Abstract
In recent decades, invasive electrophysiological study (ES) has become an important instrument to evaluate patients with conduction disturbance and cardiac arrhythmias. Using catheters placed in heart chambers via central vein and/or central arterial access, ES may evaluate sinus node function, atrioventricular conduction, and tachyarrhythmias. Cardiac arrhythmias may not always be present in the baseline condition. Therefore, it is necessary to induce these arrhythmias by programmed pacing protocols. Commonly arrhythmias are seen as chaotic alterations of the normal heart conduction and of the normal heart rhythm, and then they are defined as cardiac rhythm disorders. Cardiac arrhythmias present with a common phenotype, characterized by irregularity of the cardiac rhythm and related clinical symptoms. In this setting, ES may be performed by using different diagnostic and pacing catheters and pacing protocols. Programmed pacing protocols involve incremental pacing, coupled with the introduction of single or multiple premature stimuli during one or more drive cycles. The pacing protocols are performed with a current output of twice the diastolic threshold or more, and at one or more sites. Therefore, a great discrepancy may exist between arrhythmia induction techniques in different laboratories. However, in the majority of cases, arrhythmias are due to specific arrhythmic electrical, anatomical, and/or electroanatomical circuits. These circuits respond to specific conduction properties of the systolic and diastolic electrical phases, which are reproducible and evocable by external triggers and by specific pacing techniques. Moreover, in the light of these observations, we have to stress the concept that induction and stimulation programs have to be selected and then paced to test the arrhythmic circuits for refractoriness and to trigger the conduction properties of the arrhythmic pathways. Therefore, we have to make arrhythmia induction protocols more uniform and as standardized as possible to avoid all possible bias. Indeed, how to induce arrhythmias by atrial and ventricular stimulation remains a relevant question that needs a specific and unique response. To respond to this question, we would like to introduce the concept that a pacing protocol to induce cardiac arrhythmias may be standard and programmed. As first, by programmed pacing, physicians may study the properties of the cardiac conduction system. This may be secondarily achieved by introduction of early stimuli to determine the conduction response, as a specific arrhythmia induction protocol. As discussed earlier, the type of induction and the chosen programmed stimulation protocol may be selected with regard to the type of arrhythmia the patient is suspected to have. In fact, re-entry tachycardias may usually be triggered using extrastimuli to stimulate the conduction pathways in slow conduction and fast conduction ways. Differently, automatic tachycardias not due to re-entry mechanisms may be more easily induced by burst pacing. In this chapter we would like to introduce pacing protocols to induce cardiac arrhythmias. Apart from the similarity of the diagnostic and pacing catheters, and in the setting of programmed stimulation protocols, the differences in the paced heart chambers and in the induced tachycardias teach us to separate the discussion of atrial stimulation from ventricular stimulation protocols. Therefore, we schematically divide the arrhythmia induction protocols into two separate chapter sections, discussing atrial stimulation protocols and ventricular stimulation protocols.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others

2.1 Introduction
In recent decades, invasive electrophysiological study (ES) has become an important instrument to evaluate patients with conduction disturbance and cardiac arrhythmias [1]. Using catheters placed in heart chambers via central vein and/or central arterial access, ES may evaluate sinus node function, atrioventricular conduction, and tachyarrhythmias. Cardiac arrhythmias may not always be present in the baseline condition. Therefore, it is necessary to induce these arrhythmias by programmed pacing protocols [1]. Commonly arrhythmias are seen as chaotic alterations of the normal heart conduction and of the normal heart rhythm, and then they are defined as cardiac rhythm disorders [2]. Cardiac arrhythmias present with a common phenotype, characterized by irregularity of the cardiac rhythm and related clinical symptoms [2]. In this setting, ES may be performed by using different diagnostic and pacing catheters and pacing protocols. Programmed pacing protocols involve incremental pacing, coupled with the introduction of single or multiple premature stimuli during one or more drive cycles [3]. The pacing protocols are performed with a current output of twice the diastolic threshold or more, and at one or more sites [3]. Therefore, a great discrepancy may exist between arrhythmia induction techniques in different laboratories. However, in the majority of cases, arrhythmias are due to specific arrhythmic electrical, anatomical, and/or electroanatomical circuits [2]. These circuits respond to specific conduction properties of the systolic and diastolic electrical phases, which are reproducible and evocable by external triggers and by specific pacing techniques [2]. Moreover, in the light of these observations, we have to stress the concept that induction and stimulation programs have to be selected and then paced to test the arrhythmic circuits for refractoriness and to trigger the conduction properties of the arrhythmic pathways. Therefore, we have to make arrhythmia induction protocols more uniform and as standardized as possible to avoid all possible bias. Indeed, how to induce arrhythmias by atrial and ventricular