Abstract
Sleep apnea with periodic cessation of breathing during sleep is a highly prevalent respiratory disorder affecting an estimated 10% of adults. Patients with sleep apnea exhibit several co-morbidities including hypertension, stroke, disrupted sleep, and neurocognitive and metabolic complications. Emerging evidence suggests that a hyperactive carotid body (CB) chemo reflex is an important driver of apneas in sleep apnea patients. Gasotransmitters carbon monoxide (CO) and hydrogen sulfide (H2S) play important roles in oxygen sensing by the CB. We tested the hypothesis that an augmented CB chemo reflex stemming from disrupted CO-H2S signaling may lead to sleep apnea. This possibility was tested in mice deficient in hemeoxygenase-2 (HO-2), an enzyme involved in CO synthesis, which were shown to exhibit hyperactive CB activity due to high H2S levels. We found that HO-2−/− mice exhibit a high incidence of apneas during sleep compared to wild type mice. Blocking the CB hyperactivity with L-propargylglycine, an inhibitor of cystathionine-γ-lyase (CSE), which catalyzes H2S synthesis, prevented apneas in HO-2−/− mice. These findings suggest that targeting CB with inhibitors of CSE might be a novel therapeutic strategy for preventing sleep apnea.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Sleep disordered breathing (SDB) with obstructive (OSA) and central apnea (CSA) is a clinically prevalent respiratory disorder affecting an estimated 10% of the adult human population (Peppard et al. 2013). Patients with SDB exhibit a wide spectrum of pathologies including hypertension, stroke, neurocognitive and metabolic dysfunctions and disrupted sleep (St-Onge et al. 2016). Continuous positive airway pressure (CPAP) is the current treatment of choice for OSA (Combs et al. 2014; Ip et al. 2012; Veasey et al. 2006) and adaptive servo-ventilation is considered beneficial for CSA patients (Javaheri et al. 2016; Yamauchi et al. 2016). However, a substantial number of OSA patients do not respond to CPAP therapy (Antic et al. 2015; Gilmartin et al. 2005; Quan et al. 2012). Adaptive ventilation in heart failure patients exhibiting CSA showed 30% mortality with no demonstrable benefits for CSA (Cowie et al. 2015). These findings suggest that current treatment strategies are suboptimal for normalizing breathing in SDB patients. The estimated cost burden in unmanaged sleep apnea patients is exorbitantly high and ranges between ~$65 and 165 billion per year in the U.S. (Frost and Sullivan 2016; Harvard Medical School Division of Sleep Medicine 2010), highlighting a need for developing alternative therapeutic strategies for preventing SDB. However, development of additional therapeutic strategies critically depends on the availability of suitable animal model(s) exhibiting SDB. SDB patients often exhibit a mixed phenotype of OSA and CSA (Javaheri et al. 2009; Morgenthaler et al. 2006). Therefore, identifying animal model(s) exhibiting both OSA and CSA will facilitate the development of alternative therapeutic strategies.
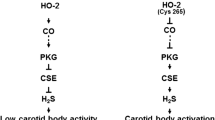
Reflex arising from the carotid body (CB), the primary sensory organ for detecting hypoxia, is a major regulator of breathing (Kumar and Prabhakar 2012). Clinical studies have implicated abnormal CB chemo reflex in both genesis and downstream pathologies caused by sleep apnea (Dempsey et al. 2012; Kara et al. 2003; Mansukhani et al. 2015; Xie et al. 1995). Emerging evidence suggests that gasotransmitters play an important role in oxygen sensing by the CB (Makarenko et al. 2012; Peng et al. 2010, 2014; Prabhakar and Peers 2014; Yuan et al. 2015). Mice lacking hemeoxygenase-2 (HO-2), an enzyme responsible for the generation of endogenous carbon monoxide (CO), exhibit exaggerated CB response to hypoxia, due to increased cystathionine-γ-lyase (CSE)-derived H2S synthesis (Yuan et al. 2015). These findings led us to hypothesize that HO-2 null mice exhibit high incidence of apnea, and pharmacologic blockade of H2S synthesis prevents apnea. We tested this hypothesis by monitoring breathing under normoxia with whole body plethysmography continuously from 10 AM to 4 PM (which is the sleep time for rodents) in adult wild type and HO-2−/− mice of both genders.
