Abstract
The carotid body (CB) is the main arterial chemoreceptor involved in oxygen sensing. Upon hypoxic stimulation, CB chemoreceptor cells release neurotransmitters, which increase the frequency of action potentials in sensory nerve fibers of the carotid sinus nerve. The identity of the molecular entity responsible for oxygen sensing is still a matter of debate; however several ion channels have been shown to be involved in this process. Connexin-based ion channels are expressed in the CB; however a definitive role for these channels in mediating CB oxygen sensitivity has not been established. To address the role of these channels, we studied the effect of blockers of connexin-based ion channels on oxygen sensitivity of the CB. A connexin43 (Cx43) hemichannel blocking agent (CHBa) was applied topically to the CB and the CB-mediated hypoxic ventilatory response (FiO2 21, 15, 10 and 5%) was measured in adult male Sprague-Dawley rats (~250 g). In normoxic conditions, CHBa had no effect on tidal volume or respiratory rate, however Cx43 hemichannels inhibition by CHBa significantly impaired the CB-mediated chemoreflex response to hypoxia. CHBa reduced both the gain of the hypoxic ventilatory response (HVR) and the maximum HVR by ~25% and ~50%, respectively. Our results suggest that connexin43 hemichannels contribute to the CB chemoreflex response to hypoxia in rats. Our results suggest that CB connexin43 hemichannels may be pharmacological targets in disease conditions characterized by CB hyperactivity.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
7.1 Introduction
The carotid body (CB) chemoreceptors are located in the bifurcation of the common carotid artery and have a primary role in sensing the partial pressure of O2, CO2 and pH in arterial blood (Iturriaga and Alcayaga 2004; Nurse and Piskuric 2013). The CB is composed of two primary cell types: the chemoreceptor cells (glomus) and the sustentacular cells (López-Barneo et al. 2008), which are believed to modulate glomus cell activity through a purinergic P2Y receptor-dependent pathway (Murali and Nurse 2016). Despite significant research devoted to understanding the function of this small but important organ, the molecular mechanisms by which glomus cells detect changes in PO2 are not well understood. The most well-accepted theory is that reductions of PO2 (directly or indirectly) decrease K+ channel (TASK) activity (Buckler et al. 2006), depolarizing the cell and leading to opening of voltage sensitive L-type Ca2+ channels, which in turn causes increases in intracellular Ca2+ which ultimately lead to the release of one or more neurotransmitters to the extracellular space (López-Barneo et al. 2016). Recent evidence also suggests a potentially important role of HIF-1α (Nurse 2017) and gaseous neurotransmitters such as CO and H2S (Prabhakar and Peng 2017) in controlling CB oxygen sensing. Once activated by low oxygen partial pressure, glomus cells release several neurotransmitters including ATP (Zhang et al. 2000), acetylcholine (Nurse and Zhang 1999) and dopamine (Buerk et al. 1998). The release of these neurotransmitters impacts the rate of discharge of petrosal ganglion neurons (Smith and Mills 1976), which deliver “PO2 –mediated sensory information” to the nucleus of the tractus solitarii (Lipski et al. 1977; Iturriaga and Alcayaga 2004).
In addition to the chemical synapses within the CB, electrical coupling between glomus cells has also been observed (Monti-Bloch et al. 1993; Abudara and Eyzaguirre 1994). The structural basis of these electrical synapses are the connexins. These proteins are a family of transmembrane proteins which contain four transmembrane segments; two extracellular loops, one intracellular loop, and both the N- and C- terminals facing the cytoplasm (Retamal et al. 2016). Connexins are named according to their predicted molecular weight, thus connexin43 (Cx43) is predicted to have a molecular weight of ~43 kDa. In 1996, Kondo and Iwasa (1996) conducted an ultra-structural study of adult rat CBs which confirmed the presence of gap junction channels (GJC)-like structures between glomus cells, between glomus cells and sustentacular cells, and between glomus cells and petrosal neuron terminals. This observation was confirmed by immunofluorescent detection of Cx43 in the CB (Abudara et al. 1999). GJCs formed by Cx43 are regulated by cAMP and pH in cultured CB cells which induced an increase and decrease in cell coupling, respectively (for further details see Abudara et al. 2000, 2001). Other studies have demonstrated that sustained stimuli may also elicit changes in GJC-like expression, in which 2 weeks of hypobaric hypoxia increased expression of Cx43 in glomus cells and in petrosal neurons (Chen et al. 2002). Interestingly, it is well known that Cx43 can also form GJCs between cells and also hemichannels in cells in vitro as well as in vivo (Sáez et al. 2005). Hemichannels are half of a GJC and are found in non-appositional plasma membranes (Sáez et al. 2005). While GJCs allow the passive flow of ions and molecules between cells, hemichannels permit the exchange of ions and small molecules between the extracellular media and the cytoplasm (Retamal 2014). In spite of their low open probability (Contreras et al. 2003), Cx43 hemichannels allow the release of paracrine signaling molecules such as ATP and glutamate (Orellana and Stehberg 2014). Therefore, Cx43 hemichannels are of potential interest as potential modulators of CB chemosensory activity (Reyes et al. 2014). Despite the fact that numerous studies suggest a potential role of Cx43-based hemichannels in regulation of CB activity, this hypothesis has not been specifically tested. Thus, the aim of this work was to test the effect of a Cx43-specific hemichannel blocker (CHBa) on CB O2 sensitivity in vivo.
