Abstract
Intracranial AVMs are complex lesions requiring multidisciplinary expertise for optimal patient outcomes. While the optimal treatment depends on AVM-specific factors such as rupture status, size, location within the brain, and the presence of deep venous drainage, the treating neurosurgeon must be able to synthesize the risks and benefits of multiple treatment options to best determine the safest treatment modality for each patient. In this chapter we present current review on best practices for the treatment of intracranial AVMs.
Would be helpful to have a table showing the different classification methods. There is too much text in this chapter dedicated to this.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Diagnosis
Intracranial vascular malformations are broadly classified into four major categories and include arteriovenous malformations (AVM), cavernous malformations, capillary telangiectasias, and developmental venous anomalies. Intracranial AVMs are the most common of these vascular malformations with a prevalence of approximately 15–18 per 100,000 people. They do not show a gender predilection [1]. Most commonly, AVMs are first diagnosed with intracerebral hemorrhage (~75%) or, to a lesser extent, seizures (~30%). The risk of morbidity or mortality is significant, ranging from 40% to 50% after rupture [2].
Diagnostic imaging is key to the diagnosis and management of intracranial AVMs. In patients presenting with symptoms concerning for an intracranial hemorrhagic process, non-contrasted computed tomography (CT) is the preferred screening modality. Non-contrasted CT will show acute hemorrhage in an AVM that has ruptured. In addition, calcifications are often visible within the AVM nidus. Magnetic resonance imaging (MRI) will show hypointense flow voids on T2-weighted imaging, which can help identify the size of the AVM nidus, as well as a preliminary analysis of feeding arteries and draining veins. MR and CT angiograms are useful diagnostic studies to better image the presence of underlying vascular abnormalities and to rule out other causes of intracranial hemorrhage when present.
The gold standard for diagnosis of intracranial AVMs is digital subtraction angiography. This allows for identification and dynamic analysis of feeding arteries, draining veins, and the AVM nidus. In addition, flow-related aneurysms or other vascular anomalies that may require additional treatment can be identified. Angiography is limited by its inability to show its three-dimensional relationship to brain parenchyma. However, a thorough understanding of the dynamics of an AVM is critical to determine appropriate treatment.
While multiple classification schemes exist for intracranial AVMs, the Spetzler-Martin (SM) classification is the most ubiquitous. This scale grades AVMs from 1 to 5 based on eloquent or noneloquent location, size, and presence of deep venous drainage . Eloquent location is defined as motor, sensory, and visual cortex, frontal and temporal cortex language centers, hypothalamus, thalamus, internal capsule, brain stem, cerebellar peduncles, and deep cerebellar nuclei. Deep venous drainage is defined as any draining vein not draining into a cortical vein or convexity sinus. This includes internal cerebral veins, basal veins, or the precentral cerebellar vein. The SM classification was developed to predict the risk of morbidity and mortality of microsurgical resection and has been applied as a surrogate to the severity of the AVM as it applies to treatment decision-making. In the original study, microsurgical outcomes were categorized into three categories of increasing neurological morbidity: no deficit, minor deficit, and major deficit. Grade I (n = 23) lesions had no minor or major neurological deficits nor any mortality associated with microsurgical resection, while grade II AVMs (n = 21) had a 5% incidence of a minor deficit. Comparatively, grade III–V AVMs were associated with higher levels of minor deficits (grade III, 12%; grade IV, 20%; grade V, 19%) as well as major deficits (grade III, 7%; grade IV, 12%; grade V, 4%) [3]. To guide management decisions, the five-tier grading system was placed into three classes (Table 22.1). Class A AVMs are thought to be best treated with microsurgery; class B with a combination of microsurgery, radiation, and/or embolization; and class C with nonsurgical methods, including embolization, radiotherapy, or conservative management [4].
Other patient factors that clearly effect surgical decision-making are not incorporated into the original SM criteria. Lawton et al. proposed a supplementary grading scale incorporating age, rupture status, and AVM nidus diffusivity. This grading system ranges from 0 to 5 and is aimed at supplementing the SM system. For example, in AVMs with low SM grades and low supplementary grades, microsurgery is associated with excellent outcomes, while AVMs with high SM grades and high supplementary scores are associated with higher levels of morbidity and mortality. In cases where the SM grade and supplementary grade are mismatched, the supplementary grade may be more accurate. For instance, high SM grade AVMs (grades IV–V) with low supplementary grades, surgical morbidity, and mortality were similar to that of a grade III AVM in the original SM scale, suggesting that these additional factors can assist treating physicians in surgical decision-making and counseling patients on operative risk with a higher degree of accuracy [5].
Additional AVM classifications have been proposed and studied. Hollerhage et al. used feeding artery distribution (ACA, MCA, PCA, rolandic MCA branches) and Hunt and Hess score and correlated this with Glasgow Outcome Scale (GOS) scores [6]. The University of Toronto AVM study group proposed a classification scheme involving eloquent cortex, diffuseness of the AVM nidus, and deep venous drainage . This classification was able to accurately predict permanent disabling neurologic outcomes with an increased area under the curve (receiver operating characteristic of 0.79) as compared to the SM scale (0.69) [7]. These alternative classification schemes are rarely used, given the ubiquity and familiarity of the SM scale.
Etiology
AVMs consist of high-pressure abnormal connections (shunts) of arteries directly into draining veins without an intervening capillary network. Feeding arteries lack a muscularis layer, and, as a result of the high-flow shunting of blood through these vessels, undergo smooth muscle hyperplasia. Unlike cavernous malformations (where there is no intervening brain parenchyma within the vascular lesion), AVMs contain gliotic parenchyma within the nidus. Because of high-pressure shunting, flow-related aneurysms and fistulas may develop.