14.2 Mice with Deficiency in HO-2 Exhibit Sleep Apnea
14.2.1 HO-2−/− Mice Exhibit High Incidence of Apnea and Hypopnea
Figure 14.1 shows examples of tracings of breathing in a wild type and a HO-2−/− mouse. Wild type mouse displayed relatively regular breathing during the 6 h period of recording. However, HO-2−/− mouse exhibited irregular breathing with apnea and hypopnea. We defined apnea as cessation of breathing for >2.5 breaths duration, excluding post sigh apnea, and hypopnea as breathing events with ≥30% reduction in tidal volume. As shown in Table 14.1, a majority of HO-2−/− mice showed greater than 20 events of apnea per hour (apnea index), ranging from 20 to 168 apneas/hour, whereas only 5% wild type mice exhibited such severe apneas. Likewise, 56% HO-2−/− mice showed greater than 80 events of hypopnea per hour (hypopnea index, Table 14.1), whereas only 2.5% wild type mice displayed similar hypopnea index. Chi-squared test showed that the distributions of apnea and hypopnea were significantly different between wild type and HO-2−/− mice (P < 0.01). These observations suggest that HO-2−/− mice exhibit high incidence of apnea and hypopnea.
14.2.2 HO-2−/− Mice Exhibit Sleep Apnea
Sleep-wake states significantly influence breathing. To examine the impact of sleep-wake states on the occurrence of apneas, we simultaneously monitored electroencephalogram (EEG), electromyogram of neck muscles (EMG) and breathing in un-sedated wild type and HO-2−/− mice. Sleep-wake states are determined based on analysis of combination of EEG spectrum and neck muscle tone. Wake state is characterized by EEG activity of mixed frequency with low amplitude and high muscle tone. Rapid eye movement (REM) sleep is identified by both the frequency of theta waves (6–9 Hz) and muscle atonia. Non-REM (NREM) sleep is characterized by high amplitude slow waves in the delta frequency range (1–4 Hz) and low muscle tone. Incidence of apnea and hypopnea during wake state were low and comparable between wild type and HO-2−/− mice (Table 14.2). NREM or REM sleep had little impact on apnea or hypopnea index in wild type mice. In striking contrast, apnea and hypopnea indices significantly increased during NREM sleep, and further increased during REM sleep in HO-2−/− mice (Table 14.2). These results demonstrate that HO-2−/− mice exhibit sleep apnea.
14.2.3 HO-2−/− Mice Exhibit Both Central and Obstructive Apnea
Apneas are classified as central and obstructive in nature. To characterize the apnea phenotype, we monitored inspiratory intercostal muscle electromyography activity with chronically implanted electrodes along with breathing in un-sedated HO-2−/− mice (n = 12). There were incidences of apnea with absence of both breathing movements and respiratory muscle activity, which were considered as central apneas. On other hand, some of the apneic events were associated with increased inspiratory muscle activity, which were considered as obstructive apneas. We found that a given HO-2−/− mouse exhibited both central and obstructive apnea during 6 h of recording. Average data showed that 59% of apnea events are of obstructive type, and 41% are of central type (Table 14.3).
14.3 Enhanced Carotid Body (CB) Chemo Reflex Contributes to Apnea in HO-2 Null Mice
We determined whether an exaggerated CB reflex contributes to high incidence of apnea in HO-2−/− mice. Hyperoxia (90% O2), which is known to inhibit the CB activity, markedly reduced the number of apneas (Table 14.4). In contrast, hypoxia (15% O2), a physiological activator of the CB, significantly increased the incidence of apnea (Table 14.4). Further experiments showed that hyperoxia reduced the incidence of both obstructive and central apneas. In contrast, hypoxic breathing increased the incidence of obstructive and central apnea by 3 and 2 fold, respectively. Although apneas could result from reduced chemosensitivity to CO2 (Kumar et al. 2015), we found that stimulation of central CO2 chemoreceptors with 2% CO2 caused only a modest reduction (~32%) in the apnea index in HO-2−/− mice, which was due to reduced incidence of central apnea. These observations suggest that the exaggerated CB reflex is a major contributor to apneas in HO-2−/− mice.
