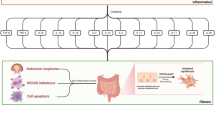
Abstract
Wound healing is a common process in the intestinal tract, in particular during chronic intestinal inflammation. Recent studies suggested a so-called regenerative inflammation that plays a crucial role for the regeneration of injured tissue. While this self-limiting acute inflammation protects the tissue, an overwhelming and chronic ongoing inflammatory process might lead to development of fibrosis or even cancer. Intestinal fibrosis and the resulting strictures represent, in addition to fistulas, frequent complications in IBD patients. To date, treatment options for fistulas and strictures are limited and no preventive treatment for intestinal fibrosis and stricture formation has been approved. As a result, irreparable organ damage and surgery is a frequent event in IBD patients. The onset of fibrosis often precedes fistula formation in the intestinal tract suggesting a pathophysiological connection between both of the processes. Nevertheless, our understanding of the pathogenetic mechanisms underlying intestinal fibrosis and fistula development is limited. An involvement of epithelial-to-mesenchymal transition (EMT) has been demonstrated for both, intestinal fibrosis as well as fistula development. It is anticipated that fistulas and fibrosis may result from chronic and severe intestinal inflammation and deregulated wound healing mechanisms. However, current knowledge also demonstrates fundamental differences between fibrosis and fistula development. Taken together, further research efforts are clearly required to gain a better understanding of the complex pathophysiology of fistula and intestinal fibrosis development. This would finally help to foster the development of novel treatment options for those intestinal complications.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
21.1 Introduction
Tissue remodeling and wound healing are crucial mechanisms how the body reacts to cell and tissue damage. This damage occurs continuously during a lifetime and is caused by infectious, toxic, neoplastic or immune-mediated events. Depending on the affected organ system, the reacting cell types, the duration, type and intensity of the damaging event the ensuing response of the body can be very different. Nevertheless, some degree of tissue inflammation is commonly involved [1]. Inflammation itself can exert protective as well as detrimental effects. If adequately controlled, inflammation protects the body from pathogens and is involved in tissue repair and regeneration. In contrast, uncontrolled, chronic or overwhelming inflammation results in cell death, tissue damage, fibrosis, autoimmunity, and tumor development [2]. Crohn’s disease as well as ulcerative colitis are both associated with chronic, possible life-long, inflammation, often resulting in severe and permanent organ dysfunction as well as tissue remodeling that is finally associated with the development of intestinal fibrosis and neoplasia [3] (Table 21.1).
21.2 Wound Healing
During IBD disease course , chronic and severe mucosal damage requires efficient wound healing mechanisms in the intestinal tract. Regeneration of the intestinal epithelium is dependent on transmembrane receptor Lgr5 expressing intestinal stem cells (ISC). Those ISC are located at the base of the intestinal crypts between the Paneth cells which produce factors being essential for ISC survival and proliferation [4, 5]. Upon tissue injury the ISC expand, repair the mucosa and restore epithelial barrier function.
Intestinal tissue damage is the result of inflammation what causes infiltration of immune cells into the mucosa. Severe inflammation results in local tissue destruction as indicated by loss of epithelial cells and degradation of extracellular matrix in the submucosa. Mainly the infiltrating mononuclear cells secrete reactive oxygen radicals and tissue-degrading enzymes, such as matrix metalloproteinases (MMP) and release pro-inflammatory cytokines, chemotactic and cell-activating peptides [6, 7]. This further enhances the extent of ongoing inflammation and tissue damage resulting in the continued infiltration of immune and non-immune cells into the inflamed tissue. In the case of severe tissue damage, finally so-called myofibroblasts migrate into the affected areas. These myofibroblasts are able to contract the wound area and to produce extracellular matrix (ECM) what is supposed to limit the extent of tissue damage [6]. Since MMPs are able to degrade secreted ECM components the balance between MMPs and their inhibitors, tissue inhibitors of MMPs (TIMP), is critical for the extent of tissue damage during inflammation [6]. A damage to the intestinal epithelium also allows the entry of commensal microbes and microbial macromolecules into the mucosa. This process leads to the generation of damage-associated molecular pattern (DAMP), pathogen-associated molecular pattern (PAMP) and reactive oxygen species (ROS), finally activating immune cells that then produce a broad number of cytokines, such as interleukin (IL)-6, IL-10, IL-17, IL-22 or tumor necrosis factor (TNF). Those cytokines not only are involved in the inflammatory response, but also in initiating the regenerative response by modulating ISC proliferation [1]. In the absence of microbiota-associated inflammation, a so-called sterile inflammation is induced by tissue damage and cell death [8], mainly mediated by DAMPs and activating pattern recognition receptors (PRR), such as toll-like receptors (TLR) and NOD-like receptors (NLR) as well as MMPs [9,10,11].