Genomic analysis of AVM tissue has shown up to 900 differences in gene expression as compared to normal brain. Genes specifically related to angiogenesis in AVMs include angiopoietin-1, angiopoietin-2, matrix metalloproteases, fibroblast growth factor, vascular endothelial growth factor and its associated receptors, Tie-1 and 2, CD31, neuronal nitric oxide synthase, and αVβ3 integrin [8]. Mutations in endoglin and TGF-beta signaling have also been implicated in murine AVM models [9].
In addition to genetic predisposition to AVMs, evidence suggests that hemodynamic factors can lead to expression of proteins involved in vascular remodeling. An increase in wall shear stress induces endothelial cells to increase surface expression of proteins previously shown to be upregulated in AVMs. These proteins include matrix metalloproteinase-9, platelet-derived growth factor, and vascular endothelial growth factor [10, 11].
The most common presentation of intracranial AVMs is neurological deficit associated with hemorrhage. While neurological symptoms may vary, acute onset of headache is nearly universal. Hemorrhage is typically within the brain parenchyma. However, hemorrhages into the subarachnoid or the intraventricular spaces are possible with lesions adjacent to the cortical surface or near the ventricles. The second most common presentation is seizures, typically occurring as a result of AVMs located in the supratentorial compartment [12, 13].
AVMs are dynamic lesions capable of growth, remodeling, and re-formation, even after surgical or radiosurgical obliteration [14,15,16,17]. Some AVMs are congenital lesions, as observed in inherited clinical syndromes where AVMs have a high prevalence. These include hereditary hemorrhagic telangiectasia, Sturge-Weber, and von Hippel-Lindau syndromes .
Treatment
Unruptured AVMs
The decision to treat unruptured AVMs is based on comparing the risk of rupture over the course of a patient’s lifetime with the risks of a proposed treatment. The natural history of intracranial AVMs suggests that the risk of rupture is approximately 2–4% per year [18]. In 2014, a prospective clinical trial, “A Randomized Trial of Unruptured Brain AVMs” (ARUBA) , [19] was designed to determine if the risk of treatment differed from the natural history of intracranial AVMs. This study enrolled patients from 39 institutions in 9 countries with unruptured AVMs in a 1:1 design to either intervention (microsurgical resection, stereotactic radiosurgery, endovascular embolization, or a combination thereof) or medical management. If randomized to the intervention arm, the intervention was not prespecified, but rather determined by treating physicians at each center.
Notable inclusion criteria included patients older than 18 years, no previous hemorrhage, no previous interventions, and radiographic characteristics that were deemed suitable for intervention. The primary outcome was death or symptomatic stroke with a secondary outcome of clinical impairment, as defined by a modified Rankin scale (mRS) of 2 or higher at 5-year follow-up. A single study neurologist who was not involved in the interventional procedures performed the clinical outcome assessment.
ARUBA centers screened and enrolled patients as shown (Fig. 22.1). Of patients having interventions, 5 underwent microsurgical resection, 30 underwent embolization, and 31 underwent radiotherapy. Embolization was combined with microsurgical resection in 12 patients or radiotherapy in 15 patients. A single patient underwent embolization, radiation therapy, and microsurgical resection. At the time of trial analysis, 53 of the above patients had ongoing treatment plans, and the remaining 20 patients that were randomized to intervention had not yet initiated treatment.
One hundred ten patients were allocated to the medical management arm, 109 of which were included in the final analysis. At the time of publication, the primary endpoint had been reached by 11 patients in the medical management arm and 35 patients in the interventional arm over a mean follow-up of 33.3 months. The study was halted prematurely as interim analysis showed that the risk of stroke or death in the medical management arm was significantly lower than the intervention arm. There were a higher number of strokes in the intervention arm (45 vs 12, p < 0.0001) and neurological deficits that were not related to stroke (14 vs 1, p < 0.0001) as compared to the medical management arm [19].
While there are multiple limitations to the ARUBA data, the randomized design of the study remains the highest level of evidence for guiding the treatment of intracranial AVMs. However, the study suffers from significant design constraints that limit its generalizability. The study design was significantly limited by a low number and heterogeneous group of enrolled patients. Of the 726 eligible patients, 323 patients refused entry into the trial and 226 were enrolled. Both the patients who refused enrollment and the 177 patients treated outside the study potentially caused a selection bias. In addition, the trial was halted early after an average follow-up of 33 months, which likely biased the results of both the primary and secondary outcome in favor of medical management, as such limited follow-up likely detected complications of those patients undergoing treatment while not detecting potential strokes or deaths as a result of the natural history of non-treated AVMs beyond the limited study period. Only 30.7% of patients randomized to treatment and 10.1% of patients randomized to medical management reached the primary endpoint of symptomatic stroke or death. The secondary endpoint of clinical impairment (mRS ≥2) was only reached by 38.6% of treated patients and 14% of patients randomized to medical management. The heterogeneity of patients included in the study also significantly limited analysis. While the study authors argued that heterogeneity of patients mimics that seen in actual clinical settings, it is clear that a SM IV or V AVM is more likely to have a poor outcome as compared to a SM I/II lesion, limiting the ability to detect a difference in low-grade AVMs compared to medical management.
