Abstract
Cytomegalovirus (CMV) is the leading cause of congenital infection in humans and constitutes a major public health problem. Congenitally infected infants, both symptomatic and asymptomatic at birth, are at risk for sequelae including sensorineural hearing loss. The virus can be transmitted to the fetus following either a primary or a non-primary maternal infection during pregnancy. Even though the transmission rate is much higher in primary infected mothers than in mothers with preconceptional immunity, routine CMV screening of pregnant women is not recommended today because no consensus exists on prenatal treatment options. Intravenous ganciclovir or oral valganciclovir are used to treat neonates with symptoms at birth. Valganciclovir treatment for 6 months is recommended for congenitally infected neonates with moderately to severely symptomatic disease. All infants with congenital CMV infection, both symptomatic and asymptomatic at birth, need a follow-up evaluation to detect sequelae promptly. For several years, a universal screening for congenital CMV infection has been suggested by many authors for early detection of sequelae and timely intervention. A real-time PCR assay of saliva specimens seems to offer the best characteristics for use in screening.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Epidemiology
Adult infection . Human cytomegalovirus (CMV) is an ubiquitous double-stranded DNA herpesvirus [1]. CMV is found only in humans; primary infection is followed by lifelong persistence of the virus in a latent phase and periodic reactivations. Reinfection with other strains of CMV may occur and can cause congenital infection in seropositive women [2]. The virus spreads from person to person via contact with infected bodily fluids, most notably saliva. CMV infection is very common; seropositivity ranges from 50 to 70% in the United States and Western Europe and is >90% in developing countries [3,4,5]. Low socioeconomic status, non-white race, sexual activity, and child care (either personally or professionally) are associated with CMV infection. This is because young children who acquire CMV infection in the first few years of life shed the virus in urine and saliva for an average of 18 months [6]. The annual rate of infection in seronegative individuals is approximately 1–2%, but seronegative women caring for young children acquire CMV at rates 10–25 times higher [7].
Congenital infection . Congenital CMV infection is the most common congenital infection by an order of magnitude [8]. Congenital CMV constitutes a major public health problem because of its frequency and its role as a cause of sensorineural hearing loss (SNHL) and central nervous system damage in children. In the United States, large prospective cohort studies suggest that approximately 0.5–0.7% (1 in 140–200) infants have congenital cytomegalovirus, which equates to approximately 25,000 infected infants annually [9]. Of these, more than 3500 will develop SNHL at some point during childhood. In developing countries, the incidence of congenital CMV is higher; rates as high as 5% have been reported in sub-Saharan Africa [10]. This may be due in part to concomitant infection with HIV; risk for congenital CMV infection is increased sixfold in infants who are also HIV-exposed. Immunosuppression is associated with increased CMV shedding in the genital tract and with a higher rate of CMV reactivation.
Postnatal infection . Postnatal infection is most commonly acquired by ingestion of CMV-positive breast milk. Blood transfusions are a theoretical route of infection, but CMV-safe transfusion practices have virtually eliminated infection of infants via blood transfusion [11]. The rate of infection among newborns fed CMV-positive breast milk varies from 15 to 70%.
Pathogenesis
CMV can be transmitted to the fetus following (1) a primary maternal infection during pregnancy, (2) reactivation of latent virus, or (3) reinfection with a new strain.
Primary infection . Primary CMV infection is reported in 1–4% of seronegative women during pregnancy, and the risk of viral transmission to the fetus is much higher in primary infected mothers than in mothers with preconceptional immunity (30–35% versus 1–2%) [7, 12].
Reactivation or reinfection . Preconceptional immunity to CMV provides substantial protection against intrauterine transmission, newborn disease, and sequelae. The relatively benign course in the infants of mothers with recurrent infection is presumably due to the modulating effect of preexisting maternal antibody. However, this protection is incomplete as intrauterine transmission and symptomatic congenital infections do occur in infants born to women who were seropositive before pregnancy. Therefore, considering the high seroprevalence in adults, congenital CMV infection occurs as a result of non-primary maternal infection in approximately two-thirds of infected infants [2].
The risk of congenital CMV infection increases with increasing gestational age, from 30 to 40% in the first trimester to 70–90% at the end of pregnancy. However, the risk of fetal damage is much higher when the fetus is infected in the early stages of pregnancy (see chapter “Pathogenesis of congenital infections”).