14.4 Pharmacologic Blockade of H2S Synthesis Prevents Apneas in HO-2 Mice
HO-2−/− mice exhibit increased CSE-derived H2S production in the CB, and lowering H2S production by genetic removal of CSE prevents the exaggerated CB response to hypoxia in HO-2−/− mice (Yuan et al. 2015). We found that apnea and hypopneas were absent in HO-2/CSE double knockout mice, suggesting that lowering H2S production may prevent apnea. While genetic removal of CSE might not be a practical therapeutic strategy to treat sleep apnea clinically, we explored whether pharmacologic blockade of CSE-derived H2S synthesis stabilizes breathing in HO-2−/− mice. Intra-peritoneal administration of L-propargylglycine (L-PAG), an inhibitor of CSE, reduced the incidence of apnea in a dose-dependent manner (1 ~ 150 mg/kg), with a median effective dose at 30 mg/kg (Table 14.5). L-PAG reduced both central and obstructive apneas. The effect of L-PAG at a dose of 30 mg.kg on apneas was seen within 2 h after administration and completely reversed after 24 h. More importantly, L-PAG (30 mg/kg) administration via oral route was equally effective in reducing the number of apneas and hypopneas. No sign of toxicity was observed with any of the doses of L-PAG tested.
14.5 Summary and Perspective
Our study establishes that HO-2 null mice exhibit a high incidence of apnea and hypopnea during sleep and might serve as a pre-clinical murine model of sleep apnea. Like patients with sleep apnea, HO-2 null mice display both central and obstructive apneas. The enhanced CB chemo reflex mediated by CSE-produced H2S contributes to high incidence of apneas. Lowing H2S production by pharmacologic blockade of CSE significantly prevent apneas in HO-2 null mice. Since sleep apnea is a multi-factorial respiratory disease, it would be important to determine whether CSE inhibitors prevent apneas in patients with sleep apnea caused by other etiologies, such as heart failure, renal insufficiency and stroke.
References
Antic NA, Heeley E, Anderson CS, Luo Y, Wang J, Neal B, Grunstein R, Barbe F, Lorenzi-Filho G, Huang S, Redline S, Zhong N, McEvoy RD (2015) The sleep apnea cardio vascular endpoints (SAVE) trial: rationale, ethics, design, and progress. Sleep 38:1247–1257
Combs D, Shetty S, Parthasarathy S (2014) Advances in positive airway pressure treatment modalities for hypoventilation syndromes. Sleep Med Clin 9:315–325
Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H (2015) Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 373:1095–1105
Dempsey JA, Smith CA, Blain GM, Xie A, Gong Y, Teodorescu M (2012) Role of central/peripheral chemoreceptors and their interdependence in the pathophysiology of sleep apnea. Adv Exp Med Biol 758:343–349
Frost & Sullivan (2016) Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. American Academy of Sleep Medicine, Darien
Gilmartin GS, Daly RW, Thomas RJ (2005) Recognition and management of complex sleep-disordered breathing. Curr Opin Pulm Med 11:485–493
Harvard Medical School Division of Sleep Medicine (2010) The price of fatigue: the surprising economic costs of unmanaged sleep apnea. Harvard Medical School Division of Sleep Medicine, Boston
Ip S, D’Ambrosio C, Patel K, Obadan N, Kitsios GD, Chung M, Balk EM (2012) Auto-titrating versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: a systematic review with meta-analyses. Syst Rev 1:20
Javaheri S, Smith J, Chung E (2009) The prevalence and natural history of complex sleep apnea. J Clin Sleep Med 5:205–211
Javaheri S, Germany R, Greer JJ (2016) Novel therapies for the treatment of central sleep apnea. Sleep Med Clin 11:227–239
Kara T, Narkiewicz K, Somers VK (2003) Chemoreflexes–physiology and clinical implications. Acta Physiol Scand 177:377–384
