Abstract
Zoonotic viruses account for 75% of emerging infectious diseases in the world [1]. Many of these zoonotic viruses are arthropod-borne viruses (arboviruses) that can be transmitted by mosquitoes, flies, and ticks [2]. Arboviral infections can produce disease in the central nervous system (CNS) with acute clinical manifestations, such as headache and nuchal rigidity suggestive of meningitis, seizures, mental confusion or coma in cases of encephalitis, and motor dysfunctions in limbs and sphincter dysfunction, related to myelitis. Arbovirus infections can also produce later manifestations in the CNS including Guillain-Barré syndrome and Parkinsonism. The Flaviviridae of Flavivirus genera Zika (ZIKV), the four types of dengue (DENV-1–4), and the Togaviridae of Alphavirus genera chikungunya (CHIKV) are all arboviruses that cause epidemics of acute febrile illnesses in tropical world and, eventually, are reported producing diseases of the CNS [3].
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Zoonotic viruses account for 75% of emerging infectious diseases in the world [1]. Many of these zoonotic viruses are arthropod-borne viruses (arboviruses) that can be transmitted by mosquitoes, flies, and ticks [2]. Arboviral infections can produce disease in the central nervous system (CNS) with acute clinical manifestations, such as headache and nuchal rigidity suggestive of meningitis, seizures, mental confusion or coma in cases of encephalitis, and motor dysfunctions in limbs and sphincter dysfunction, related to myelitis. Arbovirus infections can also produce later manifestations in the CNS including Guillain-Barré syndrome and Parkinsonism. The Flaviviridae of Flavivirus genera Zika (ZIKV) , the four types of dengue (DENV-1–4), and the Togaviridae of Alphavirus genera chikungunya (CHIKV) are all arboviruses that cause epidemics of acute febrile illnesses in tropical world and, eventually, are reported producing diseases of the CNS [3].
ZIKV and CHIKV from Africa and the four types of DENV from Southeastern Asia are viruses originally maintained in cycles involving nonhuman primates and mosquitoes of tree canopy that at some point adapted to new cycles involving humans and the anthropophilic mosquito Aedes aegypti. This African mosquito is anthropophilic and lives in cities at tropical countries worldwide completely adapted to the urban conditions [4]. Other arboviruses such as West Nile (WNV) from the Old World, Japanese encephalitis from Asia, and the Americans Saint Louis encephalitis (SLEV) and Rocio (ROCV) viruses are all Flavivirus phylogenetically grouped in the Japanese encephalitis complex, having birds as reservoirs and mosquitoes of the Culicinae subfamily as vectors [5, 6]. These viruses are commonly reported causing outbreaks or sporadic cases of meningoencephalitis. Another arbovirus that causes human infections of the CNS is the Peribunyaviridae of Orthobunyavirus genera, Oropouche (OROV) . OROV causes outbreaks of acute febrile illness in the Amazon region and Central Plateau of Brazil and neighboring countries. The virus has a sylvatic cycle involving sloths (Bradypus tridactylus), nonhuman primates, and wild birds and Ochlerotatus serratus, Coquillettidia venezuelensis, as well as other mosquitoes as vectors. The virus adapted to an urban cycle involving man, transmitted by the hematophagous midge Culicoides paraensis [7].
Arboviruses of the Flaviviridae family in Flavivirus genera, WNV, JEV, ROCV, SLEV, DENV-1–4, and ZIKV, are enveloped, positive-sense, single-stranded RNA viruses [8]. Arboviruses of Alphavirus genera in the Togaviridae family, CHIKV, are enveloped with a genome of a single positive-sense strand RNA, and three structural proteins of these viruses are translated from a subgenomic mRNA [9]. Arboviruses of Orthobunyavirus genera in the Peribunyaviridae family such as OROV have three-segmented negative-stranded RNA linear genome [10, 11]. Flaviviruses, alphaviruses, and orthobunyaviruses, when infecting humans, can cause meningitis or meningoencephalitis.
Special capabilities are necessary for an arbovirus to infect the CNS. The pathogen, besides being virulent, has to defeat general cellular and humoral immune response, be able to cross the blood-brain barrier (BBB) or the choroid plexus, and when in nervous tissue, defeat the local host defense mechanisms [12, 13].