stimulation remains a relevant question that needs a specific and unique response. To respond to this question, we would like to introduce the concept that a pacing protocol to induce cardiac arrhythmias may be standard and programmed [2]. As first, by programmed pacing, physicians may study the properties of the cardiac conduction system. This may be secondarily achieved by introduction of early stimuli to determine the conduction response [2], as a specific arrhythmia induction protocol. As discussed earlier, the type of induction and the chosen programmed stimulation protocol may be selected with regard to the type of arrhythmia the patient is suspected to have. In fact, re-entry tachycardias may usually be triggered using extrastimuli to stimulate the conduction pathways in slow conduction and fast conduction ways [2]. Differently, automatic tachycardias not due to re-entry mechanisms may be more easily induced by burst pacing [2]. In this chapter we would like to introduce pacing protocols to induce cardiac arrhythmias. Apart from the similarity of the diagnostic and pacing catheters, and in the setting of programmed stimulation protocols, the differences in the paced heart chambers and in the induced tachycardias teach us to separate the discussion of atrial stimulation from ventricular stimulation protocols. Therefore, we schematically divide the arrhythmia induction protocols into two separate chapter sections, discussing atrial stimulation protocols and ventricular stimulation protocols.
2.2 Atrial Stimulation
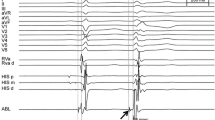
To perform atrial stimulation, the authors place diagnostic quadripolar and/or decapolar catheters in the atrial chambers. These catheters are introduced by central vein access, to map and to pace the right atrium appendage, the coronary sinus, and along the tricuspid valve annulus as indicated by a radioscopic biplane view of the heart chambers. Sometimes, pacing maneuvers may be performed by direct access to the left atrium, at the authors’ discretion. To induce atrial arrhythmias the authors perform programmed pacing protocols divided into coupled pacing protocols and a burst pacing protocol [2]. In the case of a programmed coupled pacing protocol, the authors set a standard pacing protocol choosing a drive of 8 beats as an S1 interval of 600, 500, and then 400 ms, coupled with a first extrastimulus, called S2, that is conventionally at least 60 ms higher than the documented Wenckebach interval [2] (Fig. 2.1). Normally, the authors start with an S1 of 600 ms, then decreasing to 500 and 400 ms. The choice of the S1 cycle length may also depend on the patient’s heart rate. Therefore, at the authors’ discretion the coupled S2 interval is decreased by 10–20 ms for each new pacing train, in a manual and/or automatic way [2]. During each pacing train it is relevant to observe a resting period of 4 s, and to register and to note every arrhythmic event that occurs. In the case of atrial stimulation of re-entrant arrhythmias we may assist with an increase in the supra-Hisian (AH) interval by at least 50 ms from one train to the next, and this is called an “AH jump” [4]. This phenomenon is due to the pacing of the slow pathway of a re-entrant arrhythmic circuit, during the pass on a slow conduction pathway, indicating dual AV node physiology [4]. During programmed and coupled atrial pacing, we may reach a stimulation interval where the atrial pacing is not conducted through the atrioventricular node to the ventricles. This stimulation interval is called the atrioventricular node effective refractory period (AVNERP) [2] (Fig. 2.2). To induce atrial arrhythmias during programmed pacing, we start to shorten the S2 interval until the pacing signal no longer causes the atrium to contract, reaching the atrial effective refractory period (AERP) [2]. Therefore, reaching the refractory S2 interval, we increase the S2 interval to at least 20 ms above the AVNERP, and we repeat this coupled programmed stimulation introducing the secondary (S3) and then the third (S4) coupled extrastimulus as discussed earlier for S2 [2] and/or the AVNERP (in the case of re-entrant atrial arrhythmia induction). Reaching triple refractoriness of the coupled extrastimuli (S2–S3–S4), we stop the S1 programmed stimulation, switching the S1 interval from 600 to 500 and then 400 ms [2]. Moreover, we repeat the same induction protocol until S4 refractoriness occurs and/or in the case of tachycardia induction [2] (Fig. 2.3). Completing all this programmed pacing protocol from 600 to 400 ms in the S1 interval, and until refractoriness of the triple coupled extrastimulus (S2–S3–S4) occurs, we may start burst pacing to achieve a clinical arrhythmia and especially in the case of clinical atrial tachycardia induction [5] (Fig. 2.4). This second type of programmed pacing modality is different from coupled pacing for atrial arrhythmias due to re-entry circuits, and is related to continuous atrial pacing from 300 ms and down by 10–20 ms until 200 ms [5]. In the case of atrial burst pacing there are a few rules to follow. First, the authors consider atrial burst pacing below 200 ms to be contraindicated [5]. Second, it seems intuitive and is commonly agreed to turn off the stimulator immediately in the case of atrial arrhythmia induction [6] (Fig. 2.5). During these pacing protocols we have to choose a standard value for the pacing pulse amplitude in milliamperes, and a duration in milliseconds. Normally these values have to be calculated and then chosen as the lowest values for atrial local capture [2]. All these pacing protocols are started in baseline conditions and may be repeated without coexisting contraindications during infusion of drugs interfering with vagal and sympathetic tone, and during patient maneuvers such as a hand grip [2].
