid cancer. Since BRAF is also mutated in a quarter of ATC cases, a phase II clinical trial using Sorafenib was conducted involving 20 ATC patients. Only 2 of 20 had a partial response and 5 of 20 had stable disease that was short-lived. Toxicities were manageable and included transient hypertension and rash [4]. Imatinib, a tyrosine kinase inhibitor of Bcr-ABL and PDGF, has been implicated in ATC cell proliferation pre-clinically. A phase II clinical trial (terminated early due to poor accrual) enrolled 11 patients with advanced ATC and over-expressing PDGF receptors or cABL (9 of whom had prior chemoradiation), and were started on imatinib 400 mg BID. There were no complete responses, however 75% of patients either had a partial response or stable disease and 6 month overall survival was 45% which is comparable to other cytotoxic regimens, suggesting some activity in advanced tumors [46]. A trial using Pazopanib also had disappointing results demonstrating no tumor responses among the first 14 patients enrolled such that the trial was closed [47].
Other small molecule inhibitors such as EGFR inhibitors have been studied in radio-resistant advanced thyroid cancer. A phase II trial using EGFR inhibitor Gefitinib in advanced, radio-iodine resistant thyroid cancer (19% of which of were ATC, n = 5) described a single patient who had stable disease for 12 months [13]. A phase II trial evaluating the efficacy and safety of mTOR kinase inhibitor in advanced, radio-resistant thyroid cancer (all histology) included 6 patients (15% of total) with ATC. 63% of total patients, including 1 with ATC experienced tumor shrinkage during the study [48]. Because of this unexpected finding, a non-randomized phase II clinical trial of mTOR kinase inhibitor in metastatic ATC has been designed but has not yet begun enrolling patients.
Preclinical studies demonstrated that peroxisome proliferator-activated receptor (PPAR-γ) agonist therapy inhibits ATC cell proliferation and promotes apoptosis when combined with paclitaxel [49]. Subsequently, a phase I clinical trial (which closed early due to poor accrual) treated 15 ATC patients with paclitaxel and variable doses (0.15 mg BID and 0.3 mg BID) of efatutazone. Dose-dependent biologic activity was observed and median survival was 41% greater with the 0.3 mg BID versus the 0.15 mg BID dosing (138 days vs. 98 days) [49].
Vascular disrupting agents which impact tumor blood supply are another area being actively researched. Most recently, a phase II trial of single-agent fosbretabulin in ATC was performed. Fosbretabulin destabilizes microtubules causing vascular disruption and decreased blood flow/necrosis to tumors [50]. Median survival was 4.7 months in this trial. No patients achieved the primary endpoint of the trial (doubling median survival time), however, the 6-month and 12-month survival rates were 34% and 23%, respectively. The authors concluded that these results were comparable to the aforementioned single-agent study using paclitaxel [51].
Combination Therapy
Results from trials using single selective targeted agents in ATC has been disappointing. Randomized trials using a combination of cytotoxic and targeted therapy hope to achieve improved overall survival. One of the largest randomized prospective trials in ATC (n = 80, enrollment stopped early due to low accrual) investigated the overall survival differences between carboplatin/paclitaxel with or without fosbretabulin. The median survival was 5.2 months in the carboplatin/paclitaxel + fosbretabulin arm versus 4.0 months in the carboplatin/paclitaxel arm. Overall survival rates were greater at 6 months and 1 year in the carboplatin/paclitaxel + fosbretabulin group [50]. A Radiation Therapy Oncology Group (RTOG 0912) randomized phase II clinical trial that is ongoing is evaluating IMRT, paclitaxel and pazopanib in ATC is currently open to accrual. The basis for this trial was preclinical data demonstrating that combination pazopanib with paclitaxel had synergistic anti-tumor effects and part of the mechanism was attributed to possible inhibition of aurora A kinase by pazopanib [52].
Conclusion
Anaplastic thyroid cancer (ATC) is a highly lethal disease even with aggressive multimodal treatment. The debate on initial surgical resection or chemoradiation depends on many factors including the ability to obtain a gross tumor resection with low morbidity, other comorbidities, and the patient’s wishes. Historically, palliative measures were the primary treatment options including prophylactic tracheostomy, debulking surgery, and palliative chemoradiation. Although there is a scarcity in prospective literature for ATC treatment, some evidence has suggested that resection is advised in some cases where a RO or R1 resection is possible. Furthermore, airway management has become more tailored to each patient and not the initial and only operation for a patient. Chemoradiation is often the first-line therapy due to the disease extent and invasiveness at presentation. Radiotherapy demonstrates survival benefit and local control but long-term benefit is rare. Systemic therapy has expanded into targeted agents as ATC has been studied and analyzed on a genetic level, but by itself have shown minimal benefit. For many patients who wish to proceed with treatment, chemoradiation is frequently the first-line therapy. Evidence-based literature is low quality as most studies are retrospective and have variable regimens.