Clinical Findings
Older children and adults . Manifestations of primary infection in adults and children vary with the age and the immunocompetence of the host. Asymptomatic infections are the most common, particularly in young children. In contrast, nonspecific “influenza-like” illness, prolonged fever, fatigue, and malaise mimicking infectious mononucleosis or even overt hepatitis can occur in older children and adults. Immunocompromised hosts are at risk for end-organ dysfunction including retinitis and pneumonia [1].
Symptomatic congenital infection . Approximately 10–15% of congenitally infected infants have symptoms at birth. The clinical picture varies widely, ranging from mild findings to a severe disease with multiple organ system involvement, especially reticuloendothelial system and central nervous system (Table 1) [13]. The most common findings are petechiae, jaundice, hepatomegaly, splenomegaly, intrauterine growth restriction, and neurological signs or symptoms such as microcephaly, seizures, hypotonia, and lethargy [14]. Laboratory findings include thrombocytopenia, conjugated hyperbilirubinemia, high level of transaminases, and elevated cerebrospinal fluid protein level. Ocular and auditory damage, especially chorioretinitis and SNHL, may be present at birth. Brain abnormalities include congenital malformations (e.g., ventriculomegaly, polymicrogyria, cerebellar hypoplasia) and destructive lesions (e.g., impaired myelination, calcifications, frontal/temporal/germinolytic cysts), depending on the timing of infection.
It should be highlighted that the diagnostic criteria of symptomatic infection vary widely in the literature. For example, some authors have considered neonates with isolated intrauterine growth restriction as symptomatic, whereas other authors have not. In addition, neonates with isolated SNHL are not classified as symptomatic by all authors. In 2017, a consensus recommendation from a panel of experts suggested definitions of congenital CMV infection and disease previously published, with minor adjustments (Table 2) [15]. The mortality rate in symptomatic infants is about 5–10%; approximately 50% of survivors develop sequelae, including SNHL, visual deficits, and cognitive and motor deficits [13,14,15].
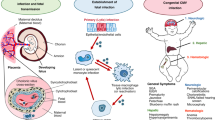
Asymptomatic congenital infection . The remaining 85–90% of infants with congenital CMV are asymptomatic at birth. The risk for long-term sequelae is reduced, but not eliminated, with asymptomatic infection. Approximately 10–15% will develop sequelae, particularly SNHL and learning disorders [16]. Notably, some infants initially classified as “asymptomatic” actually have signs of infection once a thorough evaluation is performed; the distinction has important prognostic implications. Although individual risk is greater for symptomatic infants, asymptomatic infants account for the majority of CMV-associated SNHL (Fig. 1).
Although case-by-case risk for sensorineural hearing loss (SNHL) is higher for infants with symptomatic congenital cytomegalovirus (CMV) infection, the preponderance of CMV-associated SNHL annually is actually due to asymptomatic infants due to their greater numbers. As a result, there is significant interest in research toward a universal screening program for congenital CMV infection, which would allow earlier identification, targeted screening, and prompt audiological correction of CMV-associated SNHL
Sensorineural hearing loss . SNHL is the most common sequela of congenital CMV infection, affecting about 50% of symptomatic and 10% of asymptomatic infants [17]. Congenital CMV infection is the most common cause of nongenetic hearing loss worldwide [18]. SNHL is bilateral in most cases and can vary from mild loss to profound impairment. SNHL may be present in the newborn period or appear as late as adolescence; the median age at onset is 33 months for symptomatic and 44 months for asymptomatic infants. Approximately 50% of congenitally infected infants with SNHL will have further deterioration of their hearing. The rate of progression seems to be similar regardless of whether the infant had a symptomatic or an asymptomatic infection. CMV-related hearing loss can also fluctuate from mild to profound over time, but the overall pattern is one of deterioration [19].
Postnatal infection . In extremely premature infants, postnatal CMV infection may be asymptomatic or can present with nonspecific sepsis-like illness or with focal disease (e.g., hepatitis, pneumonitis) [11]. However, in contrast to congenital CMV infection, there is no compelling evidence that risk for long-term neurologic sequelae or SNHL is increased following postnatal CMV infection [20].
Diagnosis
Diagnosis of CMV infection can be based on serology or by detection of CMV in bodily fluids by PCR or culture. Serology is most often used for screening purposes, whereas PCR or culture is favored for confirmation of congenital CMV in neonates [15].
