Abstract
REM sleep behavior disorder (RBD) has been described in the setting of some paraneoplastic neurological disorders and autoimmune diseases. Reports include small case series and single reports suggesting that the association is not strong or that it has been underestimated because physicians paid little attention to RBD features when patients presented with acute or subacute disabling neurological symptoms (e.g., mental confusion, seizures, paralysis, ataxia) linked to the underlying condition. RBD confirmed by polysomnography has been described in the paraneoplastic syndromes anti-Ma2 encephalitis and cerebellar degeneration and in the limbic encephalitis associated with LGI1 antibodies. In most of the cases reported, RBD symptoms were not prominent, but the associations reflect that the brain stem (damaged in the anti-Ma2 encephalitis), limbic system (the amygdala is impaired in the anti-LGI1 limbic encephalitis), and the cerebellum (severe Purkinje cell loss in paraneoplastic cerebellar degeneration) play a role in the pathogenesis of RBD. Some patients with Morvan syndrome present with agrypnia excitata, a form of status dissociatus. RBD is present in some autoimmune diseases including narcolepsy (probably due to lack of hypocretin input to the brain stem nuclei that regulate REM sleep) and multiple sclerosis (when plaques are located in the pontine tegmentum). REM sleep without atonia has been described in the acute phase of Guillain-Barré syndrome in some patients. RBD is very common in the anti-IgLON5 disease (a condition of probable autoimmune origin linked to neuronal antibodies and tau deposits in the brain, with a strong HLA link) in association with purposeful behaviors in NREM sleep, disorganized sleep architecture, laryngeal stridor, and obstructive apnea.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Paraneoplastic syndromes
- Anti-Ma2 encephalitis
- Limbic encephalitis associated with LGI1 antibodies
- Morvan syndrome
- Autoimmune diseases
- Multiple sclerosis
- Guillain-Barré syndrome
- Anti-IgLON5 disease
1 RBD in Paraneoplastic Neurological Syndromes
Paraneoplastic neurological syndromes (PNS) are uncommon disorders related to neoplasms outside the central nervous system, such as limbic encephalitis and subacute cerebellar syndrome [1, 2]. PNS are not caused by metastases or infiltration of a tumor in the brain. PNS are immune-mediated disorders linked to onconeuronal antibodies against neural antigens expressed by both the tumor and the nervous system. Antibodies react with an antigen located both in the tumor cells and cells of the nervous system, usually neurons and Purkinje cells. The immune system recognizes a protein expressed by the tumor as foreign and attacks this protein that is also located in the normal brain. Antibodies react with specific proteins expressed in the cytoplasm, nuclei, or surface membrane of the neuronal cells. In most PNS, the direct pathogenic role of the antibodies is debatable. Autopsy usually shows neuronal loss, gliosis, and inflammatory infiltrates of cytotoxic T lymphocytes. Interestingly, PNS may precede the diagnosis of the underlying systemic cancer. PNS are common in patients with cancers of the lung, ovary, breast, and testis and Hodgkin’s disease. The clinical course is usually subacute and progressive. Symptomatology can be severe and involve any area of the nervous system. Neurological symptomatology depends on the structures where antigens are prominently expressed in the brain. Damage of dorsal root ganglia, cerebellum, amygdala, hippocampus, brain stem, hypothalamus, and thalamus is common in PNS. Neurological symptoms include a confusional state, cognitive impairment, memory loss, seizures, movement disorders, psychosis, sensory polyneuropathy, and sleep disorders.
Sleep disorders in patients with PNS have received attention only recently [3]. Prospective and well-designed studies are lacking. Only small series and case reports have described patients with PNS suffering from sleep disturbances. They include RBD, excessive daytime sleepiness, insomnia, and central respiratory abnormalities. In some of the reported cases with sleep disturbances, video-polysomnography (V-PSG) was not performed. In the setting of PNS, sleep disorders are not the only manifestation since they coexist with limbic syndrome, brain stem symptomatology, or hypothalamic disturbances. RBD is not the most severe neurological complaint among patients with PNS. It is possible that the presence of RBD in the setting of any PNS may have been previously overlooked because physicians frequently pay little attention to sleep and because patients and bed partners usually do not spontaneously report sleep problems. This chapter reviews the medical literature where RBD was linked to PNS.
Anti-Ma2 encephalitis . This is a PNS affecting diencephalon, limbic, and brain stem structures in any combination, and it is usually linked to lung and testicular cancers [1, 2]. There are a few reports of RBD associated with anti-Ma2 encephalitis. In these cases, RBD was apparently linked to secondary narcolepsy due to an inflammatory process involving the hypothalamus, amygdala, and brainstem. RBD, in these cases, was not the sole manifestation of the anti-Ma2 encephalitis where it is associated with coexistent cognitive impairment, gaze palsy, hypersomnia, and cataplexy. In anti-Ma2 encephalitis, RBD is usually mild and not a prominent feature of the clinical picture. There are a few case reports of patients presenting with RBD in the context of anti-Ma2 encephalitis.
A 69-year-old man with anti-Ma2 encephalitis presented with a subacute onset of severe hypersomnia, memory loss, parkinsonism, and gaze palsy. Brain magnetic resonance imaging showed bilateral damage in the dorsolateral midbrain, amygdala, and paramedian thalami. V-PSG disclosed RBD and reduced sleep efficiency of 48% and absence of sleep spindles. REM sleep was characterized by increased phasic and tonic muscular activity in the submentalis muscle and the four limbs associated with kicking and prominent limb and truncal jerking. The multiple sleep latency test (MSLT) showed a mean sleep latency of 7 min and four sleep-onset REM periods. HLA typing was negative for the DQB1*0602 and DRB1*15 alleles. CSF hypocretin level was low (49 pg/mL) [4].