Recommendations
Recommendations for Operative Management as First-Line Therapy
-
Once the histopathologic diagnosis of anaplastic thyroid cancer is made, if preoperative imaging demonstrates a tumor that can be resected safely and with low morbidity (Strength of Recommendation: Moderate; Quality of Evidence: Low)
-
If operative management is selected, it should not be delayed by workup and biopsy of other potential distant tumor sites (Strength of Recommendation: Moderate; Quality of Evidence: Low)
-
The presence of distant metastases does not prohibit initial neck resection especially if the neck disease is resectable. If the tumor is confined to the thyroid or can be removed safely with minimal morbidity, neck resection can be performed even in the setting of distant disease, as this may prevent the need for a tracheostomy due to impending airway obstruction. (Strength of Recommendation: Weak; Quality of Evidence: Low)
-
Prophylactic tracheostomy is not indicated in all biopsy-proven cases of ATC. Since it is the source of much morbidity, it should be reserved for those patients with impending airway obstruction. If needed, it should be performed in the most controlled setting in the operating room, not as a bedside or intensive care unit (ICU) procedure. (Strength of Recommendation: Weak; Quality of Evidence: Low)
Recommendations for Chemoradiation as First-Line Therapy
-
If preoperative staging suggests that initial operation would be unsafe and would cause morbidity, chemoradiation is warranted for local control (Strength of Recommendation: Weak; Quality of Evidence: Low)
-
Hyperfractionated radiotherapy may provide local control for locally advanced neck disease and may make the patient a potentially surgical candidate (Strength of Recommendation: Weak; Quality of Evidence: Low)
-
In patients receiving definitive radiotherapy, a higher dose of radiation may be used for better local control and survival benefit. (Strength of Recommendation: Weak; Quality of Evidence: Low)
-
Chemotherapy should be given in conjunction with radiotherapy for synergistic anti-tumor effects. (Strength of Recommendation: Weak; Quality of Evidence: Low)
-
Multiple agents including targeted therapy along with cytotoxic agents may provide greater survival benefit in unresectable patients with distant metastases than single agent therapy. (Strength of Recommendation: Weak; Quality of Evidence: Low)
References
Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol). 2010;22(6):486–97.
Keutgen XM, Sadowski SM, Kebebew E. Management of anaplastic thyroid cancer. Gland Surg. 2015;4(1):44–51.
Derbel O, Limem S, Segura-Ferlay C, Lifante JC, Carrie C, Peix JL, et al. Results of combined treatment of anaplastic thyroid carcinoma (ATC). BMC Cancer. 2011;11:469.
Savvides P, Nagaiah G, Lavertu P, Fu P, Wright JJ, Chapman R, et al. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid. 2013;23(5):600–4.
Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005;103(7):1330–5.
Kim TY, Kim KW, Jung TS, Kim JM, Kim SW, Chung KW, et al. Prognostic factors for Korean patients with anaplastic thyroid carcinoma. Head Neck. 2007;29(8):765–72.
Kobayashi T, Asakawa H, Umeshita K, Takeda T, Maruyama H, Matsuzuka F, et al. Treatment of 37 patients with anaplastic carcinoma of the thyroid. Head Neck. 1996;18(1):36–41.
Pierie JP, Muzikansky A, Gaz RD, Faquin WC, Ott MJ. The effect of surgery and radiotherapy on outcome of anaplastic thyroid carcinoma. Ann Surg Oncol. 2002;9(1):57–64.
Besic N, Hocevar M, Zgajnar J, Pogacnik A, Grazio-Frkovic S, Auersperg M. Prognostic factors in anaplastic carcinoma of the thyroid-a multivariate survival analysis of 188 patients. Langenbecks Arch Surg. 2005;390(3):203–8.
Ito K, Hanamura T, Murayama K, Okada T, Watanabe T, Harada M, et al. Multimodality therapeutic outcomes in anaplastic thyroid carcinoma: improved survival in subgroups of patients with localized primary tumors. Head Neck. 2012;34(2):230–7.
Besic N, Gazic B. Sites of metastases of anaplastic thyroid carcinoma: autopsy findings in 45 cases from a single institution. Thyroid. 2013;23(6):709–13.
Kihara M, Miyauchi A, Yamauchi A, Yokomise H. Prognostic factors of anaplastic thyroid carcinoma. Surg Today. 2004;34(5):394–8.
Smallridge RC. Approach to the patient with anaplastic thyroid carcinoma. J Clin Endocrinol Metab. 2012;97(8):2566–72.
Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104–39.