During acute intestinal inflammation, the activation of the immune system and matrix remodelling enzymes causes limited tissue damage what frequently results in a complete restitution of the damaged tissue due to sufficient wound repair mechanisms. More severe acute or moderate chronic inflammation causes severe or chronic tissue degradation and damage. These events are regularly followed by tissue repair, what might already cause fibrosis and scars. Severe acute and continuously ongoing chronic tissue damage frequently results in severe tissue fibrosis. This finally promotes the development of intestinal strictures and clinically symptomatic intestinal obstruction as the end point of inflammation-induced tissue injury [6].
21.3 Crohn’s Disease Fistula
In contrast to the physiologic process of wound healing, intestinal fistulas represent a severe and frequent complication of CD. CD-associated fistulas occur in up to 50% of patients [12, 13]. They still represent an unresolved medical problem in the treatment of CD patients, since permanent fistula healing is hardly achievable and recurrences are frequent. Fistulas in CD patients often impair the quality of life because of the above mentioned limited treatment options. The cumulative incidence in population-based cohorts and meta-analyses of fistula formation varies widely between 17 and 50% [14,15,16,17,18,19]. Most of the fistulas are located in the perianal region (54%), 24% are entero-enteric, 9% rectovaginal and 13% involve other locations, such as entero-cutaneous, entero-vesical, and intraabdominal fistulas [16].
Morphologically, a fistula represents a tract between two epithelial-lined surfaces. The prevalence of perianal fistulas increases with disease duration and more distal localization of intestinal disease [12, 20]. Noteworthy, especially perianal fistulas are not specific for CD and also occur during infection, hidradenitis suppurativa and malignant processes [21, 22]. Histologic features of CD fistulas are nonspecific. Fistula tracts may be detectable microscopically and lined by granulation tissue and/or “squamous” epithelium. The tracts are typically filled with debris, erythrocytes and acute inflammatory cells [12]. Chronic inflammation and surrounding fibrosis are regularly visible. It is hypothesized that fistulas arise as a consequence of an acute inflammatory process with infection and suppuration [23]. A deep penetrating ulceration located in the rectum or the anus might fill with fecal material. The luminal pressure then forces this material into underlying tissue layers. Additionally, also anal gland or duct abscesses might serve as a point of origin. The process of tissue destruction may then be maintained by ongoing inflammation as well as luminal antigens and bacteria. Interestingly, CD fistulas are commonly surrounded by fibrotic tissue [12, 24].
To date, only a small number of studies have investigated the pathogenesis of CD-associated fistulas. About 30% of intestinal and perianal fistulas from CD, but also from non-CD patients feature flattened intestinal or narrow squamous epithelium and are surrounded by granulation tissue. “Non-epithelialized” fistula areas exhibit a lining of myofibroblast-like cells (so-called “transitional cells”) forming a new basement membrane (BM). Only fistulas from CD patients, but not from non-CD patients, show regions with disordered myofibroblasts and fragmented BM suggesting different mechanisms of fistula formation in CD vs. non-CD patients [12]. Additionally, a characteristic composition of inflammatory cells has been described in and around fistulas. CD fistulas typically feature a central infiltrate consisting of CD45R0+ T cells, an underlying band of CD68+ macrophages as well as a dense infiltrate of CD20+ B cells in the outer fistula wall. Fistulas from non-CD patients, in contrast, commonly present a dense macrophage infiltrate and only few CD20+ B cells and CD45R0+ T cells [12]. Recent data also suggest accumulation of CD4+ CD161+ T cells with a Th17, Th17/Th1 and Th1 phenotype in CD perianal fistulas [25].