Notably, there were no clear criteria for the application of specific interventions (i.e., microsurgical resection, embolization, or radiosurgery) which may have limited detecting a potential helpful therapy when grouped with potentially harmful therapies. Optimal design would have considered each treatment separately when compared to medical management. However, in a rare pathology, this is challenging in the time it would require to enroll enough patients to power the study to detect a significant difference and long-term funding needed to continue to follow patients for an extended period of time [20, 21].
Current best practices for unruptured AVMs must factor patient age, AVM location, associated high-risk features (flow-related aneurysms, intranidal aneurysms, or venous outflow stenosis), and patient preference. A balanced assessment and treatment approach by experienced cerebrovascular centers remain the standard of care.
Microsurgical Resection
The results of the ARUBA study challenged treating neurosurgeons to assess unruptured AVM patients that would optimally benefit from microsurgical resection. Bervini et al. reported improved surgical outcomes in Spetzler-Ponce class A AVMs as compared to conservative management in a series of 427 patients that were retrospectively reviewed. They found a 5-year risk of hemorrhage of 11.5%, and, when hemorrhage occurred, 14 cases (88%) resulted in mRS >1. Following surgery, the risk of mRS >1 was 1.6%, while the risk of mRS >2 was 0.5% at postoperative follow-up. The risk for adverse outcomes with an mRS >1 at postoperative follow-up was increased in Spetzler-Ponce class B and C AVMs: 14.0 and 38.6%, respectively [22]. In a retrospective study of 61 ARUBA -eligible patients (31 SM I/II, 20 SM III, 10 SM IV/V) undergoing microsurgical resection, Nerva et al. reported that all patients had angiographic obliteration without any associated mortality [23]. Of these patients, impaired functional outcomes (mRS ≥2) occurred in 3% of grade I/II, 25% of grade III, and 20% of grade IV/V. Long-term outcomes were similarly better for patients with lower-grade AVMs. Rutledge et al. performed a similar retrospective analysis of 43 ARUBA -eligible patients who underwent microsurgical resection. Ninety-three percent of these patients had radiographic obliteration with a 11.6% rate of stroke or death. Impaired functional outcome was observed in 4.8% of patients [24]. Javadpour reported on 34 ARUBA -eligible patients, 24 of which were SM grade I/II. Of these patients 6% had an mRS ≥ 2 following microsurgical resection [25]. Schramm et al. reported on 104 ARUBA -eligible patients, 63 of which were SM grade I/II. Of these patients 3.2% had significant permanent neurological deficit and 14.3% had mRS scores ≥ 2 [26]. Finally, Wong et al. reported on 155 ARUBA -eligible patients who underwent microsurgery with or without preoperative embolization. Complete obliteration was achieved in 98.1% of patients and 99.2% of SM I/II AVMs. For SM grade I/II AVMs, early disabling deficits (mRS ≥ 1) occurred in 9.3% of patients and permanent debilitating deficits in 3.4% of patients [27]. These retrospective studies, which more effectively control for treatment modality compared to the ARUBA clinical trial, show that microsurgical resection for low-grade (SM I/II) AVMs can effectively treat unruptured AVMs with an acceptable safety profile that exceeds the risk of hemorrhage defined by the natural history of the disease. These data should frame the context of future AVM-related clinical trials.
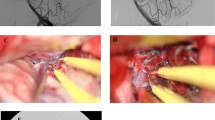
While grade I and II AVMs are associated with low surgical risk and grade IV and V AVMs are associated with high risk, grade III AVMs represent a unique management challenge. For instance, in the original SM criteria report, large (>6 cm) AVMs located in noneloquent cortex carry the same risk as small AVMs located in eloquent cortex with deep venous drainage . Lawton et al. reported the outcomes of grade III AVMs and showed that small AVMs with deep venous drainage in eloquent cortex had the lowest rate of new deficit or death (2.9%) as compared to moderate-sized (3–6 cm) AVMs in either noneloquent cortex with deep venous drainage (7.1%) or eloquent cortex without deep venous drainage (14.8%) [28]. Thus, supplementary criteria may be used to better stratify the treatment risks of various grade III AVMs. A representative case of SM III AVM treated with staged embolization, followed by microsurgical resection, is shown (Fig. 22.2).
A 13-year-old boy presented with recurrent seizures. (a) Non-contrast CT showed a hyperdensity within the right frontal lobe, consistent with an unruptured AVM. (b) Diagnostic angiography showed a SM III AVM fed primarily by the right anterior cerebral artery. (c) Staged embolization was performed. (d) Following microsurgical resection, no residual AVM was appreciated
In addition to technical excellence needed for AVM resection, appropriate postoperative care is critical to the success of surgery. Patients are admitted to the intensive care unit postoperatively for hourly neurological exams and strict blood pressure management, typically reducing the systolic pressure by 10–20% of baseline until angiographic verification of complete AVM resection. This is important as elevated blood pressure through a small, residual AVM nidus could lead to AVM rupture. Potential postoperative complications directly associated with craniotomy and microsurgical resection can include seizure, hemorrhage, edema, stroke, and infection.