Representation of programmed coupled atrial pacing for atrial arrhythmia induction. The drive of 8 beats is indicated as S1, at a cycle length of 600 ms. The coupled extrastimulus (S2) is at an interval of 450 ms. The pulse amplitude is 10 mA; the duration is 2 ms. The upper part of the figure shows the DI, DII, aVF, and V1 surface ECG derivations. The yellow color denotes the right atrium (RA: 1–2 is distal, 3–4 is proximal); the green color denotes the His bundle (HIS: d is distal, p is proximal). In this case the authors prefer to use quadripolar diagnostic catheters. (Image created by C. Sardu, WorkMate Claris polygraph, Abbot)
Representation of programmed coupled atrial pacing to evaluate the atrioventricular node effective refractory period (AVNERP). The drive of 8 beats is indicated as S1, at a cycle length of 600 ms. The coupled extrastimulus (S2) is at an interval of 400 ms. The pulse amplitude is 10 mA; the duration is 2 ms. As can be seen, we may reach the stimulation interval where the pacing interval does not conduct through the AV node to the ventricles, then called the AVNERP. The upper part of the figure shows the DI, DII, aVF, and V1 surface ECG derivations. The yellow color denotes the right atrium (RA: 1–2 is distal, 3–4 is proximal); the green color denotes the His bundle (HIS: d is distal, p is proximal). In this case the authors prefer to use quadripolar diagnostic catheters. (Image created by C. Sardu, WorkMate Claris polygraph, Abbot)
Representation of programmed coupled atrial pacing for atrial arrhythmia induction. The drive of 8 beats is indicated as S1, at a cycle length of 400 ms. The coupled extrastimuli (S2, S3, and S4) are at an interval of 200 ms. The upper part of the figure shows the DI, DII, aVF, and V1 surface ECG derivations. The yellow color denotes the right atrium (RA: 1–2 is distal, 3–4 is proximal); the green color denotes the His bundle (HIS: d is distal, p is proximal). In this case the authors prefer to use quadripolar diagnostic catheters. (Image created by C. Sardu, WorkMate Claris polygraph, Abbot)
Representation of programmed burst atrial pacing for atrial arrhythmia induction. The drive of beats is indicated as S1, at a cycle length of 300 ms. The upper part of the figure shows the DI, DII, aVF, and V1 surface ECG derivations. The pulse amplitude is 10 mA; the duration is 2 ms. The yellow color denotes the right atrium (RA: 1–2 is distal, 3–4 is proximal); the green color denotes the His bundle (HIS: d is distal, p is proximal). In this case the authors prefer to use quadripolar diagnostic catheters. (Image created by C. Sardu, WorkMate Claris polygraph, Abbot)
Representation of programmed burst atrial pacing for atrial arrhythmia induction. As indicated in the text, the authors perform gradually increasing pacing from 300 to 200 ms, and/or until induction of an atrial arrhythmia. In this case the drive of beats is indicated as S1, and the authors (as discussed in the text) stop the pacing at a cycle length of 220 ms because nonsustained atrial tachycardia is induced. The upper part of the figure shows the DI, DII, aVF, and V1 surface ECG derivations. The pulse amplitude is 10 mA; the duration is 2 ms. The yellow color denotes the right atrium (RA: 1–2 is distal, 3–4 is proximal); the green color denotes the His bundle (HIS: d is distal, p is proximal). In this case the authors prefer to use quadripolar diagnostic catheters. (Image created by C. Sardu, WorkMate Claris polygraph, Abbot)
2.3 Ventricular Stimulation
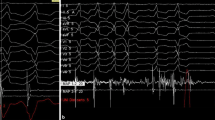