7.2 Methods
7.2.1 Animals
Adult male Sprague-Dawley rats (n = 8), weighing between 250 and 300 g, were used in these experiments. All experiments were approved by the Bioethical Committee of the Facultad de Ciencias Biológicas, P. Universidad Católica de Chile and were carried out under the guidelines of the American Physiological Society, the Guía para el Cuidado y Uso de los Animales de Laboratorio from CONICYT and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
7.2.2 CB-Mediated Ventilatory Chemoreflex Function
Rats were anaesthetized with sodium pentobarbitone (40 mg kg−1; ip) and placed in the supine position. The trachea was cannulated for airflow recording, and connected to a pneumotachograph to measure tidal volume (Vt), respiratory frequency (Rf), and minute inspiratory volume (VE). During the experiment, rectal temperature was measured and maintained at 38.0 ± 0.5 °C with a regulated heating pad. Supplemental doses of sodium pentobarbitone were administered as necessary to maintain a surgical plane of anesthesia. Signals were acquired with an analogue-digital system PowerLAB 8SP (ADInstruments Pty Ltd., Castle Hill, Australia), calibrated and analyzed with the Labchart 7-Pro software (ADInstruments Pty Ltd., Australia). CB-mediated chemoreflex function was estimated as previously described (Del Rio et al. 2010, 2013, 2014). Briefly, rats breathed hypoxic gas mixtures (FiO2 5%,10%,15%) until a semi-steady state response was achieved (20–25 s). All recordings were made at an ambient temperature of 25 ± 2 °C. During chemoreflex testing, respiratory variables (RF and Vt) were averaged over 10 consecutive breaths.
7.2.3 Acute Blockade of Connexin 43 Hemichannels
A Cx43-selective hemichannel-blocking agent (CHBa) was used as previously described (Stehberg et al. 2012; Wang et al. 2013). CHBa (based on an 11 amino acid sequence targeting the second extracellular loop of the Cx43 protein) was dissolved in Pluronic F-127 at 20 °C, for a final concentration of 25 mM. The CBs were visualized and isolated from the surrounding tissue. Then, 20 μl of CHBa solution was topically applied to the CBs. Ten minutes were allowed for Pluronic F-127 to polymerize before ventilatory recordings were initiated.
7.2.4 Cx43 Expression
CB Cx43 protein expression was assessed using immunoblot as previously described (Del Rio et al. 2017). Briefly, after euthanizing the rats (pentobarbital 100 mg/kg i.p.), the carotid sinus region containing the CBs was quickly removed and snap frozen on dry ice and stored at −80 °C. The tissue was lysed in 200 μL of RIPA buffer with fresh protease inhibitor cocktail (Sigma Aldrich, St. Louis, MO, USA) and total protein concentration was assessed using a Thermo Scientific BCA Assay kit (Waltham, MA). Samples were loaded onto a 10% SDS-PAGE gel (50 μg/20 μl per well) and electrophoresis was performed. Then, proteins were transferred to a PVDF membrane (Millipore, USA) and the membrane was probed overnight with the following antibodies: rabbit anti- Cx43 (1:750, Cat. #PPS046, Novus Biological), and mouse anti- β-actin (1:500–1:1000, Cat. #sc-47,778, Santa Cruz, USA). Following washes with PBST, the appropriate secondary antibodies were added to each membrane. Blots were developed using a ECL kit (ThermoFischer, USA) scanner, and quantitative analysis of band densitometry was performed using Li-Cor Imaging software (Li-cor, USA).
