Abstract
Cryptococcal meningitis (CM) is the most common cause of adult meningitis in human immunodeficiency virus (HIV) patients with CD4+ cell count <100 cells/μL and is frequently seen in many immunocompromised patients. The respiratory tract is usually the primary site of infection, and the central nervous system is a major site of dissemination due to Cryptococcus neurotropism. Patients with CM present with subacute fever, headache, altered mental status, and even coma. Immune reconstitution inflammatory syndrome (IRIS) in patients with HIV and CM occurs in two forms: paradoxical and unmasking. Identification of risk factors causing IRIS and timely treatment after ruling out residual CM infection are important. Also, increased intracranial pressure plays a major rule in the pathophysiology of CM and needs to be managed promptly to avoid complications. Obtaining lumbar punctures is critical to make the diagnosis and relieve increased intracranial pressure. Cerebrospinal fluid (CSF) should be sent for analysis, fungal cultures, India ink staining, and cryptococcal antigen (CrAg) testing. The use of point-of-care tests for the detection of serum CrAg has a preemptive role in resource-limited settings in ART-naïve, high-risk HIV patients. The use of Amphotericin B formulations in combination with flucytosine is the mainstay of treatment for the induction step of the course, while fluconazole is used in consolidation and maintenance of therapy. Optimizing immunity in immunocompromised patients helps to treat CM. Alternative agents can be used to manage CM or its complications including adalimumab, sertraline, interferon-γ, and new antifungal agents such as Viamet.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Ecology and Mycology of Cryptococcus
First identified in 1894, the genus of Cryptococcus comprises more than 30 known species, of which human infections are almost always caused by Cryptococcus neoformans and Cryptococcus gattii [1, 2]. Based on antigenic determinants on the polysaccharide capsule, the two varieties of C. neoformans are identified as var. grubii [serotype A] and var. neoformans [serotype D], while C. gattii includes serotypes B and C [3]. Recent genetic studies propose to redivide the two species into seven separate species and genotypes [4].
C. neoformans and C. gattii are encapsulated, heterobasidiomycetous fungi that exist in asexual or sexual stages [1]. C. neoformans was isolated from soil, avian excreta especially pigeons, and many other environmental sources, while C. gattii is restricted to red gum trees (Eucalyptus) [5,6,7,8,9]. The filaments that result from the mating of the two opposite types “alpha” and “a” have basidia that produce 1–2 micron basidiospores, thought to be the infectious propagules [10]. Most environmental and clinical isolates of C. neoformans only have the alpha mating locus shown to be more virulent in mice [11, 12]. This predominance can be explained by the yeast’s ability under certain conditions to produce haploid fruiting without mating and sexual reproduction within the same mating type which may have explained the emergence of the Vancouver Island C. gattii outbreak [11,12,13,14].
Epidemiology and Risk Factors of Cryptococcal Meningitis (CM)
Cryptococcus is not considered a part of the human normal flora [15]. Prior to the era of acquired immune deficiency syndrome (AIDS), data analyzed from 725 isolates revealed that 100% of the isolates from Europe and Japan and more than 85% of the isolates from Canada, the UK, and the USA (except Southern California and Hawaii) were C. neoformans (serotypes A, D, or AD), while 35–100% of the isolates from tropical and subtropical areas were C. gattii (serotypes B and C). Overall, C. neoformans serotypes were 86% of the isolates, and C. gattii serotypes were 13%, and 1% was not typeable [16].
Cryptococcosis remains a rare infection in normal hosts [15]. In fact, most adults and children in New York City were found to have antibodies to C. neoformans antigens, indicating that most of these infections are asymptomatic [17, 18]. In patients with AIDS, most infections are caused by C. neoformans serotype A [19], and C. gattii is much less common even in tropical and subtropical areas [20]. C. gattii is thought to cause disease predominantly in immunocompetent hosts, whereas C. neoformans mostly affects immunosuppressed patients [21], although C. neoformans (serotype A) in Vietnam has been associated with high prevalence of CM in human immunodeficiency virus (HIV)-negative, immunocompetent patients [22].
CM is the most common cause of adult meningitis in HIV patients in areas with high prevalence of HIV [23, 24]. The lower the CD4+ count in HIV patients, the higher the incidence of cryptococcosis, and that skyrockets with CD4+ count <100 cells/μL [25, 26]. The incidence of CM has declined significantly in Europe and the USA following the wide availability of antiretroviral therapy (ART) since 1997. Similarly, the rate of hospitalization in the USA declined from 16.6 million in 1997 to 7.7 million total population in 2009 [27, 28]. This was not seen in Africa as many patients present with a history of ART use and low CD4+ count due to nonadherence and loss of follow-up [29]. The updated analysis of the global burden of HIV-associated CM in 2014 estimated the global annual rate of CM as 223,100 cases and global deaths of 181,100, of which 73% and 75%, respectively, were in sub-Saharan Africa [30]. This is, however, a remarkable reduction from 957,000 annual CM cases and 600,000 deaths estimated in 2009 [31].
In HIV-negative patients, most patients with disseminated cryptococcosis have an identifiable underlying disease. For example, these infections are seen in patients with hematologic malignancies, treatment with corticosteroids, sarcoidosis with or without corticosteroids, and solid organ transplantation (SOT) but not in bone marrow transplantation likely due to the routine use of azole antifungal prophylaxis in these patients. Other populations at risk are patients with abnormalities in cell-mediated immunity [32, 33].
Of note, 51% of HIV-negative patients with cryptococcosis had central nervous system (CNS) involvement , and of that 30% had no apparent predisposing conditions [34]. The “normal host” may actually have subtle or uncommon immune abnormalities [2]. Furthermore, smoking and outdoor occupations was associated with increased risk of cryptococcal infections in HIV-infected patients [35]. Table 5.1 shows common predisposing conditions for CM [34, 36,37,38,39,40,41,42,43,44].
Clinical Manifestations of Cryptococcosis
Pathogenesis, Immune Responses, and Neurotropism
After inhaling the aerosolized basidiospores from the environment, the immune system of a normal host can efficiently kill the yeast [1]. Alternatively, the initial possibly asymptomatic infection is contained in a primary complex in the hilar lymph nodes similar to primary tuberculosis [45]. This process involves CD4+ T cells, interleukin-2 (IL-2), and tumor necrosis factor-α (TNF-α) [1, 46]. On the hand, the infection may disseminate outside the lungs in immunocompromised hosts and sometimes in normal hosts following a primary infection or a reactivation in dormant hosts after a decline of the CD4+ count or the use of corticosteroids [1, 47].
C. neoformans has many virulence factors, of which the capsule is the most defined [48]. The polysaccharide capsule helps to evade phagocytosis by macrophages [49], activates the alternative complement pathway leading to depletion of complements [50], inhibits T-cell activation and pro-inflammatory cytokines such as TNF-α [51, 52], downregulates the antigen-presentation capacity of monocytes [53], and decreases the production of interferon-γ (IFN-γ) which suppresses the IL-12 production leading to inhibition of the protective T-helper type 1 response (Th-1) against C. neoformans [54, 55]. It also enhances HIV replication and resists oxidative stress [1, 48].
Other important virulence factors in C. neoformans which can explain the yeast neurotropism are (A) a laccase enzyme that converts CNS catecholamines to melanin that protects against oxidative stress and exerts multiple cell-wall functions [56], (B) thermotolerance of C. neoformans to high temperatures up to 43 °C compared to C. gattii and serotype D that do not tolerate heat above 40° [57], (C) a urease and metalloprotease Mpr1 enzymes in C. neoformans that facilitate its transcellular migration into the mouse brain [58, 59], and (D) mechanisms in C. neoformans that allow it to survive nutrient starvation in the brain [60].