To perform ventricular stimulation the authors set a programmed pacing protocol with a catheter placed in the ventricular chambers. The choice of the catheter and the catheter position may differ between authors. Commonly, the authors prefer to perform quadripolar mapping and place a pacing catheter in at least two different right ventricular positions, such as the right ventricular apex and right ventricular outflow tract, as indicated by biplane fluoroscopic imaging of the heart chambers. Uncommonly, ventricular stimulation may be performed by left ventricular access. During ventricular pacing we study retrograde conduction of the atrioventricular node, and/or we may stimulate a concealed accessory pathway, and this may be the first relevant observation during an ES [2]. In fact, ventricular pacing may be used first to study retrograde atrioventricular node conduction, and secondarily to induce suspected arrhythmias [2]. Therefore, during ventricular pacing, and in the case of a suspected supraventricular arrhythmia, it is important to map the left atrium and left ventricle conduction, introducing a secondary diagnostic catheter into the coronary sinus (a few authors also use a third catheter placed in the right atrial appendage) to observe the retrograde atrioventricular node activation [2] (Fig. 2.6). In fact, during ventricular pacing we may observe concentric and decremental retrograde atrioventricular node conduction in the case of retrograde normal atrioventricular node activation [2]. Sometimes, during S1 ventricular pacing we may observe retrograde atrioventricular dissociation, which confirms retrograde normal atrioventricular node activation [2]. On the other hand, we may not see decremental retrograde atrioventricular node conduction, which may also be eccentric (not retroactivated as proximal to distal by local coronary sinus electrogram analysis), by concealed retrograde atrioventricular accessory pathway pacing [2] (Fig. 2.7). Therefore, during ventricular pacing, we may perform a pacing protocol to study retrograde atrioventricular node activation, and ventricular pacing to induce supraventricular and/or ventricular arrhythmias [2]. As mentioned earlier, we may perform induction pacing for ventricular arrhythmias by a programmed pacing protocol similar to that used for atrial arrhythmia induction. Moreover, we may perform coupled stimulation with eight pacing trains in the drive as S1 with progressive extrastimuli until the refractory periods are found, and/or burst pacing [7] (Figs. 2.8, 2.9, 2.10, and 2.11). During coupled pacing, one may reach the retrograde atrioventricular node effective refractory period (RAVNERP) as an interval in which ventricular pacing is not followed by a retroconducted atrial signal, and then the ventricle effective refractory period (VERP) as an interval in which the ventricular paced extrastimulus is not followed by a local ventricular electrogram [7]. The principal scope of ventricular stimulation is to induce ventricular arrhythmias by programmed pacing protocols. These pacing protocols may be coupled pacing protocols and/or a burst pacing protocol, and the choice of the correct pacing protocol to perform may represent the most relevant question in the setting of life-threatening ventricular arrhythmia induction [8]. As part of this, sometimes ventricular pacing may induce several types of supraventricular tachycardia such as atrioventricular node re-entry, atrioventricular re-entry, and even atrial fibrillation. Generally, to induce ventricular tachycardias the authors perform ventricular pacing by programmed coupled ventricular pacing, by a drive of 8 beats at an S1 interval of 600, 500, and then 400 ms (sometimes the authors prefer only two different drives), decreasing the extrastimuli by 10–20 ms for each new pacing train, in a manual and/or automatic way [7]. The extrastimuli are coupled with the pacing interval until ventricular conduction refractoriness is reached, as a conduction interval in which there is no ventricular capture with pacing [7]. Therefore, the coupled S2 interval is increased by 10–20 ms and repeated as coupled programmed stimulation introducing the secondary (S3) and then the third (S4) coupled extrastimulus in the same modality as discussed earlier in the text (Figs. 2.8 and 2.9). Similarly to atrial arrhythmia induction, during each pacing train we observe a resting period of 4 s, and we register and note every arrhythmic event that occurs. Therefore, we stop the S1 programmed stimulation, switching the S1 interval from 600 to 500 and/or 400 ms (F9), and repeat the same process until S4 (the third coupled