Diagnosis of Maternal Infection During Pregnancy
CMV screening during pregnancy remains a topic of intense interest and research, but still no consensus has been reached [21]. Therefore, universal CMV screening of pregnant women is not currently recommended as part of routine antenatal screening in any country. However, some providers view routine CMV screening at the beginning of pregnancy as useful as it can identify seronegative women that may benefit from education and preventive measures. “Serial” screening for primary infection during pregnancy in seronegative women is even more controversial, since there is no evidence-based prenatal treatment option (see Treatment, below).
Maternal primary infection is defined as detection of IgG antibody to CMV in a previously seronegative woman (seroconversion). Unfortunately, seroconversion during pregnancy is not easily documented, since maternal immunity status just before conception is often unknown. In addition, IgM antibodies to CMV may persist for months after primary infection and can reappear during reactivation or reinfection. When the exact timing of seroconversion is unknown, avidity testing can be useful [22]. The avidity test is a measure of the binding capacity of CMV-IgG antibodies. Low to moderate avidity is observed for 16–18 weeks following a primary infection. Therefore, a low-moderate IgG avidity in combination with detection of specific IgM antibodies supports a diagnosis of primary CMV infection within the preceding 3 months. There are currently no tests for diagnosing a recurrent infection during pregnancy.
If screening is not performed, serologic specific tests (IgG, IgM, IgG avidity) should be done when a pregnant woman has flu-like symptoms not attributable to another specific infection or if signs suggestive of fetal CMV infection are detected by ultrasound or MRI (e.g., cerebral ventriculomegaly, intracranial calcifications, cerebral cysts, microcephaly, fetal growth restriction, echogenic fetal bowel).
Diagnosis of Fetal Infection
Fetal infection can be confirmed by detection of virus or viral DNA from amniotic fluid. Although viral culture is 100% specific, it can be falsely negative; CMV DNA PCR is both sensitive and specific, especially when the amniotic fluid is sampled after 21 weeks gestation and at least 6 weeks after the onset of infection in the mother [23]. Cordocentesis may be performed to ascertain the presence of virus, viral DNA, and anti-CMV IgM in fetal blood. However, amniotic fluid sampling is usually sufficient, so the benefit of deriving additional information from cordocentesis must be weighed against the risk of cord injury or pregnancy loss. Targeted ultrasonography is used to identify fetal abnormalities compatible with CMV disease; fetal MRI can reveal dysgenesis or injury to the central nervous system.
Diagnosis of Neonatal Infection
Congenital CMV infection should be suspected in neonates with any of the signs described in Tables 1 and 2 or those who had fetal ultrasound findings consistent with CMV infection. Asymptomatic infants often go undetected, but testing should be considered in certain situations:
-
1.
Infants who refer their newborn hearing screen [24]
-
2.
Infants born to HIV-positive mothers [25]
-
3.
Infants with intrauterine growth restriction
Detection of CMV in urine or saliva by either real-time PCR or viral culture is the gold standard for diagnosis, with sensitivity and specificity both approaching 100% [9]. Testing should be performed within the first 3 weeks of life in order to distinguish congenital infection from postnatal infection [15]. Since most CMV-seropositive women shed CMV in breast milk, saliva samples should be obtained >1 h after feeding, and positive saliva tests in breastfeeding infants should be confirmed with a urine sample. The utility of blood or cerebrospinal fluid testing is uncertain as there is no clear association between viral load and prognosis.
For infants with confirmed congenital CMV infection, additional testing includes laboratory testing (complete cell blood count, platelet count, liver function tests), ophthalmological evaluation, and audiological evaluation by using auditory brain-stem response [15]. Neuroimaging is also recommended: cerebral ultrasound is very reliable in detecting findings associated with congenital CMV infection such as ventriculomegaly, calcifications, periventricular pseudocysts, and lenticulostriate vasculopathy; MRI is better suited to identify cortical, white matter, and cerebellar dysplasia. CT is useful to detect ventriculomegaly and calcifications but is rarely performed today due the excessive radiation exposure.
Treatment
Maternal infection . There is no consensus currently regarding treatment of primary CMV infection in pregnant women. In 2005, a nonrandomized study by Nigro et al. [26] suggested that treatment of pregnant women who seroconvert in pregnancy with intravenous CMV-specific immune globulin may be effective in the prevention and treatment of congenital CMV infection. However, a randomized, placebo-controlled, double-blind study in 2014 did not demonstrate a significant reduction in congenital infection with immune globulin therapy (30% in the immune globulin group, 44% in the placebo group, P = 0.13) [27]. Currently, two randomized, placebo-controlled trials are ongoing in the United States and in Europe to confirm this result. In the interim, experts recommend that hyperimmune globulin should not be administered to pregnant women who seroconvert during pregnancy.
