Abstract
The carotid bodies (CB) respond to changes in blood gases with neurotransmitter release, thereby increasing carotid sinus nerve firing frequency and ultimately correcting the pattern of breathing. It has previously been demonstrated that acute application of the adipokine leptin augments the hypoxic sensory response of the intact in-vitro CB (Pye RL, Roy A, Wilson RJ, Wyatt CN. FASEB J 30(1 Supplement):983.1, 2016) and isolated CB type I cell (Pye RL, Dunn EJ, Ricker EM, Jurcsisn JG, Barr BL, Wyatt CN. Arterial chemoreceptors in physiology and pathophysiology. Advances in experimental medicine and biology. Springer, Cham, 2015). This study’s aim was to examine, in-vivo, if elevated leptin modulated CB function and breathing.
Rats were fed high fat or control chow for 16-weeks. High fat fed (HFF) animals gained significantly more weight compared to control fed (CF) animals and had significantly higher serum leptin levels compared to CF. Utilizing whole-body plethysmography, HFF animals demonstrated significantly depressed breathing compared to CF at rest and during hypoxia. However, amplitudes in the change in breathing from rest to hypoxia were not significantly different between groups. CB type I cells were isolated and intracellular calcium levels recorded. Averaged and peak cellular hypoxic responses were not significantly different.
Despite a small but significant rise in leptin, differences in breathing caused by high fat feeding are unlikely caused by an effect of leptin on CB type I cells. However, the possibility remains that leptin may have in-vivo postsynaptic effects on the carotid sinus nerve; this remains to be investigated.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
17.1 Introduction
Obese individuals suffer from many pathologies, one of particular interest being obesity hypoventilatory syndrome (OHS) (Berger et al. 2009). The adipokine leptin, found in excess in the circulating serum of the obese, has been shown to have profound effects on increasing breathing in wild-type animals and those unable to produce functional leptin proteins (ob−/ob−). For example, focal injections of leptin into central breathing centers (i.e., nucleus tractus solitarius) causes dose-dependent increases in minute ventilation in wild-type rats (Inyushkina et al. 2009). Ob−/ob− mice, normally suffering from hypoventilation, also show significant increases in minute ventilation after exogenous, peripheral infusion of obese levels of leptin (O’Donnell et al. 1999). However, despite high circulating levels of this respiratory stimulant in obese individuals, hypoventilation persists (Campo et al. 2007).
Capable of crossing the blood brain barrier and interacting with central regions to trigger satiety (i.e., hypothalamus) and increase ventilation (i.e., NTS), leptin also has receptors in the periphery through which it may act to modulate breathing. It was recently demonstrated that functional leptin receptors, both long (Ob-Rb) and short isoforms (Ob-Ra – Ob-Rf) exist on the type I cell of the rat and human carotid body (Porzionato et al. 2011). Pye et al. (2016) observed that obese quantities of exogenous leptin (200 ng/mL) administered in acute doses to intact, arterially perfused carotid bodies, increased their baseline firing rates and sensory responses to hypoxia. This experimental evidence demonstrated leptin’s potential ability to modulate breathing via its actions on the carotid body.
It was the aim of the following study to build upon the work completed by Pye et al. in 2015 and 2016. In the present study, we aimed to increase circulating serum leptin levels, in-vivo, through a high fat feeding paradigm. High fat feeding has been demonstrated to lead to excessive weight gain and a subsequent rise in serum leptin levels (Van Heek et al. 1997; Knight et al. 2010). We hypothesized that chronic overstimulation of leptin receptors (i.e., due to hyperleptinemia) on carotid body type I cells would cause their downregulation and/or insensitivity to leptin, potentially leading to a blunting of the carotid body mediated hypoxic response. Whole-body plethysmography was used to test breathing parameters and measure the acute hypoxic ventilatory response (HVR). Intracellular calcium measurement, in isolated type I cells, was used to indicate whether any depression in breathing was due to leptin’s actions on these oxygen-sensing organs. We hypothesized that HFF animals would have blunted hypoxic responses due to a decrease in the hypoxic sensory responses of the type I cells of the carotid body.
17.2 Methods
Thirty-six adult, male, Sprague-Dawley rats (Envigo) were randomly assigned to either a control chow (CF; n = 18; TD.06414, Harlan) or an ingredient-matched high-fat chow (HFF; n = 18; TD.08806, Harlan) for 16 weeks ad libitum; animals weight was measured weekly.
17.2.1 Ethics
All procedures and experiments were conducted in accordance with local, state, and federal guidelines relating to animal welfare and treatment. Wright State University’s Institutional Animal Care and Use Committee approved experimental protocols described herein. Authors declare no conflict of interest.
17.2.2 Metabolism
Resting metabolism was assessed after 16 weeks of feeding using an indirect calorimetric method as previously described by Frappel et al. (1992). A respiratory exchange ratio [RER: VCO2 (mL of CO2 min−1 kg−1)/VO2 (mL of O2 min−1 kg−1)] was then calculated for each animal.