A 55-year-old man presented with hypersomnolence, cataplexy, abnormal sleep behaviors, parkinsonism, and vertical supranuclear palsy. PSG showed disrupted sleep architecture and complete loss of REM sleep atonia. During REM sleep, vocalizations (talking, laughing, and singing) and arm and leg movements were observed. MSLT demonstrated reduced sleep latency of 2 min and multiple sleep-onset REM periods without muscle atonia. The patient had positive Ma1 and Ma2 antibodies, and a tonsillar squamous cell carcinoma was discovered [5].
A 63-year-old man with anti-Ma2 antibodies had diencephalic encephalitis with excessive daytime sleepiness, cataplexy, and hypocretin deficiency. PSG demonstrated fragmented and reduced sleep efficiency and sustained muscle activity in REM sleep. MSLT showed two sleep-onset REM periods and a mean sleep latency of 7 min. Neuropathology demonstrated inflammation induced by cytotoxic CD8+ T lymphocytes and complete loss of hypocretinergic neurons within the hypothalamus [6].
Limbic encephalitis associated with LGI1 antibodies (previously termed potassium channel antibody-associated limbic encephalitis ) [7].
The antibodies specific for this syndrome are active against the leucine-rich glioma inactivated protein that is part of the voltage-gated potassium channel complex and is located in the surface of the neuron. This limbic encephalitis affects middle-aged or elderly individuals, mainly men, and it is not associated with gliomas. Indeed, it is usually not associated with malignancy, but in 10% of the cases, it is associated with thymomas. In this condition there is a primary insult in the mesial temporal lobe involving the hippocampus and the amygdala, with no apparent direct involvement of the brainstem. Patients present with subacute progressive cognitive impairment, confusion, disorientation in time and space, memory loss, hyponatremia, and seizures. Sleep disorders such as insomnia, excessive daytime sleepiness, and RBD have also been described in a few subjects with limbic encephalitis associated with LGI1 antibodies . The clinical syndrome is partially or totally reversible with immunotherapy. The association of RBD with this limbic encephalitis served to establish a pathophysiological link between this parasomnia and impairment of the limbic system, which could explain the intense emotions occurring in the RBD-related dreams (e.g., being attacked and chased). Other antibodies against the potassium channel complex bind the contactin-associated protein-2 (Caspr2), and the resulting neurological disorders consist of Morvan syndrome (described below), neuromyotonia (a form of peripheral nerve hyperexcitability that causes spontaneous muscular activation), and encephalitis. RBD has not yet been described in patients with Caspr2 antibodies. The identity of other potassium channel complex antibodies is unknown.
In one study of six patients with non-paraneoplastic limbic encephalitis associated with LGI1 antibodies, five had the typical clinical history of RBD in the context of encephalopathy, seizures, and excessive daytime sleepiness. V-PSG could be performed in three of these five patients and demonstrated RBD showing increased electromyographic activity in REM sleep linked to prominent limb jerking. In three patients, immunosuppression resulted in resolution of RBD in parallel with remission of the limbic syndrome and disappearance of mesial lobe hyperintensity. RBD persisted in two patients with partial resolution of the limbic syndrome [8].
In another study, 15 patients were identified with antibodies against voltage-gated potassium channels (specific LGI1 and Caspr2 antibodies were not analyzed in this study) and had limbic encephalitis (n = 5), Morvan syndrome (n = 4), and overlapping features (n = 6). Clinical history revealed that two patients had hypersomnia, ten had prominent insomnia, and eight had dream-enacting behaviors (four with Morvan syndrome, three with limbic encephalitis, and one with overlapping features). PSG in seven of the eight patients with dream-enacting behaviors showed normal REM sleep in one, absence of REM sleep in three, and REM sleep without atonia (but without abnormal behaviors in REM sleep) in three (one with Morvan syndrome, one with limbic encephalitis and one with overlapping clinical features) patients. NREM sleep parasomnias were not found in these patients [9].
A 62-year-old man with limbic encephalitis associated with LGI1 antibodies had RBD documented by V-PSG showing that REM sleep contained increased phasic electromyographic activity associated with multiple kicks and jerks [10].
Morvan syndrome (also known as Morvan’s fibrillary chorea and Morvan’s chorea ). Morvan syndrome is characterized by subacute onset of severe insomnia (usually not associated with hypersomnia), disrupted sleep-wake pattern, abnormal and nearly continuous motor activation, mental confusion, daytime visual hallucinations, dysautonomia (constipation, salivation, lacrimation, urinary incontinence, hyperhidrosis, increased body temperature, tachycardia, hypertension), fasciculations, myoclonic jerks, cramps, and neuromyotonia. It is associated with Caspr-2 antibodies in the context of myasthenia gravis, malignant thymoma, or small cell lung cancer. In most cases, the syndrome responds to immunotherapy, but some cases worsen and lead to death [11].