extrastimulus) no longer conducts or tachycardia is triggered [2]. Completing all this programmed pacing protocol from a 600- to 400-ms S1 interval, and until refractoriness of the triple coupled extrastimulus (S2–S3–S4) occurs, we may start burst pacing to achieve a clinical ventricular arrhythmia [9] (Figs. 2.10 and 2.11). This second type of programmed pacing modality is different from coupled pacing for ventricular arrhythmias. The burst pacing is due to continuous ventricular pacing from 300 ms and down to 10–20 ms until 200 ms [9] (Figs. 2.10 and 2.11). When pacing protocols are completed through S4 by at least two S1 cycle lengths (600 and 500 and/or 400 ms), with trough burst pacing until 200 ms, we may choose another pacing site such as the right ventricular outflow tract [10]. Indeed, the programmed pacing protocol to induce ventricular arrhythmias is started over again at the second site of pacing [10]. Few authors advocate introduction of the fourth (S5) extrastimulus during ventricular tachycardia induction [7, 11]. This is a relevant discussion point, and authors express different opinions about the fourth extrastimulus to induce ventricular tachycardias [7, 11]. In fact, we have to report a great discrepancy of results regarding the specificity and sensitivity of the fourth coupled pacing protocol, to induce a ventricular arrhythmia [7, 11]. Moreover, we may speculate that it may be enough to perform a coupled pacing maneuver from the right ventricle with two different drives (500, 500, and/or 400 ms), until refractoriness of the third coupled extrastimulus (S2, S3, and S4) occurs, and at two different sites to induce ventricular tachycardias [11]. As referred to by the authors, once the process of pacing at two different ventricular sites has been completed, Isuprel (isoproterenol/isoprenaline) may be used to enhance cardiac conduction, repeating all the programmed pacing induction protocol [12]. This is not the case for patients with known or suspected coronary artery disease, in that there is no indication to use the drug [12]. Once the pacing protocols are fully performed in baseline conditions, and during Isuprel administration, and there is no arrhythmia induction, we have to consider the study result as negative. Similarly to atrial arrhythmia induction, during these pacing protocols we have to choose a standard value of the pacing pulse amplitude in milliamperes, and a duration in milliseconds. This is really important in the case of ventricular tachycardia induction, to avoid the capture of ventricular sites not so close to the tip position of the pacing catheter. Moreover, in the case of ventricular arrhythmia induction, the values of the pacing pulse amplitude and duration have to be calculated and then chosen as the lowest values to have ventricular local capture, and to avoid the induction of nonspecific ventricular arrhythmias and/or ventricular fibrillation [9]. As part of this, we have to remember that during ventricular pacing, we may also induce supraventricular arrhythmias. In this different case, we have to look at the retrograde atrioventricular conduction during ventricular pacing, and the modality of the arrhythmia induction (Fig. 2.12). In fact, in the case of supraventricular arrhythmia induction, the relationship between the ventricular signal and the atrial signal may be diagnostic for the induced tachycardia [13] (Fig. 2.12). Moreover, added to the retrograde atrioventricular activation pathway by an accurate interpretation of endocavitary electrograms, a shorter ventricular–atrial (VA) interval of <70 ms may indicate atrioventricular node re-entry [13]. Conversely, a more prolonged VA interval, such as a VA interval of >70 ms, points toward atrioventricular re-entry tachycardia through a bypass tract and/or atrial tachycardia [13] (Fig. 2.12). In this case ventricular pacing after the arrhythmia induction may be used to perform pacing maneuvers, such as reset and/or entrainment pacing, to differentiate the clinical diagnosis for longer VA interval tachycardias. This point will be discussed in a separate chapter.