Passler C, Scheuba C, Prager G, Kaserer K, Flores JA, Vierhapper H, et al. Anaplastic (undifferentiated) thyroid carcinoma (ATC). A retrospective analysis. Langenbecks Arch Surg. 1999;384(3):284–93.
Haigh PI, Ituarte PH, Wu HS, Treseler PA, Posner MD, Quivey JM, et al. Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer. 2001;91(12):2335–42.
Akaishi J, Sugino K, Kitagawa W, Nagahama M, Kameyama K, Shimizu K, et al. Prognostic factors and treatment outcomes of 100 cases of anaplastic thyroid carcinoma. Thyroid. 2011;21(11):1183–9.
Swaak-Kragten AT, de Wilt JH, Schmitz PI, Bontenbal M, Levendag PC. Multimodality treatment for anaplastic thyroid carcinoma--treatment outcome in 75 patients. Radiother Oncol. 2009;92(1):100–4.
Tan RK, Finley RK 3rd, Driscoll D, Bakamjian V, Hicks WL Jr, Shedd DP. Anaplastic carcinoma of the thyroid: a 24-year experience. Head Neck. 1995;17(1):41–7. discussion 7–8.
Zhang ZM, Xu ZG, Tang PZ, Xue LY, Lu N. A retrospective analysis of anaplastic thyroid carcinoma. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006;28(3):322–4.
Holting T, Meybier H, Buhr H. Problems of tracheotomy in locally invasive anaplastic thyroid cancer. Langenbecks Arch Chir. 1989;374(2):72–6.
Holting T, Meybier H, Buhr H. Status of tracheotomy in treatment of the respiratory emergency in anaplastic thyroid cancer. Wien Klin Wochenschr. 1990;102(9):264–6.
Shaha AR. Airway management in anaplastic thyroid carcinoma. Laryngoscope. 2008;118(7):1195–8.
Wein RO, Weber RS. Anaplastic thyroid carcinoma: palliation or treatment? Curr Opin Otolaryngol Head Neck Surg. 2011;19(2):113–8.
Wang Y, Tsang R, Asa S, Dickson B, Arenovich T, Brierley J. Clinical outcome of anaplastic thyroid carcinoma treated with radiotherapy of once- and twice-daily fractionation regimens. Cancer. 2006;107(8):1786–92.
Levendag PC, De Porre PM, van Putten WL. Anaplastic carcinoma of the thyroid gland treated by radiation therapy. Int J Radiat Oncol Biol Phys. 1993;26(1):125–8.
Sherman EJ, Lim SH, Ho AL, Ghossein RA, Fury MG, Shaha AR, et al. Concurrent doxorubicin and radiotherapy for anaplastic thyroid cancer: a critical re-evaluation including uniform pathologic review. Radiother Oncol. 2011;101(3):425–30.
Nutting CM, Convery DJ, Cosgrove VP, Rowbottom C, Vini L, Harmer C, et al. Improvements in target coverage and reduced spinal cord irradiation using intensity-modulated radiotherapy (IMRT) in patients with carcinoma of the thyroid gland. Radiother Oncol. 2001;60(2):173–80.
Posner MD, Quivey JM, Akazawa PF, Xia P, Akazawa C, Verhey LJ. Dose optimization for the treatment of anaplastic thyroid carcinoma: a comparison of treatment planning techniques. Int J Radiat Oncol Biol Phys. 2000;48(2):475–83.
Dandekar P, Harmer C, Barbachano Y, Rhys-Evans P, Harrington K, Nutting C, et al. Hyperfractionated Accelerated Radiotherapy (HART) for anaplastic thyroid carcinoma: toxicity and survival analysis. Int J Radiat Oncol Biol Phys. 2009;74(2):518–21.
De Crevoisier R, Baudin E, Bachelot A, Leboulleux S, Travagli JP, Caillou B, et al. Combined treatment of anaplastic thyroid carcinoma with surgery, chemotherapy, and hyperfractionated accelerated external radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60(4):1137–43.
Wong CS, Van Dyk J, Simpson WJ. Myelopathy following hyperfractionated accelerated radiotherapy for anaplastic thyroid carcinoma. Radiother Oncol. 1991;20(1):3–9.
Tennvall J, Lundell G, Wahlberg P, Bergenfelz A, Grimelius L, Akerman M, et al. Anaplastic thyroid carcinoma: three protocols combining doxorubicin, hyperfractionated radiotherapy and surgery. Br J Cancer. 2002;86(12):1848–53.
Pudney D, Lau H, Ruether JD, Falck V. Clinical experience of the multimodality management of anaplastic thyroid cancer and literature review. Thyroid. 2007;17(12):1243–50.
Besic N, Auersperg M, Us-Krasovec M, Golouh R, Frkovic-Grazio S, Vodnik A. Effect of primary treatment on survival in anaplastic thyroid carcinoma. Eur J Surg Oncol. 2001;27(3):260–4.
