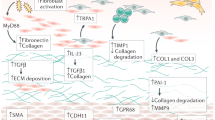
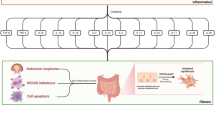
Abstract
Intestinal fibrosis in inflammatory bowel disease (IBD) leading to stricture formation, intestinal obstruction and need for surgical intervention remains one of the largest unresolved clinical challenges in IBD. Despite the emergence of novel anti-inflammatory drugs the incidence of stricture formation and surgery remained largely unchanged. Challenges in testing anti-fibrotic compounds have so far prevented progress in this area, but recent development put clinical trials for anti-fibrotic compounds into reach.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Intestinal fibrosis remains one of the largest unresolved problems in the field of inflammatory bowel diseases (IBD). It is estimated that more the half of the patients with Crohn’s disease (CD) develop fibrostenosing complications over their lifetime leading to intestinal obstruction and need for resection [1]. This importance is emphasized by the belief that strictures precede internal penetrating disease, which is based on the observation that isolated stricturing disease is common, but internal penetrating disease is associated with strictures in >85% of cases and located upstream of strictures in the area of pre-stenotic dilation [1, 2]. More than 80% of patients with Crohn’s disease undergo surgery at least once during their lifetime with strictures being a major indication [3]. In addition, accumulating evidence suggests that fibrosis may be a clinically relevant factor in Ulcerative colitis, where it can be found in 100% of colectomy specimen [4] and could potentially lead to clinical symptoms such as urgency or diarrhea.
Fibrosis is considered the final pathological outcome of the majority of chronic inflammatory diseases [5] and consequences in other organs, such as liver, lung, kidney and pancreas are well documented. There is a robust understanding of its pathophysiology, leading to excessive accumulation of extracellular matrix (ECM). This led to approval of now two drugs for the use in idiopathic pulmonary fibrosis [6, 7].
Fibrosis in IBD, to the contrary, to date has not yet been substantially explored. No specific anti-fibrotic therapy is available. Disease progression to complications may be slightly delayed by immunomodulatory or biologic therapy in IBD [8], but this does not lead to a robust reduction in the need for intestinal surgery [9]. Endoscopic therapy as well as bowel resection are still the major therapeutic modalities for CD patients with clinically symptomatic fibrostenosis [10]. Strikingly, fibrosis has been reported to be reversible and may not represent a unidirectional process from tissue damage, abnormal repair over excessive ECM accumulation to clinical symptoms and surgery. When patients undergo strictureplasty for established strictures in CD the surgical recurrence rate was 39% for jejunoileal strictures. The reason for re-surgery, however, was at site of the original strictureplasty in only 3% of the cases [11]. When examining the site of the original strictureplasty, a regression of fibrosis was noted, which is consistent with cross sectional imaging studies following strictureplasties [12]. This model is now used prospectively for strictureplasties over the ileocecal valve [13].
Multiple mechanisms may be exploited for a therapeutic intervention that are multifactorial and dynamic, meaning dependent on the quality, quantity and timing of the inflammatory process [1]. Genetic and epigenetic factors may play a role, as do cytokines and numerous growth factors [1]. The gut is unique in its exposure to environmental factors and we now begin to understand that those, including the microbiota, smoking or dietary components, could also drive fibrogenesis [14]. It is apparent that tissue damage, once established, may progress in the absence of inflammation [1, 15]. This is highly relevant as it may explain, why conventional anti-inflammatory therapies do not seem to be able to prevent fibrostenosis. Understanding mechanisms mediating this process, such as cell to matrix interactions or tissue mechanoproperties could offer future therapies. The chief pro-fibrotic cell type, the mesenchymal cell, can arise from a variety of sources in IBD, including cellular transformation, proliferation of local fibroblasts, intestinal stellate cells or circulating precursors, so called fibrocytes [1]. Preventing the accumulation of mesenchymal cells, rather than controlling their activation could be used to prevent or treat fibrosis.
Despite the large clinical problem and possible reversibility of fibrosis, as well as known mechanisms of fibrosis the progress of developing novel anti-fibrotic drugs in IBD has been slow. This could be explained by multiple obstacles: we are missing accurate biomarkers to predict, which patients develop fibrostenosis or what the fibrotic burden of each individual patient is at any given time [16]. The current phenotype classifications are only grouping patients, based on their clinical symptoms, therefore missing clinically silent fibrostenosis. Biomarker studies are based on patient populations using solely clinical classifications and hence are bound to be inaccurate. We are currently lacking imaging tools to quantify fibrosis or separate fibrosis from inflammation [17]. Major progress is occurring, including validation of radiologic endpoints for clinical trials. The time from disease diagnosis to stricture detection is long and hence a clinical trial would need to be large, long and hence very expensive, an investment that pharmaceutical companies were shying back from to date.