21.4 Epithelial to Mesenchymal Transition (EMT) and CD Fistulas
Current knowledge suggests that the driving force for the development of CD-associated fistulas is EMT. While EMT is as a physiological process involved in embryogenesis, organ development, wound healing and tissue remodelling, it also plays an important role for pathological processes such as tissue fibrosis and cancer progression [26, 27]. During EMT, a differentiated, resident epithelial cell loses its epithelial cell shape, down-regulates epithelial-cell specific proteins as E-cadherin or claudin-4. And acquires a mesenchymal cell shape accompanied by the upregulation of mesenchymal proteins, such as vimentin [26].
The process of EMT has been clearly demonstrated in pathogenesis of CD-associated fistulas [12, 24]. In particular, tracts of CD fistulas are covered by intestinal epithelial cells (IEC) as well as by “transitional cells” (TC). The transitional cells develop from intestinal epithelial cells via EMT and express typical mesenchymal cell markers, such as vimentin and alpha smooth-muscle-actin (α-SMA), in addition to their epithelial markers, such as cytokeratins (CK)-8, CK-20 or E-cadherin [24]. Additionally, in cells undergoing EMT, nuclear localization of β-catenin and of the EMT-associated transcription factor SLUG, can be detected. A further hint for an involvement of EMT in fistula development is the strong expression of transforming growth factor (TGF) β , the most powerful driving force for EMT, in cells along as well as surrounding the CD fistula tracts [24, 28]. Immunohistochemical studies have also detected Snail family transcription factors in cells lining the fistula tract as well as around CD-associated fistulas. On the one hand, SNAIL1 is detected in nuclei of transitional cells lining the fistula tracts. On the other hand, SLUG (SNAIL2) is expressed in cells of fistula surrounding tissue, but almost absent in transitional cells [29].
21.5 Molecules Involved in CD Fistula Formation
IL-13 is strongly expressed in cells lining the fistula tract and also, to some lesser extent, in fistula surrounding fibrotic tissue layers. On a molecular level, TGFβ is able to induce IL-13 secretion from colonic lamina propria fibroblasts (CLPF) derived from CD patients with fistulizing disease, but not from non-IBD control patients or CD patients without fistulas. This suggests a specific amplification loop in CD fistula tissue [13, 24]. Recent data have also shown that IL-13 induces expression of the EMT transcription factor SLUG as well as of β6-Integrin, a protein that is associated with cell invasiveness, in an in vitro model of EMT using HT29 IEC spheroids [13]. Further support for the “amplification loop” theory is given by the fact that TNF and TNF-receptor 1 are also strongly detectable in cells lining the CD-associated fistula tracts [29]. We and others have demonstrated that TNF induces EMT and the expression of EMT-associated genes in IEC spheroids [30, 31]. TNF and TGFβ also induce the expression of the Wnt-antagonist, Dickkopf-homolog 1 (DKK-1) in CLPF derived from CD patients with fistulas. DKK-1 is expressed along fistula tracts in CD patients and limits TGFβ-induced IL-13 expression (32). Support for the hypothesis that the intestinal microbiota is somehow involved in fistula formation in CD patients is provided by the observation that the bacterial wall component, muramyl-dipeptide (MDP), induces not only EMT in IEC, but also the expression of fistula-associated molecules in IEC and fistula CLPF [30].
With respect to matrix remodelling enzymes, a strong expression of MMP-3 and MMP-9 can be observed in CD fistulae, while levels TIMP-1, TIMP-2 and TIMP-3 are lower compared to their expression level in colon tissue from non-IBD patients. This observation supports the assumption that fistula formation is associated with a dysbalance of matrix remodelling enzymes, in particular of MMPs and TIMPs , what contributes to the development of fistulas through enhanced ECM degradation [32]. All of those observations strongly suggest EMT-like processes in the pathogenesis of CD-associated fistulae [12, 13, 24, 29, 30, 33]. Additionally, fistula-associated molecules seem to be associated with the development of so-called fistula-carcinomas in CD patients, a very severe and important complication of CD and CD-associated fistulas [34,35,36].
