Abstract
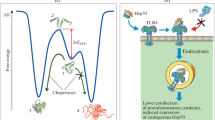
The HSP70 is a chaperon protein that is expressed during stress conditions that participates in many biological processes, including protein trafficking, nascent polypeptide folding and the refolding of the wrong proteins and cleaning of the misfolded ones. The expression is increased during various pathological conditions such as cerebral ischemia, neurodegenerative diseases, epilepsy, and trauma. They are found in both intracellular and extracellular compartments. HSP70 exhibits different functions in accordance with its location. Intracellular HSP70 exerts cytoprotective functions as a chaperone protein, whereas extracellular HSP70 exerts immunomodulatory functions that trigger immunological responses. They play an auxiliary role in antigen presentation in the appearance of immunological response in multiple sclerosis. Epilepsy is thought to have emerged as a stressor. HSP overexpression is proposed as a potential therapy for neurodegenerative diseases characterized by the accumulation or aggregation of abnormal proteins. In this chapter, we wanted to summarize the recent studies on the role of HSP70 in neurological disorders.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Heat shock protein (HSP) is a survival protein that acts as a molecular chaperone. When the metabolism is in danger, HSP70 expression increases in order to remove the unwanted, unfolded proteins, to repair of the damaged proteins and to help the synthesis of new polypeptides. HSP70 binds to protein substrates to stabilize them to avoid denaturation and apoptosis, assists the maintenance of cellular integrity. In the central nervous system, it has been found that HSP is produced in many cell types, including neurons, glia and endothelial cells. In this chapter we review the role of HSP70 in common neurological disorders and discuss the therapeutic interventions.
HSP70 in Alzheimer Disease
Alzheimers Disease is the most common cause of fatal neurodegenerative disease characterized by progressive memory loss, language disorders, cognitive function impairment. Advancing age, mitochondrial DNA mutations, oxidative stress are the factors that facilitate the development of the disease. The amyloid plaques caused by β Amyloid (Aβ) peptide aggregation inside the cell and the neurofibrillary tangles formed by phosphorylated tau aggregation outside the cell are the pathologic hallmarks of the disease. Hippocampus and cerebral cortex that is responsible for memory and cognition are the most affected parts (Ciechanover and Kwon 2015). Efforts to treat the disease is focused on the accumulation of Aβ and hyperphosphorylated tau proteins. Pathological processes resulting incorrectly folded proteins leads to the clinical manifestation of the disease (Desler et al. 2017). In the normal cell life, the nascent (newly formed) proteins about 30%of them are misfolded and they are destroyed quickly in the cell. Misfolded and aggregated proteins pass from quality control but some mutant proteins escape from proteolysis and form intracellular inclusions and extracellular plaques (Pratt et al. 2015). As the proteasome activities diminish at the older ages, the accumulation of protein aggregate gets easier.
Protein folding is required for a functional and stable protein. Synthesized polypeptide chains pass from thermodynamically unstable, α-helix form to a stable three-dimensional tertiary structure. The most important reason of misfolding of proteins is mutations rather than posttranslational protein modifications, oxidative stress, environmental conditions like pH and heat (Ho et al. 2015). Aβ proteins can accumulate as oligomer, and aggregate as amyloid fibril forms. HSP70 can selectively recognize the Aβ oligomers which are the most toxic form (Whyte et al. 2017). Therefore, HSP70 overexpression inhibits Aβ aggregation that may lead to the clinical improvement. On the other hand, HSP 70 has an important role on tau hemostasis by helping degradation of tau by either proteasomal pathway or ubiquitination (Patterson et al. 2011). HSP70 prevents cell death by weakening the caspase-dependent and independent pathways (Sabirzhanov et al. 2012). For this reason, treatment approaches to elevate the HSP70 levels are under investigation. Geldamycin which is a HSP70 inducer prevents the formation of neurofibrillary tangles by inhibiting tau accumulation (Lu et al. 2014; Hung and Fu 2017). We think neuroprotective therapy approaches on the chaperone system based studies must be executed.
