Abstract
Ischemic heart disease (IHD) is the most common cause of death worldwide and its prevalence is increasing. The electrocardiogram (EKG) is a fundamental diagnostic tool in the setting of suspected myocardial ischemia. Ischemia produces complex changes in the EKG, involving the QRS complex and, mostly, the repolarization phase (ST-segment). In this chapter we will review the basic mechanisms underlying these EKG changes, focusing on the theories of diastolic and systolic currents of injury. Furthermore, starting from clinical cases, we will try to give the clinician practical and useful tips for the correct interpretation of the EKG in order to identify the area of the myocardium involved by ischemia, the occluded coronary artery and also to capture prognostic information. Our final goal is being to improve patient management and help choosing the most appropriate therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ischemic Heart Disease
- ST-segment Elevation MI (STEMI)
- Left Anterior Descending
- Left Main Trunk
- Left Anterior Fascicular Block
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
9.1 Case 1
A 61-year-old man with a history of arterial hypertension, obesity (BMI = 42 kg/m2), and tobacco smoking presented to ER complaining of severe oppressive chest pain for the past 2 h, radiated to the left upper arm and to the back side associated with profuse and cold sweating. The pain started at rest, worsened with minimal exertion and was unaffected by respirations.
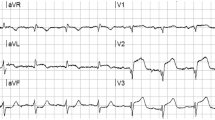
An EKG was recorded (Fig. 9.1) and, given the persistence of symptoms, the patient was sent to cath lab for coronary angiography.
9.1.1 EKG Analysis
Sinus tachycardia, 105 bpm. P waves show a prominent negative terminal component in V1, which, together with a prolonged (110 ms) P wave duration in DII, is diagnostic for a left atrial strain. The PR segment is isoelectric and of normal duration (160 ms). QRS has a left anterior fascicular block morphology, with an axis of −45°; there is a slow progression of the R wave in the precordial leads and QS complexes in V1 and V2, possibly secondary to the left anterior fascicular block. ST segment is quite abnormal with an almost 1.5 mm ST-segment elevation (ST↑) in aVR and a 1 mm ST↑ in V1 together with reciprocal ST-segment depression (ST↓) in DII, aVF, DIII and from V3 to V6; negative T wave is recorded in DI and VL.
This EKG, coupled with the clinical presentation, is strongly suggestive for an acute coronary syndrome secondary to either acute subocclusion or even occlusion with good collateral circulation of the left main trunk or its equivalent (left anterior descending plus left circumflex coronary artery). The clues are the marked ST↑ in VR more than in V1, the reciprocal ST↓ in at least 7 other leads and the left anterior fascicular block.
A similar EKG may however be of difficult interpretation in all the patients with an acute coronary syndrome because of large interindividual variability of coronary anatomy, a possible presence of a well-developed collateral circulation, pre-existent myocardial infarction (MI), or a prior coronary artery bypass surgery [1].
The coronary angiogram showed as a culprit lesion an acute thrombotic occlusion of the proximal left anterior descending coronary artery distal to the origin of the first diagonal branch.
It was also evident a severe (70%) stenosis of the first diagonal branch, an 80% proximal stenosis of the left circumflex coronary artery, and a 50% stenosis of the right coronary artery. This was a left main trunk equivalent picture.
The patient was treated with optimal medical therapy (aspirin, heparin, ticagrelor, atorvastatin) together with PCI on the proximal left anterior descending coronary artery; the pain solved. A week later a second PCI on the circumflex and on the first diagonal branch was performed. The echocardiogram upon presentation showed a dilated left ventricle with an ejection fraction (EF) = 35%, an akinesia of the inter-ventricular septum and of the anterolateral wall together with a moderate dilation of the left atrium.
During hospitalization, after PCI, the patient improved clinically and echocardiographically, with EF reaching 45% prior to dismission.
A left main trunk disease ( or equivalent) may be somewhat nonspecific but an ST↑ in lead aVR > V1 should raise the suspicion for this severe condition, especially if accompanied by a diffuse ST↓ in at least 7 leads and presence of a conduction disturbance such as right bundle branch block or left anterior fascicular block.