17.2.3 Whole-Body Plethysmography
Breathing parameters (tidal volume, frequency) were measured using whole-body plethysmography (DSI Finepointe) as previously described by Nirogi et al. (2012). Briefly, acclimated animals had baseline breathing recordings collected in room air before exposure to an acute hypoxic stimulus (10% O2).
17.2.4 Carotid Body Dissection & Type I Cell Isolation
After 16-weeks of feeding, unfasted, adult Sprague-Dawley rats were deeply anesthetized (isoflurane: 4.5% in O2) before euthanasia via decapitation. Carotid bodies were then dissected and type I cells isolated as previously described (Burlon et al. 2009).
17.2.5 Blood Collection & Leptin Assay
Immediately post-decapitation, whole blood was collected passively by draining of the euthanized animal’s trunk. Blood was centrifuged and serum aliquoted and frozen −15 °C (<4 months) before being thawed, diluted both 5- and 20-fold, and used for quantification of leptin via ELISA (KRC2281; Novex). Samples were diluted and tested (Syngery H1; BioTek) in duplicates while following manufacturers protocol, equating to four tests per sample of serum.
17.2.6 Carotid Body Type I Cell Ca2+-Imaging
A ratiometric calcium-sensitive fluorescent dye, FURA-2AM (Invitrogen) was used to visualize and quantify intracellular calcium in isolated carotid body type I cells as previously described (Pye et al. 2015).
17.2.7 Statistics
Two-tailed Student’s unpaired t-tests were used to analyze data with significance set at p < 0.05. Data are presented as means ± the standard error of the mean. One star: p < .05; two stars: p < .01; three stars: p < .001; four stars: p < .0001.
17.3 Results
17.3.1 Effects of Diet on Physiology
Thirty-six male Sprague-Dawley rats, separated into two equal groups (n = 18), were fed either a high fat or control chow for 16 weeks. After 16 weeks, HFF animals weighed significantly more than CF (512.6 ± 14.70 vs. 444.1 ± 7.090 g, p < .001, Fig. 17.1a). Respiratory exchange ratios (RER) at rest were calculated using an indirect calorimetric method. After 16 weeks on the diet HFF animals’ metabolic fuel shifted toward the use of fatty acids (0.77 ± 0.018 vs. 0.88 ± 0.037 RER, p < .01); a function of lowered CO2-production in HFF animals (10.02 ± 0.1609 vs. 12.15 ± 0.418 mL CO2 min−1 kg−1, p < .0001). Oxygen consumption between groups was not significantly different.
After the 16th week on the diet regimen, animals were sacrificed, blood collected and processed, before being used for quantification of endogenous leptin levels via ELISA. Standards ran in duplicate were significantly correlated (r2: 0.997, p < .05). Sixteen weeks of HFF resulted in significantly higher leptin levels in HFF animals compared to CF (3.05 ± 0.24 vs. 1.29 ± 0.12 ng/mL, p < 0.0001, Fig. 17.1b).
17.3.2 Effects of Diet on Breathing
Whole-body plethysmography was used to measure breathing parameters in unrestrained, conscious animals during rest and acute exposure to hypoxia. HFF animals demonstrated significantly depressed baseline breathing compared to CF (373.9 ± 13.46 vs. 442.8 ± 15.29 mL min−1 kg−1, p < .01). HFF animals also exhibited attenuated breathing during exposure to hypoxia (1084.1 ± 46.25 vs. 1298.2 ± 52.61 mL min−1 kg−1, p < .01, Fig. 17.2a). Attenuated minute ventilation was a function of lowered tidal volume, not frequency, among HFF animals compared to CF during rest (6.412 ± 0.212 vs. 8.163 ± 0.263 mL breath−1 kg−1, p < .0001) and hypoxia (8.587 ± 0.287 vs. 10.63 ± 0.354 mL breath−1 kg−1, p < .0001).
Assessment of oxygen sensitivity at the level of the whole animal was accomplished by subtracting the VE values during hypoxia from those taken during baseline. This simple transformation allows for comparison of the absolute change in breathing between groups. Oxygen sensitivity at the level of the whole animal remained unchanged between CF and HFF groups, data shown in Fig. 17.2b .
17.3.3 Effects of Diet on CB Type I Cell O2-Sensing
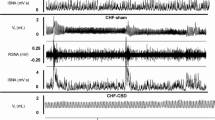
A calcium-sensitive dye, FURA-2, was used to measure intracellular calcium levels in isolated CB type I cells from HFF and CF animals. Analyzing both the peak cellular responses (Fig. 17.3c) and the averaged cellular responses (Fig. 17.3d) during hypoxic exposure shows nearly identical responses from cells of HFF (n = 16) and CF (n = 16) animals. Cellular data is time-matched to the whole-animal breathing data; both analyses concur in suggesting O2-sensitivity is not changed by a high fat diet at the level of the whole-animal and CB type I cell.