7.2.5 Statistical Analysis
Data was expressed as means ± S.E.M. The differences in hypoxic ventilatory response was analyzed by paired t-test. A p value of <.05 was considered statistically significant.
7.3 Results
7.3.1 Expression of Cx43 in the Carotid Body
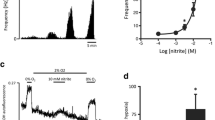
Figure 7.1 illustrates representative immunoblots of Cx43 expression in homogenates of rat CBs. Two bands close to 43 kDa were observed, however, bands with higher electrophoretic mobility were more abundant, indicating that in the CB, Cx43 is mainly in the dephosphorylated form.
7.3.2 Effects of CHBa on Resting Breathing Parameters
The effects of CHBa on resting Vt, Rf and VE are summarized in Table 7.1. CHBa did not induce significant changes in VE when compared to values obtained before CHBa exposure (p > 0.05). However, we did observe a small trend for increasing Vt values following CHBa (Table 7.1).
7.3.3 Effects of Cx43 Hemichannel Blockade on the Hypoxic Ventilatory Response
In response to acute hypoxic stimulation, rats increased both Vt and Rf (Fig. 7.2). We observed a maximal response of >200% increase in VE following exposure to FiO2 5% (Fig. 7.3). Remarkably, CHBa significantly reduced the hypoxic ventilatory response (HVR) in rats (Fig. 7.2). As shown in Fig. 7.3, CHBa markedly attenuated the Vt and Rf responses to several levels of hypoxia when compared to the values obtained before CHBa administration. Furthermore, CHBa reduced both the HVR gain and the maximum HVR by ~25% and ~50%, respectively. No effects on hyperoxic ventilatory responses were found (Fig. 7.3).
Topical application of a connexin hemichannel blocking agent (CHBa) reduce the hypoxic ventilatory response. (a) Representative traces of tidal volume (Vt), respiratory frequency (Rf) and minute ventilation (VE) during FiO2 100, 21, 15, 10 and 5% in one Control animal. (b) Representative traces of tidal volume (Vt), respiratory frequency (Rf) and minute ventilation (VE) during FiO2 100, 21, 15, 10 and 5% after CHBa. Note that the ventilatory response to hypoxia is significantly decreased compared to the Control condition
Summary data showing the effect of CHBa on the CB-mediated ventilator chemoreflex response to hypoxia. (a) Effects of CHBa on Vt. Note that Vt responses in hypoxia were slightly reduced after CHBa application. (b) Effects of CHBa application on hypoxic Rf response. Note that CHBa markedly attenuate the Rf response to hypoxia. (c) After CHBa application the VE response to hypoxia was significantly reduced. Holm-Sidak posthoc after two ways ANOVA. *, p < 0.05 vs. CHBa FiO2 5%, n = 4
7.4 Discussion
The major findings of these studies are: (i) Cx43 is constitutively expressed in CB tissue and (ii) specific blockade of Cx43 hemichannels within the CB reduces the ventilatory response to hypoxia without changing resting breathing parameters. Taken together, our results strongly suggest that Cx43 hemichannels contribute to/modulate the acute CB-mediated ventilatory responses to hypoxia.
Previous studies indicate that CB glomus cells express gap junction-like structures (Kondo and Iwasa 1996), and the work of Abudara et al. (1999) showed that CBs express Cx43. Our results confirm these previous observations and indicate a functional role for Cx43 in the CB. Activated Cx43 hemichannels allow the exchange of small molecules (~1 kDa) from extracellular media to the cytoplasm (Sáez et al. 2005). It is worth noting that our data showed that resting breathing parameters were not affected by Cx43 hemichannel blockade, which suggests that these hemichannels are not normally open in the CB during normoxic conditions. This finding is consistent with the observations of Contreras and co-workers in 2003 who found that Cx43 hemichannels have a very low open probability under physiological conditions (Contreras et al. 2003). In contrast to the lack of an effect observed in normoxia, our results show that CHBa markedly reduced the CB-mediated ventilatory response to hypoxia. This effect strongly suggests that Cx43 hemichannels are activated at low oxygen partial pressures. Cx43 hemichannel opening has been observed under hypoxic conditions or metabolic stress in other experimental models (John et al. 1999; Wang et al. 2014). Interestingly, it has been shown that stimulation of CB glomus cells with hypoxia induces the release of several small molecules to the extracellular milieu (Zhang et al. 2000). Thus we postulate that Cx43 hemichannels may participate in this process. Future studies are needed to address the biophysical properties of Cx43 hemichannels in the CB under normoxic and hypoxic conditions.