HSP70 in Parkinson’s Disease
Parkinson’s disease (PD) is second most common movement disorder, and it affects nearly 1% of the population over the age of 60. PD is characterized mainly by progressive and selective loss of dopaminergic neurons in the substantia nigra pars compacta, with the subsequent dopamine (DA) decline in the nigrostriatal pathway, and by the presence of intracytoplasmic fibrillar α-Syn protein aggregates (Lewy Bodies) in the remaining nigral neurons (Halliday et al. 2011). Increased levels of α-synuclein or α-synuclein-containing aggregates are also characteristic of other neurodegenerative diseases, including Lewy body dementia, multiple system atrophy and AD. This group of diseases termed “synucleinopathies”. Motor impairments, bradykinesia, rigidity, and resting tremor are clinical characteristic features of PD. These impairments are result of loss of dopaminergic neuron loss in substantia nigra. Although the mechanism is not fully understood it is hypothesized that disease arise from combination of genetic and environmental factors. Oxidative stress associated with mitochondrial dysfunction, proteolytic stress due to dysfunction of the ubiquitin-proteasome system (UPS), and local inflammation are pathogenic pathways that have been concerned. Although the majority of cases of PD appear to be sporadic when the age of symptom onset is younger than 50 years genetic factors play more role. Glucocerebrosidase, Alpha-synuclein (SNCA), leucine-rich repeat kinase-2 (LRRK2), Parkin, PTEN-induced putative kinase 1 (PINK1; PARK6) mitochondrial DJ-1 (PARK7) are the genes that was described which has role in PD (Krüger et al. 1998).
Correct protein folding is essential for proteins’ biological functions. Heat shock proteins are critical elements of the cellular response to unfolded proteins. HSP are involved in promoting proper protein folding and preventing aggregation, as well as promoting ubiquitination and degradation of misfolded proteins. Under certain pathological conditions the protein quality control machinery is not sufficient to prevent the accumulation of misfolded proteins and these accumulations may lead to neurodegenerative disorders including PD (Kalmar and Greensmith 2017). The role of molecular chaperones in PD was first suggested by the detection of HSP90, HSP70, HSP60, HSP40, and HSP27 molecules in lewy bodies. In 1991 Namba Y et al. worked on brain tissues, which were obtained from the autopsy of patients who had neurodegenerative diseases including PD. They performed immunohistochemical studies on brain tissues from patients with various neurodegenerative conditions by using specific polyclonal antibody to HSP 70 and found HSP 70 association with abnormal cytoplasmic inclusions, which are characteristic for neurodegenerative diseases (Namba et al. 1991).
Fiszer U et al. worked on CSF of patients PD. They measured IgG levels against anti-HSP 70 molecules and found significantly increased levels in patients with PD compared to patients who had non-inflammatory neurological diseases (Fiszer et al. 1996). It has been shown that neuron cells, are postmitotic cells and they are susceptible to misfolded proteins (Muchowski and Wacker 2005). The misfolded aggregates are immunoreactive for ubiquitin, and most have been reported to contain molecular chaperones and components of the proteasome (Davies et al. 1997). Molecular chaperones and components of the proteasome can also be found in aggregates formed in transgenic animal models and transfected cell cultures (Suhr et al. 2001). Sheng Chen and Ian R. Brown demonstrated intermediate Hsc70 levels in neurons of the substantia nigra affected as PD in a rat model (Chen and Brown 2007). In this way determining these proteins, suggest that protein aggregates are recognized as targets and cellular protein quality control mechanisms are activated in an attempt to prevent their accumulation (Kazemi-Esfarjani and Benzer 2002). α-Syn is a 140-amino acid neuronal protein probably involved in regulating cell differentiation, synaptic plasticity, and dopaminergic neurotransmission.It has been demonstrated that HSP70 overexpression reduced α-Syn accumulation and toxicity in both mouse and Drosophila models of PD. (Klucken et al. 2004; Auluck et al. 2002). Other experiments using heat shock induced expression of chaperones demonstrate that HSP70 supplies protection against cytotoxicity of PD-inducing pesticide rotenone and reduced alpha synuclein aggregation in a cellular model (Zhou et al. 2004). In the light of this information’s Huang et al. showed that HSP70 inhibits as fibril formation via preventing the formation of prefebrillar α-syn, binding with these species to inhibit nuclei formation, as well as binding with nuclei to retard fibril elongation (Huang et al. 2006).