9.1.2 Introduction and Definition
The term MI should be used when there is evidence of myocardial necrosis in a clinical setting consistent with acute myocardial ischemia. The recently revised universal definition of MI [2] focuses on the rise and/or fall of cardiac troponins with at least one value above the 99th percentile upper reference limit (URL) as a mandatory sign together with at least another criterion among symptoms of myocardial ischemia, new or presumed new significant ST-segment–T wave (ST–T) changes or new left bundle branch block (LBBB), development of pathological Q waves, evidence of new loss of viable myocardium or new regional wall motion abnormality, or identification of an intracoronary thrombus by angiography or autopsy.
9.1.3 Classification
The EKG allows clinicians to distinguish between ST segment elevation MI (STEMI) [3] and non-ST elevation acute coronary syndromes (NSTEMI, a broad term which encompasses non-ST elevation MI and unstable angina according to whether or not there’s a rise and/or fall of cardiac troponins in the blood) [4]. This is a simple yet crucial distinction in that it influences the treatment, with the first group of patients going necessarily straight to the cath-lab.
The most widely accepted clinical/pathological classification distinguishes 5 types of MI:
-
Type 1: MI is the spontaneous one, related to atherothrombosis;
-
Type 2: MI is secondary to an imbalance between myocardial oxygen supply and/or demand;
-
Type 3: MI is the one resulting in death when biomarker values are unavailable;
-
Type 4a: MI is the one related to percutaneous coronary intervention (PCI);
-
Type 4b: MI is the one related to stent thrombosis;
-
Type 5: MI is related to coronary artery bypass grafting (CABG).
9.1.4 Epidemiology
Ischemic heart disease (IHD) is the most common cause of death worldwide. Its prevalence is increasing, even though IHD related mortality has been decreasing in Europe over the past three decades. IHD now accounts for 20% of all deaths in Europe. The relative incidences of STEMI and NSTEMI are decreasing and increasing, respectively, with NSTEMI presentation being more diffused nowadays.
9.1.5 Mechanisms of EKG Changes Under Ischemic Conditions
EKG is a fundamental diagnostic tool in the setting of suspected myocardial ischemia. Ischemia produces complex changes in the EKG, involving the QRS complex and, mostly, the repolarization phase (ST-segment).
It was since the 1960s [5] that the theories of diastolic and systolic currents were proposed to explain the ST-segment displacement, based on animal experiments. These theories rely on the observation that under ischemic conditions, the cardiac action potential is modified with shortening of the action potential duration, and a slowing of the rapid 0 phase of the action potential (upstroke); also an increase of the baseline potential (which becomes less negative) was demonstrated [6, 7]. This last is due to: (1) the depletion of ATP in the ischemic tissues, which determines an opening of ATP regulated K channels, thus increasing the extracellular K+ content and (2) by the increased H+ ions concentration in the cells due to the production of lactic acid (which is necessary to keep glycolysis active when the cell gets short of oxygen); the H+ ions will then be exchanged for Na+ ions, which in turn will be exchanged for Ca++ ions by the Na+/Ca++ exchanger.
The increased intracellular Ca++ concentration will render the diastolic membrane potential less negative, thus provoking the inactivation of Na+ channels; the result is a slowing of phase 0 (upstroke). This mechanism leads to the ischemic slowing of conduction, which may facilitate re-entry circuits and thus ventricular arrhythmias.
Moreover, the repolarization phase will be accelerated, because of a reduction of Ca++ current flowing into the cell (due to a reduced outer to inner Ca++ gradient) and an increase in K+ current flowing out of the cells, mainly because of the opening of the ATP regulated K+ channel.
These changes inevitably produce an electric heterogeneity between the ischemic myocardium and the normally perfused myocardium, which provides the basis for both the electrocardiographic changes and the arrhythmic risk inherent to ischemia.
In particular, during diastole, there’s a current flowing from the ischemic region (which is less negative) and the normally perfused myocardium; this is called the diastolic current of injury and is responsible for the TQ segment depression. In turn, this TQ segment depression will appear as an -ST↑ since EKG recorders used in clinical practice automatically compensate for any shift of the -TQ segment by placing it at the isoelectric line. In other words, according to the diastolic current of injury hypothesis, the ST↑ observed during transmural ischemia is an apparent phenomenon, reflecting the depression of the TQ segment.