Genomic mutations can increase the virulence of flaviviruses, alphaviruses, and orthobunyaviruses. An artificial example of that has been seen in ROCV and WNV, two neurovirulent flaviviruses. A chimeric WNV containing prM-E genes of ROCV replicated in mammalian cells more efficiently than WNV or chimeric ROCV containing WNV prM-E genes. WNV containing ROCV prM-E genes was as virulent as ROCV in adult mice. Proteins prM and E of ROCV showed major virulence determinants and inhibited type I interferon response which could potentially enhance neurovirulence [14]. Glycosylation and amino acid changes of the envelope (E) and membrane (M) proteins of WNV and JEV viruses can also increase their neuroinvasive capacities [15, 16].
Innate and adaptive immune system responses to Flavivirus infection can prevent CNS invasion. Immune deficiencies related to age, diabetes mellitus, hypertension, or other chronic diseases increase risk of CNS disease in infections by SLEV and WNV. Infection of the CNS in individuals infected by JEV can be prevented by their normal immune systems. The maturation of defense mechanisms in adolescents can explain the decline of JEV neurological infections compared to children [17]. Activation of MAVS, IRF-3, and IRF-7 and production of type I IFN are able to control OROV replication and restrict tissue injury in mouse experimental model. In infections by OROV, IFN signaling in nonmyeloid cells, probably, contributes to the host defense [10].
Despite not completely understood, factors responsible for neuroinvasion in flaviviruses, alphaviruses, and orthobunyaviruses bypass the BBB by infecting endothelial cells and emerging their viral progenies at the opposite side, inside the CNS. Likewise, viruses can infect leukocytes that migrate to the CNS. Some viruses, after introduced by arthropod bite in subcutaneous tissue, migrate through peripheral axonium reaching the neuron in the CNS [14]. A study in a mouse model showed that OROV, after subcutaneous infection, accesses nervous receptor and neural routes, reaches the spinal cord, and ascends toward the brain producing little inflammation [18].
The local CNS inflammation produces brain edema and ischemia that damages nervous tissues. Blood cells migrate induced by chemokines produced after viral presence in the CNS, and it produces and/or aggravates inflammation. The importance of the impact of macrophages on the severity of the encephalitis has been clearly shown in a study on macrophage chemokines, CCR5 (CC-chemokine receptor 5) and MIP-1 (chemokine receptor that binds to macrophage inflammatory protein), using knockout mice to genes of these chemokines. The knockout animals, after infected with ROCV, survived longer to the meningoencephalitis and had reduced inflammation in the brain than wild-type (WT) mice infected with ROCV. Knockout mice also required a higher lethal dose of ROCV than wild-type mice [19].
In the CNS, the BBB, neurons, and glial and endothelial perivascular cells all have cell membrane receptors for infection by Flavivirus and other arboviruses [14]. In an experiment infecting Balb/C adult mice with ROCV, brain inflammatory changes were produced by immune response induced by Th1 and Th2 cytokines. ROCV encephalitis evolved with irreversible damage of nervous tissues due to neuronal degeneration and apoptosis [20]. Likewise, studies with WNV show that E, M, NS3, and NS4 (the last two are nonstructural) viral proteins elicit response from CD8 T cells in the CNS producing pro-inflammatory cytokines such as interferon (IFN), granzymes A and B, and perforins that lyse infected cells [21]. CD8 T cells also induce apoptosis of neural cells infected by WNV through the Fas ligand, CD40-CD 40 ligand, and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). This is an important measure to prevent persistence of the virus in the CNS [22]. Apoptosis also occurs in mice OROV-infected neurons. Besides that, CNS infection by OROV produces astrocyte activation and glial reaction [18].
Neurologic features of infections by Flavivirus start after 3–15 days of incubation period and usually succeed a nonspecific febrile illness. Neurologic manifestations depend on the affected part of the nervous system, resulting in meningitis, encephalitis, or myelitis [5]. In many cases of encephalitis, overlap reduced level of consciousness, seizures, and flaccid paralysis.
A ZIKV Asian strain from Polynesia has produced large outbreaks of acute febrile illness with rash and conjunctivitis in Brazil since 2015. However, during these outbreaks, reports of severe forms of Zika fever surprised the world. First, it was reported that ZIKV infections increased the number of cases of Guillain-Barré (GBS) muscle paralysis syndrome, a serious neurological disease caused by autoantibodies that damage axons of motor neurons as a consequence of a viral infection [23]. Furthermore, an increase in the incidence of fetal microcephaly was observed in the Northeast Region of Brazil, which was soon associated with maternal infection by ZIKV [24]. Congenital disease caused by ZIKV , having microcephaly as severe manifestation, resulted in an international concern followed by a research effort to obtain information on the different aspects of the disease [25].