21.6 Pathogenetic Differences Between Stricture and Fistula Formation
Inflammation is a crucial trigger for tissue regeneration, but nowadays knowledge suggest that uncontrolled or chronic inflammation might also be an important trigger for both fibrosis and fistula formation [1, 37]. Unfortunately, to date, this has not been demonstrated formally in mammalian animal models. Mouse and rat models only rarely and late develop fibrotic alterations in the intestine and the onset of clinically relevant strictures or fistulas is very rare. Nevertheless, the general concept of an inflammatory trigger for development of fibrosis and fistulas is generally accepted.
From a clinical point of view, there is no medication available that directly targets fibrosis and anti-inflammatory treatment in IBD patients at the same time and is also sufficient to treat fibrosis once excessive ECM deposition has occurred [38, 39]. Subsequently pathophysiological mechanisms perpetuating fistula and/or fibrosis formation may be distinct from the ones regulating the onset of fibrosis and fistula formation. In particular, inflammation seems to play an important role in the beginning of fibrotic tissue alterations and fistula development. However, the impact of inflammation in later stages of the disease is unclear. This aspect becomes very interesting when considering novel therapeutic strategies, such as SMAD7 antisense oligonucleotides that affect TGFβ function.
While treatment options for intestinal inflammation in IBD patients become more and more sophisticated, options for treatment and prevention of intestinal fibrosis or fistula formation are still very limited. This is also due to the fact that our understanding of the pathophysiological mechanisms of fistula and fibrosis development is scarce. Of course, fistulas and fibrosis share some common pathogenetic features, such as EMT, but have also clearly distinct pathways and triggers. Currently, the most promising approach to prevent fibrosis and likely also fistula development might be to control inflammation before the complication has occured. Therapeutic interventions to control inflammation once stenosis/fibrosis or fistulas have been formed are in general not successful [38].
From a molecular perspective, the development of fibrosis is defined as the excessive accumulation of ECM what finally causes organ dysfunction or even organ failure [26]. Current knowledge suggests that the key factors for fibrosis are chronic tissue damage due to chronic inflammation, overwhelming or defective wound healing mechanisms and expanding mesenchymal cells, mostly fibroblasts, myofibroblasts and smooth muscle cells [40]. Fibroblasts are continuously producing ECM as part of continuously ongoing tissue regeneration mechanisms. Following injury or inflammation, mesenchymal cells are able to rapidly proliferate and to invade the affected sites of injury or inflammation from within and without the intestinal tract. Hereby they follow a chemical gradient which is produced by growth factors. Finally, the fibroblasts become activated by a cocktail of cytokines that is produced by and secreted from immune as well as non-immune cells [40]. As a consequence, the mesenchymal cells produce excessive amounts of collagen and other components of the ECM [41]. Nevertheless, expression and activity of MMPs and their inhibitors TIMPs are elevated in the intestine of CD and UC patients. This suggests that the development of intestinal fibrosis in IBD patients is not only due to excessive ECM production, but rather dependent on an imbalance in regular tissue-remodeling processes [6]. Here, a clear correlation to the development of CD-associated fistulas is seen. Aberrant matrix remodeling, production of ECM components and a deregulated ECM turn-over are characteristic features of the development of both, fistulas and fibrosis. Noteworthy, CD fistulas are commonly surrounded by fibrotic tissue: A possible explanation for this observation might be the fact that the body aims at initiating wound healing around the fistula tracts. Since the fistula itself can be already result from defective wound healing mechanisms, the onset of fibrosis and the development of fibrotic tissue around the fistula tract might serve to limit ongoing tissue damage as well as further fistula growth. In this regard, the onset of fibrosis around fistula tracts would represent a rescue mechanism of the intestinal tissue. A further hint to his theory is the fact that fibroblasts which are isolated from dense fibrosis tissue reveal a clearly higher migratory potential as colonic lamina propria fibroblasts (CLPF) isolated from fistulas. These observations suggest that fibroblasts in fistula areas might exert a lower capacity to repair tissue defects. As a compensatory mechanism, intestinal epithelial cells might be reprogrammed via EMT into mesenchymal cells. This allows them to migrate to the affected tissue regions what finally promotes fistula formation [42].