However, there’s also evidence for systolic currents of injury, because of the systolic voltage gradient that determines a current flow from the normally perfused myocardium to the ischemic region. This happens mainly during phase 2 (plateau) of the action potential, since this phase is shorter and during this phase the myocardium is less positive in the ischemic region, but also during phase O (upstroke), which is slower in the ischemic myocardium as compared to the normally perfused one. This will be recorded as a positive displacement of the ST segment and as hyperacute giant T waves by an electrode exploring the ischemic region.
In addition to these diastolic and systolic currents, which flow parallel to the epicardial surface, there are several experiments that have suggested the presence of transmural currents, directed perpendicularly to the epicardial surface. This is due to the transmural differences in those cellular mechanisms that were explained previously; most notably, in particular, the Na+ current mediating phase 0 shows different inactivation kinetics between the endocardium and the epicardium [8], inactivating earlier, at more negative membrane voltages in the latter as compared to the former. This produces a dramatic slowing of the conduction between the endocardium and epicardium and creates, once again, a voltage gradient, this time between the endocardium and the epicardium, which will contribute to the ST↑ as seen by electrodes over the transmurally ischemic area.
From these basic observations, we can understand how the correct EKG interpretation, focusing on repolarization alterations, may aid to identify the ischemic segment(s) in case of transmural ischemia and thus the vessel most likely involved.
9.1.6 Left Main Disease or Its Equivalents
There are two very different clinical and electrocardiographic presentations of patients with an acute coronary syndrome secondary to a left main trunk disease or its equivalents (proximal left anterior descending coronary artery plus left circumflex) (Fig. 9.2):
-
1.
The occlusion of the left main trunk with a poorly developed collateral circulation;
-
2.
The subocclusion with scarce collaterals or the occlusion with well-developed collaterals [9].
9.1.6.1 Occlusion of the Left Main Trunk with Poorly Developed Collaterals
In this case, patients usually show a significant hemodynamic compromise or even die before reaching the hospital because of cardiac arrest. When these patients survive, they usually show a cardiogenic shock and an EKG STEMI pattern with a significant ST↑ in multiple precordial leads starting from V2. This pattern differs from what is usually recorded in case of an acute occlusion of the left anterior descending artery proximal to the origin of the first septal and the first diagonal branches, in which there’s a significant ST↑ even in V1 and VR. This difference is due to the concurrent involvement of left circumflex artery territory in case of a left main trunk occlusion with poorly developed collaterals [10].
Thus, an isoelectric ST segment in V1 (and usually aVR) in a patient with ST↑ in multiple precordial leads (V2 to V4–V6) and DI/VL and ST↓ in the inferior leads [11] suggests an acute complete occlusion of the left main coronary artery with a poor collateral circulation, especially if accomplished with a newly developed right bundle branch block and/or left anterior fascicular block.
These conduction disturbances are due to the poor blood flow circulation to the right bundle and left anterior fascicle, which relies on the septal perforator branches (usually the first septal branch, S1) of the proximal left anterior descending artery. The development of a bifascicular block in patients with STEMI portends a poor prognosis, with a 30% risk of complete heart block [12, 13], that by itself carries an almost 80% risk of mortality in this setting [14].
9.1.6.2 Subocclusion of the Left Main Trunk or Occlusion of the Left Main Trunk with Developed Collaterals
The clinical and EKG picture of patients with an acute coronary syndrome secondary to a subocclusion of the left main trunk or to an occlusion of this artery with well-developed collaterals is that of an NSTEMI with an ST↓ in usually at least 7 leads and an ST↑ ≥1 mm in VR and often V1, with the ST↑ in VR > V1 (because the electrical forces due to posterior wall ischemia, secondary to left circumflex artery involvement, counterbalance the forces due to anterior wall ischemia secondary to left anterior descending artery involvement and thus bring the ST segment in lead V1 closer to the isoelectric) [15].