Endovascular Embolization
Endovascular embolization provides both a primary treatment and, more often, an adjuvant treatment option in patients undergoing microsurgical resection or radiosurgery. Current embolic agents include N-butyl cyanoacrylate (NBCA), ethylene-vinyl alcohol copolymer (Onyx), polyvinyl alcohol particles, or platinum coils. Embolization as monotherapy is rarely curative. In an extensive review of 1246 patients, a 5% cure rate was achieved with embolization alone [29]. More recent studies by Katsaridis et al. and Saatci et al. showed complete occlusion with embolization alone in 54% and 51% of patients, respectively [30, 31]. However, despite these cure rates, Saatci et al. reported a permanent morbidity rate of 7.1% and a mortality rate of 1.4%. Often, embolization is an adjunctive treatment with either microsurgical resection or radiosurgery (Fig. 22.3). Embolization can eliminate deep arterial pedicles that would either be difficult to access or would be encountered in the latter stages of microsurgical resection. In addition, high-risk features can be treated to theoretically decrease the risk of intraoperative rupture [32]. Similarly, in patients undergoing radiosurgery, preoperative embolization can eliminate high-risk features, as the curative effects of radiosurgery take several years to manifest. Notably, while embolization has previously been used to decrease the volume of the AVM nidus in patients undergoing radiosurgery, studies suggest that long-term obliteration rates are actually worse as compared to patients who did not undergo preoperative embolization; partial embolization followed by radiosurgery alone is not recommended [33].
A 49-year-old man presented with seizures. (a) Contrast-enhanced MRI showed a left frontal lobe SM I AVM. (b) The AVM nidus was fed primarily by branches of the anterior cerebral artery. (c) Embolization was performed with near obliteration of the AVM nidus. (d) Following microsurgical resection, no residual AVM was present
Radiosurgery
Stereotactic radiosurgery represents the only noninvasive method of treating intracranial AVMs. While different delivery platforms are available, including Gamma Knife, proton beam, and linear accelerator-based technologies, all aim to direct focused ionizing radiation to obliterate the AVM nidus. Radiosurgery is primarily implemented in high-grade AVMs that are deemed non-resectable due to significant risk to adjacent neural parenchyma or in patients with significant surgical comorbidities (Fig. 22.4). Compared to microsurgical resection which aims for an immediate cure, the preventative treatment effect of radiosurgery takes approximately 2–4 years to manifest. Thus, the patient is exposed to the standard 2–4% risk of rupture in the interval between radiosurgery and cure. In addition, the normal parenchyma near the AVM may be exposed to radiation with varying clinical effects including edema, radiation necrosis, or, rarely, radiation-induced neoplasms [34].
A 43-year-old man presented with acute onset of headache. (a) Non-contrast CT showed intraventricular hemorrhage. (b) Diagnostic angiography showed a SM III AVM with dysplastic veins draining into the vein of Galen. Arterial supply was from the bilateral superior cerebellar arteries, left posterior cerebral artery, and right posterior communicating artery. This patient was referred for Gamma Knife radiosurgery
Obliteration of AVMs following radiosurgery occurs from endothelial proliferation and fibroblast proliferation of the intimal layer leading to progressive stenosis of the AVM and eventual resolution [35]. Typical radiation dose ranges from 12 to 30 Gy delivered to the margin of the AVM nidus [36, 37]. While the earliest radiological evidence of AVM obliteration can be seen 2–3 months following treatment, up to 3 years are typically allotted before assessing for complete obliteration and treatment success. Noninvasive imaging studies such as CTA or MRA may be used to evaluate treatment effects. However, angiography remains the gold standard to assess the response to treatment.
Obliteration rates following stereotactic radiosurgery range between 60% and 90% with negative predictors of obliteration being lower marginal dose, prior hemorrhage, eloquent location, larger nidus volume, and increased numbers of isocenters [38,39,40]. Treatment failure may be the result of insufficient dose, either due to reduction of dose to important adjacent structures (such as the optic nerves or pituitary stalk) or recanalization of previously treated AVM not included in the original radiosurgical treatment plan.
Though the ARUBA study suggested that stereotactic radiosurgery for unruptured AVMs was inferior to medical management, multiple studies have demonstrated its safety and efficacy. Nerva et al. reported 30 patients (SM I/II, 12; SM III, 11; SM IV/V, 7) who underwent stereotactic radiosurgery. Complications were identified in 33% of grade I/II, 9% of grade III, and 14% of grade IV/V AVMs. Radiographic cure was shown in 80% of grade I/II, 67% of grade III, and 25% of grade IV/V AVMs [23]. In 174 ARUBA -eligible patients (SM I/II, 85; SM III, 55; SM IV/V, 34) treated with stereotactic radiosurgery, Pollock et al. reported an 8.7% rate of adverse radiation effects and 6.9% rate of posttreatment neurological deficit. The rate of stroke or death was 10.3% and 11.5% at 5 and 10 years, respectively, with a lower rate for lower-grade AVMs [41]. In a multicenter retrospective study of 509 ARUBA -eligible patients (SM I/II, 232; SM III, 245; SM IV/V, 32), complete AVM obliteration was obtained in 75% of cases [42]. Permanent neurological morbidity occurred in 4.5% and there was a 4.3% mortality rate. Of those patients that died, 22.7% (0.98% of overall patients) were related to posttreatment hemorrhage, whereas the remaining 77.3% of mortalities were due to other medical causes or remained unknown. While direct comparisons to the ARUBA study are difficult to make, these retrospective studies suggest a low rate of hemorrhage risk that is comparable to the medical treatment arm of patients in the ARUBA study, with the eventual advantage of AVM resolution and reduction in long-term annual hemorrhage risk.