While the involvement of cytokines and growth factors, such as IL-13, TNF or TGFβ in the pathogenesis of intestinal fibrosis has been well documented [6, 40], recent studies also suggest an involvement of TNF and IL-13 as well as of their receptors in fistula formation. Those molecules are highly expressed in TC lining fistula tracts. These observations support the hypothesis that comparable mechanisms might contribute to the onset fistulas and fibrosis in the intestine. This assumption is even more underlined by the observation that EMT is crucial for fistula development and that hallmarks of EMT are be detected in areas of intestinal fibrosis in CD patients [28, 43]. TGFβ , the most powerful inducer of EMT, is highly detectable in fistula as well as fibrotic regions of IBD patients [24, 44]. Additionally, the EMT-associated molecule, β-catenin, is less expressed in the membrane, but strongly expressed in the nucleus, in fibrotic areas and fistulas hinting at its enhanced transcriptional activity. While the EMT-related transcription factor SNAIL1 is strongly expressed in both, fistula tissue and fibrotic tissue, expression of SLUG transcription factor can only be observed in the nuclei of mesenchymal cells in fibrotic areas. In transitional cells along CD fistulas SLUG expression is only poorly detectable [28, 29]. Interestingly, in a case report of a patient with a fistula-associated anal adenocarcinoma, a remarkable staining of SLUG transcription factor was shown not only in TC lining the fistula tract, but also in the carcinoma tissue originating from those cells [34]. As a limitation, however, one must mention that all of the current literature studying the pathogenic role for EMT in Crohn’s disease intestinal fistulas and fibrosis are based on descriptive results obtained by haematoxylin-eosin staining, immunohistochemistry and electron microscopy only. Due to this lack of functional studies and in particular in vivo studies on this topic, the true relevance for EMT in fistula and fibrosis development in CD patients warrants further confirmation.
Increased levels of IL-13 in the fibrotic intestine of CD patients are produced by a population of cells expressing high levels of IL-13Rα1 [45]. According to their phenotype, those cells (KIR+ CD45+ CD56± CD3− IL-13Rα1+) might be innate lymphoid cells (ILC). Fibroblasts down-regulate levels of MMP-2 as well as of TNF-induced MMP-1 and MMP-9 protein in response to IL-13 [45]. A very interesting observation in fibrosis and fistula development are the effects of IL-13 and TGFβ. In the pathogenesis of fibrosis, IL-13 induces TGFβ secretion. In contrast, in fistula development fistula-derived myofibroblasts secrete IL-13 following stimulation with TGFβ [13, 44]. Nevertheless, conflicting results have been demonstrated with respect to the impact of IL-13 in the development of intestinal fibrosis in stricturing CD [46] suggesting that the possible pro-fibrogenic role of IL-13 in CD needs to be critically reassessed. A further hint to the complexity of fistula and fibrosis development is the observation that IFNγ is able to induce fibroblast apoptosis following co-treatment with TNF in an in vitro model of fibrosis [47, 48]. However, TNF is able to induce intestinal fibrosis by inducing collagen accumulation and inflammation [48].
Fistula formation in CD patients and development of intestinal fibrosis exhibit several similarities, but they also features remarkable differences. In the pathogenesis of CD fistulas, less migratory potential of myofibroblasts and an aberrant ECM production occurs, while as a compensatory mechanism, intestinal epithelial cells invade the wounded area to close the wound area. In contrast, increased proliferation as well as migration of myofibroblasts followed by enhanced matrix synthesis might be the critical mechanism in the development of intestinal fibrosis [6].
21.7 Summary
A well-balanced wound healing response represents a critical repair mechanism of the intestinal tract during acute and chronic intestinal inflammation. Defective wound healing promotes the onset of fistulas or fibrosis, the latter possibly resulting in clinically relevant stenosis or strictures. On a molecular level, EMT might play a crucial role for the development of both, fistulas and fibrosis. However, the exact mechanisms for their development are not yet determined. This clearly suggests that further studies and if possible in vivo studies, are needed to gain a better understanding of both pathologies, which would be essential for the development of novel therapeutic strategies aiming at preventing and healing fistulas and stenosis. It is necessary to consider fistula formation as a pathological process which is distinct from inflammation. This has also been discussed for the development of intestinal fibrosis. It will certainly be a great achievement to better understand the pathways of fistula formation and to compare those mechanisms to those of frequently coincident fibrosis formation. Since treatment options for fistula and fibrosis therapy are limited to date, this represents one of the big unmet goals in IBD therapy and further research is clearly needed [37].