The lead VR is often ignored in EKG interpretation; it may be referred to as “the neglected lead” in past; however, in the ischemic condition may be of great diagnostic importance. Regarding the electrophysiological mechanisms underlying ST↑ in lead VR, there are two possibilities: the first is that a diffuse anterolateral sub-endocardial ischemia produces an ST↑ in VR as a reciprocal change; the other is that this may represent an acute transmural ischemic injury of the walls directly explored by lead VR, including the basal portion of the interventricular septum [16].
As for the prognostic significance of ST↑ in VR, there is discordance in the medical literature, with some but not all data suggesting a strong correlation to the 30-day mortality [17, 18]. Clinicians should be alert and aware of the clinical relevance of this EKG sign.
9.2 Case 2
A 57-year-old man, cigarette smoker, without any other risk factor, awakened early morning with a severe substernal chest pain, neck, shoulder and left arm radiated, associated with dyspnea. Because of pain persistence and its increasing intensity he called the emergency medical care.
9.2.1 EKG Analysis
The electrocardiogram was the following: irregular rhythm at 87 bpm. Absence of P waves and presence of small irregular oscillation in all leads, typical of atrial fibrillation. Narrow QRS complex. Evident ST segment elevation, of 6 mm, in lead V1–V3, I and aVL associated with reciprocal ST-depression in inferior leads (II, III, aVF) and V6 (Fig. 9.3).
Clinical presentation and electrocardiogram both suggest an extensive acute anterior myocardial infarction.
A relevant ST elevation (>2.5 mm) in lead V1 and reciprocal changes in inferior leads with high ST deviation (>1 mm) suggest a proximal left anterior descending occlusion.
An echocardiography was performed and revealed hypokinesia of left ventricle anterior wall and apex. After 30 min the patient underwent coronary angiography which disclosed only a distal occlusion of the left anterior descending artery. Aspiration thrombectomy and angioplasty was performed with subsequent stent implant.
The discrepancy between the angiography result and the electrocardiographic pattern suggests that other mechanisms might be suspected such coronary vasospasm with possible occlusion, at the time of infarction, that lately may disappear due to recanalization or lysis of the thrombus. Moreover, atrial fibrillation is the most frequent cause of coronary artery embolism, a rare but important nonatherosclerotic cause of acute myocardial infarction.
9.2.2 Anterior Myocardial Infarction
Anterior myocardial infarction is the most relevant form of infarct because it is burdened by a high risk of short-term mortality and also of subsequent deterioration of left ventricular function. Prognosis following MI is mostly related to the infarct size rather than to infarct location and the more proximal the occlusion the less favorable is prognosis. Anterior MI is generally associated with the most extensive left ventricular damage [19].
Anterior wall infarct is due to occlusion of the left anterior descending artery (LAD), the most important of the three main coronary arteries that supplies over half of the heart muscle.
LAD gives rise to septal and diagonal branches and occasionally to intermediate branches. The septal branches supply the anterior two-thirds of the interventricular septum and the diagonal branches supply the anterolateral ventricular wall. Distal LAD supplies the inferoapical part and, when wrapped around the apex, supplies the area beyond that.
According to Engelen et al. [10], LAD occlusions at different sites led to electrocardiographically four different patterns (Fig. 9.4):
-
1.
Proximal to the septal and diagonal branches;
-
2.
Before the first diagonal but distal to the first septal branch;
-
3.
Before the first septal but distal to the first diagonal branch;
-
4.
Distal to the first septal and diagonal branches.
The ST↑ in leads V2 and V3 indicates occlusion of the LAD, especially when V3 ST↑ > V1 ST↑. These findings represent a specific marker of anterior MI and are common to all the above-mentioned cases.
-
1.
The very proximal LAD occlusion, before the septal and diagonal branches, results in ischemia of all areas perfused by the left anterior descending coronary. Injury current vector is directed towards the damaged myocardium area, so it pointing superiorly (>−80 < −100°) and forward.
EKG shows:
-
ST↑ in leads V1-V3 (usually V1 ST↑ >2.5 mm);
-
ST↑ in leads VR and VL;
-
Reciprocal ST↓ II, III, VF (ST↓ >1.0 mm);
-
Reciprocal ST↓ in leads V5-V6;
-
Additionally, a new right bundle branch block (RBBB).
