Abstract
Chronic hepatitis B virus (HBV) is a significant threat to public health, with an estimated 240 million people living with the disease worldwide (http://www.who.int/mediacentre/factsheets/fs204/en/). HBV and the associated complications, such as cirrhosis and hepatocellular carcinoma, cause >600,000 deaths yearly (GBD 2013 Mortality and Causes of Death Collaborators, Lancet 385:117–71, 2015). Since the development of the HBV vaccine in the 1980s, new cases have decreased, yet the disease remains a global health concern, especially due to high rates of mother-to-child transmission (MTCT) in many countries (http://www.who.int/mediacentre/factsheets/fs204/en/). The focus of this chapter is to explore the current management of hepatitis B in pregnancy, with an emphasis on treatment and prevention of transmission to the neonate.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Epidemiology
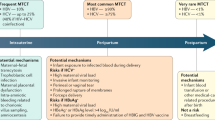
The prevalence of chronic HBV varies globally, from <2% in low-prevalence areas such as North America, Australia, and Western Europe to 5–10% in East Asia and sub-Saharan Africa [3]. The variation in prevalence is predominantly due to age of infection, and in high-prevalence areas, the most common mode of transmission is mother-to-child, or transmission in early childhood [1, 4]. This is in contrast to low-prevalence areas, where the common modes of transmission are through sexual contact and intravenous drug use [1, 5]. The risk of developing chronic HBV depends on the age of infection, with rates of 80–90% in individuals infected in the first year of life, 30–50% in children <6 years of age, and <5% in healthy adults [1, 2, 6].
Since implementation of the HBV vaccine in the 1980s, new cases of HBV have drastically decreased. The vaccine is 95% effective in preventing new infection with HBV [1]. In 2014 in the United States, the incidence of HBV was 0.9 cases per 100,000 persons—an 82% decline in new infections since 1991, when childhood vaccination started [7]. The World Health Organization promotes universal immunization programs beginning at birth. As of 2015, 185 countries have adopted vaccine programs for infants, and global coverage with the recommended three-dose HBV vaccine is approximately 83% [8]. Ninety-six countries are now vaccinating newborns within 24 h of life. Even with these improvements, only 39% of newborns worldwide are receiving the first dose of the vaccine in the recommended timeframe [8].
Pathogenesis
Hepatitis B virus is a member of the Hepadnaviridae family and a double-stranded DNA virus that mainly infects cells in the liver but has also been found in bile duct epithelium, pancreas, kidneys, and lymphoid tissues [9, 10]. HBV is spread through exposure to blood and bodily fluids including saliva, semen, and vaginal secretions [1, 7]. It can be contracted percutaneously, or through mucous membranes [1, 7]. Our focus here is on mother-to-child transmission, where the primary route is through mucous membranes during passage through the birth canal [7]. Only a small percentage of cases are acquired intrauterine, likely from transplacental “leakage” of maternal blood during a threatened abortion or preterm labor [11, 12].
A maternal HBV DNA level of >100,000 IU/mL is the most important independent risk factor for MTCT; transmission rates are reportedly 90% if the viral load is greater than 105 copies/mL [13, 14]. These rates are decreased with infant immunoprophylaxis (see Prevention, below). However, even with immunoprophylaxis, transmission rates of 8–30% have been reported from women with high viral loads [13, 15, 16]. In a retrospective study by Zou et al. [16], immunoprophylaxis failure rates for HBV DNA levels of <6, 6–6.99, 7–7.99, and ≥8 log10 copies/mL were 0%, 3.2%, 6.7%, and 7.6%, respectively. Rates of transmission also vary depending on the presence of the hepatitis B e antigen (HBeAg) , a marker of infectivity. Without measures to prevent transmission, the rates of MTCT from mothers who are HBeAg positive are 70–90% [4, 17]. In mothers without HBeAg, rates of MTCT are 10–40% [4]. Timing of maternal acquisition of acute HBV also affects the risk of vertical transmission, with the highest risk in the third trimester or near the time of delivery [18].