Treatment of Ruptured AVMs
The primary management of a ruptured AVM focuses on managing intracranial pressure. In the setting of intraventricular hemorrhage, an external ventricular drain can be placed both for intracranial pressure monitoring and CSF diversion to prevent the development of hydrocephalus. Hypertonic and hyperosmolar solutions can be used as treatment for intermittent increases in intracranial pressure. However, once intracranial pressure is refractory to CSF diversion and hypertonic/hyperosmolar therapy, the treating physician must weigh the risks and benefits of decompressive hemicraniectomy and possible judicious implementation of hematoma evacuation in selected cases. If the underlying AVM angioarchitecture has not been well-characterized (typically by catheter angiography), emergency surgery for refractory intracranial pressure should consist of decompressive hemicraniectomy only, and AVM resection should be considered in a second-stage operation, if required.
Following optimization of intracranial pressure, a diagnostic cerebral angiogram is obtained to identify the size and location of the AVM nidus as well as identify feeding arteries, adjacent arteries, en passage vessels (arteries that both supply the AVM and normal brain parenchyma), and draining veins. High-risk features can also be identified.
Treatment of the AVM is based on the understanding of the natural history of ruptured lesions. Longitudinal studies suggest approximately a 6–7.5% risk of re-rupture within 1 year of initial rupture, decreasing to approximately 2–3% per year following 1 year after the initial rupture [43,44,45]. Both the options and rationale for each treatment option are similar to those in cases of unruptured AVMs.
Microsurgery is often recommended in low-grade (SM I/II) ruptured AVMs in noneloquent/superficial regions of the brain (Fig. 22.5). In many cases, embolization is often used to treat intranidal aneurysms (to protect against re-rupture during surgery) and deep feeding arterial pedicles (as these are generally the last vessels that can be seen during resection and are often the most complicated to dissect and ligate intraoperatively) (Fig. 22.6). In low-grade AVMs that are located deep within the brain or in patients that are poor candidates for surgery, radiosurgery is often recommended with adjuvant embolization of high-risk features if necessary.
A 73-year-old woman presented with acute decline in mental status. (a) Non-contrast CT showed a cerebellar intraparenchymal hemorrhage. (b) CT angiography showed a superficial SM I AVM. (c) A suboccipital craniotomy was performed for resection of the AVM and intraparenchymal clot. (d) Postoperative angiography showed no residual AVM
A 35-year-old woman presented with an acute episode of worst headache of life. (a) Non-contrast CT showed a right temporal intraparenchymal hemorrhage. (b) Diagnostic angiography showed a superficial SM I AVM with feeding vessels from the inferior division of the middle cerebral artery. Embolization was performed followed immediately by microsurgical resection. (c) Postoperative angiography shows complete obliteration of the AVM
Ruptured SM III AVMs present a similar treatment challenge as microsurgery is associated with slightly higher risk of morbidity as compared to low-grade AVMs. However, microsurgery in this setting can provide an immediate cure of an AVM without a latent period that is seen with AVMs treated with radiosurgery. In general, superficial AVMs are treated with microsurgery and embolization. AVMs that are located deep in the brain or in eloquent cortex are often best treated with radiosurgery with embolization if high-risk features exist.
High-grade AVMs (SM IV/V) are most often observed unless high-risk features are present, in which case targeted therapy is performed. If repeat hemorrhages occur and treatment is necessary, then staged radiosurgery is considered. Partial treatment or surgical resection has not been proven to be an effective treatment strategy without high risk of permanent morbidity and mortality.
Adverse Treatment Effects
Postoperative Epilepsy
Seizures represent a common presenting symptom of patients with both unruptured and ruptured AVMs. Risk factors for developing preoperative epilepsy include younger age, cortical location, and increasing size. Following treatment, approximately 40% of patients will be seizure-free and approximately 70% will be seizure-free or have improvement in their seizure frequency compared to pretreatment [46].
Approximately 2% of patients will experience worsening of seizure frequency following treatment. Seizures often occur within 1 year of treatment, but 25% of seizures can occur beyond 1 year. In addition, approximately 5–20% of patients will develop new seizures after surgical resection [47, 48]. Anti-epileptic medications are commonly prescribed following treatment, and their discontinuation and recommendations regarding activities of daily living must take into account the risk of delayed seizures.
Re-rupture of Recently Treated AVMs
Hemorrhage remains an uncommon yet potentially devastating complication of AVM treatment. This can be due to rupture of sub-totally resected AVM, normal perfusion pressure breakthrough, arterial hemorrhage from feeding arteries, or occlusive hyperemia. These causes are grouped into a syndrome termed arterial-capillary-venous hypertensive (ACVH) syndrome and likely represent a constellation of pathological changes to surrounding vessels and the brain that can lead to postoperative hemorrhage. It reportedly occurs in less than 5% of AVMs undergoing microsurgical resection with risk factors including nidus size >4 cm and postoperative hypertension [49].
Normal perfusion pressure breakthrough was first described in 1978 in which Spetzler et al. postulated that small arteries that fed an AVM, and were located in adjacent normal brain, lost their cerebral autoregulatory properties causing them to become dilated due to hyperemia from the adjacent AVM [50]. Following AVM resection, these arteries would continue to experience perfusion but would be unable to autoregulate in the setting of hyper- or hypotension. Hyperemia through this compromised vasculature would lead to postoperative edema and hemorrhage. Clinical as well as rat models of hypoperfusion suggest that arteries can vasodilate but are unable to vasoconstrict, providing indirect evidence to alterations in vasomotor reactivity following changes in hemodynamics [51, 52]. However, this theory has been challenged. Young et al. showed intact autoregulation in adjacent parenchyma both pre- and post-AVM resection, while Ogasawara et al. showed impaired autoregulation in adjacent parenchyma in areas that had normal autoregulation prior to resection [53,54,55]. In addition, while Spetzler hypothesized that it was adjacent feeding arteries that had lost the ability to autoregulate, increases in cerebral blood flow maximally occur several centimeters from the AVM nidus and can be seen throughout the entire brain [49, 56].