Congenital infection . Infants with confirmed symptomatic congenital CMV infection should be treated with 6 months of antiviral therapy [28]. Options include oral valganciclovir or intravenous ganciclovir for infants who cannot be fed enterally [28, 29]. At present, there is no recommendation to treat asymptomatic or mildly symptomatic infants (Table 2), as this group has not been extensively studied. However, some providers prefer treatment for neonates with an abnormal hearing assessment, regardless of the presence or absence of other signs or symptoms [30]. Ultimately, treatment of congenital CMV is a prolonged process that requires significant caregiver effort, laboratory monitoring, and follow-up, and therefore decisions regarding treatment in borderline cases should be made in consultation with the family and a pediatric infectious disease specialist.
All infants with congenital CMV infection, whether symptomatic or asymptomatic at birth, require close, multidisciplinary follow-up. This includes the primary care physician, audiology, ophthalmology, and developmental surveillance at a minimum. Since hearing loss can present later in life, hearing screening is recommended every 6 months until the child is in school, then annually. Ophthalmologic examinations should occur annually. Close attention to speech delay, impaired socialization, motor delays, or other signs of abnormal neurodevelopment should be investigated promptly.
Postnatal CMV infection . Data on treatment of postnatal CMV infection is limited to case reports. Expert opinion varies, but some providers would recommend a short course (e.g., 2–3 weeks) of antiviral therapy for preterm infants with pneumonitis, hepatitis, or sepsis-like syndrome secondary to postnatal CMV [31]. In reality, it is often difficult to differentiate postnatal from congenital CMV infection if the infant does not have a negative test during the first 3 weeks of life.
Prevention
Congenital CMV prevention . Given the burden of congenital CMV infection following primary maternal infection, the development of an effective CMV vaccine likely would markedly reduce the burden of congenital CMV infection. Unfortunately, despite continued advances in CMV vaccine research, no product is yet under consideration for licensure [32]. Therefore, the most effective current prevention strategy is education of pregnant women (Table 3). This includes education regarding CMV awareness, modes of transmission, and preventive measures (e.g., handwashing after contact with urine or saliva from young children). Unfortunately, despite being by far the most common congenital infection, knowledge of congenital CMV among women of childbearing age is extremely low. A survey by Jeon et al. [33] demonstrated that few women of childbearing age (22%) had heard of congenital CMV, and even fewer were aware of prevention strategies. CMV education is viewed favorably by pregnant women and has been shown to reduce the risk for primary infection during pregnancy [34].
Postnatal CMV prevention . Preterm infants requiring transfusion should receive CMV-safe packed red blood cells. CMV-safe techniques, including irradiation and leukoreduction, minimize the risk of transfusion-associated postnatal CMV. Currently, there is no technique that eliminates CMV from breast milk without also interfering with its immunologic and nutritional value [35]. Since the short- and long-term risks of postnatal CMV are thought to be minimal, there is no recommendation to withhold fresh human milk from preterm infants [36].
References
Plosa EJ, Esbenshade JC, Fuller MP, Weitkamp JH. Cytomegalovirus infection. Pediatr Rev. 2012;33:156–63.
Ross SA, Arora N, Novak Z, Fowler KB, Britt WJ, Boppana SB. Cytomegalovirus reinfections in healthy seroimmune women. J Infect Dis. 2010;201:386–9.
Lanzieri TM, Kruszon-Moran D, Gambhir M, Bialek SR. Influence of parity and sexual history on cytomegalovirus seroprevalence among women aged 20-49 years in the USA. Int J Gynaecol Obstet. 2016;135:82–5.
Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50:1439–47.
Lanzieri TM, Dollard SC, Bialek SR, Grosse SD. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int J Infect Dis. 2014;22:44–8.
Cannon MJ, Stowell JD, Clark R, et al. Repeated measures study of weekly and daily cytomegalovirus shedding patterns in saliva and urine of healthy cytomegalovirus-seropositive children. BMC Infect Dis. 2014;14:569.
Hyde TB, Schmid DS, Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol. 2010;20:311–26.