ST↑ in VR is specific for LAD occlusion proximal to S1 and it is consequent to transmural ischemia of the basal part of the septum. However, this finding could be absence due to the counterbalance of the septal ischemia by ischemia in other larger areas perfused by the LAD.
ST↓ in the inferior leads represents reciprocal changes associated with ischemia in the anterobasal region. It is the most important sign of a proximal occlusion of the left anterior descendant. The amount of deviation result is significantly higher in proximal LAD occlusion and ST↓ >1.0 mm is strongly predictive of a culprit lesion proximal to the origin of the first diagonal branch [20].
During an anterior MI, a new onset of right bundle branch block (RBBB) with a Q wave preceding the R wave in lead V1 is a specific marker of extensive myocardial damage and identify high risk patients. S1 supplies the distal part of the bundle of His and proximal bundle branches, thus RBBB may be a consequence of LAD occlusion proximally to S1. When RBBB occurs in association with a left anterior hemiblock, the risk of progression to a complete AV block is high [21].
-
2.
LAD occlusion of the first diagonal (distal to septal branches) or intermediate branches results in ischemia of anterolateral wall. Therefore, ST segment vector is directed toward left leads. EKG pattern is characterized by:
-
ST↑ in V2-V4 leads;
-
ST↑ mainly in I and aVL, fewer in V5 and V6 leads and possibly in II lead (unlike the left circumflex artery (LCx) occlusion, when the anterior descending artery is involved, ST segment elevation is evident not only in the lateral leads, but also in anterior leads) [22].
-
Mild reciprocal ST↓ in III and aVF
-
-
3.
The occlusion of the LAD proximal to the main septal branch but distal to first diagonal branch preserves the areas perfused by D1 and leads to the ischemia of the septum and inferoapical ventricular wall. This type of occlusion results in inferior and rightward direction of injury current vectors.
-
ST↑ in V2–V4 leads;
-
ST↑ in inferior leads (II, III, aVF);
-
Reciprocal ST↓ in I and VL leads (unlike the inferior myocardial infarction supported by right coronary artery (RCA) occlusion, when the anterior descending artery is involved, ST segment elevation is evident not only in the inferior leads, but also in anterior) [10, 22].
-
-
4.
Occlusion of left anterior descending artery after the origin of the S1 and D1 produces ischemia of inferoapical ventricular area [8]. ST segment vector is directed inferiorly and leftward.
EKG shows:
-
ST↑ in V1-V3;
-
Reciprocal ST↓ <1 mm in II, III, aVF, sometimes ST↑ in inferior leads
-
Pathological Q waves in V4-V5 and possibly in V6 leads identify a preserved early septal activation with the vector directed away from the left leads. However, these waves have pathological characteristics consequent to the slowed electrical activity in the ischemia areas [22].
9.3 Case 3
A 72-year-old man was referred to our hospital because of sudden onset of chest pain at rest irradiated to the neck and to the left arm lasting 30 min. The patient was also sweaty and dyspneic. He had a history of diabetes mellitus type II and was a tobacco smoker (20 cigarettes/day). A surface EKG was obtained (Fig. 9.5).
9.3.1 EKG Analysis
Sinus rhythm, heart rate 88 bpm, normal atrioventricular conduction (PQ 160 ms), electrical axis +75°, normal interventricular conduction (QRS width 90 ms), a high and unexpected high R wave in lead V3. There are significant ST-segment elevations in all inferior leads: 2 mm in lead III, 1 mm in lead aVF and 0.5 mm in lead II. In the peripheral lateral leads is visible an ST↓: 1.5 mm in lead VL and 1 mm in lead I. In VL the ST↓ is followed by a T wave inversion.
In lead I a biphasic (negative-positive) T wave is present. In the precordial leads V2, V3, and V4 an ST↓ is visible: 1.2 mm in lead V2, 2.2 mm in lead V3, and 0.8 in lead V4. QTc 436 ms.
This EKG together with the patient’s symptoms was strongly suggestive of inferior acute ST-elevation MI.