Hemorrhage from feeding arteries is thought to occur from the loss of low-resistance vessels from the AVM nidus following resection resulting in an increase in arterial pressure as well as increased pulsatility in the feeding artery [56, 57]. These changes in hemodynamics can lead to significant stress on both feeding arteries and aneurysms associated with these vessels.
The theory of occlusive hyperemia postulates that slow or stagnant flow occurring in either the arterial or venous system leads to changes in intracranial hemodynamics and subsequent postsurgical edema and hemorrhage. Abnormalities in venous drainage are reported to occur in 30–100% of AVMs, and those with less than three draining veins and/or deep locations are at risk for hemorrhage [58, 59].
Vasospasm
Vasospasm is a rare complication of ruptured AVMs as compared to aneurysmal subarachnoid hemorrhage. Vasospasm from intraventricular or intraparenchymal hemorrhage is thought to arise via re-circulation of heme breakdown products via cerebrospinal fluid into the subarachnoid space [60, 61]. Of note, the treatment of ACVH syndrome can worsen the effects of cerebral vasospasm as the inability to elevate the patient’s blood pressure can lead to ischemia. Treatment of delayed cerebral vasospasm includes hypertension, intra-arterial nicardipine, and balloon angioplasty.
Conclusion
Intracranial AVMs represent a complex lesion requiring the expertise of multiple clinical teams for optimal patient outcomes. While the optimal treatment depends on AVM-specific factors such as rupture status, size, location within the brain, and the presence of deep venous drainage , the treating neurosurgeon must be able to synthesize the risks and benefits of multiple treatment options to best determine the safest treatment modality for each patient. While the ARUBA study concluded that medical management was superior to intervention, multiple limitations exist. In high-volume stroke centers, microsurgical resection is considered safe for low-grade and some intermediate-grade AVMs. What is clear is a multidisciplinary approach is needed to maximally treat intracranial AVMs to prevent rupture while limiting treatment-associated morbidity.
References
Al-Shahi R, Fang JSY, Lewis SC, Warlow CP. Prevalence of adults with brain arteriovenous malformations: a community based study in Scotland using capture-recapture analysis. J Neurol Neurosurg Psychiatry. 2002;73:547–51. https://doi.org/10.1136/jnnp.73.5.547.
Hartmann A, Mast H, Mohr JP, et al. Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke. 1998;29:931–4.
Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476–83. https://doi.org/10.3171/jns.1986.65.4.0476.
**Spetzler RF, Ponce FA. A 3-tier classification of cerebral arteriovenous malformations. Clinical article. J Neurosurg. 2011;114:842–9. https://doi.org/10.3171/2010.8.JNS10663.
*Lawton MT, Kim H, McCulloch CE, et al. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery. 2010;66:702–13 discussion 713. https://doi.org/10.1227/01.NEU.0000367555.16733.E1.
Höllerhage HG. Cerebral arteriovenous malformations: factors influencing surgical difficulty and outcome. Neurosurgery. 1992;31:604–5.
Spears J, Terbrugge KG, Moosavian M, et al. A discriminative prediction model of neurological outcome for patients undergoing surgery of brain arteriovenous malformations. Stroke. 2006;37:1457–64. https://doi.org/10.1161/01.STR.0000222937.30216.13.
Hashimoto T, Lawton MT, Wen G, et al. Gene microarray analysis of human brain arteriovenous malformations. Neurosurgery. 2004;54:410–25. https://doi.org/10.1227/01.NEU.0000103421.35266.71.
Satomi J, Mount RJ, Toporsian M, et al. Cerebral vascular abnormalities in a murine model of hereditary hemorrhagic telangiectasia. Stroke. 2003;34:783–9. https://doi.org/10.1161/01.STR.0000056170.47815.37.
Girerd X, London G, Boutouyrie P, et al. Remodeling of the radial artery in response to a chronic increase in shear stress. Hypertension. 1996;27:799–803. https://doi.org/10.1161/01.HYP.27.3.799.
Hashimoto T, Emala CW, Joshi S, et al. Abnormal pattern of Tie-2 and vascular endothelial growth factor receptor expression in human cerebral arteriovenous malformations. Neurosurgery. 2000;47:910–9. https://doi.org/10.1097/00006123-200010000-00022.
Thorpe ML, Cordato DJ, Morgan MK, Herkes GK. Postoperative seizure outcome in a series of 114 patients with supratentorial arteriovenous malformations. J Clin Neurosci. 2000;7:107–11. https://doi.org/10.1054/jocn.1999.0159.
Englot DJ, Young WL, Han SJ, et al. Seizure predictors and control after microsurgical resection of supratentorial arteriovenous malformations in 440 patients. Neurosurgery. 2012;71:572–80. https://doi.org/10.1227/NEU.0b013e31825ea3ba.
Higuchi M, Bitoh S, Hasegawa H, et al. Marked growth of arteriovenous malformation 19 years after resection: a case report. No Shinkei Geka. 1991;19:75–8.
Fuwa I, Wada H, Matsumoto T. Recurrence of AVM after disappearing on postoperative angiography – report of two cases. No Shinkei Geka. 1988;16:887–91.