Infections of the CNS by ZIKV not associated with congenital disease also occurred. A prospective study in Rio de Janeiro, Southeast of Brazil, among hospitalized adults analyzed 40 patients infected by ZIKV (15 women and 25 men; median age, 44 years) including 27 cases of GBS, 5 cases of encephalitis, 2 of transverse myelitis, and 1 with chronic demyelinating polyneuropathy. Nine patients were admitted to the intensive care unit, and among them, five required mechanical ventilation. After 3 months, two patients died (6%) including one with encephalitis [26]. In Ribeirão Preto, also in the Southeast of Brazil, a 2-year-old girl became ill in March 2016, during a ZIKV epidemic. The girl had a fever (up to 38.5 °C) for 8 days and a macular rash of extremities in the last 2 days. She also presented with irritability, weakness, myalgias, and dysuria. Finally, 9 days after the onset of illness, she developed ataxic gait with normal reflexes and a positive Babinski sign. The cerebrospinal fluid (CSF) had no cells but 6400 erythrocytes/mm3, 102.6 mg/dl of protein, and 35 mg/dl of glucose. RT-PCR for ZIKV was positive in plasma and CSF. Magnetic resonance imaging (MRI) demonstrated rhombencephalitis with lesions in the vermix and left cerebellar hemisphere. The ataxic gait disappeared after 3 days, and the patient recovered without sequelae (unpublished data). A 36-year-old man heart transplant recipient from Ribeirão Preto was also reported to have ZIKV encephalitis. This patient presented with a 2-day history of high-grade fever, malaise, headache, and seizures that evolved with progressive hemodynamic instability, mental deterioration, and finally death. CSF had 58 leukocytes (100% lymphocytes)/mm3 and 105.37 mg/dl of protein. MRI revealed extensive cortical encephalitis with image suggestive of necrosis of the brain parenchyma and vasogenic edema. ZIKV genome was detected in the CSF by reverse transcriptase polymerase chain reaction (RT-PCR) and also by immunohistochemistry, by immunofluorescence, and by electron microscopy of brain tissue. The amplicon of viral genome was sequenced confirming the ZIKV infection. A pseudotumoral form of ZIKV meningoencephalitis was confirmed at autopsy [27].
The incidence of encephalopathy in patients with dengue ranges between 0.5% and 6.2% [28]. In a study of 49 patients with lymphocytic meningitis in Manaus City, North of Brazil, four of them had dengue fever, with headache, myalgias, and arthralgias that evolved to neck stiffness and impaired consciousness that healed without sequelae. Based on viral genomes detected in the CSF by RT-PCR, three patients had DENV-2 and one had DENV-1 [29]. Another study reported 498 DENV-3 cases from Goiania City in the Brazilian Central Plateau, in 2005–2006. Nine patients had clinical features compatible with infection of the CNS, five women and four men, mostly teenagers and young adults. These patients had encephalopathy (presented seizures or paresis) or meningoencephalitis, and two of them died. Fatal cases were of a 15-year-old girl with meningoencephalitis, whose CSF had 76 leukocytes (80% mononuclear cells)/μL, 64 mg/dL of glucose, and 127 mg/dL of protein. Her computed tomography scans revealed sulci effacement and intracranial hypertension, and DENV-3 was detected in brain tissues collected at necropsy. The other fatal case was of a 41-year-old woman that 10 days after onset of disease had tonic-clonic seizures and acute liver failure. PCR tests of blood were positive for DENV-3, and lumbar puncture was contraindicated due to thrombocytopenia [30]. In Ribeirão Preto City, a 65-year-old woman had acute meningoencephalitis by DENV. After 4 days with headache, fever, myalgia, and vomiting, the patient started with myopathy, seizures, and decreased level of consciousness. CSF showed normal leukocyte count, 72–86 mg/dL of protein, and immunoglobulins M (IgM) and G (IgG) positive to dengue. Brain MRI revealed acute central pattern encephalitis affecting internal capsule and nuclei of the base [31].