Pulmonary, Disseminated Disease and Atypical Sites of Infections
Cryptococcus causes a wide spectrum of infections with two major sites: the lungs and the CNS [15]. In immunocompetent hosts, pulmonary infections may be asymptomatic or may present with fever, chills, cough, chest pain, productive cough, hemoptysis, weight loss, and night sweats [61]. C. neoformans may colonize the respiratory tract of patients with chronic lung disease without underlying immune dysfunction. Infection may only involve the lungs associated with negative serum cryptococcal antigen (CrAg), but serum CrAg positivity should prompt ruling out an extrapulmonary focus of infection [62].
Most immunosuppressed patients present symptomatically, and pneumonia may progress faster and cause acute respiratory distress syndrome [63]. These patients may present with meningeal rather than pneumonia symptoms despite having both infections. Other coinfections have to be considered in AIDS patients with CD4+ count <100 cells/μL especially cytomegalovirus (CMV), Nocardia, Pneumocystis, and typical and atypical mycobacteria [1, 64].
Cryptococcus can infect any organ system of the body. Noteworthy, skin involvement is almost exclusively associated with disseminated disease, and lesions can be of any type. Lesions may mimic bacterial cellulitis or abscess, acne vulgaris, molluscum contagiosum, and squamous or basal carcinoma and may originate deeper from the underlying bone or subcutaneous tissue [1, 65, 66]. Of note, SOT recipients on tacrolimus were found to have more skin and soft tissue infections than CNS infections. This may be explained by the antifungal activity of tacrolimus at 37–39 °C and the lower skin temperatures [67]. Another site of the infection is the prostate which is usually asymptomatic, and the isolation of Cryptococcus in the urine indicates disseminated disease [1]. Of note, the prostate may be a reservoir for the yeast which may grow in the urine even after the successful treatment of CM in AIDS patients [68].
CNS and Ocular Disease
CM may present with fever, headache, altered mental status, cranial nerve palsies, lethargy, coma, and memory loss [1, 15]. HIV patients with CM usually present after 2 weeks of the onset of symptoms and have a more disseminated disease, while non-HIV patients with CM may present after 6–12 weeks of the onset, a diagnosis often delayed by the absence of fever in non-HIV patients [2]. HIV patients with CM have more yeast burden, higher CSF CrAg titers, and higher rates of increased cerebrospinal fluid (CSF) pressure [1, 69]. In fact, 51% of HIV patients with CM have an opening pressure of >250 mm H2O [70]. Also, HIV patients are also more likely to have other infections such as Toxoplasma gondii or CNS lymphomas [1]. Interestingly, C. gattii is associated with more cryptococcomas and hydrocephalus than C. neoformans [71].
Ocular disease is frequently seen in patients with CM. The most common findings are papilledema, cranial nerve palsies, and decreased visual acuity due to raised intracranial pressure [72, 73]. Visual loss may occur secondary to optic neuritis or endophthalmitis [74]. Furthermore, ocular coinfection may be seen with CMV and HIV [75]. In addition, compression of the ophthalmic artery may occur during the antifungal therapy due to raised intracranial pressure [1].
Outcomes and Prognostic Factors of CM
A study in Botswana showed no significant difference between the presentation and outcome in HIV-associated CM due to C. neoformans or C. gattii [76]. The updated analysis of the global burden of HIV-associated CM in 2014 estimated the 1-year mortality in patients in care to be 70% in low-income countries, 40% in middle-income countries, 20% in North America, and 30% in Europe, with 1.5 times higher mortality in patients not in care in these regions [30]. Risk factors that influence mortality in HIV-associated CM are CSF fungal burden, decreased sensorium, and the rate of clearance of infection [70]. In addition, HIV infection and cryptococcemia were associated with higher mortality rates, whereas hematologic malignancy and organ failure were not associated with mortality [77]. Also, low CSF white cell count (WCC) (<20 cell/μL) and high CSF CrAg titers >1:1024 were associated with worse outcomes [78].
In the USA, the mortality of HIV-negative patients was higher than HIV-positive patients (35% vs 26%) [77]. This may be attributed to the late presentation, delayed diagnosis, and possibly subtle immune dysfunction [77, 79]. Furthermore, the predictors of mortality of cryptococcosis in HIV-negative patients were shown to be age ≥60 years, hematologic neoplasm and organ dysfunction [34]. Also, a study of C. gattii CM in Australia showed that a CSF CrAg titer of ≥256 was associated with worse neurological consequences and death [80].
Cryptococcal Immune Reconstitution Inflammatory Syndrome (IRIS) in Patients with CM
Although the association between IRIS and CM in HIV patients is well established [81], IRIS has been described also in normal hosts, solid and bone marrow transplant recipients, and hematological malignancy patients on chemotherapy [1, 2, 82] after the immunosuppressive or antirejection regimens have been reduced to strengthen the immune system [83]. In apparently immunocompetent hosts, post-infectious inflammatory response syndrome (PIIRS) happens when cerebral edema and neurological damage are exacerbated by the immune response [79]. Two forms of IRIS identified in HIV patients are paradoxical IRIS in CM patients responding to antifungal therapy who relapse after initiating ART and unmasking IRIS in patients developing CM after starting ART [84].
IRIS may present with relapsing aseptic meningitis, abscess development, increased intracranial pressure, new focal findings, cryptococcomas, or other CNS findings [85, 86]. Risk factors for CM-IRIS include high fungal burden which inhibits leukocyte migration into the CNS [87]; low initial CSF WCC and CSF protein levels as well as lower CSF IFN-γ, TNF-α, IL-2, IL-6, IL-8, and IL-17 cytokines; and higher CSF chemokines of macrocyte chemotactic protein-1, macrophage inflammatory protein-1α, and granulocyte macrophage colony-stimulating factor (GM-CSF). In addition, a rapid improvement of low CD4+ cell count after starting ART is another major risk factor [88,89,90,91]. Predictors of IRIS in transplant patients include host immune responses and discontinuation of calcineurin inhibitors which causes a five times increased risk for IRIS [83]. The optimal time of starting ART and management options of IRIS will be discussed below in the management of CM section.
Diagnosis of CM
CSF Findings
HIV-negative patients with CM have increased CSF protein levels and WCC, while HIV patients have lower CSF protein levels and CSF WCC (median 15 × 106 cells/L) [70]. Low glucose levels and lymphocytic predominance are seen in both groups [2, 92]. India ink staining is a rapid tool for the diagnosis of CM and has a sensitivity of 50–70% in HIV-negative patients [93, 94] and a sensitivity/specificity of 84%/53% in HIV patients [95]. The performance of this test is highly operator-dependent.
CSF Cultures
Most bacterial and fungal media cultures of the CSF can detect the yeast in 3–7 days, with a sensitivity of 50–80% [93]. Biochemical reactions and DNA-based methods can help to identify isolates and distinguish between C. neoformans and C. gattii [96, 97]. Quantitative fungal cultures have been used to assess the rate of clearance and the fungicidal activity of various antifungal drugs [98]. Recently, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) has been studied and can rapidly identify species and genotypes of Cryptococcus [99].