Gabriel EM, Sampson JH, Wilkins RH. Recurrence of a cerebral arteriovenous malformation after surgical excision. Case Rep J Neurosurg. 1996;84:879–82. https://doi.org/10.3171/jns.1996.84.5.0879.
Freudenstein D, Duffner F, Ernemann U, et al. Recurrence of a cerebral arteriovenous malformation after surgical excision. Cerebrovasc Dis. 2001;11:59–64.
*Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387–91. https://doi.org/10.3171/jns.1990.73.3.0387.
***Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614–21. https://doi.org/10.1016/S0140-6736(13)62302-8.
Magro E, Gentric J-C, Darsaut TE, et al. Responses to ARUBA: a systematic review and critical analysis for the design of future arteriovenous malformation trials. J Neurosurg. 2016:1–9. https://doi.org/10.3171/2015.6.JNS15619.
Russin J, Spetzler R. Commentary: the ARUBA trial. Neurosurgery. 2014;75:E96–7. https://doi.org/10.1227/NEU.0000000000000357.
Bervini D, Morgan MK, Ritson EA, Heller G. Surgery for unruptured arteriovenous malformations of the brain is better than conservative management for selected cases: a prospective cohort study. J Neurosurg. 2014;121:878–90. https://doi.org/10.3171/2014.7.JNS132691.
Nerva JD, Mantovani A, Barber J, et al. Treatment outcomes of unruptured arteriovenous malformations with a subgroup analysis of ARUBA (a randomized trial of unruptured brain arteriovenous malformations)-eligible patients. Neurosurgery. 2015;76:563–70. https://doi.org/10.1227/NEU.0000000000000663.
Rutledge WC, Abla AA, Nelson J, et al. Treatment and outcomes of ARUBA-eligible patients with unruptured brain arteriovenous malformations at a single institution. Neurosurg Focus. 2014;37:E8. https://doi.org/10.3171/2014.7.FOCUS14242.
Javadpour M, Al-Mahfoudh R, Mitchell PS, Kirollos R. Outcome of microsurgical excision of unruptured brain arteriovenous malformations in ARUBA-eligible patients. Br J Neurosurg. 2016;30:619–22. https://doi.org/10.1080/02688697.2016.1181153.
Schramm J, Schaller K, Esche J, Boström A. Microsurgery for cerebral arteriovenous malformations: subgroup outcomes in a consecutive series of 288 cases. J Neurosurg. 2011:1–8. https://doi.org/10.3171/2016.4.JNS153017.
Wong J, Slomovic A, Ibrahim G, et al. Microsurgery for ARUBA trial (a randomized trial of unruptured brain arteriovenous malformation)-eligible unruptured brain arteriovenous malformations. Stroke. 2017;48:136–44. https://doi.org/10.1161/STROKEAHA.116.014660.
Lawton MT, UCSF Brain Arteriovenous Malformation Study Project. Spetzler-Martin grade III arteriovenous malformations: surgical results and a modification of the grading scale. Neurosurgery. 2003;52:740–8. discussion 748–9.
Frizzel RT, Fisher WS III. Cure, morbidity, and mortality associated with embolization of brain arteriovenous malformations. Neurosurgery. 1995;37:1031–40. https://doi.org/10.1227/00006123-199512000-00001.
Saatci I, Geyik S, Yavuz K, Cekirge HS. Endovascular treatment of brain arteriovenous malformations with prolonged intranidal Onyx injection technique: long-term results in 350 consecutive patients with completed endovascular treatment course. J Neurosurg. 2011;115:78–88. https://doi.org/10.3171/2011.2.JNS09830.
Katsaridis V, Papagiannaki C, Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50:589–97. https://doi.org/10.1007/s00234-008-0382-x.
Alexander MD, Cooke DL, Hallam DK, et al. Less can be more: targeted embolization of aneurysms associated with arteriovenous malformations unsuitable for surgical resection. Interv Neuroradiol. 2016;22:445–51. https://doi.org/10.1177/1591019916641316.
***Xu F, Zhong J, Ray A, et al. Stereotactic radiosurgery with and without embolization for intracranial arteriovenous malformations: a systematic review and meta-analysis. Neurosurg Focus. 2014;37:E16. https://doi.org/10.3171/2014.6.FOCUS14178.
Xhumari A, Rroji A, Enesi E, et al. Glioblastoma after AVM radiosurgery. Case report and review of the literature. Acta Neurochir. 2015;157:889–95. https://doi.org/10.1007/s00701-015-2377-9.
Chang SD, Shuster DL, Steinberg GK, et al. Stereotactic radiosurgery of arteriovenous malformations: pathologic changes in resected tissue. Clin Neuropathol. 1997;16:111–6.
Flickinger JC, Kondziolka D, Maitz AH, Lunsford LD. An analysis of the dose-response for arteriovenous malformation radiosurgery and other factors affecting obliteration. Radiother Oncol. 2002;63:347–54.
Schlienger M, Lefkopoulos D, Mérienne L, et al. Linac radiosurgery for cerebral arteriovenous malformations (AVM): results in 173 patients. Int J Radiat Oncol Biol Phys. 1998;42:212. https://doi.org/10.1016/S0360-3016(98)80277-0.
Flickinger JC, Pollock BE, Kondziolka D, Lunsford LD. A dose-response analysis of arteriovenous malformation obliteration after radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36:873–9.
Friedman WA, Bova FJ, Mendenhall WM. Linear accelerator radiosurgery for arteriovenous malformations: the relationship of size to outcome. J Neurosurg. 1995;82:180–9. https://doi.org/10.3171/jns.1995.82.2.0180.