An Asian genotype of CHIKV was introduced into the Americas through the Caribbean in December 2013, and the first autochthonous Brazilian cases were reported in September 2014 in the State of Amapá, in the North, and at the same time, another genotype of CHIKV, the East, Central, and South African and Asian strain (ECSA), was found in Bahia State at the Northeast of Brazil. CHIKV ECSA rapidly spread through other northeastern states causing outbreaks [32]. During these large-scale outbreaks, uncommon severe clinical features of CHIKV infection were observed such as meningitis, encephalitis, GBS, and retrobulbar neuritis [33]. Two cases of encephalitis by CHIKV were reported in the Ceará State, Northeast of Brazil. These were a 55- and a 74-year-old man. Both patients tested positive for anti-chikungunya IgM in their serum. The first patient had acute febrile illness and disorientation in time and space. His CSF had 61 cells (71% lymphocytes)/mm3, 98 mg/dL of protein, and 62 mg/dL of glucose. His MRI showed acute bilateral encephalitis affecting the white matter in nuclei of the base and brainstem and also extending to the internal capsule and the cerebellum. The second patient had temporal and spatial disorientation with fluctuating level of consciousness, progressive lower extremity weakness, and diffuse areflexia. He had 90 leukocytes (91% lymphocytes)/mm3 and a protein of 179 mg/dL in CSF. MRI showed acute encephalitis affecting extensive area including the central white matter and cerebellum [34]. Another case of encephalitis by CHIKV was reported in Recife City, also in the Northeast of Brazil, a 48-year-old woman with fever, headache, muscle aches, skin rash, and painful polyarthralgia with edema that progressed to temporary functional impairment. The patient also presented a persistent hyponatremia with a plasma osmolality below 275 mOsm/kg of H2O, compatible with a syndrome of inappropriate antidiuretic hormone secretion (SIADH). The patient evolved to cognitive impairment and apraxia of speech and had 90 leukocytes/mm3 and a protein 68 mg/dL in CSF. MRI showed acute encephalitis involving putamen and nuclei of the base bilaterally but discrete edema [35].
In 2002, in Cordoba, Argentina, a 61-year-old businessman with headache, fever with chills, nausea, vomiting, unstable gait, left hand tremors, and diplopia was admitted to a hospital. He was lethargic and had neck rigidity. His CSF revealed 18 leukocytes (80% lymphocytes)/mm3, 87 mg/dL of protein, and 48 mg/dL of glucose. Meningeal signs evolved with frank cervical stiffness, positive Kerning sign, and photophobia. Lower extremities were spastic with bilateral Babinski sign. He had a wide-based gait and had dysdiadochokinesia. All symptoms disappeared after 5 days [36]. Diagnosis of infection was made using the hemagglutination inhibition test whose titers to SLEV increased between acute- (320) and convalescent-phase (1280) samples. A serological survey of horses from various regions of Brazil showed that 415 (55.1%) of the 753 studied horses were seropositive for flavivirus and, among them, monotypic reactions were observed to SLEV in 93 (12.3%). These results suggest SLEV is infecting horses in Southeast, Pantanal, and Northeast of Brazil [37]. In 2007, during a large DENV-3 epidemic, six patients were found infected by SLEV in the city of São José do Rio Preto, southeastern Brazil. SLEV genome was detected by RT-PCR in CSF of all six patients. From these, two children had acute febrile illness, one of them had facial palsy, and both were diagnosed with meningoencephalitis (the first cases of meningoencephalitis by SLEV reported in Brazil). Their CSFs showed 12 (100% lymphocytes) and 286 (60% lymphocytes) leukocytes/mm3 and both survived [38]. Therefore, SLEV is endemic in South America infecting horses and also producing small human outbreaks and sporadic cases of meningitis and encephalitis, probably misdiagnosed as dengue or other viral diseases.