Serum and CSF CrAg
Detection of cryptococcal capsular polysaccharide (glucuronoxylomannan, GXM) Ag in serum and CSF by latex agglutination (LA) and enzyme-linked immunosorbent assay (ELISA) has a sensitivity of 100% for disseminated disease and 94% for meningeal disease [100]. Specificity for CSF and serum CrAg was at least 90% for both LA and ELISA regardless of HIV status [101, 102]. In addition, cross-reactive serum CrAg has been seen in infections with Trichosporon beigelii [103] and Stomatococcus mucilaginosus [104].
A simple, quick, and cheap point-of-care test for the detection of CSF and serum CrAg has been developed; this new bedside lateral flow assay (LFA) has a sensitivity and specificity of 99% [105]. It has a preemptive role in resource-limited settings in the early diagnosis of asymptomatic CM and prevention of IRIS after starting ART [106] and in ART-naïve patients [107]. In addition, LFA’s improved sensitivity offers an advantage over LA and cultures in diagnosing HIV-negative C. gattii meningitis [108].
Radiographic Findings
Brain magnetic resonance imaging (MRI) is more sensitive than computed tomography (CT) in CM, but there are no pathognomonic findings. Findings include lesions in the basal ganglia and midbrain that hyperenhance with T2-weighted images but do not enhance with T1-weighted postcontrast images [1]. Also, findings include hydrocephalus, single or multiple nodules with or without enhancement, dilated Virchow-Robin spaces, pseudocysts, masses, gyral enhancement, cryptococcomas, and lacunar and cortical infarcts [109, 110]. Even with the initiation of ART, these lesions may not resolve in months or years after successful treatment [111]; thus, cultures, symptoms, and clinical findings have to be considered before declaring treatment failure, and in the case of CNS parenchymal lesions, CNS lymphoma and coinfections with Nocardia or Toxoplasma should be ruled out [1]. Chest radiographs (chest X-ray, CT) can show single to multiple, well-defined noncalcified nodules in normal hosts diagnosed by lung biopsy. Other findings include lobar and mass-like infiltrates, hilar lymphadenopathy, lung cavities, and pleural effusions [112]. Disease may progress more rapidly in immunosuppressed patients such as AIDS patients or those receiving high-dose corticosteroids [63].
Management and Complications of CM
Antifungal Therapy
If untreated, CM can progress to altered sensorium, seizures, coma, and even death [2]. The rate of progression of the disease depends on host factors and fungal burden of Cryptococcus. The management of CM according to the practice guidelines of the Infectious Disease Society of America (IDSA) is based on three risk groups: HIV-infected individuals, organ transplant recipients, and non-HIV-infected nontransplant hosts [113]. The course is divided into three steps: induction, consolidation, and maintenance.
The use of Amphotericin B (AmB) has been imperative in the management of CM [114], and its combination with flucytosine (5-Fluorocytosine, 5-FC) was shown to be more fungicidal than AmB alone in sterilizing CSF [115]. The combination of AmB/5-FC was also associated with improved survival [116], reduced nephrotoxicity, shorter hospitalization [117], and prevention of relapse [118].
Patients with or predisposed to renal dysfunction or organ transplant recipients should not receive AmB deoxycholate (AmBd) but should be placed on lipid formulations of AmB (LFAmB) either with liposomal AmB (L-AmB) or AmB lipid complex (ABLC). Monitoring the kidney function is important during therapy with AmBd or LFAmB, and the dose of 5-FC has to be adjusted accordingly [113].
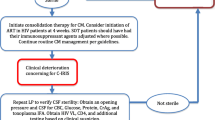
HIV-infected individual should receive induction with AmB/5-FC for at least 2 weeks until clinical response is seen, followed by consolidation with 400–800 mg fluconazole daily for 8–10 weeks and maintenance with 200 mg fluconazole daily for at least 1 year which can be stopped when CD4 count is ≥100 cells/μL, viral load is low or undetectable for ≥3 months, and serum CrAg is negative or low [113]. This requires the successful introduction of ART with the possibility of inducing paradoxical IRIS [113]. Current IDSA 2010 practice guidelines for CM recommend to start ART in 2–10 weeks after initiating induction, although more recent studies suggested 4–6 weeks as the most optimal time to start ART and prevent IRIS [70, 119]. Please see Table 5.2 for detailed recommendations of treatment of CM in HIV patients, SOT recipients, and HIV-negative patients.
5-FC is used in combination with one of AmB formulations for induction for at least 2 weeks (a dose of 100 mg/kg/day or renally adjusted) and should not be used alone as monotherapy can lead to resistance [120]. Monitoring of complete blood counts for bone marrow suppression is important, but it is not necessary to monitor serum drug levels [116, 121]. Also, monitoring of hypokalemia, hypomagnesemia, and acute kidney injury is essential in patients on AmB, and routine intravenous hydration and preemptive electrolyte replacement reduced the rates of hypokalemia and renal toxicity [122].
Azoles such as fluconazole have been used in the management of CM due to its safe profile and excellent penetration into the brain [123, 124]. However, due to its fungistatic properties, fluconazole should not be used in the induction phase when there is a high fungal burden in the CSF [1]. Itraconazole, although has less CSF penetration, was shown to successfully treat CM [125]. When 5-FC is not available, AmB plus fluconazole (800 mg/day superior to 400 mg/day) can be used [126]. Furthermore, fluconazole 1200 mg daily was shown to be more fungicidal than 800 mg daily in HIV-associated CM [127], and its combination with 5-FC (100 mg/kd/day) had early fungicidal activity close to that of AmB alone [128]. Also, voriconazole, posaconazole, and isavuconazole were used as salvage therapy in refractory cases with 38–60% response rates [129,130,131]. Echinocandins are not effective against Cryptococcus [132]. CNS cryptococcomas are treated similarly to CM but may require longer duration and surgical resection is rarely needed [133].
Persistence of CM Infection
Studies showed that in patients with AIDS and CM, at 10 weeks of therapy with AmB or fluconazole alone, 60–65% did not have a successful outcome compared to 35–45% failure rate in those who received a combination of AmB/5-FC or fluconazole/5-FC [134,135,136,137].
Persistence or relapse of infection may be difficult to identify but should be considered after at least 4 weeks of therapy with new signs or symptoms or repeat positive cultures and should not be based only on the persistence of positive India ink staining or CSF CrAg titers [1]. Also, a diagnosis of unmasking IRIS has to be considered in these settings. Most initial isolates of C. neoformans and C. gattii have low minimal inhibitory concentrations (MICs) to AmB, 5-FC, and azoles by in vitro susceptibility testing [138]. Mechanisms of drug resistance in C. neoformans were described [139], and in fact, the clinical response may correlate with MIC levels [140]. Of note, molecular testing confirmed that most recurrent infections represented relapse of the initial strain rather than a new strain [141].
Management of IRIS in Patients with CM
The percentage of patients who develop IRIS (including paradoxical and unmasking) was shown to be 30% by 30 days of starting ART [142]. For patients who are worsening despite a sterile CSF, the IDSA guidelines recommend continuing antifungal therapy and using corticosteroids (0.5–1 mg/kg/day of prednisone equivalent) or dexamethasone at higher doses for severe CNS signs and symptoms [113]. Doses are to be tapered over the next 2–6 weeks. Nonsteroidal anti-inflammatory drugs and thalidomide were used but data is limited [113]. Also, recent cases suggest using TNF-α blockade with adalimumab in patients with severe CM-associated IRIS [143, 144]. Of note, chloroquine was used successfully to treat IRIS that resulted from withdrawal of corticosteroids due to its antifungal effects on Cryptococcus [145].