21.8 Conflict of Interest Statement
No conflicts of interest exist.
References
Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529(7586):307–15.
Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–35.
Rieder F, Fiocchi C. Mechanisms of tissue remodeling in inflammatory bowel disease. Dig Dis. 2013;31(2):186–93.
Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154(2):274–84.
Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–8.
Rieder F, Brenmoehl J, Leeb S, Scholmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut. 2007;56(1):130–9.
Vaday GG, Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J Leukoc Biol. 2000;67(2):149–59.
Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–42.
Stevens LJ, Page-McCaw A. A secreted MMP is required for reepithelialization during wound healing. Mol Biol Cell. 2012;23(6):1068–79.
Chalaris A, Adam N, Sina C, Rosenstiel P, Lehmann-Koch J, Schirmacher P, et al. Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J Exp Med. 2010;207(8):1617–24.
Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32(8):380–7.
Bataille F, Klebl F, Rummele P, Schroeder J, Farkas S, Wild PJ, et al. Morphological characterisation of Crohn’s disease fistulae. Gut. 2004;53(9):1314–21.
Scharl M, Frei S, Pesch T, Kellermeier S, Arikkat J, Frei P, et al. Interleukin-13 and transforming growth factor beta synergise in the pathogenesis of human intestinal fistulae. Gut. 2013;62(1):63–72.
McKee RF, Keenan RA. Perianal Crohn’s disease--is it all bad news? Dis Colon Rectum. 1996;39(2):136–42.
van Dongen LM, Lubbers EJ. Perianal fistulas in patients with Crohn’s disease. Arch Surg. 1986;121(10):1187–90.
Schwartz DA, Loftus EV Jr, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122(4):875–80.
Judge TA, Lichtenstein GR. Treatment of fistulizing Crohn’s disease. Gastroenterol Clin N Am. 2004;33(2):421–54, xi-xii.
Hellers G, Bergstrand O, Ewerth S, Holmstrom B. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut. 1980;21(6):525–7.
Solomon MJ. Fistulae and abscesses in symptomatic perianal Crohn’s disease. Int J Color Dis. 1996;11(5):222–6.
Gecse KB, Bemelman W, Kamm MA, Stoker J, Khanna R, Ng SC, et al. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63(9):1381–92.
Yu H, Liu Y, Wang Y, Peng L, Li A, Zhang Y. Clinical, endoscopic and histological differentiations between Crohn’s disease and intestinal tuberculosis. Digestion. 2012;85(3):202–9.
Makharia GK, Srivastava S, Das P, Goswami P, Singh U, Tripathi M, et al. Clinical, endoscopic, and histological differentiations between Crohn’s disease and intestinal tuberculosis. Am J Gastroenterol. 2010;105(3):642–51.
Plesec TP, Owens SR. Inflammatory and neoplastic disorders of the anal canal. In: Odze RD, Goldblum JR, editors. Surgical pathology of the GI tract, liver, biliary tract and pancreas. 3rd ed. Philadelphia: Elsevier; 2015. p. 887–920.
Bataille F, Rohrmeier C, Bates R, Weber A, Rieder F, Brenmoehl J, et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn’s disease. Inflamm Bowel Dis. 2008;14(11):1514–27.
Maggi L, Capone M, Giudici F, Santarlasci V, Querci V, Liotta F, et al. CD4+CD161+ T lymphocytes infiltrate Crohn’s disease-associated perianal fistulas and are reduced by anti-TNF-alpha local therapy. Int Arch Allergy Immunol. 2013;161(1):81–6.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8.
Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–84.
Scharl M, Rogler G, Biedermann L. Fistulizing Crohn’s disease. Clin Transl Gastroenterol. 2017;8(7):e106. https://doi.org/10.1038/ctg.2017.33.
Scharl M, Weber A, Furst A, Farkas S, Jehle E, Pesch T, et al. Potential role for SNAIL family transcription factors in the etiology of Crohn’s disease-associated fistulae. Inflamm Bowel Dis. 2011;17(9):1907–16.
Frei SM, Pesch T, Lang S, Weber A, Jehle E, Vavricka SR, et al. A role for tumor necrosis factor and bacterial antigens in the pathogenesis of Crohn’s disease-associated fistulae. Inflamm Bowel Dis. 2013;19(13):2878–87.