ROCV has produced an outbreak of encephalitis in the southern coast of São Paulo State in 1973–1978, with 1021 reported cases [39]. ROCV patients presented acutely with fever, headache, anorexia, nausea, vomiting, myalgia, and malaise. Encephalitis signs appeared later, including confusion, reflex disturbances, motor impairment, meningeal irritation, and cerebellar syndrome. Some patients presented convulsions. The disease produced sequelae such as visual, olfactory, and auditory disturbances, lack of motor coordination, equilibrium disturbance, swallowing difficulties, incontinence, and impaired memory in 20% of the survivors. The case fatality rate of this ROCV outbreak was 10% [40]. After this outbreak, serologic evidence of ROCV circulation in the original area as well as in other parts of Brazil have been reported, and public health authorities are always concerned about reappearance of ROCV outbreaks in Brazil [6]. A serological survey of horses from various Brazilian regions showed that 415 (55.1%) of the 753 studied horses were seropositive for flavivirus, and among them, a monotypic reaction to ROCV was found in 46 animals (6.1%). These results suggest that ROCV, or other closely related virus, is infecting horses in Southeast, Pantanal, and Northeast of Brazil [38]. Besides that, in 2010, testing 23 CSF samples from human patients from Manaus City by RT-PCR, amplicons of ROCV genome from two patients were amplified and sequenced. These were a 53-year-old man with seizures and abnormal conscientiousness and a 30-year-old woman with headache, vomiting, and signs of intracranial hypertension. Curiously, both had AIDS and the woman also had tuberculosis. CSFs showed 45 and 29 lymphocytic cells/mm3, 153 and 328 protein mg/dl, and 48 and 56 mg/dl of glucose, respectively. Both survived after 20 days of hospitalization (unpublished data, 2010). Interestingly, these human cases occurred in the North of Brazil, more than 2000 km from where the virus was originally isolated. Serologic evidence in horses and the casual finding of human cases in Manaus show that ROCV circulates unrecognized in Brazil and possibly in other South American countries, producing human infections including those of the CNS, particularly affecting immunodeficient individuals, such as AIDS patients.
WNV is a pathogen from Africa and Asia that emerged in North America in 1999 and spread toward all the Americas. This virus causes a severe encephalitis in humans and in horses. In North America, WNV has caused dozens of thousands of clinical cases and some thousands of deaths of humans and horses [41]. WNV was isolated in Argentina, in 2006, from the brain of three horses [42]. A serologic survey to WNV including sera of 753 healthy horses from Central-West, Northeast, and Southeast Brazil showed 79 seropositive horses to WNV, and among them, 9 sera expressed WNV-specific neutralizing antibodies. Eight of these animals were from the western Pantanal region in the State of Mato Grosso do Sul, and one was from the State of Paraíba in the Northeast of Brazil [43]. Based on the virus introduction in Argentina as well as on the results of serologic surveys shown above, WNV has been introduced in Brazil, and it is probably spreading in the country based on a transmission network that involves mosquitoes, birds, and horses. The first human case of encephalitis by WNV in Brazil was reported in 2014. A 52-year-old agricultural worker man from Piauí State, Northeast of Brazil, presented an acute febrile illness with headache, neck pain, vomiting, diarrhea, abdominal pain, and severe muscle weakness. The patient evolved with a tonic-clonic seizure and confusional state. Physical exam revealed nuchal rigidity, bilateral facial palsy, flaccid symmetrical tetraparesis and abolished myotatic reflexes. CSF showed 14 leukocytes (85% lymphomonocytic cells)/mm3, 274 mg/dl of protein, and 59 mg/dl of glucose. Diagnosis of WNV infection was made by serologic tests including positive neutralization test to the virus. MRI images were normal. The patient recovered partially and was not able to walk after discharge [44]. It is probable that other WNV infections have occurred unrecognized in Brazil, particularly in Northeast and Pantanal regions.
OROV has caused large outbreaks of acute febrile illness with sporadic cases of meningitis, in Amazon region and Central Plateau of Brazil as well as in Peru and other South American countries [7, 10]. In a study performed from 2005 to 2010 in Manaus City, in the Amazonas State, North Region of Brazil, CSF samples of 110 patients with meningoencephalitis were submitted to RT-PCR in order to identify infecting viruses. OROV was found in three CSF samples. Patients were a 20-year-old agricultural worker man, a 54-year-old fisherman man, and a 37-year-old domestic worker woman. All patients referred headache; one patient had dizziness, cloud vision, and Romberg sign; the second had fever, chills, and malaise; and the third had nausea, vomiting, and paraplegia. CSFs showed 6, 134, and 533 lymphocytic cells/mm3; 40, 107, and 136 mg/dl of protein; and 40, 50, and 107 mg/dl of glucose, respectively. All patients survived after hospitalization. Interestingly, two of these patients had other diseases affecting the CNS or immune system; the first had neurocysticercosis and the last had AIDS [29].