Box 1 Risk Factors for Mother-to-Child Transmission of Hepatitis B Virus
Maternal
High maternal viremia
HBeAg positive
Maternal infection near time of delivery
Neonatal
Failure to receive appropriate passive-active immunoprophylaxis (hepatitis B vaccine and immune globulin)
Other
Invasive testing (i.e., chorionic villi sampling, amniocentesis) when viral load is high
Preterm labor
Threatened abortion
Data surrounding the risk of MTCT with invasive tests such as amniocentesis and chorionic villus sampling are limited. A case-control study in 2014 showed a significant increase in rates of vertical transmission in women undergoing amniocentesis versus controls when stratified by HBV DNA levels ≥7 log10 copies/mL (50% vs. 4.5%, respectively) [19]. Women considering invasive testing should be counseled about the possible increased risk of transmission with high viral loads, but if genetic testing is indicated, it can be offered [20]. These women should be counseled on available noninvasive screening options [21].
Clinical Findings
Acute infection in adults . After an incubation period of approximately 75 days (range, 30–180 days), symptoms of acute HBV may present in 30–50% of infected individuals; however, most people with acute HBV, including pregnant women, are asymptomatic [1]. Of those who do exhibit symptoms, they include nausea, vomiting, abdominal pain, fatigue, jaundice, loss of appetite, and joint pain [1, 7]. Extrahepatic signs such as a cutaneous rash may also occur [22]. The duration of these signs and symptoms ranges from only a few weeks up to 6 months [7]. The risk of an acute infection causing liver failure (acute fulminant hepatitis) is low, but when this occurs, it can lead to death, with fatality rates of 0.5–1.5% [22].
Acute infection in children . Infants and young children infected with HBV typically have no signs or symptoms [22]. This highlights the importance of serologic follow-up testing after perinatal HBV exposure (see Prevention, below).
Chronic infection . Adults and children with chronic HBV are usually asymptomatic, until development of liver cirrhosis, hepatocellular carcinoma, or liver failure [1, 7, 22].
Diagnosis
Diagnosis of HBV starts with a thorough history and physical exam. Both the American College of Obstetricians and Gynecologists and the US Preventive Services Task Force recommend universal testing for hepatitis B virus in pregnancy [21, 23]. Screening is based on detection of hepatitis B surface antigen (HBsAg) . HBsAg can be detected beginning 30–60 days after infection [1]. A positive test requires further testing to evaluate for acute versus chronic infection (Table 1). Testing includes hepatitis B surface antibody (anti-HBs), total hepatitis B core antibody (anti-HBc), and hepatitis B core IgM antibody (IgM anti-HBc). Presence of the IgM anti-HBC indicates acute infection. Positive results for the HBsAg must be reported to the state health department, based on state reporting requirements [21, 24].
Once a diagnosis of HBV is made, additional laboratory testing includes evaluation of HBV viral load and HBeAg, liver function testing (i.e., aminotransferases, alkaline phosphatase, coagulation studies), and a complete blood count. These women should also be screened for coinfection with hepatitis C virus, hepatitis delta virus, and human immunodeficiency virus. Hepatitis A testing can also be done to evaluate for a need for vaccination. Imaging studies, such as a liver ultrasound, should also be performed in all patients [25].
Treatment
All pregnant women should be screened for HBsAg at their first prenatal visit. In women who engage in high-risk behaviors (i.e., injection drug use, HBsAg-positive sexual partner), testing should also be done when admitted for delivery [22]. A positive test requires follow-up, as noted in the section on “Diagnosis.”
Women who have not previously been vaccinated for HBV should receive the vaccine if they are at high risk of infection. High-risk individuals include injection drug users, HIV-positive persons, household contacts of persons with chronic HBV, health-care workers, those with >1 sexual partner in the past 3 months, recent diagnosis of a sexually transmitted infection, developmentally delayed persons in a long-term care facility, hemodialysis patients, and those traveling to high-prevalence regions [21, 26]. Currently available adult vaccines include two single-antigen vaccines (Engerix-B® and Recombivax HB®) and one combination hepatitis A and B vaccine (Twinrix®) [21, 26]. Each of these is to be administered as a three-dose vaccine series, by intramuscular injection.