Roodveldt et al. showed that HSP70 depletion can be a direct result for the presence of aggregation-prone polypeptides. They found that a nucleotide-dependent interaction between HSP70 and αSyn, which leads to the aggregation of HSP70, with the presence of ADP along with αSyn. Such a co-aggregation phenomenon could be prevented in vitro by the co-chaperone Hip (ST13). Their findings indicated that Hip utilizes stabilization of HSP70 and helps chaperone mediated amyloid formation inhibition. Another finding of this study was that ADP-bound HSP70 has a very high tendency to co-aggregate with α-syn, suggesting that chaperone depletion favored under certain conditions could be an important feature in the onset and progression of amyloid disorder (Roodveldt et al. 2009). DnaK/DnaJ/GrpE of HSP70 system model used by Ahamad et al. By this system they showed that HSP70 inhibits αSyn fibrillar assembling but cannot activate refolding process (Ahmad 2010). For all these reasons misfolded proteins are considered a common therapeutic target in PD and many studies have focused on the neuroprotective role of HSP.
HSP70 in Amyotrophic Lateral Sclerosis
Tar DNA binding protein 43 (TDP-43) neuronal cytoplasmic inclusion aggregations are thought to be the key in some neurodegenerative disease (amyotrophic lateral sclerosis, frontotemporal lobar degeneration) Inhibition or the clearance of this toxic aggregation is, therefore, a strategy for therapeutic intervention. The ubiquitin-proteasome system or the autophagy pathway participates in resolving potentially detrimental protein aggregates. HSP might refold TDP-43 and return it to its natural physiological state. Either transgenic TDP-43 mouse model or sporadic ALS patients reduced HSP70 levels are assessed. Strategies against HSP activation be may be an important therapeutic challenge for the TDP-43 proteinopathies. Arimoclomol that is a co-inducers of heat shock protein 70 and 90 expressions under cellular stress, is neuroprotective in a number of neurodegenerative disease models, including familial Amyotrophic Lateral Sclerosis (ALS). Superoxide Dismutase 1 transgenic mice (an animal model of ALS), Arimoclomol has effects on the prevention of neuronal loss and the promotion of motor neuron survival. The therapeutic potential of Arimoclomol is currently under investigation in Phase II/III clinical trials for familial ALS patients with SOD1 mutations. The HSP70 co-inducer drugs like Arimoclomol may be a hope for ALS and other neurodegenerative disorders.
HSP in Huntington Disease
Huntington disease (HD) is a non curable, adult-onset, autosomal dominant inherited disorder associated with cell loss within a specific subset of neurons in the basal ganglia and cortex. The disease named by the physician who described it as hereditary chorea in 1872 (Huntington 1872). Involuntary movements, dementia, and behavioral changes are HD characteristic hallmarks. Formation of intracellular inclusions composed primarily of the ubiquitous protein huntingtin, the subsequent death of striatal medium spiny neurons and cortical pyramidal neurons are responsible for disease symptoms and signs (Vonsattel and DiFiglia 1998). Electron microscopy reveals both cytoplasmic and nuclear abnormalities, including the presence of large neuronal intra nuclear inclusions or aggregates similar to those in other polyglutamine disorders. The aggregates are also found in dystrophic neurites. The genetic basis of HD is the expansion of a cysteine-adenosine-guanine repeat encoding a polyglutamine tract in the N-terminus of the protein product called huntingtin. This leads to a mutant protein that contains an expanded poly-glutamine (polyQ) sequence (Ho and Hocaoglu 2011). The length of the polyQ sequence determines the age of disease onset and severity (Zoghbi and Orr 2000). In HD pathologic examination we can see cytoplasmic and nuclear aggregates, including large neuronal inclusions like other polyglutamine disorders (Davies et al. 1998).