The sleep abnormalities that characterize Morvan syndrome are insomnia and an extreme expression of status dissociatus due to breakdown of the sleep-wake boundaries [12,13,14,15,16]. This state is termed agrypnia excitata which also is present in fatal familial insomnia and alcohol withdrawal syndrome (delirium tremens) [16]. It is thought that agrypnia excitata represents a thalamo-limbic system dysfunction, although brain imaging is normal in Morvan syndrome. In agrypnia excitata , patients present with severe insomnia, generalized sympathetic dysautonomia, and nocturnal motor overactivation where subjects perform simple or complex behaviors mimicking daily-life activities such as eating, setting up a device, dressing, buttoning the pajamas, or pointing at something on the wall. This nocturnal purposeful activity is labelled oneiric stupor and is nearly continuous during the entire night, with quiet pauses lasting several minutes. Episodes of oneiric stupor can occur with open or closed eyes. If questioned during one of these episodes, patients may respond that they are awake, although they seem to be behaving in the context of a dream or a hallucination. The oneiric stupor episodes can emerge from any stage (relaxed wakefulness, NREM sleep, and REM sleep). In agrypnia excitata the circadian rhythmicity is lost, and motor activity is increased throughout the 24 h without any circadian pattern. Electrophysiological recordings show an extreme disorganized pattern with reduced sleep time, brief fragments of alpha-theta activity without sleep elements (probably representing wakefulness), reduction or absence of K complexes and spindles, loss or small amounts of N3 stage, and brief intrusions or clusters of REM sleep bursts without muscle atonia. Rapid shifts across subwakefulness, NREM sleep features and REM sleep without atonia are typical during 24 h recordings [12,13,14,15,16].
Paraneoplastic cerebellar degeneration. Paraneoplastic cerebellar degeneration is characterized by rapid progressive pancerebellar syndrome (trunk and limb ataxia, dysarthria, nystagmus) due to loss of the Purkinje cells in the cerebellum. It is mostly associated with breast and ovarian cancers, small cell lung cancer, and Hodgkin’s disease. Brain neuroimaging is usually normal. Onconeuronal antibodies in paraneoplastic cerebellar degeneration are anti-Yo, anti-Ro, anti-Gad65, anti-Tr, anti-amphiphysin, anti-Hu, anti-CARP, and anti-GluR1. However, in a number of cases, antibodies are not found [1, 2].
There is a report of two patients with paraneoplastic cerebellar degeneration and RBD [17]. Two women of 43 and 66 years of age with breast cancer and paraneoplastic cerebellar degeneration (where no onconeuronal antibodies were found) presented with PSG-confirmed RBD. It is notable that the 43-year-old woman developed symptoms of RBD for more than 2 years before the rapid onset of cerebellar symptoms, with vivid dreams and dream enactment, and her sister observed restless sleep with kicking and screaming. Immunotherapy improved RBD symptomatology in both patients, but not the cerebellar syndrome. The association of a cerebellar syndrome with RBD suggests that the cerebellum may be implicated in the pathogenesis of RBD in at least some cases. In fact, other disorders where the cerebellum is damaged, such multiple system atrophy [18] and some spinocerebellar ataxias [19], are strongly linked to RBD. Furthermore, paraneoplastic cerebellar degeneration with RBD joins Wilson’s disease and the alpha-synucleinopathy neurodegenerative disorders as a neurologic disorder in which RBD in adults under the age of 50 years can precede the emergence of frank symptoms of the neurologic disorder by years, as discussed further in Chap. 15.
Anti-NMDA receptor encephalitis . Patients typically present with hallucinations, delusions, seizures, short-term memory loss, movement disorders, decreased level of consciousness, and central hypoventilation requiring mechanical support. It is usually associated with young women afflicted with ovarian teratomas. Brain magnetic resonance imaging is normal or shows abnormalities in the mesial temporal lobes, basal ganglia, and brain stem. Autopsies reveal extensive gliosis, rare T-cell infiltrates, and neuronal degeneration in the hippocampus and other regions including the brain stem. Patients can recover after tumor removal and immunotherapy [20, 21].
The most common sleep abnormality in anti-NMDA receptor encephalitis is insomnia during the acute phase, usually in combination with psychosis. It is not clear if RBD accompanies this paraneoplastic encephalitis. It has been speculated that the movement disorders seen in the anti-NMDA receptor encephalitis (complex bilateral antigravity stereotyped movements of the arms, with perioral and eye movements and less frequently involvement of the legs) represent status dissociatus, but this has never been proved by PSG [22]. A case report described a 58-year-old man with anti-NMDA receptor encephalitis who presented with a 4-month symptomatology somewhat resembling dementia with Lewy bodies, characterized by memory loss, aggressive behavior, visual hallucinations, and urinary incontinence. His wife reported acting out violent dreams, vocalizations, kicking, biting, and screaming during sleep occurring one or two times every night. PSG could not be performed to detect RBD or to exclude one of its mimics (e.g., confusional awakenings, severe obstructive sleep apnea) [23].
2 RBD in Autoimmune Disorders
Autoimmune disorders of the central nervous system are characterized by an abnormal immune-mediated response (humoral and/or cellular) against antigens expressed in the cells of the central nervous system. RBD has been described in the following autoimmune disorders: narcolepsy, multiple sclerosis, Guillain-Barré syndrome, and anti-IgLON5 disease.
Narcolepsy [24] (Chap. 11 covers the topic of narcolepsy-RBD). This disease, when linked to cataplexy and low cerebrospinal fluid levels of hypocretin, can often be associated with RBD. The severity of RBD in narcolepsy is less intense than RBD associated with the idiopathic form or secondary to a neurodegenerative disease. In very rare cases, RBD can be the first manifestation of narcolepsy, including in children. It is unknown why RBD occurs in narcolepsy, but the decreased input of hypocretinergic innervation from the hypothalamus to the limbic system and brain stem nuclei that regulate REM sleep muscle tone may play a crucial role.