Cantey JB, Sanchez PJ. Overview of congenital infections: the prominence of cytomegalovirus. Infect Disord Drug Targets. 2011;11:426–31.
Boppana SB, Ross SA, Shimamura M, et al. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N Engl J Med. 2011;364:2111–8.
Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26:86–102.
Josephson CD, Caliendo AM, Easley KA, et al. Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: a prospective cohort study. JAMA Pediatr. 2014;168:1054–62.
Kagan KO, Hamprecht K. Cytomegalovirus infection in pregnancy. Arch Gynecol Obstet. 2017;296:15–26.
Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57:S178–81.
Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93–9.
Rawlinson WD, Boppana SB, Fowler KB, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis. 2017;17:177–88.
Fowler KB, McCollister FP, Dahle AJ, et al. Progressive and fluctuating sensorineural hearing loss in infants with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130:624–30.
Goderis J, De Leenheer E, Smets K, Van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics. 2014;134:972–82.
Kenna MA. Acquired hearing loss in children. Otolaryngol Clin N Am. 2015;48:933–53.
Fowler KB. Congenital cytomegalovirus infection: audiologic outcome. Clin Infect Dis. 2013;57:S182–4.
Gunkel J, de Vries LS, Johngmans M, et al. Outcome of preterm infants with postnatal cytomegalovirus infection. Pediatrics. 2018;141:e20170635.
Mosca F, Pugni L. Cytomegalovirus infection: the state of the art. J Chemother. 2007;19:46–8.
Kaneko M, Ohhashi M, Minematsu T, Muraoka J, Kusumoto K, Sameshima H. Maternal immunoglobulin G avidity as a diagnostic tool to identify pregnant women at risk of congenital cytomegalovirus infection. J Infect Chemother. 2017;23:173–6.
Goegebuer T, Van Meensel B, Beuselinck K, et al. Clinical predictive value of real-time PCR quantification of human cytomegalovirus DNA in amniotic fluid samples. J Clin Microbiol. 2009;47:660–5.
Stehel EK, Shoup AG, Owen KE, et al. Newborn hearing screening and detection of congenital cytomegalovirus infection. Pediatrics. 2008;121(5):970.
Duryea EL, Sanchez PJ, Sheffield JS, et al. Maternal human immunodeficiency virus infection and congenital transmission of cytomegalovirus. Pediatr Infect Dis J. 2010;29:915–8.
Nigro G, Adler SP, La Torre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–62.
Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316–26.
Kimberlin DW, Jester PM, Sánchez PJ, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372:933–43.
Kimberlin DW, Lin CY, Sánchez PJ, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143:16–25.
Lim Y, Lyall H. Congenital cytomegalovirus – who, when, what-with, and why to treat? J Infect. 2017;74:S89–94.
Gunkel J, Wolfs TF, de Vries LS, Nijman J. Predictors of severity for postnatal cytomegalovirus infection in preterm infants and implications for treatment. Expert Rev Anti-Infect Ther. 2014;12:1345–55.
Schleiss MR, Permar SR, Plotkin SA. Progress toward development of a vaccine against congenital cytomegalovirus infection. Clin Vaccine Immunol. 2017;24:e00268-17.
Jeon J, Victor M, Adler SP, et al. Knowledge and awareness of congenital cytomegalovirus among women. Infect Dis Obstet Gynecol. 2006;2006:80383.
Vauloup-Fellous C, Picone O, Cordier AG, et al. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. J Clin Virol. 2009;46:S49–53.
Hamprecht K, Goelz R. Postnatal cytomegalovirus infection through human milk in preterm infants: transmission, clinical presentation, and prevention. Clin Perinatol. 2017;44:121–30.
American Academy of Pediatrics, Policy Statement. Breastfeeding and the use of human milk. Pediatrics. 2012;129:827–41.
Amir J, Schwarz M, Levy I, Haimi-Cohen Y, Pardo J. Is lenticulostriated vasculopathy a sign of central nervous system insult in infants with congenital CMV infection? Arch Dis Child. 2011;96:846–50.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Ronchi, A., Pugni, L., Mosca, F. (2018). Cytomegalovirus Infection. In: Cantey, J. (eds) Neonatal Infections. Springer, Cham. https://doi.org/10.1007/978-3-319-90038-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-90038-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90037-7
Online ISBN: 978-3-319-90038-4
eBook Packages: MedicineMedicine (R0)