One of the most critical adaptive physiological responses to hypoxic challenges is an increase in pulmonary ventilation (Moore et al. 1986). Importantly, this ventilatory response is almost entirely dependent on functional CBs as denervation of the CBs totally abolishes the hypoxic ventilatory response (Del Rio et al. 2013). Despite this appreciation of the CBs as the primary mediator of the hypoxic ventilatory response, the precise molecular mechanism(s) underpinning oxygen sensing in the CB chemoreceptors is still debated. In these studies, we show that topical application of a Cx43 hemichannel blocker to the CB, significantly reduces the hypoxic ventilatory response. These results strongly suggest that Cx43 hemichannels modulate CB chemotransduction of hypoxic stimuli. Considering that these hemichannels allow the exchange of ions and other small molecules (i.e. ATP and glutamate) between the extracellular media and the cytoplasm (Sáez et al. 2005), and that CB chemoreceptor cells release ATP to the extracellular milieu (Zhang et al. 2000), it is likely that Cx43 hemichannels are activated by low oxygen partial pressure and that this results in the release of one or several neuromodulators within the CB. Further studies are needed to establish a causal relationship between Cx43 hemichannel function and CB hypoxic sensitivity.
In summary, our data indicate that topical application of a Cx43 hemichannel blocking agent (CHBa) in the CB significantly attenuates the hypoxic ventilatory response. Taking into account that Cx43 protein is expressed in the CB and that upon activation Cx43 hemichannels allow the release of several molecules from glomus cells, it is plausible that one or more neuromodulators are released by CB glomus cells through hemichannels during hypoxic stimulation. Future studies should focus on the functional role of connexin-based hemichannels in CB oxygen sensing and mediation of the hypoxic ventilatory response.
References
Abudara V, Eyzaguirre C (1994) Electrical coupling between cultured glomus cells of the rat carotid body: observations with current and voltage clamping. Brain Res 664:257–265
Abudara V, Garcés G, Sáez JC (1999) Cells of the carotid body express connexin43 which is up-regulated by cAMP. Brain Res 849:25–33
Abudara V, Eyzaguirre C, Sáez JC (2000) Short- and long-term regulation of rat carotid body gap junctions by cAMP. Identification of connexin43, a gap junction subunit. Adv Exp Med Biol 475:359–369
Abudara V, Jiang RG, Eyzaguirre C (2001) Acidic regulation of junction channels between glomus cells in the rat carotid body. Possible role of [Ca(2+)]. Brain Res 916:50–60
Buckler KJ, Williams BA, Orozco RV, Wyatt CN (2006) The role of TASK-like K+ channels in oxygen sensing in the carotid body. Novartis Found Symp 272:73–85
Buerk DG, Osanai S, Mokashi A, Lahiri S (1998) Dopamine, sensory discharge, and stimulus interaction with CO2 and O2 in cat carotid body. J Appl Physiol 85:1719–1726
Chen J, He L, Dinger B, Stensaas L, Fidone S (2002) Chronic hypoxia upregulates connexin43 expression in rat carotid body and petrosal ganglion. J Appl Physiol 92:1480–1486
Contreras JE, Sáez JC, Bukauskas FF, Bennett MV (2003) Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci U S A 100(20):11388–11393
Del Rio R, Moya EA, Iturriaga R (2010) Carotid body and cardiorespiratory alterations in intermittent hypoxia: the oxidative link. Eur Respir J 36(1):143–150
Del Rio R, Marcus NJ, Schultz HD (2013) Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol 62(25):2422–2430
Del Rio R, Moya EA, Iturriaga R (2014) Carotid body potentiation during chronic intermittent hypoxia: implication for hypertension. Front Physiol 5:434
Del Rio R, Andrade DC, Toledo C et al (2017) Carotid body-mediated Chemoreflex drive in the setting of low and high output heart failure. Sci Rep 7:8035
Iturriaga R, Alcayaga J (2004) Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res Rev 46:46–53
John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN (1999) Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem 274:236–240