Management of Increased Intracranial Pressures in Patients with CM
Elevated intracranial pressure plays a critical role in the initial management of HIV-associated CM and improvement clinically and microbiologically [146]. Patients with severe CM often have CSF opening pressure >250 mm, acutely worsening brain edema, and possible development of CSF outflow obstruction [147]. Increased intracranial pressure may cause uncal herniation, tonsillar-cerebellar herniation, or compression of the midbrain [1]. During the early phase of treatment, controlling the increased intracranial pressure may be critical with external drainage by repeat lumbar punctures and ventricular or lumbar drains [148]. Hydrocephalus in patients with CM can be managed safely with placement of ventriculoperitoneal (VP) shunt even with positive CSF cultures [149]. Corticosteroids should only be used in patients with concomitant increased intracranial pressure and IRIS and not without IRIS [150].
Salvage and Adjunctive Immunomodulating Therapy
The use of adjunctive corticosteroids during initial combination antifungal treatment of CM was associated with increased disability, adverse side effects, and decreased rates of fungal clearance of CSF [150]. Stopping corticosteroids in SOT recipients is recommended to optimize immunity [83]; however, discontinuing calcineurin agents was associated with IRIS owing to the synergistic activity of calcineurin inhibitors with antifungals against Cryptococcus [151]. The HIV-negative, apparently immunocompetent host may develop CM due to virulent strains; however, ruling our subtle immunodeficiencies (as outlined in Table 5.1) or idiopathic lymphopenia is recommended [152].
Adding sertraline to AmB and fluconazole has been associated with increased rates of CSF fungal clearance and less IRIS incidence and relapse [153]. Mycograb® which is a humanized Ab against heat shock protein 90 (hsp90) was shown to synergistically render AmB fungicidal and mirror the effects of 5-FC on the killing of C. neoformans [154]. Cytokine therapy with IFN-γ improved the fungal CSF clearance of Cryptococcus without increased adverse events [155]. Also, GM-CSF may enhance the anti-cryptococcal activity of monocytes and neutrophils in HIV patients [156, 157]. In addition, a new oral tetrazole, cytochrome P51 (CYP51) inhibitor Viamet 1129 showed potent in vitro activity against C. neoformans and C. gattii in animal models and may be used for fluconazole-resistant isolates [158, 159].
Screening and Prevention of CM
The goal of this approach is early diagnosis of HIV patients at high risk of developing CM through early detection of CrAg in the blood [106], detectable at median of 22 days before CNS symptoms [160]. The 100% negative predictive value supports its use in screening and preemptive fluconazole in CrAg-positive patients [2, 161]. In fact, the World Health Organization (WHO) supports its use in screening ART-naïve HIV patients with CD4+ count <100 cells/μL and high prevalence of cryptococcal antigenemia (≥3%) [106, 162].
Research Gaps
Despite the use of combination therapy with AmB and 5-FC, CM mortality remains significantly high [30]. Many agents with potential antifungal properties remain under investigation [152]. A vaccine against cryptococcal GXM-tetanus toxoid conjugate was developed and elicited protective antibodies in mice [163]; however, human trials are yet to be conducted. The use of monoclonal antibodies could be promising but is to be further investigated and may require repeated injections [164]. Viamet 1129 is a new oral azole-like agent and may be promising but needs to be evaluated clinically. Further investigations of subtle immune deficiencies that predispose apparently immunocompetent hosts to cryptococcal infections may lead to the discovery of new agents and new mechanisms to better target and treat Cryptococcus.
Abbreviations
- 5-FC:
-
Flucytosine, 5-fluorocytosine
- ABLC:
-
Amphotericin B lipid complex
- AIDS:
-
Acquired immune deficiency syndrome
- AmB:
-
Amphotericin B
- ART:
-
Antiretroviral therapy
- CM:
-
Cryptococcal meningitis
- CMV:
-
Cytomegalovirus
- CNS:
-
Central nervous system
- CrAg:
-
Cryptococcal antigen
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- CYP51:
-
Cytochrome P51
- ELISA:
-
Enzyme-linked immunosorbent assay
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- HIV:
-
Human immunodeficiency virus
- Hsp90:
-
Heat shock protein 90
- IDSA:
-
Infectious Disease Society of America
- IFN-γ:
-
Interferon-γ
- IL:
-
Interleukin
- IRIS:
-
Immune reconstitution inflammatory syndrome
- LA:
-
Latex agglutination
- LFA:
-
Lateral flow assay
- LFAmB:
-
Lipid formulations of AmB
- MALDI-TOF-MS:
-
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- MIC:
-
Minimal inhibitory concentrations
- MRI:
-
Magnetic resonance imaging
- PIIRS:
-
Post-infectious inflammatory response syndrome
- SOT:
-
Solid organ transplantation
- Th-1:
-
T-helper type 1 response
- TNF-α:
-
Tumor necrosis factor-α
- VP:
-
Ventriculoperitoneal
- WCC:
-
White cell count
- WHO:
-
World Health Organization
References
Perfect JR. Cryptococcosis (Cryptococcus neoformans and Cryptococcus gattii). In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 8th ed. Philadelphia: Elsevier; 2015. p. 2934–48.
Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol. 2017;13(1):13–24.
Kwon-Chung K, Boekhout T, Fell J, Diaz M. Proposal to conserve the name Cryptococcus gattii against C. hondurianus and C. bacillisporus (Basidiomycota, Hymenomycetes, Tremellomycetidae). Taxon. 2002;51(4):804–6.
Hagen F, Khayhan K, Theelen B, Kolecka A, Polacheck I, Sionov E, et al. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol. 2015;78:16–48.
Emmons CW. Isolation of Cryptococcus neoformans from soil. J Bacteriol. 1951;62(6):685–90.
Emmons CW. Saprophytic sources of Cryptococcus neoformans associated with the pigeon (Columba livia). Am J Hyg. 1955;62(3):227–32.
Muchmore HG, Rhoades ER, Nix GE, Felton FG, Carpenter RE. Occurrence of Cryptococcus neoformans in the environment of three geographically associated cases of Cryptococcal meningitis. N Engl J Med. 1963;268(20):1112–4.
Bauwens L, Swinne D, De Vroey C, De Meurichy W. Isolation of Cryptococcus neoformans var. neoformans in the aviaries of the Antwerp zoological gardens. Mykosen. 1986;29(7):291.
Levitz SM. The ecology of Cryptococcus neoformans and the epidemiology of cryptococcosis. Rev Infect Dis. 1991;13(6):1163–9.
Botts MR, Giles SS, Gates MA, Kozel TR, Hull CM. Isolation and characterization of Cryptococcus neoformans spores reveal a critical role for capsule biosynthesis genes in spore biogenesis. Eukaryot Cell. 2009;8(4):595–605.
Kwon-Chung KJ, Bennett JE. Distribution of alpha and alpha mating types of Cryptococcus neoformans among natural and clinical isolates. Am J Epidemiol. 1978;108(4):337–40.
Kwon-Chung KJ, Edman JC, Wickes BL. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect Immun. 1992;60(2):602–5.
Wickes BL, Mayorga ME, Edman U, Edman JC. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the alpha-mating type. Proc Natl Acad Sci U S A. 1996;93(14):7327–31.
Fraser JA, Giles SS, Wenink EC, Geunes-Boyer SG, Wright JR, Diezmann S, et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature. 2005;437(7063):1360–4.