Bates RC, Mercurio AM. Tumor necrosis factor-alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Mol Biol Cell. 2003;14(5):1790–800.
Kirkegaard T, Hansen A, Bruun E, Brynskov J. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn’s disease. Gut. 2004;53(5):701–9.
Frei SM, Hemsley C, Pesch T, Lang S, Weber A, Jehle E, et al. The role for dickkopf-homolog-1 in the pathogenesis of Crohn’s disease-associated fistulae. PLoS One. 2013;8(11):e78882.
Scharl M, Frei P, Frei SM, Biedermann L, Weber A, Rogler G. Epithelial-to-mesenchymal transition in a fistula-associated anal adenocarcinoma in a patient with long-standing Crohn’s disease. Eur J Gastroenterol Hepatol. 2014;26(1):114–8.
Kim J, Lee HS, Park SH, Yang SK, Ye BD, Yang DH, et al. Pathologic features of colorectal carcinomas associated with Crohn’s disease in Korean population. Pathol Res Pract. 2017;213(3):250–5.
Maejima T, Kono T, Orii F, Maemoto A, Furukawa S, Liming W, et al. Anal canal adenocarcinoma in a patient with longstanding Crohn’s disease arising from rectal mucosa that migrated from a previously treated rectovaginal fistula. Am J Case Rep. 2016;17:448–53.
Siegmund B, Feakins RM, Barmias G, Ludvig JC, Teixeira FV, Rogler G, et al. Results of the fifth scientific workshop of the ECCO (II): pathophysiology of perianal fistulizing disease. J Crohns Colitis. 2015;10(4):377–86.
Latella G, Rogler G, Bamias G, Breynaert C, Florholmen J, Pellino G, et al. Results of the 4th scientific workshop of the ECCO (I): pathophysiology of intestinal fibrosis in IBD. J Crohns Colitis. 2014;8(10):1147–65.
Lawrance IC, Rogler G, Bamias G, Breynaert C, Florholmen J, Pellino G, et al. Cellular and molecular mediators of intestinal fibrosis. J Crohns Colitis. 2015;11(12):1491–503.
Rieder F. The gut microbiome in intestinal fibrosis: environmental protector or provocateur? Sci Transl Med. 2013;5(190):190ps10.
Burke JP, Mulsow JJ, O’Keane C, Docherty NG, Watson RW, O’Connell PR. Fibrogenesis in Crohn’s disease. Am J Gastroenterol. 2007;102(2):439–48.
Meier JK, Scharl M, Miller SN, Brenmoehl J, Hausmann M, Kellermeier S, et al. Specific differences in migratory function of myofibroblasts isolated from Crohn’s disease fistulae and strictures. Inflamm Bowel Dis. 2011;17(1):202–12.
Scharl M, Rogler G. Pathophysiology of fistula formation in Crohn’s disease. World J Gastrointest Pathophysiol. 2014;5(3):205–12.
Rieder F, Fiocchi C. Intestinal fibrosis in IBD--a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6(4):228–35.
Bailey JR, Bland PW, Tarlton JF, Peters I, Moorghen M, Sylvester PA, et al. IL-13 promotes collagen accumulation in Crohn’s disease fibrosis by down-regulation of fibroblast MMP synthesis: a role for innate lymphoid cells? PLoS One. 2012;7(12):e52332.
Biancheri P, Di Sabatino A, Ammoscato F, Facciotti F, Caprioli F, Curciarello R, et al. Absence of a role for interleukin-13 in inflammatory bowel disease. Eur J Immunol. 2014;44(2):370–85.
Bettenworth D, Rieder F. Reversibility of Stricturing Crohn’s disease-fact or fiction? Inflamm Bowel Dis. 2015;22(1):241–7.
Speca S, Giusti I, Rieder F, Latella G. Cellular and molecular mechanisms of intestinal fibrosis. World J Gastroenterol. 2012;18(28):3635–61.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Scharl, M. (2018). What Distinguishes Mechanisms of Fistula and Stricture Formation. In: Rieder, F. (eds) Fibrostenotic Inflammatory Bowel Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-90578-5_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-90578-5_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90577-8
Online ISBN: 978-3-319-90578-5
eBook Packages: MedicineMedicine (R0)