Example calcium-imaging recordings are shown in (a) and (b). High fat feeding over 16-weeks did not change oxygen-sensitivity of isolated CB type I cells (n = 16/n = 16) when analyzed as either peak cellular responses (c) or averaged cellular responses (d) (70-second data bin: gray box in a and b) during hypoxia
17.4 Conclusion
These experiments demonstrated that a high fat diet in rats led to significant physiological changes including weight gain, hyperleptinemia, lowered carbohydrate metabolism (decreased RER), and depression of breathing during baseline and hypoxic exposure. Although respiratory depression is evident, the mechanism underpinning this attenuation does not appear to be mediated by the CB type I cell. Similar hypoxic responses (ΔVE) between groups (see Fig. 17.2b), during the CB-mediated phase of the biphasic hypoxic response (≤90 s; Cummings and Wilson 2005), provide further evidence for lack of involvement of the carotid bodies in the development of respiratory depression.
Future experiments would aim to further increase the animal weight on HFF, and thus leptin levels, through addition of simple sugars to drinking water, lengthening the time on the diet, and/or supplementing the chow with ingredient-matched treats. Leptin levels in the experiments by Pye et al.’s (2015, 2016) were nearly 65-fold larger than measured in our HFF animals and likely explain why differences were not seen at the CB type I cellular level.
References
Berger KI, Goldring RM, Rapoport DM (2009) Obesity hypoventilation syndrome. Semin Respir Crit Care Med 30(3):253–261
Burlon DC, Jordan HL, Wyatt CN (2009) Presynaptic regulation of isolated neonatal rat carotid body type I cells by histamine. Respir Physiol Neurobiol 168(3):218–223
Campo A, Frühbeck G, Zulueta JJ, Iriarte J, Seijo LM, Alcaide AB, Salvador J (2007) Hyperleptinaemia, respiratory drive and hypercapnic response in obese patients. Eur Respir J 30(2):223–231
Cummings KJ, Wilson RJ (2005) Time-dependent modulation of carotid body afferent activity during and after intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 288(6):1571–1580
Frappell PB, Dotta A, Mortola JP (1992) Metabolism during normoxia, hyperoxia, and recovery in newborn rats. Can J Physiol Pharmacol 70(3):408–411
Inyushkin AN, Inyushkina EM, Merkulova NA (2009) Respiratory responses to microinjections of leptin into the solitary tract nucleus. Neurosci Behav Physiol 39:231–240
Knight ZA, Hannan KS, Greenberg ML, Friedman JM (2010) Hyperleptinemia is required for the development of leptin resistance. PLoS One 5(6):1–8
Nirogi R, Shanmuganathan D, Jayarajan P, Abraham R, Kancharla B (2012) Comparison of whole body and head out plethysmography using respiratory stimulant and depressant in conscious rats. J Pharmacol Toxicol Methods 65(1):37–43
O’Donnell CP, Schaub CD, Haines AS, Berkowitz DE, Tankersley CG, Schwartz AR, Smith PL (1999) Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med 159(5):1477–1484
Porzionato A, Rucinski M, Macchi V, Stecco C, Castagliuolo I, Malendowicz LK, De Caro R (2011) Expression of leptin and leptin receptor isoforms in the rat and human carotid body. Brain Res 1385:56–67
Pye RL, Dunn EJ, Ricker EM, Jurcsisn JG, Barr BL, Wyatt CN (2015) Acutely administered leptin increases [Ca2+]i and BKCa currents but does not alter chemosensory behavior in rat carotid body Type I cells. In: Peers C, Kumar P, Wyatt C, Gauda E, Nurse C, Prabhakar N (eds) Arterial chemoreceptors in physiology and pathophysiology. Advances in experimental medicine and biology, vol 860. Springer, Cham, pp 61–67
Pye RL, Roy A, Wilson RJ, Wyatt CN (2016) The effect of obese levels of leptin on peripheral chemoreception. FASEB J 30(1 Supplement):983.1
Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Davis HR Jr (1997) Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Investig 99(3):385–390
Acknowledgements
We would like to thank Dr. Lynn Hartzler, Lori and Dale Goss, and the Wright State University LAR staff and veterinarians for their support with this project. This work was funded in part by NIH grant RO1HL091836.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this paper
Cite this paper
Rakoczy, R.J., Pye, R.L., Fayyad, T.H., Santin, J.M., Barr, B.L., Wyatt, C.N. (2018). High Fat Feeding in Rats Alters Respiratory Parameters by a Mechanism That Is Unlikely to Be Mediated by Carotid Body Type I Cells. In: Gauda, E., Monteiro, M., Prabhakar, N., Wyatt, C., Schultz, H. (eds) Arterial Chemoreceptors. Advances in Experimental Medicine and Biology, vol 1071. Springer, Cham. https://doi.org/10.1007/978-3-319-91137-3_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-91137-3_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91136-6
Online ISBN: 978-3-319-91137-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)