Perfect JR, Casadevall A. Cryptococcosis. Infect Dis Clin N Am. 2002;16(4):837–74, v–vi.
Kwon-Chung KJ, Bennett JE. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol. 1984;120(1):123–30.
Chen LC, Goldman DL, Doering TL, Pirofski L, Casadevall A. Antibody response to Cryptococcus neoformans proteins in rodents and humans. Infect Immun. 1999;67(5):2218–24.
Goldman DL, Khine H, Abadi J, Lindenberg DJ, Pirofski L, Niang R, et al. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics. 2001;107(5):E66.
Steenbergen JN, Casadevall A. Prevalence of Cryptococcus neoformans var. neoformans (serotype D) and Cryptococcus neoformans var. grubii (serotype A) isolates in New York City. J Clin Microbiol. 2000;38(5):1974–6.
Morgan J, McCarthy KM, Gould S, Fan K, Arthington-Skaggs B, Iqbal N, et al. Cryptococcus gattii infection: characteristics and epidemiology of cases identified in a South African province with high HIV seroprevalence, 2002–2004. Clin Infect Dis. 2006;43(8):1077–80.
Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21(1):28–34; discussion 5–6.
Chau TT, Mai NH, Phu NH, Nghia HD, Chuong LV, Sinh DX, et al. A prospective descriptive study of cryptococcal meningitis in HIV uninfected patients in Vietnam—high prevalence of Cryptococcus neoformans var grubii in the absence of underlying disease. BMC Infect Dis. 2010;10:199.
Rajasingham R, Rhein J, Klammer K, Musubire A, Nabeta H, Akampurira A, et al. Epidemiology of meningitis in an HIV-infected Ugandan cohort. Am J Trop Med Hyg. 2015;92(2):274–9.
Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis. 2010;10:67.
Crowe SM, Carlin JB, Stewart KI, Lucas CR, Hoy JF. Predictive value of CD4 lymphocyte numbers for the development of opportunistic infections and malignancies in HIV-infected persons. J Acquir Immune Defic Syndr. 1991;4(8):770–6.
Sorvillo F, Beall G, Turner PA, Beer VL, Kovacs AA, Kerndt PR. Incidence and factors associated with extrapulmonary cryptococcosis among persons with HIV infection in Los Angeles County. AIDS. 1997;11(5):673–9.
Dromer F, Mathoulin-Pelissier S, Fontanet A, Ronin O, Dupont B, Lortholary O. Epidemiology of HIV-associated cryptococcosis in France (1985–2001): comparison of the pre- and post-HAART eras. AIDS. 2004;18(3):555–62.
Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. Epidemiology of cryptococcal meningitis in the US: 1997–2009. PLoS One. 2013;8(2):e56269.
Wall EC, Everett DB, Mukaka M, Bar-Zeev N, Feasey N, Jahn A, et al. Bacterial meningitis in Malawian adults, adolescents, and children during the era of antiretroviral scale-up and Haemophilus influenzae type b vaccination, 2000–2012. Clin Infect Dis. 2014;58(10):e137–45.
Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17(8):873–81.
Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–30.
Diamond RD, Bennett JE. Disseminated cryptococcosis in man: decreased lymphocyte transformation in response to Cryptococcus neoformans. J Infect Dis. 1973;127(6):694–7.
Graybill JR, Alford RH. Cell-mediated immunity in Cryptococcosis. Cell Immunol. 1974;14(1):12–21.
Pappas PG, Perfect JR, Cloud GA, Larsen RA, Pankey GA, Lancaster DJ, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33(5):690–9.
Hajjeh RA, Conn LA, Stephens DS, Baughman W, Hamill R, Graviss E, et al. Cryptococcosis: population-based multistate active surveillance and risk factors in human immunodeficiency virus-infected persons. Cryptococcal Active Surveillance Group. J Infect Dis. 1999;179(2):449–54.
Zonios DI, Falloon J, Huang CY, Chaitt D, Bennett JE. Cryptococcosis and idiopathic CD4 lymphocytopenia. Medicine (Baltimore). 2007;86(2):78–92.
Nath DS, Kandaswamy R, Gruessner R, Sutherland DE, Dunn DL, Humar A. Fungal infections in transplant recipients receiving alemtuzumab. Transplant Proc. 2005;37(2):934–6.
Tsiodras S, Samonis G, Boumpas DT, Kontoyiannis DP. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83(2):181–94.
Rosen LB, Freeman AF, Yang LM, Jutivorakool K, Olivier KN, Angkasekwinai N, et al. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol. 2013;190(8):3959–66.
Saijo T, Chen J, Chen SC, Rosen LB, Yi J, Sorrell TC, et al. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. MBio. 2014;5(2):e00912–4.
Lee YC, Chew GT, Robinson BW. Pulmonary and meningeal cryptococcosis in pulmonary alveolar proteinosis. Aust NZ J Med. 1999;29(6):843–4.
Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367(8):725–34.
Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115(8):1519–29.
Hu XP, Wu JQ, Zhu LP, Wang X, Xu B, Wang RY, et al. Association of Fcgamma receptor IIB polymorphism with cryptococcal meningitis in HIV-uninfected Chinese patients. PLoS One. 2012;7(8):e42439.
Salyer WR, Salyer DC, Baker RD. Primary complex of Cryptococcus and pulmonary lymph nodes. J Infect Dis. 1974;130(1):74–7.
Hill JO. CD4+ T cells cause multinucleated giant cells to form around Cryptococcus neoformans and confine the yeast within the primary site of infection in the respiratory tract. J Exp Med. 1992;175(6):1685–95.
Garcia-Hermoso D, Janbon G, Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol. 1999;37(10):3204–9.
Zaragoza O, Chrisman CJ, Castelli MV, Frases S, Cuenca-Estrella M, Rodriguez-Tudela JL, et al. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol. 2008;10(10):2043–57.
Kozel TR, Gotschlich EC. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982;129(4):1675–80.
Levitz SM. Overview of host defenses in fungal infections. Clin Infect Dis. 1992;14(Suppl 1):S37–42.
Mody CH, Syme RM. Effect of polysaccharide capsule and methods of preparation on human lymphocyte proliferation in response to Cryptococcus neoformans. Infect Immun. 1993;61(2):464–9.
Vecchiarelli A, Retini C, Pietrella D, Monari C, Tascini C, Beccari T, et al. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1 beta secretion from human monocytes. Infect Immun. 1995;63(8):2919–23.
Retini C, Vecchiarelli A, Monari C, Bistoni F, Kozel TR. Encapsulation of Cryptococcus neoformans with glucuronoxylomannan inhibits the antigen-presenting capacity of monocytes. Infect Immun. 1998;66(2):664–9.
Pietrella D, Monari C, Retini C, Palazzetti B, Kozel TR, Vecchiarelli A. HIV type 1 envelope glycoprotein gp120 induces development of a T helper type 2 response to Cryptococcus neoformans. AIDS. 1999;13(16):2197–207.
Decken K, Kohler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, et al. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66(10):4994–5000.
Salas SD, Bennett JE, Kwon-Chung KJ, Perfect JR, Williamson PR. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med. 1996;184(2):377–86.
Martinez LR, Garcia-Rivera J, Casadevall A. Cryptococcus neoformans var. neoformans (serotype D) strains are more susceptible to heat than C. neoformans var. grubii (serotype A) strains. J Clin Microbiol. 2001;39(9):3365–7.
Shi M, Li SS, Zheng C, Jones GJ, Kim KS, Zhou H, et al. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest. 2010;120(5):1683–93.