Kumar P, Prabhakar NR (2012) Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2:141–219
Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, Ludwig MG, Perez-Reyes E, Mohebbi N, Bettoni C, Gassmann M, Suply T, Seuwen K, Guyenet PG, Wagner CA, Bayliss DA (2015) Regulation of breathing by CO2 requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 348:1255–1260
Makarenko VV, Nanduri J, Raghuraman G, Fox AP, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR (2012) Endogenous H2S is required for hypoxic sensing by carotid body glomus cells. Am J Physiol Cell Physiol 303:C916–C923
Mansukhani MP, Wang S, Somers VK (2015) Chemoreflex physiology and implications for sleep apnoea: insights from studies in humans. Exp Physiol 100:130–135
Morgenthaler TI, Kagramanov V, Hanak V, Decker PA (2006) Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep 29:1203–1209
Peng Y-J, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR (2010) H2S mediates O-2 sensing in the carotid body. Proc Natl Acad Sci U S A 107:10719–10724
Peng YJ, Makarenko VV, Nanduri J, Vasavda C, Raghuraman G, Yuan G, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR (2014) Inherent variations in CO-H2S-mediated carotid body O2 sensing mediate hypertension and pulmonary edema. Proc Natl Acad Sci U S A 111:1174–1179
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM (2013) Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177:1006–1014
Prabhakar NR, Peers C (2014) Gasotransmitter regulation of ion channels: a key step in O2 sensing by the carotid body. Physiology (Bethesda) 29:49–57
Quan SF, Awad KM, Budhiraja R, Parthasarathy S (2012) The quest to improve CPAP adherence–PAP potpourri is not the answer. J Clin Sleep Med 8:49–50
St-Onge MP, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, Bhatt DL, American Heart Association Obesity B. C., D.abetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health, Young C. o. C. D. i. t., Cardiology C. o. C., Council a. S (2016) Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation 134:e367–e386
Veasey SC, Guilleminault C, Strohl KP, Sanders MH, Ballard RD, Magalang UJ (2006) Medical therapy for obstructive sleep apnea: a review by the Medical Therapy for Obstructive Sleep Apnea Task Force of the Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 29:1036–1044
Xie A, Rutherford R, Rankin F, Wong B, Bradley TD (1995) Hypocapnia and increased ventilatory responsiveness in patients with idiopathic central sleep apnea. Am J Respir Crit Care Med 152:1950–1955
Yamauchi M, Combs D, Parthasarathy S (2016) Adaptive servo-ventilation for central sleep apnea in heart failure. N Engl J Med 374:689
Yuan G, Vasavda C, Peng YJ, Makarenko VV, Raghuraman G, Nanduri J, Gadalla MM, Semenza GL, Kumar GK, Snyder SH, Prabhakar NR (2015) Protein kinase G-regulated production of H2S governs oxygen sensing. Sci Signal 8:ra37
Acknowledgements
This work was supported by National Institutes of Health grants P01-HL-90554 and UH2-HL-123610.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this paper
Cite this paper
Peng, YJ., Zhang, X., Nanduri, J., Prabhakar, N.R. (2018). Therapeutic Targeting of the Carotid Body for Treating Sleep Apnea in a Pre-clinical Mouse Model. In: Gauda, E., Monteiro, M., Prabhakar, N., Wyatt, C., Schultz, H. (eds) Arterial Chemoreceptors. Advances in Experimental Medicine and Biology, vol 1071. Springer, Cham. https://doi.org/10.1007/978-3-319-91137-3_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-91137-3_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91136-6
Online ISBN: 978-3-319-91137-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)