Representation of programmed and coupled ventricular pacing to study retrograde atrioventricular node conduction. As indicated in the text, the authors perform 8-beat drive pacing as S1 (in this case 500 ms) coupled with gradually increasing pacing as S2 (in this case S2 is 210 ms). It can be seen that S2 is not conducted to the right ventricle, reaching the effective refractory period of the right ventricle. More than this, retrograde normal nodal conduction can be seen as atrioventricular retrograde dissociation during the drive pacing. The upper part of the figure shows the DI, DII, aVF, and V1 surface ECG derivations. The pulse amplitude is 10 mA; the duration is 2 ms. The yellow color denotes the right atrium (RA: 1–2 is distal, 3–4 is proximal); the green color denotes the His bundle (HIS: d is distal, p is proximal), which in this image is placed in the right ventricular apex. In this case the authors prefer to use quadripolar diagnostic catheters. (Image created by C. Sardu, WorkMate Claris polygraph, Abbot)
Representation of programmed and coupled ventricular pacing to study retrograde atrioventricular node conduction. As indicated in the text, the authors perform 8-beat drive pacing as S1 (in this case 600 ms) coupled with gradually increasing pacing until retrograde atrioventricular refractoriness occurs, as S2 (in this case S2 is 350 ms). The figure is stopped at the S1 drive because during S1 pacing, retrograde eccentric nodal conduction occurs via a concealed retrograde bypass tract. The upper part of the figure shows the DI, DII, aVF, and V1 surface ECG derivations. The pulse amplitude is 10 mA; the duration is 2 ms. The yellow color denotes the His bundle (HIS: 1–2 is distal, 3–4 is proximal) placed in the right ventricular apex; the red color denotes the coronary sinus (CS: d is distal, p is proximal), in this case placed in the CS ostium. In this case the authors prefer to use a quadripolar diagnostic catheter for HIS and a decapolar catheter for CS (discussed in the text). (Image created by C. Sardu, WorkMate Claris polygraph, Abbot)
Representation of programmed and coupled ventricular pacing to induce ventricular arrhythmias. As indicated in the text, the authors perform 8-beat drive pacing as S1 (in this case 500 ms) coupled with gradually increasing pacing by triple coupled beats as S2, S3, and S4 until refractoriness occurs (in this case S2, S3, and S4 are 200 ms). The upper part of the figure shows the DI, DII, aVF, and V1 surface ECG derivations. The pulse amplitude is 5 mA; the duration is 2 ms (the refractory ventricular conduction is at 2 mA with a duration of 2 ms). The yellow color denotes the right atrium (RA: 1–2 is distal, 3–4 is proximal); the green color denotes the His bundle (HIS: d is distal, p is proximal), which in this image is in the right ventricular apex. In this case the authors prefer to use quadripolar diagnostic catheters. (Image created by C. Sardu, WorkMate Claris polygraph, Abbot)
Representation of programmed and coupled ventricular pacing to induce ventricular arrhythmias. As indicated in the text, the authors perform 8-beat drive pacing as S1 (in this case 400 ms) coupled with gradually increasing pacing until retrograde atrioventricular refractoriness occurs, by triple coupled beats as S2, S3, and S4 until refractoriness occurs (in this case S2, S3, and S4 are 200 ms). The upper part of the figure shows the DI, DII, aVF, and V1 surface ECG derivations. The pulse amplitude is 5 mA; the duration is 2 ms (the refractory ventricular conduction is at 2 mA with a duration of 2 ms). The yellow color denotes the right atrium (RA: 1–2 is distal, 3–4 is proximal); the green color denotes the His bundle (HIS: d is distal, p is proximal), which in this image is in the right ventricular apex. In this case the authors prefer to use quadripolar diagnostic catheters. (Image created by C. Sardu, WorkMate Claris polygraph, Abbot)
Representation of programmed burst ventricular pacing for ventricular arrhythmia induction. As indicated in the text, the authors perform gradually increasing and continuous burst pacing from 300 to 200 ms and/or until induction of a ventricular arrhythmia. In this case the burst pacing is indicated as S1 at 300 ms. The upper part of the figure shows the DI, DII, aVF, and V1 surface ECG derivations. The pulse amplitude is 5 mA; the duration is 2 ms. The yellow color denotes the right atrium (RA: 1–2 is distal, 3–4 is proximal); the green color denotes the His bundle (HIS: d is distal, p is proximal) placed in the right ventricular apex. In this case the authors prefer to use quadripolar diagnostic catheters. (Image created by C. Sardu, WorkMate Claris polygraph, Abbot)
Representation of programmed burst ventricular pacing for ventricular arrhythmia induction. As indicated in the text, the authors perform gradually increasing pacing from 300 to 200 ms and/or until induction of a ventricular arrhythmia. In this case the burst pacing is indicated as S1 at 200 ms. The upper part of the figure shows the DI, DII, aVF, and V1 surface ECG derivations. The pulse amplitude is 5 mA; the duration is 2 ms. The yellow color denotes the right atrium (RA: 1–2 is distal, 3–4 is proximal); the green color denotes the His bundle (HIS: d is distal, p is proximal) placed in the right ventricular apex. In this case the authors prefer to use quadripolar diagnostic catheters. (Image created by C. Sardu, WorkMate Claris polygraph, Abbot)
Representation of induction of supraventricular tachycardia by programmed ventricular pacing. The induced arrhythmia has a mean cycle length of 358 ms, with eccentric retrograde atrioventricular activation and first atrial retroactivation in the proximal coronary sinus (CS). As can be seen, this tachycardia has an atrioventricular conduction of 1/1, with a retrograde ventricular–atrial (VA) interval > 70 ms. This is the case in supraventricular tachycardia induced via a concealed retrograde bypass accessory pathway (discussed in the text). The upper part of the figure shows the DI, DII, aVF, and V1 surface ECG derivations. The pulse amplitude is 10 mA; the duration is 2 ms. The yellow color denotes the His bundle (HIS: 1–2 is distal, 3–4 is proximal) placed in the right ventricular apex; the red color denotes the CS (d is distal, p is proximal), in this case placed in the CS ostium. In this case the authors prefer to use a quadripolar diagnostic catheter for HIS and a decapolar catheter for CS (discussed in the text). (Image created by C. Sardu, WorkMate Claris polygraph, Abbot)
References
Scheinman MM, Morady F. Invasive cardiac electrophysiologic testing: the current state of art. Circulation. 1983;67:1169–73.