Vu K, Tham R, Uhrig JP, Thompson GR 3rd, Na Pombejra S, Jamklang M, et al. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. MBio. 2014;5(3):e01101–14.
Coelho C, Bocca AL, Casadevall A. The tools for virulence of Cryptococcus neoformans. Adv Appl Microbiol. 2014;87:1–41.
Warr W, Bates JH, Stone A. The spectrum of pulmonary cryptococcosis. Ann Intern Med. 1968;69(6):1109–16.
Randhawa HS, Pal M. Occurrence and significance of Cryptococcus neoformans in the respiratory tract of patients with bronchopulmonary disorders. J Clin Microbiol. 1977;5(1):5–8.
Henson DJ, Hill AR. Cryptococcal pneumonia: a fulminant presentation. Am J Med Sci. 1984;288(5):221–2.
Riley E, Cahan WG. Pulmonary cryptocococcis followed by pulmonary tuberculosis. A case report. Am Rev Respir Dis. 1972;106(4):594–9.
Schupbach CW, Wheeler CE Jr, Briggaman RA, Warner NA, Kanof EP. Cutaneous manifestations of disseminated cryptococcosis. Arch Dermatol. 1976;112(12):1734–40.
Pema K, Diaz J, Guerra LG, Nabhan D, Verghese A. Disseminated cutaneous cryptococcosis. Comparison of clinical manifestations in the pre-AIDS and AIDS eras. Arch Intern Med. 1994;154(9):1032–4.
Odom A, Del Poeta M, Perfect J, Heitman J. The immunosuppressant FK506 and its nonimmunosuppressive analog L-685,818 are toxic to Cryptococcus neoformans by inhibition of a common target protein. Antimicrob Agents Chemother. 1997;41(1):156–61.
Larsen RA, Bozzette S, McCutchan JA, Chiu J, Leal MA, Richman DD. Persistent Cryptococcus neoformans infection of the prostate after successful treatment of meningitis. California Collaborative Treatment Group. Ann Intern Med. 1989;111(2):125–8.
Jongwutiwes U, Sungkanuparph S, Kiertiburanakul S. Comparison of clinical features and survival between cryptococcosis in human immunodeficiency virus (HIV)-positive and HIV-negative patients. Jpn J Infect Dis. 2008;61(2):111–5.
Jarvis JN, Bicanic T, Loyse A, Namarika D, Jackson A, Nussbaum JC, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated Cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis. 2014;58(5):736–45.
Chen S, Sorrell T, Nimmo G, Speed B, Currie B, Ellis D, et al. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis. 2000;31(2):499–508.
Okun E, Butler WT. Ophthalmologic complications of cryptococcal meningitis. Arch Ophthalmol. 1964;71:52–7.
Johnston SR, Corbett EL, Foster O, Ash S, Cohen J. Raised intracranial pressure and visual complications in AIDS patients with cryptococcal meningitis. J Infect. 1992;24(2):185–9.
Rex JH, Larsen RA, Dismukes WE, Cloud GA, Bennett JE. Catastrophic visual loss due to Cryptococcus neoformans meningitis. Medicine. 1993;72(4):207–24.
Doft BH, Curtin VT. Combined ocular infection with cytomegalovirus and cryptococcosis. Arch Ophthalmol. 1982;100(11):1800–3.
Steele KT, Thakur R, Nthobatsang R, Steenhoff AP, Bisson GP. In-hospital mortality of HIV-infected cryptococcal meningitis patients with C. gattii and C. neoformans infection in Gaborone, Botswana. Med Mycol. 2010;48(8):1112–5.
Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. PLoS One. 2013;8(3):e60431.
Anekthananon T, Manosuthi W, Chetchotisakd P, Kiertiburanakul S, Supparatpinyo K, Ratanasuwan W, et al. Predictors of poor clinical outcome of cryptococcal meningitis in HIV-infected patients. Int J STD AIDS. 2011;22(11):665–70.
Panackal AA, Williamson KC, van de Beek D, Boulware DR, Williamson PR. Fighting the monster: applying the host damage framework to human central nervous system infections. MBio. 2016;7(1):e01906–15.
Chen SC, Slavin MA, Heath CH, Playford EG, Byth K, Marriott D, et al. Clinical manifestations of Cryptococcus gattii infection: determinants of neurological sequelae and death. Clin Infect Dis. 2012;55(6):789–98.
Sungkanuparph S, Filler SG, Chetchotisakd P, Pappas PG, Nolen TL, Manosuthi W, et al. Cryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in AIDS patients with cryptococcal meningitis: a prospective multicenter study. Clin Infect Dis. 2009;49(6):931–4.
Singh N, Lortholary O, Alexander BD, Gupta KL, John GT, Pursell K, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40(12):1756–61.
Sun HY, Alexander BD, Huprikar S, Forrest GN, Bruno D, Lyon GM, et al. Predictors of immune reconstitution syndrome in organ transplant recipients with cryptococcosis: implications for the management of immunosuppression. Clin Infect Dis. 2015;60(1):36–44.
Haddow LJ, Colebunders R, Meintjes G, Lawn SD, Elliott JH, Manabe YC, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10(11):791–802.
Meya DB, Okurut S, Zziwa G, Rolfes MA, Kelsey M, Cose S, et al. Cellular immune activation in cerebrospinal fluid from Ugandans with cryptococcal meningitis and immune reconstitution inflammatory syndrome. J Infect Dis. 2015;211(10):1597–606.
Boulware DR, Meya DB, Bergemann TL, Wiesner DL, Rhein J, Musubire A, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7(12):e1000384.
Dong ZM, Murphy JW. Intravascular cryptococcal culture filtrate (CneF) and its major component, glucuronoxylomannan, are potent inhibitors of leukocyte accumulation. Infect Immun. 1995;63(3):770–8.
Longley N, Harrison TS, Jarvis JN. Cryptococcal immune reconstitution inflammatory syndrome. Curr Opin Infect Dis. 2013;26(1):26–34.
Boulware DR, Bonham SC, Meya DB, Wiesner DL, Park GS, Kambugu A, et al. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J Infect Dis. 2010;202(6):962–70.
Chang CC, Lim A, Omarjee S, Levitz SM, Gosnell BI, Spelman T, et al. Cryptococcosis-IRIS is associated with lower cryptococcus-specific IFN-gamma responses before antiretroviral therapy but not higher T-cell responses during therapy. J Infect Dis. 2013;208(6):898–906.
Jarvis JN, Meintjes G, Bicanic T, Buffa V, Hogan L, Mo S, et al. Cerebrospinal fluid cytokine profiles predict risk of early mortality and immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis. PLoS Pathog. 2015;11(4):e1004754.
Liu Y, Kang M, Wu SY, Ma Y, Chen ZX, Xie Y, et al. Different characteristics of cryptococcal meningitis between HIV-infected and HIV-uninfected patients in the southwest of China. Med Mycol. 2017;55(3):255–61.
Saha DC, Xess I, Jain N. Evaluation of conventional & serological methods for rapid diagnosis of cryptococcosis. Indian J Med Res. 2008;127(5):483–8.
Chen M, Zhou J, Li J, Li M, Sun J, Fang WJ, et al. Evaluation of five conventional and molecular approaches for diagnosis of cryptococcal meningitis in non-HIV-infected patients. Mycoses. 2016;59(8):494–502.