Multiple sclerosis (MS) . This is a common disabling neurological disease characterized by an inflammatory autoimmune demyelinating process of central nervous system. It is often seen in young people with usual onset between the ages of 20 and 45 years, with clinical relapsing-remitting and chronic progressive forms. An impressive variety of symptoms and signs (motor, sensitive, visual, dysautonomic, etc.) affecting different regions of the brain and spinal cord are characteristic. Sleep disorders can also be seen in patients with MS, particularly insomnia. Narcolepsy-like cases occur when the demyelinating plaques damage the hypothalamus. RBD has been described in a few patients with MS, particularly when the demyelinating plaque is located in the pontine tegmentum, presumably impairing the nuclei and pathways that regulate REM sleep muscle atonia.
In a study comprising 135 consecutive MS patients, the individuals were interviewed for symptoms suggestive of RBD using a semi-structured questionnaire [25]. Four of the 135 patients reported symptoms suggestive of RBD that were later reevaluated by a sleep disorder specialist. V-PSG confirmed RBD in three of these four patients, giving an estimated frequency of RBD of 1.4% in MS. None of these three patients had previously consulted their doctors because of RBD-related symptoms. Two of the three RBD patients were taking antidepressants, and RBD onset coincided with the introduction of the antidepressant drug in one of these two cases. Because the antidepressant drug in this patient could not be withdrawn due to severe depression, the cause of RBD was presumably linked to the antidepressant intake, within the context of MS. In the second patient, RBD onset was not related to a previous relapse of MS, and a second V-PSG study confirmed the presence of RBD after withdrawing the antidepressant drug for 1 month. The third patient was the only patient in whom brain magnetic resonance imaging showed a demyelinating plaque in the dorsal pons. Treatment with clonazepam at bedtime completely resolved the RBD symptoms in this third patient.
RBD can rarely occur as the presenting symptom of MS. RBD was reported as the initial manifestation in a 25-year-old woman with a 6 month history of dream-enacting behaviors. She had sudden awakenings from fearful dreams with crying, screaming, kicking, falling out of bed, and running to the door or to the window, resulting in injuries. If awakened, she always recalled a fighting dream. RBD was confirmed by V-PSG, and brain imaging disclosed multiple cerebral periventricular and pontine demyelinating plaques consistent with a diagnosis of probable MS. RBD episodes disappeared after immunotherapy, thus reinforcing how the pathogenesis of RBD was tightly linked with MS [26].
In another case report, a 51-year-old woman with MS developed acute vertigo, ataxia, diplopia, dysarthria, and bifacial weakness. Her husband described how she exhibited nightly sleep-related groaning, screaming, limb jerking and flailing, and violent thrashing. She did not recall these events or any unpleasant dreams. Brain magnetic resonance imaging showed a large confluent area of increased T2 signal in the dorsal pons. V-PSG showed excessive tonic and phasic muscle activity in the chin, arms, and legs electromyographic leads during REM sleep which was associated with vocalizations, arms and legs jerking, and flailing. Clonazepam resulted in substantial improvement in the frequency and severity of RBD symptoms [27].
Guillain-Barré syndrome. This is an acute autoimmune demyelinating polyradiculoneuritis causing peripheral paralysis. Central nervous system impairment may occur in a few cases during the acute phase of the attack consisting of psychosis and mental confusion. In one study, 13 patients with Guillain-Barré syndrome admitted in the intensive care unit were studied with a portable PSG montage including mental electromyography (but not electromyographic leads in the limbs or video recording). Patients were evaluated during the acute attack, seven had abnormal mental status and six had normal mental status. PSG recordings showed disorganized sleep pattern in the patients with abnormal mental status characterized by disorganized sleep (frequent shifts between wakefulness, NREM sleep, and REM sleep), short REM sleep latency, and REM sleep without atonia. Dream-enacting behaviors were not described. REM sleep muscle tone normalized after immunotherapy against the Guillain-Barré attack [28]. Finally, it should be noted that in the original description of RBD involving five patients, one patient had RBD emerging with the Guillain-Barré syndrome [29].
Anti-IgLON5 disease . This is a novel neurological disease initially described in 2014 in eight unrelated individuals [30]. It is characterized by a heterogeneous clinical presentation: distinct sleep pattern that includes RBD; serum and cerebrospinal fluid autoantibodies against the neuronal protein IgLON5; a strong HLA association; absence of any coexistent autoimmune disorders, neoplasms, or neurodegenerative diseases; and a neuropathological pattern characterized by tau deposits in the brain stem and hypothalamus impairing some of the nuclei that regulate sleep [30,31,32,33,34,35,36,37,38,39,40,41,42,43].