A summary of reported infections of the central nervous system (CNS) caused by arboviruses in Brazil is shown in Table 15.1.
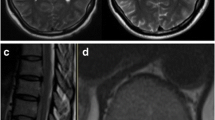
Cases of encephalitis by DENV, ZIKV, and other flaviviruses show a central pattern affecting neurons in the substantia nigra nucleus, thalamus, cerebellum, and cerebral cortex, correlating with the neuroradiological findings in MRI, as shown in Fig. 15.1 [45, 46]. These infections, particularly in immunodeficient individuals can extend to other areas of the brain. CHIKV, an Alphavirus, also produces encephalitis affecting the white matter in nuclei of the base and brainstem, and it can extend to the internal capsule and the cerebellum, as shown in Fig. 15.1.
(a) Magnetic resonance imaging (MRI) of a case of dengue encephalitis showing small areas of hyperintensity on T2/fluid attenuation inversion recovery (FLAIR), bilaterally, on the internal capsule (adapted of Queiroz et al. [31]); (b) MRI of a case of chikungunya encephalitis showing multiple supratentorial FLAIR hyperintense foci (arrowheads) distributed randomly in the white matter (adapted of Pereira et al. [34])
As final remarks on arboviruses producing infection of the CNS:
-
There is no specific antiviral treatment for arboviruses that cause meningoencephalitis. The treatment seeks to reduce cerebral edema by avoiding excessive hydration and eventually using corticosteroids, in addition to providing supportive care.
-
It is probable that serological diagnosis of ZIKV, ROCV, and SLEV infections may be confused with dengue due to cross-reactivity with this virus.
-
Our data suggest that CNS invasion by ZIKV, ROCV, OROV, and probably other arboviruses could be facilitated by immunodeficiency or by prior damage of the BBB. Arboviruses should be considered and tested in differential diagnosis of patients with CNS infections that have underlying diseases such as immunodeficiency or by prior neurologic pathologies.
References
Hubalek Z. Emerging human infectious diseases: anthroponoses, zoonoses, and sapronoses. Emerg Infect Dis. 2003;9:403–4.
Meltzer E. Arboviruses and viral hemorrhagic fevers (VHF). Infect Dis Clin N Am. 2012;26:479–96.
Figueiredo LTM. The recent arbovirus disease epidemic in Brazil. Rev Soc Bras Med Trop. 2015;48:233–4.
Patterson J, Sammon M, Garg M. Dengue, Zika and Chikungunya: emerging arboviruses in the new world. West J Emerg Med. 2016;17:671–9.
Solomon T. Flavivirus encephalitis. N Engl J Med. 2004;351:370–8.
Figueiredo LTM. The Brazilian flaviviruses. Microbes Infect. 2000;2:1643–9.
Vasconcelos HB, Nunes MR, Casseb LM, Carvalho VL, Pinto da Silva EV, Silva M, Casseb SM, Vasconcelos PF. Molecular epidemiology of Oropouche virus, Brazil. Emerg Infect Dis. 2016;17:800–6.
Brinton MA. Replication cycle and molecular biology of the West Nile virus. Viruses. 2013;6:13–53.
Jose J, Snyder JE, Kuhn RJ. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol. 2009;4:837–56.
Proenca-Modena JL, Sesti-Costa R, Pinto AK, Richner JM, Lazear HM, Lucas T, Hyde JL, Diamond MS. Oropouche virus infection and pathogenesis are restricted by MAVS, IRF-3, IRF-7, and type I interferon signaling pathways in nonmyeloid cells. J Virol. 2015;89(9):4720–37. https://doi.org/10.1128/JVI.00077-15.
Taxonomy—International Committee on Taxonomy of Viruses (ICTV). Virus taxonomy: 2016 release, Budapest, Hungary. 2016. https://talk.ictvonline.org/taxonomy.
Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe. 2013;13:379–93.
Amarilla AA, Setoh YX, Periasamy P, Pali G, Figueiredo LT, Khromykh AA, Aquino VH. Chimeras between Rocio and West Nile viruses reveal the role for Rocio virus prM and E proteins in virulence and inhibition of type I interferon signaling. Sci Rep. 2017;7:44642.
Neal JW. Flaviviruses are neurotropic, but how do they invade the CNS? J Infect. 2014;69:203–15.