Kondo H, Iwasa H (1996) Re-examination of the carotid body ultrastructure with special attention to intercellular membrane appositions. Adv Exp Med Biol 410:45–50
Lipski J, McAllen RM, Spyer KM (1977) The carotid chemoreceptor input to the respiratory neurones of the nucleus of tractus solitarus. J Physiol 269:797–810
López-Barneo J, Ortega-Sáenz P, Pardal R, Pascual A, Piruat JI (2008) Carotid body oxygen sensing. Eur Respir J 32:1386–1398
López-Barneo J, González-Rodríguez P, Gao L, Fernández-Agüera MC, Pardal R, Ortega-Sáenz P (2016) Oxygen sensing by the carotid body: mechanisms and role in adaptation to hipoxia. Am J Physiol Cell Physiol 310:629–642
Monti-Bloch L, Abudara V, Eyzaguirre C (1993) Electrical communication between glomus cells of the rat carotid body. Brain Res 622:119–131
Moore LG, Harrison GL, McCullough RE, McCullough RG, Micco AJ, Tucker A, Weil JV, Reeves JT (1986) Low acute hypoxic ventilatory response and hypoxic depression in acute altitude sickness. J Appl Physiol 60:1407–1412
Murali S, Nurse CA (2016) Purinergic signalling mediates bidirectional crosstalk between chemoreceptor type I and glial-like type II cells of the rat carotid body. J Physiol 594:391–406
Nurse CA (2017) A sensible approach to making sense of oxygen sensing. J Physiol 595:6087–6088
Nurse CA, Piskuric NA (2013) Signal processing at mammalian carotid body chemoreceptors. Semin Cell Dev Biol 24:22–30
Nurse CA, Zhang M (1999) Acetylcholine contributes to hypoxic chemotransmission in co cultures of rat type 1 cells and petrosal neurons. Respir Physiol 115:189–199
Orellana JA, Stehberg J (2014) Hemichannels: new roles in astroglial function. Front Physiol 17:193
Prabhakar NR, Peng YJ (2017) Oxygen sensing by the carotid body: past and present. Adv Exp Med Biol 977:3–8
Retamal MA (2014) Connexin and Pannexin hemichannels are regulated by redox potential. Front Physiol 25:80
Retamal MA, García IE, Pinto BI, Pupo A, Báez D, Stehberg J, Del Rio R, González C (2016) Extracellular cysteine in connexins: role as redox sensors. Front Physiol 28:1
Reyes EP, Cerpa V, Corvalán L, Retamal MA (2014) Cxs and Panx- hemichannels in peripheral and central chemosensing in mammals. Front Cell Neurosci 8:123
Sáez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV (2005) Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta 1711:215–224
Smith PG, Mills E (1976) Autoradiographic identification of the terminations of petrosal ganglion neurons in the cat carotid body. Brain Res 113:174–178
Stehberg J, Moraga-Amaro R, Salazar C, Becerra A, Echeverría C, Orellana JA, Bultynck G, Ponsaerts R, Leybaert L, Simon F, Sáez JC, Retamal MA (2012) Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J 26:3649–3657
Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Bultynck G, Leybaert L (2013) Connexin targeting peptides as inhibitors of voltage- and intracellular Ca2+−triggered Cx43hemichannel opening. Neuropharmacology 75:506–516
Wang J, Ma A, Xi J, Wang Y, Zhao B (2014) Connexin 43 and its hemichannels mediate hypoxia-ischemia-induced cell death in neonatal rats. Child Neurol Open 1:2329048x14544955
Zhang M, Zhong H, Vollmer C, Nurse CA (2000) Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol 525:143–158
Acknowledgments
This study was supported by Fondecyt 1140275 and 1180172 (RDR) and Fondecyt 1160227 (MAR). NJM is supported by a grant from the National Institutes of Health (HL-138600-01).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this paper
Cite this paper
Andrade, D.C. et al. (2018). Topical Application of Connexin43 Hemichannel Blocker Reduces Carotid Body-Mediated Chemoreflex Drive in Rats. In: Gauda, E., Monteiro, M., Prabhakar, N., Wyatt, C., Schultz, H. (eds) Arterial Chemoreceptors. Advances in Experimental Medicine and Biology, vol 1071. Springer, Cham. https://doi.org/10.1007/978-3-319-91137-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-91137-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91136-6
Online ISBN: 978-3-319-91137-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)