PolyQ-expanded Htt cooperate differently with the proteostasis network compared to other disease-associated proteins. Bersuker K et al. show that the heat shock response is not activated by mutant huntingtin gene even in cells selected for the highest expression levels and for the presence of inclusion bodies containing aggregated protein (Bersuker et al. 2013). Tagawa K et al. worked on tree types of neuron and found different response to mutant polyQ proteins. They established that HSP70 was up regulated only by mutant htt and selectively in the granule cells within the cerebellum. They also found that granule cells, that are unresponsive to degeneration in HD pathology, lost their resistance by suppressing HSP70 with siRNA, on the other hand cortical neurons, affected in human HD, gained resistance by over expressing HSP70. This indicates that induction levels of HSP70 are a critical factor for determining vulnerabilities to mutant htt among neuronal subtypes (Tagawa et al. 2007). The recent studies show that HSP70 and its co-chaperone HSP40 inhibits huntingtin exon 1 fragment aggregation and modifies the structural, seeding, and infectious properties of the resulting fibrils in a polyQ-independent manner (Monsellier et al. 2015).
HSP70 in Stroke
The effect of HSP70 is studied in many brain ischemia models. In a global cerebral ischemia model, like the brain damage after cardiac arrest, HSP70 mRNA expression increased in hours. HSP70 expression was observed within astrocytes, neurons by glial transfer to protect the neurons from damage. Ischemia in rodents showed neuroprotection after different kinds of nerve injuries in astrocyte cultures of HSP70 transgenic mice. HSP70 was used as a therapeutic agent in a focal ischemia model which was formed with 4-min middle cerebral artery occlusion. Twenty minutes before occlusion some mice were given intravenous HSP70. They reported that the ischemic zone was little about the group treated with HSP70 (Shevtsov et al. 2014). Gomez-Choco M et al. studied the existence of HSP70 in lymphoid tissue of acute stroke patients and exhibited that highly immunoreactive HSP70 was associated with smaller infarct size and better outcome (Gomez-Choco et al. 2014). Geldanamycin is a HSP inhibitor that is emerged as a therapeutic agent with the ability of upregulation of HSP70. Pretreated astrocytes and glial cells with geldamycin were more preserved from cell death (Kacimi and Yenari 2015). Another experiment on a model of brain ischemia was done with a protein called Dynamin that triggers apoptosis leads to caspase-dependent cell death. Kim et al. showed that HSP70 transgenic mice had a better outcome and dynamin inhibitor dynasore also protected the mice from the stroke. They drew attention to HSP70 and dynamin interaction may be worthy and target strategies on dynamin inhibition may protect the brain from the ischemic stroke (Kim et al. 2016).