Demographic and clinical findings. The anti-IgLON5 disease occurs in adults with a similar distribution in both genders. It has not been described in children. Mean age at diagnosis is around 65 years with an age range between 45 and 83 years. Cases described were born in several countries from Europe (Spain, Austria, Germany, Italy, France, UK), Azerbaijan, Brazil, China, Australia, the Philippines, and the USA [30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Clinical course at presentation is usually chronic (years) but the subacute form (months) is not rare. Median interval between symptom onset and diagnosis is 2.5 years (range, 2 months to 18 years) [39]. The onset of the symptomatology is insidious, and progression of the disease can be slow or fast. At referral, patients consult either to the general neurologist or to the sleep specialist, depending on the most predominant symptoms and severity. Reasons for referral are sleep symptoms, gait imbalance, dysphagia, and cognitive decline. At the initial visit, four distinct clinical phenotypes, according to symptom predominance, have been identified, but overlapping features among them are the rule. The four clinical presentations are the following: (1) a sleep disorder consisting of abnormal sleep behaviors, insomnia, excessive daytime sleepiness, sleep attacks, and sleep breathing symptoms, in any combination; (2) a bulbar syndrome that may include dysphagia, dysarthria, hypersalivation, and acute respiratory insufficiency that may require intubation and tracheotomy; (3) a progressive supranuclear palsy-like syndrome with abnormal gait, falls, and gaze palsy; and (4) a Huntington disease-like syndrome with cognitive impairment and choreic movements of the limbs and face. Other signs and symptoms are a variety of oculomotor abnormalities (nystagmus, vertical and horizontal gaze paresis, abnormal saccadic pursuit eye movements), movement disorders (orolingual, foot, and brachial dystonia, mild rigid-akinetic parkinsonism), dysautonomic features (anhidrosis, hypersalivation, diarrhea, constipation, weight gain or loss, urinary urgency, orthostatic hypotension, syncope, cardiac arrhythmias, bradycardia, takotsubo cardiomyopathy, episodic perspiration), neuropsychiatric symptoms (anxiety, depression, compulsions, confusion, disorientation, delirium, hallucinations), neck pain, frontal lobe seizures, and “stiff-person”-like syndrome with cramps and limb stiffness. In the anti-IgLON5 disease, the symptoms occur with different severity and appear in different combinations and time periods of sequence.
Sleep findings . At the initial visit, about two thirds of the reported patients complain of sleep symptoms, namely, continuous excessive daytime sleepiness, sleep attacks, insomnia affecting sleep onset and sleep maintenance, witnessed apneic events, stridor, and abnormal sleep behaviors. Our impression, however, is that most, if not all, patients suffer from sleep disorders. Sleep problems can be overlooked because other symptoms are prominent and patients and bed partners do not report sleep symptoms and also because doctors did not ask about them. Direct questioning often reveals sleep problems. Most of the patients are unaware of their abnormal sleep behaviors that are only noted and reported by the bed partners. They include prominent jerks, vocalizations such as talking, and purposeful behaviors such as manipulating imaginary objects. Stridor and breathing pauses are reported only by the bed partners. Some patients may complain that they have multiple nightly episodes of enuresis. Patients with the anti-IgLON5 disease have no previous history of disorders of arousal (sleepwalking, sleep terrors, confusional arousals), RBD, or sleep-related epilepsy.
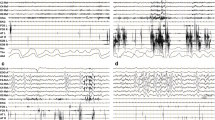
Sleep architecture shows a very complex and novel pattern that for its identification needs a full PSG with time-synchronized audiovisual recording and electromyographic leads in the four limbs (Fig. 8.1) [29]. This V-PSG pattern is characterized by (1) normal occipital alpha rhythm during wakefulness; (2) slight reduction of total sleep time and sleep efficiency; (3) a distinctive temporal sequence of sleep abnormalities, from being most abnormal at the beginning of the night to normalization at the end of the night; (4) initiation of sleep characterized by theta activity with rapid repetitive leg movements that do not fit criteria for periodic leg movements in sleep; (5) N1 sleep and N2 sleep that can be normal for some periods; (6) poorly structured stage N2 sleep characterized by spindles and K complexes with frequent vocalizations (e.g., talking, laughing, crying), simple motor activity (e.g., raising the arm, punching), and purposeful behaviors (e.g., goal-directed behaviors such as sucking the thumb while apparently eating, salting food, dabbing on perfume, manipulating wires, picking up objects, knitting); (7) periods of diffuse delta activity, typical of normal N3 sleep mixed with spindles; (8) normal N3 sleep that can be seen in the second half of the night; (9) RBD; (10) obstructive sleep apnea with an apnea-hypopnea index ranging from 15 to 80/h associated with oxyhemoglobin desaturations; and (11) inspiratory stridor. Longitudinal follow-up by V-PSG has shown no dramatic deterioration of these sleep features with time, the stability of which is another feature of this unique disorder. Obstructive sleep apnea and stridor respond both to continuous positive air pressure therapy and tracheotomy. However, these treatments do not also improve the abnormal sleep electroencephalographic pattern and motor behaviors. Central apneas are much less common than obstructive apneas.
Panel (a) represents a 30-s epoch of poorly structured non-REM stage 2 sleep (N2) with K complexes and spindles and increased phasic electromyographic activity in all four limbs and mentalis muscle associated with motor behaviors and vocalizations in a 53-year-old male patient with anti-IgLON5 disease. Panel (b) represents a 30-s epoch of REM sleep behavior disorder from the same anti-IgLON5 disease patient showing rapid eye movements, desynchronized electroencephalographic activity, and excessive phasic electromyographic activity in the mentalis muscle and the four limbs, particularly in the lower extremities. Abbreviations (from top to bottom): EOG electrooculogram, F frontal electroencephalographic lead, C central electroencephalographic lead, O occipital electroencephalographic lead, Ment electromyography of mentalis muscle, EKG electrocardiogram, FDS flexor digitorum superficialis muscle, L left, R right, AT anterior tibialis muscle, Nas nasal air flow, Tho thoracic respiratory movement, Abd abdominal respiratory movement
RBD can be detected in most of the patients. REM sleep is characterized by increased tonic and phasic electromyographic activity in the mentalis and excessive phasic electromyographic activity in the four limbs. The most frequent RBD manifestations are body and limb jerks. Aggressive behaviors such as punching and shouting in REM sleep occur in only a few instances. It has to be noted that the most complex sleep behaviors are seen in non-REM sleep (and not in REM sleep) and they are different from the classical NREM sleep parasomnias (sleepwalking, sleep terrors, confusional awakenings) since they are not abrupt and do not emerge from normal N3. Thus, anti-IgLON5 disease is not a typical overlap parasomnia, which is the topic covered in Chap. 27. However, it does comprise a unique set of combined RBD-atypical NREM sleep motor-behavioral parasomnias affecting most patients with anti-IgLON5 disease.