Sugino K, Ito K, Mimura T, Nagahama M, Fukunari N, Kubo A, et al. The important role of operations in the management of anaplastic thyroid carcinoma. Surgery. 2002;131(3):245–8.
Heron DE, Karimpour S, Grigsby PW. Anaplastic thyroid carcinoma: comparison of conventional radiotherapy and hyperfractionation chemoradiotherapy in two groups. Am J Clin Oncol. 2002;25(5):442–6.
Troch M, Koperek O, Scheuba C, Dieckmann K, Hoffmann M, Niederle B, et al. High efficacy of concomitant treatment of undifferentiated (anaplastic) thyroid cancer with radiation and docetaxel. J Clin Endocrinol Metab. 2010;95(9):E54–7.
Nehs MA, Nagarkatti S, Nucera C, Hodin RA, Parangi S. Thyroidectomy with neoadjuvant PLX4720 extends survival and decreases tumor burden in an orthotopic mouse model of anaplastic thyroid cancer. Surgery. 2010;148(6):1154–62. discussion 62.
Seto A, Sugitani I, Toda K, Kawabata K, Takahashi S, Saotome T. Chemotherapy for anaplastic thyroid cancer using docetaxel and cisplatin: report of eight cases. Surg Today. 2015;45(2):221–6.
Guerra A, Di Crescenzo V, Garzi A, Cinelli M, Carlomagno C, Tonacchera M, et al. Genetic mutations in the treatment of anaplastic thyroid cancer: a systematic review. BMC Surg. 2013;13(Suppl 2):S44.
Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16(1):17–44.
Ragazzi M, Ciarrocchi A, Sancisi V, Gandolfi G, Bisagni A, Piana S. Update on anaplastic thyroid carcinoma: morphological, molecular, and genetic features of the most aggressive thyroid cancer. Int J Endocrinol. 2014;2014:790834.
Pinto N, Black M, Patel K, Yoo J, Mymryk JS, Barrett JW, et al. Genomically driven precision medicine to improve outcomes in anaplastic thyroid cancer. J Oncol. 2014;2014:936285.
Nehs MA, Nucera C, Nagarkatti SS, Sadow PM, Morales-Garcia D, Hodin RA, et al. Late intervention with anti-BRAF(V600E) therapy induces tumor regression in an orthotopic mouse model of human anaplastic thyroid cancer. Endocrinology. 2012;153(2):985–94.
Ha HT, Lee JS, Urba S, Koenig RJ, Sisson J, Giordano T, et al. A phase II study of imatinib in patients with advanced anaplastic thyroid cancer. Thyroid. 2010;20(9):975–80.
Bible KC, Suman VJ, Menefee ME, Smallridge RC, Molina JR, Maples WJ, et al. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J Clin Endocrinol Metab. 2012;97(9):3179–84.
Lim SM, Chang H, Yoon MJ, Hong YK, Kim H, Chung WY, et al. A multicenter, phase II trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Ann Oncol. 2013;24(12):3089–94.
Smallridge RC, Copland JA, Brose MS, Wadsworth JT, Houvras Y, Menefee ME, et al. Efatutazone, an oral PPAR-gamma agonist, in combination with paclitaxel in anaplastic thyroid cancer: results of a multicenter phase 1 trial. J Clin Endocrinol Metab. 2013;98(6):2392–400.
Sosa JA, Elisei R, Jarzab B, Balkissoon J, Lu SP, Bal C, et al. Randomized safety and efficacy study of fosbretabulin with paclitaxel/carboplatin against anaplastic thyroid carcinoma. Thyroid. 2014;24(2):232–40.
Mooney CJ, Nagaiah G, Fu P, Wasman JK, Cooney MM, Savvides PS, et al. A phase II trial of fosbretabulin in advanced anaplastic thyroid carcinoma and correlation of baseline serum-soluble intracellular adhesion molecule-1 with outcome. Thyroid. 2009;19(3):233–40.
Isham CR, Bossou AR, Negron V, Fisher KE, Kumar R, Marlow L, et al. Pazopanib enhances paclitaxel-induced mitotic catastrophe in anaplastic thyroid cancer. Sci Transl Med. 2013;5(166):166ra3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Abadin, S., Suman, P., Hwang, J., Thakrar, A., Patel, S. (2018). First-Line Therapy for Anaplastic Thyroid Cancer: Operation Versus Medical Management. In: Angelos, P., Grogan, R. (eds) Difficult Decisions in Endocrine Surgery. Difficult Decisions in Surgery: An Evidence-Based Approach. Springer, Cham. https://doi.org/10.1007/978-3-319-92860-9_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-92860-9_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92858-6
Online ISBN: 978-3-319-92860-9
eBook Packages: MedicineMedicine (R0)