In order to find an ischemic involvement of the right ventricle, even if the ST-segment in lead V1 was not elevated, the right precordial leads (Fig. 9.6) were recorded. An EKG with posterior precordial leads (Fig. 9.7) was also recorded in order to exclude a posterior MI. This EKG showed an ST↑ of 0.5 mm in leads V8 and V9.
The patient was urgently brought to the cath lab for a coronarography and possibly PCI. We suggested the right coronary to be the culprit lesion.
-
1.
ST↑ is higher in lead III than in lead II;
-
2.
There is a reciprocal ST↓ in leads I and VL and the depression is deeper in lead aVL;
-
3.
S/R-wave ratio in lead aVL is 1.4 (see below in the text);
-
4.
The T-wave amplitude in lead III is greater than in lead II and there is a positive biphasic T wave in lead V5R (see below in the text).
All these signs are highly suggestive for a culprit lesion localized in RCA. Furthermore, we suspected the occlusion to be in the distal part of RCA (after the origin of acute marginal artery) since the right ventricle was not involved:
-
1.
ST↑ in lead V1;
-
2.
Not ST↑ in precordial right leads;
-
3.
V3/III ratio 1.1 (see below in the text).
We also hypothesized that the RCA gave origin to the interventricular posterior artery (right dominancy). We thought of that because of the involvement of the posterior wall visible with a high R in V3 greater than in V4, ST-depression in leads V2 and V3 and a mild ST↑ in the posterior precordial leads V8 and V9. The coronary angiography revealed an acute thrombotic occlusion of a dominant RCA in the intermediate portion (after the origin of the marginal artery). The patient underwent a PCI with implant of two drug eluting stents (DES). The other major coronary arteries were free from severe stenosis. Transthoracic echocardiography (TTE) showed an akinesia of the inferior wall and of the basal segment of the posterior wall of the left ventricle and the LVEF was 41%. The right ventricle dimension and function was normal.
9.3.2 Acute Inferior Myocardial Infarction
The acute inferior MI is usually characterized by the clinical symptoms and signs together with electrocardiographic modifications: ST↑ >1 mV in ≥2 inferior leads (II, III, VF). Sometimes other ST-segment modifications in I, aVL and precordial leads might be present. The vascularization of the inferior wall of the heart is often given by the right coronary artery (RCA) and in a less number of cases (10–18%) by the left circumflex artery (LCx). In 1% of cases the left anterior descending artery is responsible for this area [22, 23]. Prognosis is poorer in ACS due to RCA occlusion than when ACS is due to LCx. RCA is responsible for vascularization of sinus node in 60% of cases meanwhile LCx only in 40%; the atrioventricular node is vascularized by RCA in 90% of patients and by LCx in 10%; the RCA often supplies the bundle of His. An inferior infarction consequence of RCA occlusion can be more frequently complicated by conduction abnormalities such as sinus bradycardia, sinus-atrial blocks, and different degrees of atrioventricular block. Furthermore, an inferior ST-elevation ACS due to RCA occlusion may involve also the right ventricle especially when the occlusion is in the first part of RCA, before the origin of the marginal acute artery [8, 24].
When the RCA is involved the ST↑ vector is directed downwards and toward the right (Fig. 9.8). At the EKG this direction is visible as:
-
ST↑ in lead III > lead II;
-
ST↓ in leads I and VL;
-
ST↓ in lead VL is greater than in lead I;
-
Additional ST↑ in lead V1 can be a sign of a proximal occlusion of the RCA with involvement of the right ventricle.
On the other hand, when the inferior MI is due to an occlusion of LCx the ST↑ vector is directed downwards and towards left. This orientation means:
-
ST↑ in lead II > lead III;
-
Usually ST↑ in lead I;
-
ST-segment isoelectric or ST↑ in lead VL;
-
ST↑ in leads V5 and V6 can be present [8];
-
ST↓ in leads V2 and V3, more frequently when the culprit artery is LCx or not-proximal RCA [24].
Not all these ST-segment variations are always present at the same time, but when more than one is visible the predictive power increases. There are some EKG-tricks that can help us to identify the culprit vessel more surely.
-
ST↑ in lead III > II associated with ST↓ in lead VL > I is predictive of RCA occlusion. Conversely when the previous criteria are both absent the culprit lesion is LCx [25].