Shirato K, Miyosh H, iGoto A, Ako Y, Ueki T, Kariwa HA, Takashima I. Viral envelope protein glycosylation is a molecular determinant of neuro invasiveness of the New York strain of West Nile virus. J Gen Virol. 2004;85:3637–45.
Lee E, Lobbigs M. Mechanisms of virulence attenuation of glycosaminoglycan-binding variants of Japanese encephalitis virus and Murray Valley encephalitis virus. J Virol. 2002;76:4901–11.
Misra KM, Kalita J. Overview: Japanese encephalitis. Prog Neurobiol. 2010;91:108–20.
Santos RI, Bueno-Júnior LS, Ruggiero RN, Almeida MF, Silva ML, Paula FE, Correa VM, Arruda E. Spread of Oropouche virus into the central nervous system in mouse. Viruses. 2014;6:3827–36.
Chávez JH, França RFO, Oliveira CJ, Aquino MTP, Farias KJ, Machado PR, Yokosawa J, Silva JS, Fonseca BAL, Figueiredo LTM. CCR-5/MIP-1 alpha affect the pathogenesis of Rocio virus encephalitis in a mouse model. Am J Trop Med Hyg. 2013;89:1013–8.
Barros VD, Penharvel S, Forjaz J, Saggioro FP, Neder L, Figueiredo LTM. An experimental model of meningoencephalomyelitis by Rocio virus in Balb-C mice: hystopathology, inflammatory response and cytokine production. Am J Trop Med Hyg. 2011;85:363–73.
Shrestha B, Samuel MS, Diamond MS. CD8fl T cells require perforin to clear West Nile fever from infected neurons. J Virol. 2006;80:119–29.
Shresta B, Pinto AK, Green S, Bosch I, Diamond MS. CD8fl T cells use TRAIL to restrict West Nile virus pathogenesis by controlling infection in neurons. J Virol. 2012;86:8937–48.
Brazilian Ministry of Health. Dengue, Chikungunya, Zika, Syndrome of Guillain-Barré. 2016.
Butler D. Zika virus: Brazil’s surge in small-headed babies questioned by report. Nature. 2016;530:13–4.
Macciocchi D, Lanini S, Vairo F, Zumla A, Figueiredo LTM, Lauria FN, Strada G, Brouqui P, Puro V, Krishna S, Kremsner P, Scognamiglio P, Köhler C, Nicastri E, Di Caro A, Cieri RM, Ioannidis JPA, Kobinger G, Burattini MN, Ippolito G. Short-term economic impact of the Zika virus outbreak. New Microbiol. 2016;39:287–9.
Silva IRF, Frontera JA, Filippis AMB, Nascimento OJM, RIO-GBS-ZIKV Research Group. Neurologic complications associated with the Zika virus in Brazilian adults. JAMA Neurol. 2017;74:1190–8.
Schwartzmann PV, Vilar FC, Takayanagui OM, Santos AC, Ayub-Ferreira SM, Ramalho LNZ, Neder L, Romeiro MF, Maia FGM, Tollardo AL, Zapata PM, Figueiredo LTM, Schmidt A, Simões MV. Zika virus associated encephalitis in immunocompromised patient. Mayo Clin Proc. 2017;92:460–6.
Misra UK, Kalita J, Syam UK, Dhole TN. Neurological manifestations of dengue virus infection. J Neurol Sci. 2006;244:117–22.
Bastos MS, Figueiredo LT, Naveca F, Figueiredo R, Oliveira CM, Gimaque JB, Assis K, Monte RL, Mourão MP. Infection of central nervous system by Oropouche Orthobunyavirus in three Brazilian patients. Am J Trop Med Hyg. 2012;86:732–5.
Tassara MP, Guilarde AO, Rocha BAM, Féres VCR, Martelli CMT. Neurological manifestations of dengue in Central Brazil. Rev Soc Bras Med Trop. 2017;50:379–82.
Queiroz RM, Prado RMA, Abud LG. Acute dengue encephalitis in a female Brazilian adult. Rev Soc Bras Med Trop. 2017;50:431.
Figueiredo MLG, Figueiredo LTM. Emerging alphaviruses in the Americas: Chikungunya and Mayaro. Rev Soc Bras Med Trop. 2014;47:677–83.