The sleep pattern seen in the anti-IgLON5 disease cannot be considered status dissociatus because (1) wakefulness can be distinguished from sleep clinically and by PSG; (2) K complexes, spindles, and delta waves are present in NREM sleep; and (3) NREM sleep can be distinguished from REM sleep. Anti-IgLON5 disease cannot be considered a form of agrypnia excitata because (1) there is no loss of sleep (total sleep time is mildly reduced but discernible); (2) K complexes, spindles, and delta waves and REM sleep are always present and do not decrease with the progression of the disease; (3) episodes of REM sleep are not short; (4) circadian sleep-wake pattern is normal; (5) dysautonomia is not prominent; and (6) neuropathology shows that the thalamus is not damaged.
Immunological findings . The antigen Iglon5 is a normal cell adhesion protein located on the surface of the neurons. Its function is unknown. In the anti-IgLON5 disease , autoantibodies against IgLON5 are always found in the serum and very frequently in the cerebrospinal fluid. They represent the immune hallmark of the disease. IgG4 subclass antibodies predominate over IgG1 [39]. These IgLON5 antibodies are not found in idiopathic RBD, neurological autoimmune disorders (e.g., multiple sclerosis), and neurodegenerative diseases (e.g., multiple system atrophy, Parkinson disease) [30]. Antibodies against IgLON5 have been found in one patient who fulfilled clinical diagnostic criteria for progressive supranuclear palsy but with an unusual clinical course of more than 20 years [30]. Thus, it may represent another case of anti-IgLON5 disease. Onconeuronal antibodies (e.g., LGI1, Caspr2, Ma2, Hu, amphiphysin, NMDA, AMPA, mGLuR1, mGluR5, DPX, GABAB) are absent in patients with the anti-IgLON5 disease.
The haplotypes DRB1*1001 and DQB1*0501 are detected in almost 90% of the patients tested. They are uncommon in the general population. DRB1*1001 is 36 times more frequent in patients with anti-IgLON5 disease than in the general population. The DQB1*0501 is 3.5 times more frequent in the anti-IgLON5 disease than in the general population [39].
Ancillary tests . Cerebrospinal fluid can be either normal or show mild pleocytosis and increased protein concentration. Hypocretin levels in the cerebrospinal spinal fluid are normal and oligoclonal bands are absent. Electroencephalography during wakefulness and brain magnetic resonance imaging, diffusion tensor imaging, and dopamine transporter imaging SPECT are unremarkable. Cerebral 18-FDG PET is normal or shows hypermetabolism in the basal ganglia, cortex, and cerebellum [35, 36]. Electromyography may be normal or shows multiple mononeuritis and peripheral neuropathy [40]. Neuropsychological tests may show impairment of executive function, visuospatial function, and episodic memory [32, 36]. In patients with stridor during sleep, laryngoscopy during wakefulness may be normal or shows unilateral or bilateral vocal cord abductor paresis or paralysis [30, 31].
Clinical course and therapy . The introduction of anticholinergics such as clomipramine may dramatically worsen the symptomatology. Vocal cord palsy and central hypoventilation are the causes of respiratory failure, a situation that requires intensive care support and tracheotomy. The prognosis of the disease seems to be poor in many cases. Immunotherapy (cycles of intravenous steroids, intravenous immunoglobulins, pulses of cyclophosphamide, rituximab, and plasmapheresis) is usually not helpful [30, 34, 43]. Some cases, however, have been described to improve partially after immunotherapy [36, 37, 41, 43]. Most of the patients die suddenly from wakefulness or from sleep and from aspiration pneumonia.
Neuropathology . The initial description of the disease included the postmortem examination of two patients [30]. Neuropathology showed the absence of inflammatory infiltrates and the presence of neuronal loss, moderate gliosis, and extensive deposits of abnormal hyperphosphorylated tau (with the presence of three-repeat and four-repeat tau isoforms) mainly involving the neurons of the tegmentum of the brain stem and the hypothalamus. The glia are spared. Deposits of beta-amyloid and alpha-synuclein are not seen. The nuclei damaged in the brain stem are the laterodorsal tegmental area and periaqueductal gray matter (which may explain the abnormal sleep pattern), pedunculopontine nucleus (that may cause disequilibrium with gait abnormalities and falls), and nucleus ambiguous (producing vocal cord palsy leading to stridor). The subcoeruleus nucleus is preserved. Damage of the magnocellularis nucleus in the medulla may explain the occurrence of RBD in view of the preservation of the subcoeruleus region. Other structures affected are the hippocampus, hypothalamus, and amygdala. The cortex, thalamus, substantia nigra, basal ganglia, and cerebellum are preserved or mildly affected. The anatomical distribution of this taupathy in the brain is different from the primary taupathies (e.g., Alzheimer’s disease, progressive supranuclear palsy, corticobasal syndrome).