HSP70 in Epilepsy
Epilepsy is a paroxysmal disorder that is characterized by repeated seizures, which may be idiopathic, symptomatic or cryptogenic in origin. The repeated seizures induce apoptosis in the neurons within the brain especially hippocampus. Stress proteins might be assumed to counteract the pathology of increased neuronal excitation. HSP70 is the most studied neuroprotective chaperon on epilepsy. Seizures are triggered by hyperexcitability that is caused by the excessive calcium influx into the cells. HSP70 has an influence of the decrease of the calcium influx to the neurons thus prevents brain cells from seizure-induced apoptosis. Consequently, HSP70 might be involved in endogenous cellular preservation during seizures. Many animal experiments demonstrate overexpression of HSP70 exerts protective effects during kindling. According to recent studies HSP70, transgenic mice were more resistant to kindling with chemical agents. Seizure threshold and survival during kindling were higher in HSP70 transgenic mice as compared to wild-type mice (Ammon-Treiber et al. 2007). Thus, overexpression of HSP70 exerts protective effects on seizure severity and overall survival during PTZ kindling and decreases the development of kindling. Mesial temporal sclerosis (MTS) is the most known cause of intractable epilepsy that can be treated by hippocampal resection. In the surgery material of human MTS cases, Kandratavicius et al. showed increased expression of HSP70 in the hippocampal formation. After successful surgery, they saw decreased levels of HSP70 and HSP90. Surgical excision of the hippocampus with more HSP expression showed poorer outcome compared with the hippocampus with less HSP expression (Kandratavicius et al. 2014). There was a positive correlation between seizure frequency, duration of epilepsy and HSP70 expression as in many studies.
HSP 70 in Migraine
Migraine is the most common primary headache that affects many people in the world. The World Health Organization estimates the prevalence of migraine %14. It is characterized by recurrent episodes of headache, mostly unilateral throbbing or pulsatile intensified with movement and accompanied by nausea or vomiting. The pathogenesis is explained by the neurovascular theory which is a primarily neurogenic process, and secondary change takes place in cerebral perfusion. In aura phase, Neuronal hyperexcitability usually begins in the occipital cortex and spreading cortical depression affects the cortex and activates the trigeminovascular system by stimulation of nociceptive neurons. The sterile inflammation is accompanied with vasodilatation that causes the headache. In our paper, we examined serum HSP27 levels during the migraine attack and during remission phase and compared with control subjects. We couldn’t show any difference between attack and remission. We showed a positive correlation of HSP27 with headache severity scores during the migraine attack (Coban et al. 2011). Similarly, Yön et al. found no statistical difference in chronic migraine patients (Yon et al. 2016).
HSP70 in Myasthenia Gravis
Myasthenia gravis (MG) is an autoimmune neuromuscular disease. There is a limited number of studies into the role of HSP70 in MG. Munakata, et al. studied serum levels of MG patients just before and after treatment. They found a significant increase in the patient group. After treatment, in the group which responded therapy, the HSC71 levels decreased. In the therapy-resistant group, they found no change. They revealed that heat shock cognate protein 71 elevations may be a useful marker for the disease prognosis (Munakata et al. 2008). Helgeland et al. showed anti-HSP70 level elevation in Myasthenia Gravis and Guillain Barre syndrome (Helgeland et al. 2010).
HSP70 in Multiple Sclerosis
Multiple sclerosis (MS) is an autoimmune disease characterized by inflammation, demyelination, and axonal injury in central nervous system that leads disability in one-third of the patients. Between the ages of 20–40, it is the most common cause of disability after trauma. Myelin sheaths, oligodendrocytes and less frequently axon and the nerve cell itself are damaged by inflammation (Lucchinetti et al. 2000). The hallmarks of MS pathology are multifocal demyelination that is characterized by inflammation and gliosis. These areas are seen as MS plaques, which are diagnostic for MS. According to the disease status, MS plaques may be active, including the more inflammatory infiltrates, T lymphocytes, macrophages fewer B-lymphocytes, plasma cells, immunoglobulins, and complements (Lucchinetti et al. 2000). Critical approaches to MS therapy focus on the protection of oligodendrocytes and neurons by immunomodulation of T-cells against myelin. As a consequence, the activation of myelin-specific CD4 T-cells by secretion of Inflammatory cytokines(such as IFN-γ, TNF-α, IL-1β, and IL-6) leads to release of adhesion molecules from the vascular endothelial cells, help the migration of lymphocytes across the blood-brain barrier. CD8+ T cells, B cells, antibodies, natural killer cells, join the complex immune response and responsible for the axonal injury (Mansilla et al. 2014).