How do we make progress in the area of fibrostenosing IBD: we need to continue to discover novel mechanisms of fibrogenesis. Learning from other intestinal diseases with wound healing abnormalities, such as intraabdominal adhesions or fistulizing disease, or from fibrotic disease of other organs will fuel progress. International interest groups are forming to develop and validate biomarkers and clinical trial endpoints, with the goal to use those in proof of concept clinical trials and make the field of intestinal fibrogenesis a ‘Cinderella-story’. This book is intended to discuss all the above-mentioned concepts in depth and provide the reader with the necessary tools to understand obstacles and promises in the area of stricture formation in IBD.
In summary, intestinal stricture formation due to fibrosis remains one of largest unresolved obstacles in IBD. Current therapy is insufficient and no specific anti-fibrotic approach is available. Significant progress is made to overcome the challenges to develop novel anti-fibrotic bringing anti-fibrotic therapies within reach.
References
Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:340–350.e6.
Oberhuber G, Stangl PC, Vogelsang H, et al. Significant association of strictures and internal fistula formation in Crohn’s disease. Virchows Arch. 2000;437:293–7.
Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn’s disease. Relationship between the clinical pattern and prognosis. Gastroenterology. 1985;88:1818–25.
Gordon IO, Agrawal N, Goldblum JR, et al. Fibrosis in ulcerative colitis: mechanisms, features, and consequences of a neglected problem. Inflamm Bowel Dis. 2014;20:2198–206.
Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–40.
Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82.
Hughes G, Toellner H, Morris H, et al. Real world experiences: Pirfenidone and Nintedanib are effective and well tolerated treatments for idiopathic pulmonary fibrosis. J Clin Med. 2016;5. https://doi.org/10.3390/jcm5090078.
Magro F, Rodrigues-Pinto E, Coelho R, et al. Is it possible to change phenotype progression in Crohn’s disease in the era of immunomodulators? Predictive factors of phenotype progression. Am J Gastroenterol. 2014;109:1026–36.
Chatu S, Subramanian V, Saxena S, et al. The role of thiopurines in reducing the need for surgical resection in Crohn’s disease: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:23–34. quiz 35.
Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62:1072–84.
Yamamoto T, Fazio VW, Tekkis PP. Safety and efficacy of strictureplasty for Crohn’s disease: a systematic review and meta-analysis. Dis Colon Rectum. 2007;50:1968–86.
Maconi G, Sampietro GM, Cristaldi M, et al. Preoperative characteristics and postoperative behavior of bowel wall on risk of recurrence after conservative surgery in Crohn’s disease: a prospective study. Ann Surg. 2001;233:345–52.
de Buck van Overstraeten A, Vermeire S, Vanbeckevoort D, et al. Modified side-to-side isoperistaltic strictureplasty over the ileocaecal valve: an alternative to ileocaecal resection in extensive terminal ileal Crohn’s disease. J Crohns Colitis. 2016;10:437–42.
Rieder F. The gut microbiome in intestinal fibrosis: environmental protector or provocateur? Sci Transl Med. 2013;5:190ps10.
Johnson LA, Luke A, Sauder K, et al. Intestinal fibrosis is reduced by early elimination of inflammation in a mouse model of IBD: impact of a “Top-Down” approach to intestinal fibrosis in mice. Inflamm Bowel Dis. 2012;18:460–71.
Rieder F, de Bruyn JR, Pham BT, et al. Results of the 4th scientific workshop of the ECCO (Group II): markers of intestinal fibrosis in inflammatory bowel disease. J Crohns Colitis. 2014;8:1166–78.
Stidham RW, Higgins PD. Imaging of intestinal fibrosis: current challenges and future methods. United European Gastroenterol J. 2016;4(4):515–22.
Financial Support
This work was supported by grants from the National Institutes of Health [T32DK083251, P30DK097948 Pilot Feasibility Study, K08DK110415] and the European Crohn’s and Colitis Foundation to F.R.
Conflicts of Interest
F.R. Consulting: UCB, Celgene, Samsung, Roche, Pliant, Thetis, Boehringer-Ingelheim, Helmsley; AdBoards: AbbVie, UCB, Receptos, RedX, Celgene; Speakers Bureau: AbbVie.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Rieder, F. (2018). Fibrostenotic Inflammatory Bowel Disease: A Cinderella Story. In: Rieder, F. (eds) Fibrostenotic Inflammatory Bowel Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-90578-5_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-90578-5_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90577-8
Online ISBN: 978-3-319-90578-5
eBook Packages: MedicineMedicine (R0)