Gal AA, Evans S, Meyer PR. The clinical laboratory evaluation of cryptococcal infections in the acquired immunodeficiency syndrome. Diagn Microbiol Infect Dis. 1987;7(4):249–54.
el-Zaatari M, Pasarell L, McGinnis MR, Buckner J, Land GA, Salkin IF. Evaluation of the updated Vitek yeast identification data base. J Clin Microbiol. 1990;28(9):1938–41.
Mitchell TG, Freedman EZ, White TJ, Taylor JW. Unique oligonucleotide primers in PCR for identification of Cryptococcus neoformans. J Clin Microbiol. 1994;32(1):253–5.
Bicanic T, Muzoora C, Brouwer AE, Meintjes G, Longley N, Taseera K, et al. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis. 2009;49(5):702–9.
Firacative C, Trilles L, Meyer W. MALDI-TOF MS enables the rapid identification of the major molecular types within the Cryptococcus neoformans/C. gattii species complex. PLoS One. 2012;7(5):e37566.
Kauffman CA, Bergman AG, Severance PJ, McClatchey KD. Detection of cryptococcal antigen. Comparison of two latex agglutination tests. Am J Clin Pathol. 1981;75(1):106–9.
Panackal AA, Dekker JP, Proschan M, Beri A, Williamson PR. Enzyme immunoassay versus latex agglutination cryptococcal antigen assays in adults with non-HIV-related cryptococcosis. J Clin Microbiol. 2014;52(12):4356–8.
Feldmesser M, Harris C, Reichberg S, Khan S, Casadevall A. Serum cryptococcal antigen in patients with AIDS. Clin Infect Dis. 1996;23(4):827–30.
McManus EJ, Jones JM. Detection of a Trichosporon beigelii antigen cross-reactive with Cryptococcus neoformans capsular polysaccharide in serum from a patient with disseminated Trichosporon infection. J Clin Microbiol. 1985;21(5):681–5.
Chanock SJ, Toltzis P, Wilson C. Cross-reactivity between Stomatococcus mucilaginosus and latex agglutination for cryptococcal antigen. Lancet. 1993;342(8879):1119–20.
Boulware DR, Rolfes MA, Rajasingham R, von Hohenberg M, Qin Z, Taseera K, et al. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg Infect Dis. 2014;20(1):45–53.
Meya DB, Manabe YC, Castelnuovo B, Cook BA, Elbireer AM, Kambugu A, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis. 2010;51(4):448–55.
Letang E, Muller MC, Ntamatungiro AJ, Kimera N, Faini D, Furrer H, et al. Cryptococcal antigenemia in immunocompromised human immunodeficiency virus patients in rural Tanzania: a preventable cause of early mortality. Open Forum Infect Dis. 2015;2(2):ofv046.
Jitmuang A, Panackal AA, Williamson PR, Bennett JE, Dekker JP, Zelazny AM. Performance of the cryptococcal antigen lateral flow assay in non-HIV-related cryptococcosis. J Clin Microbiol. 2016;54(2):460–3.
Loyse A, Moodley A, Rich P, Molloy SF, Bicanic T, Bishop L, et al. Neurological, visual, and MRI brain scan findings in 87 South African patients with HIV-associated cryptococcal meningoencephalitis. J Infect. 2015;70(6):668–75.
Zhong Y, Zhou Z, Fang X, Peng F, Zhang W. Magnetic resonance imaging study of cryptococcal neuroradiological lesions in HIV-negative cryptococcal meningitis. Eur J Clin Microbiol Infect Dis. 2017;36(8):1367–72.
Hospenthal DR, Bennett JE. Persistence of cryptococcomas on neuroimaging. Clin Infect Dis. 2000;31(5):1303–6.
Feigin DS. Pulmonary cryptococcosis: radiologic-pathologic correlates of its three forms. AJR Am J Roentgenol. 1983;141(6):1262–72.
Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010;50(3):291–322.
Sarosi GA, Parker JD, Doto IL, Tosh FE. Amphotericin B in cryptococcal meningitis. Long-term results of treatment. Ann Intern Med. 1969;71(6):1079–87.
Brouwer AE, Rajanuwong A, Chierakul W, Griffin GE, Larsen RA, White NJ, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004;363(9423):1764–7.
Day JN, Chau TT, Wolbers M, Mai PP, Dung NT, Mai NH, et al. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med. 2013;368(14):1291–302.
Utz JP, Garriques IL, Sande MA, Warner JF, Mandell GL, McGehee RF, et al. Therapy of cryptococcosis with a combination of flucytosine and amphotericin B. J Infect Dis. 1975;132(4):368–73.
van der Horst CM, Saag MS, Cloud GA, Hamill RJ, Graybill JR, Sobel JD, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. N Engl J Med. 1997;337(1):15–21.
Boulware DR, Meya DB, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370(26):2487–98.
Utz JP. Flucytosine. N Engl J Med. 1972;286(14):777–8.
Loyse A, Dromer F, Day J, Lortholary O, Harrison TS. Flucytosine and cryptococcosis: time to urgently address the worldwide accessibility of a 50-year-old antifungal. J Antimicrob Chemother. 2013;68(11):2435–44.
Bicanic T, Bottomley C, Loyse A, Brouwer AE, Muzoora C, Taseera K, et al. Toxicity of amphotericin B deoxycholate-based induction therapy in patients with HIV-associated cryptococcal meningitis. Antimicrob Agents Chemother. 2015;59(12):7224–31.
Stern JJ, Hartman BJ, Sharkey P, Rowland V, Squires KE, Murray HW, et al. Oral fluconazole therapy for patients with acquired immunodeficiency syndrome and cryptococcosis: experience with 22 patients. Am J Med. 1988;85(4):477–80.
Yamaguchi H, Ikemoto H, Watanabe K, Ito A, Hara K, Kohno S. Fluconazole monotherapy for cryptococcosis in non-AIDS patients. Eur J Clin Microbiol Infect Dis. 1996;15(10):787–92.
Denning DW, Tucker RM, Hanson LH, Hamilton JR, Stevens DA. Itraconazole therapy for cryptococcal meningitis and cryptococcosis. Arch Intern Med. 1989;149(10):2301–8.
Pappas PG, Chetchotisakd P, Larsen RA, Manosuthi W, Morris MI, Anekthananon T, et al. A phase II randomized trial of amphotericin B alone or combined with fluconazole in the treatment of HIV-associated cryptococcal meningitis. Clin Infect Dis. 2009;48(12):1775–83.
Longley N, Muzoora C, Taseera K, Mwesigye J, Rwebembera J, Chakera A, et al. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis. 2008;47(12):1556–61.
Nussbaum JC, Jackson A, Namarika D, Phulusa J, Kenala J, Kanyemba C, et al. Combination flucytosine and high-dose fluconazole compared with fluconazole monotherapy for the treatment of cryptococcal meningitis: a randomized trial in Malawi. Clin Infect Dis. 2010;50(3):338–44.
Perfect JR, Marr KA, Walsh TJ, Greenberg RN, DuPont B, de la Torre-Cisneros J, et al. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis. 2003;36(9):1122–31.
Pitisuttithum P, Negroni R, Graybill JR, Bustamante B, Pappas P, Chapman S, et al. Activity of posaconazole in the treatment of central nervous system fungal infections. J Antimicrob Chemother. 2005;56(4):745–55.
Thompson GR 3rd, Rendon A, Ribeiro Dos Santos R, Queiroz-Telles F, Ostrosky-Zeichner L, Azie N, et al. Isavuconazole treatment of cryptococcosis and dimorphic mycoses. Clin Infect Dis. 2016;63(3):356–62.