Neuropathological criteria have been established for this new entity after the postmortem study of four additional cases [38]. Definite diagnosis of the anti-IgLON5 disease requires detection of serum or cerebrospinal fluid IgLON5 antibodies plus neuronal loss, gliosis, and tau deposits in the neurons. Probable diagnosis is defined when the antibody status is unknown, but there is a compatible clinical picture, HLA DRB1*1001 and DQB1*0501, and positive neuropathology. Possible diagnosis is considered in cases with compatible neuropathology but without information of the clinical features and immunological status (antibodies and HLA genotype) [38]. An additional postmortem study of a single case showed brain stem and hypothalamus tau deposits in addition to microglial and neuronal TDP-43 pathology in regions without tau involvement (e.g., thalamus and basal ganglia) [40].
In the anti-IgLON5 disease, there is no evidence of malignancy. It is still unclear if we are facing a neurodegenerative and/or an autoimmune disease. On the one hand, some features suggest that the disease has an autoimmune origin (e.g., antibodies against a neuronal surface antigen, the fact that other antibodies against other members of IgLON protein family are involved in autoimmune diseases such as multiple sclerosis, and the strong HLA association). Alternatively, other findings suggest a neurodegenerative basis (e.g., no marked clinical improvement with immunosuppressive therapy, a chronic and progressive clinical course, and evidence of neuronal loss, tau deposits, and absence of inflammatory infiltrates). The anti-IgLON5 disease suggests an intriguing link between autoantibodies and abnormal deposits of tau in the brain. An experimental study with rat hippocampus showed that IgLON5 antibodies recognized the antigen on the neuron surface. Antibodies produce the internalization of the antigen, suggesting a pathogenic role of the antibodies [44]. It has been speculated that the antibodies interfere the interaction of IgLON5 with the internal cytoskeletal network, leading to abnormal tau accumulation and ultimately neuronal loss [39]. Further studies are needed to clarify the origin and pathogenesis of the disease.
References
Höftenberfg R, Rosenfeld M, Dalmau J. Update on neurological paraneoplastic syndromes. Curr Opin Oncol. 2015;27:489–95.
Graus F, Dalmau J. Paraneoplastic neurological syndromes: diagnosis and treatment. Curr Opin Neurol. 2007;20:732–7.
Silber MH. Autoimmune sleep disorders. Handb Clin Neurol. 2016;133:317–26.
Compta Y, Iranzo A, Santamaria J, Casamitjana R, Graus F. REM sleep behavior disorder and narcolpetic features in anti-Ma2 encephalitis. Sleep. 2007;30:767–9.
Adams C, McKeon A, Silber MH, Kumar R. Narcolepsy, REM sleep behavior disorder, and supranuclear gaze palsy associated with Ma1 and Ma2 antibodies and tonsillar carcinoma. Arch Neurol. 2011;68:521–4.
Dauvilliers Y, Bauer J, Rigau V. Hypothalamic immunopathologyin anti-Ma-associated diencephalitis with narcolepsy-cataplexy. Hypothalamic immunopathology in anti-Ma-associated diencephalitis with narcolepsy-cataplexy. JAMA Neurol. 2013;70:1305–10.
Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133:2734–274.
Iranzo A, Graus F, Clover L, et al. Rapid eye movement sleep behavior disorder and potassium channel antibody–associated limbic encephalitis. Ann Neurol. 2006;59:178–82.
Cornellius JR, Pittock SJ, McKeom A, et al. Sleep manifestations of voltage-gated potassium channel complex autoimmunity. Arch Neurol. 2011;68:733–8.
Tezer I, Erdener E, Sel CC, Mendikanova L, Sagy S, Topcuoglu M. Daytime polysomnography recording in LIG1-related limbic encephalitis. Arch Neurol. 2012;69:145–6.
Leypoldt F, Armangue T, Dalmau J. Autoimmune encephalopathies. Ann N Y Acad Sci. 2015;1338:94–114.
Lugaresi E, Provini F. Agrypnia excitata: clinical features and pathophysiological implications. Sleep Med Rev. 2001;5:313–22.
Liguori R, Vincent A, Clover L, Avoni P, Plazzi G, Cortelli P, et al. Morvan’s syndrome: peripheral and central nervous system and cardiac involvement with antibodies to voltage-gated potassium channels. Brain. 2001;124:2417–26.
Guaraldi P, Calandra-Buonaura G, Terlizzi R, et al. Oneiric stupor: the peculiar behaviour of agrypnia excitata. Sleep Med. 2011;12:S64–7.
Provini P, Marconi M, Amadori M, et al. Morvan chorea and agrypnia excitata: when video-polysomnographic recording guides the diagnosis. Sleep Med. 2011;12:1041–3.
Antelmi E, Ferri R, Iranzo A, et al. From state dissociation to status dissociatus. Sleep Med Rev. 2016;28:1–13.
Cardoso Vale T, Bizari Fernanes do Prado L, Fernnades Do Prado G, Grazian Povoas Barsittini O, Pedroso JL. Rapid eye movement sleep behavior disorder in paraneoplastic cerebellar degeneration: improvement with immunotherapy. Sleep. 2016;39:117–20.
Iranzo A, Santamaria J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005;65:247–52.
Iranzo A, Muñoz E, Santamaría J, Vilaseca I, Milà M, Tolosa E. REM sleep behavior disorder and vocal cord paralysis in Machado-Joseph disease. Mov Disord. 2003;18:1179–83.
Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfield MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74.
Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–8.
Stamelou M, Plazzi G, Lugaresi E, Edwards MJ, Bathia KP. The distinct movement disorder in anti-NMDA receptor encephalitis may be related to status dissociates: a hypothesis. Mov Disord. 2012;27:1360–3.