Pollock BE, Flickinger JC, Lunsford LD, et al. Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery. 1998;42:1239–44. discussion 1244–7.
Pollock BE, Link MJ, Brown RD. The risk of stroke or clinical impairment after stereotactic radiosurgery for ARUBA-eligible patients. Stroke. 2013;44:437–41. https://doi.org/10.1161/STROKEAHA.112.670232.
**Ding D, Starke RM, Kano H, et al. Radiosurgery for cerebral arteriovenous malformations in a randomized trial of unruptured brain arteriovenous malformations (ARUBA)-eligible patients. Stroke. 2016;47:342–9. https://doi.org/10.1161/STROKEAHA.115.011400.
Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331–7. https://doi.org/10.3171/jns.1983.58.3.0331.
Halim AX, Johnston SC, Singh V, et al. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke. 2004;35:1697–702. https://doi.org/10.1161/01.STR.0000130988.44824.29.
da Costa L, Wallace MC, Brugge Ter KG, et al. The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke. 2009;40:100–5. https://doi.org/10.1161/STROKEAHA.108.524678.
Chen C-J, Chivukula S, Ding D, et al. Seizure outcomes following radiosurgery for cerebral arteriovenous malformations. Neurosurg Focus. 2014;37:E17. https://doi.org/10.3171/2014.6.FOCUS1454.
Hoh BL, Chapman PH, Loeffler JS, et al. Results of multimodality treatment for 141 patients with brain arteriovenous malformations and seizures: factors associated with seizure incidence and seizure outcomes. Neurosurgery. 2002;51:303–9. discussion 309–11.
de Los Reyes K, Patel A, Doshi A, et al. Seizures after Onyx embolization for the treatment of cerebral arteriovenous malformation. Interv Neuroradiol. 2011;17:331–8.
Young WL, Kader A, Ornstein E, et al. Cerebral hyperemia after arteriovenous malformation resection is related to “breakthrough” complications but not to feeding artery pressure. The Columbia University arteriovenous malformation study project. Neurosurgery. 1996;38:1085–93. discussion 1093–5.
Spetzler RF, Wilson CB, Weinstein P, et al. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651–72.
Batjer HH, Devous MD. The use of acetazolamide-enhanced regional cerebral blood flow measurement to predict risk to arteriovenous malformation patients. Neurosurgery. 1992;31:213–7. discussion 217–8.
Irikura K, Morii S, Miyasaka Y, et al. Impaired autoregulation in an experimental model of chronic cerebral hypoperfusion in rats. Stroke. 1996;27:1399–404.
Young WL, Prohovnik I, Ornstein E, et al. The effect of arteriovenous malformation resection on cerebrovascular reactivity to carbon dioxide. Neurosurgery. 1990;27:257–66. discussion 266–7.
Young WL, Solomon RA, Prohovnik I, et al. 133Xe blood flow monitoring during arteriovenous malformation resection: a case of intraoperative hyperperfusion with subsequent brain swelling. Neurosurgery. 1988;22:765–9.
Ogasawara K, Yoshida K, Otawara Y, et al. Cerebral blood flow imaging in arteriovenous malformation complicated by normal perfusion pressure breakthrough. Surg Neurol. 2001;56:380–4.
Barnett GH, Little JR, Ebrahim ZY, et al. Cerebral circulation during arteriovenous malformation operation. Neurosurgery. 1987;20:836–42.
Sorimachi T, Takeuchi S, Koike T, et al. Blood pressure monitoring in feeding arteries of cerebral arteriovenous malformations during embolization: a preventive role in hemodynamic complications. Neurosurgery. 1995;37:1041–7. discussion 1047–8.
Viñuela F, Nombela L, Roach MR, et al. Stenotic and occlusive disease of the venous drainage system of deep brain AVM’s. J Neurosurg. 1985;63:180–4. https://doi.org/10.3171/jns.1985.63.2.0180.
al-Rodhan NR, Sundt TM, Piepgras DG, et al. Occlusive hyperemia: a theory for the hemodynamic complications following resection of intracerebral arteriovenous malformations. J Neurosurg. 1993;78:167–75. https://doi.org/10.3171/jns.1993.78.2.0167.
Tseng W-L, Tsai Y-H. Vasospasm after intraventricular hemorrhage caused by arteriovenous malformation. Asian J Neurosurg. 2015;10:114–6. https://doi.org/10.4103/1793-5482.154984.
Dull C, Torbey MT. Cerebral vasospasm associated with intraventricular hemorrhage. Neurocrit Care. 2005;3:150–2. https://doi.org/10.1385/NCC:3:2:150.
To assist the reader in gaining familiarity with available evidence, the following rating system has been used to indicate key references for each chapter’s content:
***: Critical material. Anyone dealing with this condition should be familiar with this reference.
**: Useful material. Important information that is valuable in in clinical or scientific practice related to this condition.
*: Optional material. For readers with a strong interest in the chapter content or a desire to study it in greater depth.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Ruzevick, J., White-Dzuro, G., Levitt, M., Kim, L., Ferreira, M. (2018). Intracranial Arteriovenous Malformations. In: Perkins, J., Balakrishnan, K. (eds) Evidence-Based Management of Head and Neck Vascular Anomalies. Springer, Cham. https://doi.org/10.1007/978-3-319-92306-2_22
Download citation
DOI: https://doi.org/10.1007/978-3-319-92306-2_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92305-5
Online ISBN: 978-3-319-92306-2
eBook Packages: MedicineMedicine (R0)