In the third trimester, women with known HBV infection should have repeat viral load testing completed in order to determine if they would benefit from antiviral therapy. The American Association for the Study of Liver Diseases and the Society for Maternal-Fetal Medicine propose antiviral therapy in pregnant women with >6 log10 copies/mL (1 million copies/mL, or 200,000 IU/mL) [20, 25]. Due to its safety in pregnancy and the low risk of resistance, the first-line antiviral therapy is tenofovir [20, 25]. Alternative therapies include telbivudine and lamivudine. Timing of initiation of therapy has not been well studied, but many suggest starting at 28–32 weeks gestation [25]. Upon discontinuation of therapy, women must be monitored closely for aminotransferase flares [25].
Several studies have been performed to evaluate the benefit of HBIG administration to pregnant women infected with HBV. A Cochrane review determined that because of the low quality of the studies, no benefit could be shown [27].
While there have been studies to evaluate the benefit of elective cesarean delivery in reducing the risk of HBV transmission, the data are conflicting and the quality is low. Cesarean delivery solely for prevention of HBV transmission is not recommended [20, 25]. In addition, there is not enough data to make recommendations on the use of internal fetal monitoring during the intrapartum course [21].
Prevention
In order to prevent MTCT, it is recommended that children born to mothers with HBV infection receive passive-active immunoprophylaxis. This is a combination of HBIG (the passive component) and the single-antigen hepatitis B vaccine (Recombivax HB® or Engerix-B® ) (the active component) [21, 22]. When given as soon as possible (no later than 12 h of life), followed by completion of the three- or four-dose vaccine series, immunoprophylaxis is 85–95% effective in preventing transmission from mothers with HBsAg and HBeAg positivity [4, 28]. Passive-active prophylaxis should also be given to newborns of mothers with unknown HBsAg status (Table 2) [20].
Women with HBV, but with no other contraindications to breastfeeding, should be encouraged to do so, as long as the infant receives passive-active immunoprophylaxis at birth [20, 21, 29]. Women on antiviral therapy should be counseled that although drug labels may recommend against breastfeeding while on these medications, the American Association for the Study of Liver Diseases reports that there is minimal excretion of the drugs in breast milk. The overall risk of exposure is unknown [25].
Follow-up of neonates born to mothers with HBV involves completion of the vaccine series and postvaccination testing (see chapter “Immunizations in the Nursery”). The recommended schedule for the vaccine series with the single-antigen vaccine is dose #1 within 12–24 h of life, dose #2 at 1–2 months of age, and dose #3 at 6 months of age (not sooner than 24 weeks of age) [22]. If the series is completed with a combination vaccine, an “extra” dose is often given at 4 months but does not preclude the need for the 6-month dose. For HBV-exposed infants with a birth weight of <2 kg who receive HBV vaccine at birth, the immune response to the first dose may not be adequate; those infants should receive three more doses, starting after 1 month of age [22].
At 9–12 months of age, postvaccination testing for HBsAg and anti-HBs can be completed to rule out infection and to evaluate for protective titers [22]. If positive for HBsAg, the proper testing and follow-up should be done in a timely manner. If HBsAg is negative and anti-HBs is ≥10 mIU/mL, the infant is considered protected and no intervention is needed. If anti-HBs is <10 mIU/mL, additional immunization and follow-up testing should be completed (see chapter “Immunizations in the Nursery”) [22].
References
World Health Organization. Media Centre. Hepatitis B. Fact sheet. Updated July 2016. http://www.who.int/mediacentre/factsheets/fs204/en/. Accessed 6 Apr 2017.
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385:117–71.
Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212.
Tran TT. Hepatitis B in pregnancy. Clin Infect Dis. 2016;62:S314–7.