-
The ratio between the magnitude of ST↓ in lead V3 and ST↑ in lead III (V3/III ratio) <0.5 is predictive for a proximal RCA occlusion (before the marginal artery), V3/III ratio ≥0.5 and ≤1.2 for distal RCA occlusion and V3/III ratio >1.2 for an LCx occlusion [24].
-
The ratio between the S wave and the R wave in lead aVL (S/R-wave ratio) ≤0.33 associated to ST↓ in lead VL ≤1 mm is suggestive of LCx-related infarction. An S/R-wave ratio >0.33 and an ST↓ in lead VL >1 mm is a marker for RCA-related infarction [26].
-
In the early phases of the infarction a T-wave amplitude in lead III ≥ lead II and an upright or positive biphasic T wave in leads V4R and/or V5R are predictive for RCA-related infarction [8, 27].
9.4 Acute Lateral Myocardial Infarction
The acute lateral MI usually is caused by the occlusion of the LCx or one of its principal branches as the first obtuse marginal artery. In a smaller number of cases the culprit lesion is in the first diagonal branch of the LAD. At the EKG it is characterized by:
-
ST↑ >1 mV in leads aVL and I and/or in leads V5 and V6.
When all the lateral leads are not involved we can distinguish:
-
High lateral infarction: ST↑only in leads VL and I;
-
Low lateral infarction: ST↑only in leads V5 and V6.
Sometimes reciprocal ST↓ in inferior leads might be present. Usually ST in right precordial leads (V1-V3) is isoelectric or depressed [22, 23]. Sometimes an ST↑ can be visible in lead V3 when the first diagonal branch is involved [28]. The acute lateral infarction can often be a portion of larger infarctions such as the extended anterior infarctions or inferior-posterior-lateral.
9.5 Acute Right Ventricle Myocardial Infarction
The acute right ventricle MI is due to occlusion of the proximal RCA (before the acute marginal artery) or in case of isolated occlusion of the acute marginal artery. In the first option the EKG showed the already described modifications of the RCA-related inferior MI with the involvement of the right ventricle. The second and rarer option configures the “isolated” right ventricle MI.
This infarction can be recognized thorough EKG by some typical signs:
References
Bayés de Luna A, Fiol-Sala M. Where is the culprit lesion? Circulation. 2016;134(19):1507–9.
Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. ESC Committee for Practice Guidelines (CPG). Eur Heart J. 2012;33(20):2551–67.
Ibanez B, James S, Agewall S, Antunes MJ, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2017;39(2):119–77.
Roffi M, Patrono C, Pet CJ, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315.
Samson WE, Scher AM. Mechanism of ST segment alteration during acute myocardial injury. Circ Res. 1960;8:780–7.
Di Diego JM, Antzelevitch C. Acute myocardial ischemia: cellular mechanisms underlying ST segment elevation. J Electrocardiol. 2014;47(4):486–90.
Krishnan SC, Antzelevitch C. Sodium channel block produces opposite electrophysiological effects in canine ventricular epicardium and endocardium. Circ Res. 1991;69:277–91.
Zimetbaum PJ, Josephson ME. Use of the electrocardiogram in acute myocardial infarction. N Engl J Med. 2003;348:933–40.
Fiol M, Carrillo A, Rodríguez A, Pascual M, et al. Electrocardiographic changes of ST-elevation myocardial infarction in patients with complete occlusion of the left main trunk without collateral circulation: differential diagnosis and clinical considerations. J Electrocardiol. 2012;45(5):487–90.
Engelen DJ, Gorgels AP, Cheriex EC, et al. Value of the electrocardiogram in localizing the occlusion site in the left anterior descending coronary artery in acute anterior myocardial infarction. J Am Coll Cardiol. 1999;34:389–95.
Hindman MC, Wagner GS, JaRo M, et al. The clinical significance of bundle branch block complicating acute myocardial infarction. 1. Clinical characteristics, hospital mortality, and one-year follow-up. Circulation. 1978;58:679–88.
Sgarbossa EB, Pinski SL, Topol EJ, et al. Acute myocardial infarction and complete bundle branch block at hospital admission: clinical characteristics and outcome in the thrombolytic era. J Am Coll Cardiol. 1998;31:105–10.