Feldmesser M, Kress Y, Mednick A, Casadevall A. The effect of the echinocandin analogue caspofungin on cell wall glucan synthesis by Cryptococcus neoformans. J Infect Dis. 2000;182(6):1791–5.
Fujita NK, Reynard M, Sapico FL, Guze LB, Edwards JE Jr. Cryptococcal intracerebral mass lesions: the role of computed tomography and nonsurgical management. Ann Intern Med. 1981;94(3):382–8.
Bennett JE, Dismukes WE, Duma RJ, Medoff G, Sande MA, Gallis H, et al. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptoccal meningitis. N Engl J Med. 1979;301(3):126–31.
Larsen RA, Bozzette SA, Jones BE, Haghighat D, Leal MA, Forthal D, et al. Fluconazole combined with flucytosine for treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1994;19(4):741–5.
Larsen RA, Leal MA, Chan LS. Fluconazole compared with amphotericin B plus flucytosine for cryptococcal meningitis in AIDS. A randomized trial. Ann Intern Med. 1990;113(3):183–7.
Robinson PA, Bauer M, Leal MA, Evans SG, Holtom PD, Diamond DA, et al. Early mycological treatment failure in AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1999;28(1):82–92.
Espinel-Ingroff A, Aller AI, Canton E, Castanon-Olivares LR, Chowdhary A, Cordoba S, et al. Cryptococcus neoformans-Cryptococcus gattii species complex: an international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrob Agents Chemother. 2012;56(11):5898–906.
Perfect JR, Cox GM. Drug resistance in Cryptococcus neoformans. Drug Resist Updat. 1999;2(4):259–69.
Aller AI, Martin-Mazuelos E, Lozano F, Gomez-Mateos J, Steele-Moore L, Holloway WJ, et al. Correlation of fluconazole MICs with clinical outcome in cryptococcal infection. Antimicrob Agents Chemother. 2000;44(6):1544–8.
Spitzer ED, Spitzer SG, Freundlich LF, Casadevall A. Persistence of initial infection in recurrent Cryptococcus neoformans meningitis. Lancet. 1993;341(8845):595–6.
Shelburne SA 3rd, Darcourt J, White AC Jr, Greenberg SB, Hamill RJ, Atmar RL, et al. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40(7):1049–52.
Gaube G, De Castro N, Gueguen A, Lascoux C, Zagdanski AM, Alanio A, et al. Treatment with adalimumab for severe immune reconstitution inflammatory syndrome in an HIV-infected patient presenting with cryptococcal meningitis. Med Mal Infect. 2016;46(3):154–6.
Scemla A, Gerber S, Duquesne A, Parize P, Martinez F, Anglicheau D, et al. Dramatic improvement of severe cryptococcosis-induced immune reconstitution syndrome with adalimumab in a renal transplant recipient. Am J Transplant. 2015;15(2):560–4.
Narayanan S, Banerjee C, Holt PA. Cryptococcal immune reconstitution syndrome during steroid withdrawal treated with hydroxychloroquine. Int J Infect Dis. 2011;15(1):e70–3.
Graybill JR, Sobel J, Saag M, van Der Horst C, Powderly W, Cloud G, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis. 2000;30(1):47–54.
Denning DW, Armstrong RW, Lewis BH, Stevens DA. Elevated cerebrospinal fluid pressures in patients with cryptococcal meningitis and acquired immunodeficiency syndrome. Am J Med. 1991;91(3):267–72.
Rolfes MA, Hullsiek KH, Rhein J, Nabeta HW, Taseera K, Schutz C, et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis. 2014;59(11):1607–14.
Park MK, Hospenthal DR, Bennett JE. Treatment of hydrocephalus secondary to cryptococcal meningitis by use of shunting. Clin Infect Dis. 1999;28(3):629–33.
Beardsley J, Wolbers M, Kibengo FM, Ggayi AB, Kamali A, Cuc NT, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374(6):542–54.
Kontoyiannis DP, Lewis RE, Alexander BD, Lortholary O, Dromer F, Gupta KL, et al. Calcineurin inhibitor agents interact synergistically with antifungal agents in vitro against Cryptococcus neoformans isolates: correlation with outcome in solid organ transplant recipients with cryptococcosis. Antimicrob Agents Chemother. 2008;52(2):735–8.
Coelho C, Casadevall A. Cryptococcal therapies and drug targets: the old, the new and the promising. Cell Microbiol. 2016;18(6):792–9.
Rhein J, Morawski BM, Hullsiek KH, Nabeta HW, Kiggundu R, Tugume L, et al. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis. 2016;16(7):809–18.
Nooney L, Matthews RC, Burnie JP. Evaluation of mycograb, amphotericin B, caspofungin, and fluconazole in combination against Cryptococcus neoformans by checkerboard and time-kill methodologies. Diagn Microbiol Infect Dis. 2005;51(1):19–29.
Jarvis JN, Meintjes G, Rebe K, Williams GN, Bicanic T, Williams A, et al. Adjunctive interferon-gamma immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS. 2012;26(9):1105–13.
Tascini C, Vecchiarelli A, Preziosi R, Francisci D, Bistoni F, Baldelli F. Granulocyte-macrophage colony-stimulating factor and fluconazole enhance anti-cryptococcal activity of monocytes from AIDS patients. AIDS. 1999;13(1):49–55.
Coffey MJ, Phare SM, George S, Peters-Golden M, Kazanjian PH. Granulocyte colony-stimulating factor administration to HIV-infected subjects augments reduced leukotriene synthesis and anticryptococcal activity in neutrophils. J Clin Invest. 1998;102(4):663–70.
Lockhart SR, Fothergill AW, Iqbal N, Bolden CB, Grossman NT, Garvey EP, et al. The investigational fungal Cyp51 inhibitor VT-1129 demonstrates potent in vitro activity against Cryptococcus neoformans and Cryptococcus gattii. Antimicrob Agents Chemother. 2016;60(4):2528–31.
Nielsen K, Vedula P, Smith KD, Meya DB, Garvey EP, Hoekstra WJ, et al. Activity of VT-1129 against Cryptococcus neoformans clinical isolates with high fluconazole MICs. Med Mycol. 2017;55(4):453–6.
French N, Gray K, Watera C, Nakiyingi J, Lugada E, Moore M, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16(7):1031–8.
Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48(7):856–62.
WHO Guidelines Approved by the Guidelines Review Committee. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. Geneva: World Health Organization; 2011. http://www.who.int/hiv/pub/cryptococcal_disease2011/en/. Accessed 19 Dec 2017.
Devi SJ, Schneerson R, Egan W, Ulrich TJ, Bryla D, Robbins JB, et al. Cryptococcus neoformans serotype A glucuronoxylomannan-protein conjugate vaccines: synthesis, characterization, and immunogenicity. Infect Immun. 1991;59(10):3700–7.
Mukherjee J, Zuckier LS, Scharff MD, Casadevall A. Therapeutic efficacy of monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan alone and in combination with amphotericin B. Antimicrob Agents Chemother. 1994;38(3):580–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Al Hammadi, A., Ostrosky-Zeichner, L. (2018). Cryptococcal Meningitis. In: Hasbun, R. (eds) Meningitis and Encephalitis. Springer, Cham. https://doi.org/10.1007/978-3-319-92678-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-92678-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92677-3
Online ISBN: 978-3-319-92678-0
eBook Packages: MedicineMedicine (R0)