Coban A, Kücükali CI, Yalcinkaya N, et al. Evaluation of incidence and clinical features of antibody-associated autoimmune encephalitis mimicking dementia. Behav Neurol. 2014;2014:935379.
Schenck CH, Mahowald MW. Motor dyscontrol in narcolepsy: rapid-eye-movement (REM) sleep without atonia and REM sleep behavior disorder. Ann Neurol. 1992;32:3–10.
Gomez-Choco M, Iranzo A, Blanco Y, Graus F, Santamaria J, Saiz A. Prevalence of restless legs syndrome and REM sleep behavior disorder in multiple sclerosis. Mult Scler. 2007;13:805–8.
Plazzi G, Montagna P. Remitting REM sleep behaviour disorder as the initial sign of multiple sclerosis. Sleep Med. 2002;3:437–9.
Tippmann-Peikert M, Boeve BF, Keegan BM. REM sleep behavior disorder initiated by acute brainstem multiple sclerosis. Neurology. 2006;66:1277–9.
Cochen V, Arnulf I, Demeret S, et al. Vivid dreams, hallucinations, psychosis and REM sleep in Guillain-Barré syndrome. Brain. 2005;128:2535–45.
Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308.
Sabater L, Gaig C, Gelpi E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13:575–86.
Högl B, Heidbreder A, Santamaria J, Graus F, Poewe W. IgLON5 autoimmunity and abnormal behaviours during sleep. Lancet. 2015;385:1590.
Simabukuro MM, Sabater L, Adoni T, et al. Sleep disorder, chorea, and dementia associated with IgLON5 antibodies. Neurol Neuroimmunol Neuroinflamm. 2015;e136:2.
Montojo MT, Piren V, Benkhadra F, et al. Mimicking progressive supranuclear palsy and causing Tako-Tsubo syndrome: a case report on IgLON5 encephalopathy [abstract]. Mov Disord. 2015;30(Suppl 1):710.
Brüggemann N, Wandinger KP, Gaig C, et al. Dystonia, lower limb stiffness, and upward gaze palsy in a patient with IgLON5 antibodies. Mov Disord. 2016;31:762–4.
Schröder JB, Melzer N, Ruck T, et al. Isolated dysphagia as initial sign of anti-IgLON5 syndrome. Neurol Neuroimmunol Neuroinflamm. 2016 Nov 22;4(1):e302.
Haitao R, Yingmai Y, Yan H, et al. Chorea and parkinsonism associated with autoantibodies to IgLON5 and responsive to immunotherapy. J Neuroimmunol. 2016;300:9–10.
Zhang W, Niu N, Cui R. Serial 18F-FDG PET/CT findings in a patient with IgLON5 encephalopathy. Clin Nucl Med. 2016;41:787–8.
Gelpi E, Höftberger R, Graus F, et al. Neuropathological criteria of anti-IgLON5-related tauopathy. Acta Neuropathol. 2016;132:531–43.
Gaig C, Graus F, Compta Y, et al. Clinical manifestations of the anti-IgLON5 disease. Neurology. 2017;88:1736–43.
Cagnin A, Mariotto S, Fiorini M, et al. Microglial and neuronal TDP-43 pathology in anti-IgLON5-related taupathy. J Clin Alzheimer’s Dis. 2017;59:13–20.
Honorat JA, Lomorowski L, Josephs KA, et al. IgLON5 antibody. Neurological accompaniments and outcomes in 20 patients. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e385. https://doi.org/10.1212/NXI.0000000000000385.
Bahtz R, Teegen B, Borowski K, et al. Autonatibodies against IgLON5: two new cases. J Neuroimmunol. 2014;275:8.
Bonello M, Jacob A, Ellul MA, et al. IgLON5 disease responsive to immunotherapy. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e383. https://doi.org/10.1212/NXI.0000000000000383.
Sabater L, Planagumà J, Dalmau J, Graus F. Cellular investigations with human antibodies associated with the anti-IgLON5 syndrome. J Neuroinflammation. 2016 Sep 1;13(1):226. https://doi.org/10.1186/s12974-016-0689-1.
Acknowledgment
To Dr. Carles Gaig for reviewing the anti-IgLON5 disease section of this chapter and providing the figure.
Note Added in Proof: RBD has also been described in the setting of subjects with systemic autoimmune conditions such as Behcet’s disease, Sjoegren’s syndrome and rheumatoid arthritis. However, these patients also presented the cardinal symptomatology of the synucleinopathies, namely parkinsonism and cognitive impairment. (1) Fulong X, Jun Z, Waner W, Xuehua W, Wei Z, Liyue X, Fang H. A case report of REM sleep behavior disorder, Bechet’s disease, Sjoegren’s syndrome and cognitive dysfunction. BMC Rheumatology 2018 (in press). (2) Cosentino FII, Distefano A, Plazzi G, Schenck CH, Ferri R. A case of REM sleep behavior disorder, narcolepsy-cataplexy, parkinsonism and rheumatoid arthritis. Behavioral Neurology; 2014; 2014:572931. doi:10.1155/2014/572931.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Iranzo, A. (2019). RBD Associated with Paraneoplastic Neurological Syndromes and Autoimmune Disorders. In: Schenck, C., Högl, B., Videnovic, A. (eds) Rapid-Eye-Movement Sleep Behavior Disorder. Springer, Cham. https://doi.org/10.1007/978-3-319-90152-7_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-90152-7_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90151-0
Online ISBN: 978-3-319-90152-7
eBook Packages: MedicineMedicine (R0)