Harpaz D, Behar S, Gottleib S, Boyko V, Kishon Y, Eldar M. Complete atrioventricular block complicating acute myocardial infarction in the thrombolytic era. J Am Coll Cardiol. 1999;34:1721–8.
Yamaji H, Iwasaki K, Kusachi S, et al. Prediction of acute left main coronary artery obstruction by 12-lead electrocardiography. ST segment elevation in lead aVR with less ST segment elevation in lead V(1). J Am Coll Cardiol. 2001;38(5):1348–54.
Kireyev D, Arkhipov MV, Zador ST, et al. Clinical utility of aVR-The neglected electrocardiographic lead. Ann Noninvasive Electrocardiol. 2010;15:175–80.
Jong GP, Ma T, Chou P, et al. Reciprocal changes in 12-lead electrocardiography can predict left main coronary artery lesion in patients with acute myocardial infarction. Int Heart J. 2006;47:13–20.
Szymański FM, Grabowski M, Filipiak KJ, et al. Admission ST-segment elevation in lead aVR as the factor improving complex risk stratification in acute coronary syndromes. Am J Emerg Med. 2008;26:408–12.
Yan AT, Yan RT, Kennelly BM, GRACE Investigators, et al. Relationship of ST elevation in lead aVR with angiographic findings and outcome in non-ST elevation acute coronary syndromes. Am Heart J. 2007;154:71–8.
Baldi C, Polito MV, Citro R, et al. Prognostic value of clinical, echocardiographic and angiographic indicators in patients with large anterior ST-segment elevation myocardial infarction as a first acute coronary event. J Cardiovasc Med. 2017;18(12):946–53.
Tamura A, Kataoka H, Mikuriya Y, et al. Inferior ST segment depression as a useful marker for identifying proximal left anterior descending artery occlusion during acute anterior myocardial infarction. Eur Heart J. 1995;16:1795–9.
Ricou F, Nicod P, Gilpin E, et al. Influence of right bundle branch block on short- and long-term survival after acute anterior myocardial infarction. J Am Coll Cardiol. 1991;17:858–63.
Oreto G, et al. L’elettrocardiogramma: un mosaico a 12 tessere. Milano: Centro scientifico editore Srl; 2010. p. 139–42.
Surawicz B, Knilans T. Chou’s electrocardiography in clinical practice. 6th ed. Philadelphia: Saunders Elsevier; 2008. p. 133–7.
Kosuge M, Kimura K, Ishikawa T, et al. New electrocardiographic criteria for predicting the site of coronary artery occlusion in inferior wall acute myocardial infarction. Am J Cardiol. 1998;82:1318–22.
Herz I, Assali AR, Adler Y, et al. New electrocardiographic criteria for predicting either the right and left circumflex artery as the culprit coronary artery in inferior wall acute myocardial infarction. Am J Cardiol. 1997;80:1343–5.
Assali AR, Herz I, Vaturi M, et al. Electrocardiographic criteria for predicting the culprit artery in inferior wall acute myocardial infarction. Am J Cardiol. 1999;84:87–8.
Wong TW, Huang XH, Liu W, et al. New electrocardiographic criteria for identifying the culprit artery in inferior wall acute myocardial infarction-Usefulness of T-wave amplitude ratio in leads II/III and T-wave polarity in the right V5 lead. Am J Cardiol. 2004;94:1168–71.
Birnbaum Y, Hasdai D, Sclarovsky S, et al. Acute myocardial infarction entailing ST-segment elevation in lead aVL: electrocardiographic differentiation among occlusion of the left anterior descending, first diagonal, and first obtuse marginal coronary arteries. Am Heart J. 1996;131:38–42.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Baiocco, E., Compagnucci, P., Contadini, D. (2019). Does EKG Favor a Correct Localization of the Ischemic Areas?. In: Capucci, A. (eds) New Concepts in ECG Interpretation. Springer, Cham. https://doi.org/10.1007/978-3-319-91677-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-91677-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91676-7
Online ISBN: 978-3-319-91677-4
eBook Packages: MedicineMedicine (R0)