Figueiredo LTM. Chikungunya virus emerged in Brazil producing large outbreaks that revealed uncommon clinical features and fatalities. Rev Soc Bras Med Trop. 2017;50:583–4.
Pereira LP, Villas-Bôas R, Scott SSO, Nóbrega PR, Sobreira-Neto MA, Castro JDV, Cavalcante B, Braga-Neto P. Encephalitis associated with the chikungunya epidemic outbreak in Brazil: report of 2 cases with neuroimaging findings. Rev Soc Bras Med Trop. 2017;50:413–6.
Lucena-Silva N, Assunção MELSM, Ramos FAP, Azevedo F, Lessa Junior R, Cordeiro MT, Brito CAA. Encephalitis associated with inappropriate antidiuretic hormone secretion due to chikungunya infection in Recife, state of Pernambuco, Brazil. Rev Soc Bras Med Trop. 2017;50:417–22.
Spinsanti L, Basquiera AL, Bulacio S, Somale V, Kim CH, Ré V, Rabbat D, Zárate A, Zlocowski JC, Mayor CQ, Contigiani M, Palacio S. St. Louis encephalitis in Argentina: the first case reported in the last seventeen years. Emerg Infect Dis. 2003;9:271–3.
Silva JR, Romeiro MF, Sousa WM, Munhoz TD, Borges GP, Soares OAB, Campos CHC, Machado RZ, Silva MLCR, Faria JLM, Chávez JH, Figueiredo LTM. Serosurvey of Saint Louis encephalitis and Rocio viruses in horses of Brazil. Rev Soc Bras Med Trop. 2014;47:414–7.
Mondini A, Lázaro E, Cardeal ILS, Nunes SH, Moreira CC, Rahal P, Figueiredo LTM, Bronzoni RVM, Chiaravalloti Neto F, Nogueira ML. Saint Louis encephalitis virus, Brazil. Emerg Infect Dis. 2007;13:176–8.
Lopes OS, Coimbra TLM, Sacchetta LA, Calisher CH. Emergence of a new arbovirus disease in Brazil. 1. Isolation and characterization of the etiologic agent, Rocio virus. Am J Epidemiol. 1978;107:444–9.
Tiriba AC, Miziara AM, Lourenço R, Costa CRB, Cota CS, Pinto GH. Encefalite humana primária epidêmica por arbovirus observada no litoral sul do Estado de São Paulo. Rev Assoc Med Bras. 1976;22:415–20.
Roehrig JT. West Nile virus in the United States—a historical perspective. Viruses. 2013;5:3088–108.
Morales MA, Barrandeguy M, Fabbri C, Garcia JB, Vissani A, Trono K, Gutierrez G, Pigretti P, Menchaca H, Garrido N, Taylor N, Fernandez F, Levis S, Enría D. West Nile virus isolation from equines in Argentina, 2006. Emerg Infect Dis. 2006;12:1559–61.
Silva JR, Chávez JH, Munhoz TD, Borges GP, Soares OAB, Machado RZ, Valadão CAA, Silva MLCR, Faria JLM, Silva EE, Figueiredo LTM. Serologic survey for West Nile virus in Brazilian horses. Mem Inst Osvaldo Cruz. 2013;108:921–3.
Vieira MA, Romano AP, Borba AS, Silva EV, Chiang JO, Eulálio KD, Azevedo RS, Rodrigues SG, Almeida-Neto WS, Vasconcelos PF. West Nile virus encephalitis: the first human case recorded in Brazil. Am J Trop Med Hyg. 2015;93:377–9.
Bosanko CM, Gilroy J, Wang A-M, Sanders WS, Dulai M, Wilson J, Blum K. West Nile virus encephalitis involving the substantia nigra. Arch Neurol. 2003;60:1448–52.
Knox J, Cowan RV, Dayle JS, Liqtermont MK, ArcherJS Burrow JN, et al. Murray Valley encephalitis; a review of clinical features diagnosis and treatment. Med J Aust. 2012;196:322–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
de Figueiredo, M.L.G., Figueiredo, L.T.M. (2018). Emerging Causes of Encephalitis: Zika, Dengue, Chikungunya, and Beyond. In: Hasbun, R. (eds) Meningitis and Encephalitis. Springer, Cham. https://doi.org/10.1007/978-3-319-92678-0_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-92678-0_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92677-3
Online ISBN: 978-3-319-92678-0
eBook Packages: MedicineMedicine (R0)