Josephson ME. Clinical cardiac electrophysiology: techniques and interpretations. 4th ed. Philadelphia: Lippincott Williams & Williams; 2008.
Manolis AS, Cameron J, Deering T, Han EH, Estes NAM. Sensitivity and specificity of programmed atrial stimulation for induction of supraventricular tachycardias. Clin Cardiol. 1988;11:307–10.
Csanadi Z, Klein GJ, Yee R, Thakur RK, Li H. Effect of dual atrioventricular node pathways on atrioventricular reentrant tachycardia. Circulation. 1995;91:2614–8.
Brignole M, Menozzi C, Sartore B, Barra M, Monducci I. The use of atrial pacing to induce atrial fibrillation and flutter. Int J Cardiol. 1986;12:45–54.
Calvo D, Atienza L, Jalife J, Martínez-Alzamora N, Bravo L, Almendral J, et al. High-rate pacing-induced atrial fibrillation effectively reveals properties of spontaneously occurring paroxysmal atrial fibrillation in humans. Europace. 2012;14:1560–6.
Brugada P, Green M, Abdollah H, Wellens HJ. Significance of ventricular arrhythmias initiated by programmed ventricular stimulation: the importance of the type of ventricular arrhythmia induced and the number of premature stimuli required. Circulation. 1984;69:87–92.
Wellens HJJ, Brugada P, Stevenson WG. Programmed electrical stimulation of the heart in patients with life-threatening ventricular arrhythmias: what is the significance of induced arrhythmias and what is the correct stimulation protocol? Circulation. 1985;72:1–7.
Spielman SR, Farshidi A, Horowitz LN, Josephson ME. Ventricular fibrillation during programmed ventricular stimulation: incidence and clinical implications. Am J Cardiol. 1978;42:913–8.
Mann DE, Luck JC, Griffin JC, Herre JM, Limacher MC, Magro SA, et al. Induction of ventricular tachycardia using programmed stimulation: value of third and fourth extrastimuli. Am J Cardiol. 1983;52:501–6.
Herre JM, Mann DE, Luck JC, Magro SA, Figali S, Breen T, et al. Effect of increased current, multiple pacing sites and number of extrastimuli on induction of ventricular tachycardia. Am J Cardiol. 1986;57:102–7.
Sardu C, Carreras G, Katsanos S, Kamperidis V, Pace MC, Passavanti MB, et al. Metabolic syndrome is associated with a poor outcome in patients affected by outflow tract premature ventricular contractions treated by catheter ablation. BMC Cardiovasc Disord. 2014;14:176.
Platia EV, Reid PR. Nonsustained ventricular tachycardia during programmed ventricular stimulation: criteria for a positive test. Am J Cardiol. 1985;56:79–83.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sardu, C. et al. (2019). How to Induce Arrhythmias by Atrial and Ventricular Programmed Stimulation?. In: Cismaru, G. (eds) Arrhythmia Induction in the EP Lab. Springer, Cham. https://doi.org/10.1007/978-3-319-92729-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-92729-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92728-2
Online ISBN: 978-3-319-92729-9
eBook Packages: MedicineMedicine (R0)