Goldstein ST, Alter MJ, Williams IT, et al. Incidence and risk factors for acute hepatitis B in the United States, 1982–1998: implications for vaccination programs. J Infect Dis. 2002;185:713–9.
Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis. 1995;20:992–1000.
Centers for Disease Control and Prevention. Hepatitis B FAQs for health professionals. Updated August 4, 2016. https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm. Accessed 6 Apr 2017.
World Health Organization. Media Centre. Immunization coverage. Fact sheet. Reviewed March 2017. http://www.who.int/mediacentre/factsheets/fs378/en/. Accessed 6 Apr 2017.
Pontisso P, Poon MC, Tiollais P, Brechot C. Detection of hepatitis B virus DNA in mononuclear blood cells. Br Med J Clin Res. 1984;288:1563–6.
Halpern MS, England JM, Deery DT, et al. Viral nucleic acid synthesis and antigen accumulation in pancreas and kidney of Pekin ducks infected with duck hepatitis B virus. Proc Natl Acad Sci U S A. 1983;80:4865–9.
Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. 2002;67:20–6.
Lin HH, Lee TY, Chen DS, et al. Transplacental leakage of HBeAg-positive maternal blood as the most likely route in causing intrauterine infection with hepatitis B virus. J Pediatr. 1987;111:877.
Pan CQ, Duan ZP, Bhamidimarri KR, et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol. 2012;10:452–9.
Pawlowska M, Pniewska A, Pilarczyk M, Kozielewicz D, Domagalski K. Prophylaxis of vertical HBV infection. Expert Opin Drug Saf. 2016;15:1361–8.
Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;109:489–92.
Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat. 2012;19:e18–25.
Chang M-H. Hepatitis B virus infection. Semin Fetal Neonatal Med. 2007;12:160–7.
Jonas MM. Hepatitis B and pregnancy: an underestimated issue. Liver Int. 2009;29:133.
Yi W, Pan CQ, Hao J, Hu Y, Liu M, Liang D. Risk of vertical transmission of hepatitis B after amniocentesis in HBs antigen-positive mothers. J Hepatol. 2014;60:523–9.
Dionne-Odom J, Tita A, Silverman N. Society for Maternal-Fetal Medicine (SMFM) consult series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6–14.
American College of Obstetricians and Gynecologists. ACOG practice bulletin no 86. Viral hepatitis in pregnancy. Obstet Gynecol. 2007;110:941–55.
Mast EE, Margolis HS, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices (ACIP) part 1: immunization of infants, children, and adolescents. Advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2005;54:1–31.
Lin K, Vickery J. Screening for hepatitis B virus injection in pregnancy women: evidence for the US preventive services task force reaffirmation recommendation statement. Ann Intern Med. 2009;150:874–6.
Centers for Disease Control and Prevention. 2017 nationally notifiable infectious diseases. https://wwwncdcgov/nndss/conditions/notifiable/2017/infectious-diseases/ Accessed 12 Apr 2017.
Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83.
Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices (ACIP) part 2: immunization of adults. MMWR Recomm Rep. 2006;55:1–33.
Eke AC, Eleje GU, Eke UA, Xia Y, Liu J. Hepatitis B immunoglobulin during pregnancy for prevention of mother-to-child transmission of hepatitis B virus. Cochrane Database Syst Rev. 2017;(2):CD008545.
Andre FE, Zuckerman AJ. Review: protective efficacy of hepatitis B vaccines in neonates. J Med Virol. 1994;44:144–51.
American Academy of Pediatrics. Transmission of infectious agents via human milk. In: Pickering LK, editor. Red book: 2003 report of the committee on infectious diseases. 26th ed. Elk Grove Village: American Academy of Pediatrics; 2003. p. 118–21.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Pierce-Williams, R.A.M., Sheffield, J.S. (2018). Hepatitis B in the Perinatal Period. In: Cantey, J. (eds) Neonatal Infections. Springer, Cham. https://doi.org/10.1007/978-3-319-90038-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-90038-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90037-7
Online ISBN: 978-3-319-90038-4
eBook Packages: MedicineMedicine (R0)