The exact role of HSP70 in MS pathogenesis is still debated as the effect changes with the localization of the protein. While the intracellular HSP70 is neuroprotective, the HSP70 released into the extracellular space seems to be an antigen-adjuvant and may have a role in antigen presenting. Selmaj demonstrated the colocalization of T-cell receptor gamma delta cells with HSP65 and HSP70 in MS lesions postmortem (Selmaj et al. 1991). Boiocchi C et al. studied genetic polymorphism of HSP70 159 relapsing-remitting and 36 secondary progressive MS patients and compared with 586 healthy controls. They hypothesized that over expression of HSP70 as in AA genotype may have less inflammation, and better prognosis (Boiocchi et al. 2016).
Experimental allergic encephalomyelitis (EAE) is an animal model of MS. After the injection of a myelin protein (myelin associated protein, myelin basic protein, proteolipid protein, etc.), a cell-mediated immune reaction against myelin develops and causes disease like MS (Hernandez-Pedro et al. 2013). Studies with EAE showed HSP70 over expression seems to be beneficial for the recovery from the disease. Conversely, the HSP70 knockout mice were significantly resistant to EAE development (Mansilla et al. 2014). Talla et al. demonstrated that mitochondrial HSP70 elevation in retinal ganglion cells, which preserves vision by preventing neuronal apoptosis, and axonal damage in the EAE model with mice. HSP70 can also act via an anti-inflammatory mechanism by inducing the expression of anti-inflammatory cytokines and inhibiting inflammatory ones (Talla et al. 2014). In Boiocchi’s study, Genotyping of HSP70–2 + 1267 A/G polymorphism was found in 195 MS patients. In addition, HSP70-2 protein content in vitro from PBMC was meaningfully lower in MS patients with GG genotype compared to AA genotype, indicating an implication of the G allele of HSP70-2 gene polymorphism in the development of MS (Boiocchi et al. 2016). Caussi et al. found that humoral response to HSP70 was meaningfully high in a large group of MS patients (Cassu et al. 2013). Significantly elevated antibody titers against HSP70 proteins were not only found in peripheral blood but also in the cerebrospinal fluid sample of MS patients (Chiba et al. 2006). The mitochondrial dysfunction has been proposed to be the key of neurodegeneration in MS. There are studies targeting on mitochondrial HSP70 enhancement as a treatment of choice. Gene therapy to enhance the HSP70 in mitochondria is under investigation.
HSP70 in Prion Disease
Prion diseases are neurodegenerative diseases that have long incubation periods and once clinical symptoms destructively progress. Five human prion diseases are currently recognized: kuru, Creutzfeldt-Jakob disease (CJD), variant Creutzfeldt-Jakob disease (vCJD also known as new variant CJD), Gerstmann-Sträussler-Scheinker syndrome (GSS), and fatal familial insomnia (FFI) (Prusiner 2001). In prion diseases cellular prion associated proteins(PrPC) are changed into disease associated prion proteins(PrPSc) and those misfolded proteins accumulate within the neural tissue (Budka 2003). This accumulation gives rise to common features to prion diseases including neuronal loss, proliferation of glial cells, absence of an inflammatory response, and the presence of small vacuoles within the neurons, which produces a spongiform appearance. For understanding role of chaperons in prion multiplication yeast eukaryotic models have been used. Transmission, self propagating and aggregation are properties of yeast prions (PSI+) (King et al. 1997). Jones et al. worked with yeast cells and showed that a mutation in the SSA1 HSP70 allele (SSA1–21p) significantly impaired PSI+ self-replication and propagation (Jones et al. 2004). Propagation of Saccharomyces cerevisiae [PSI+] prion is impaired by factors that regulate HSP70 substrate binding. Diedrich et al. show elevation in protein levels of inducible HSP70 in active astrocytes with a C57BL6 mice model injected with 22L strain of scrapie (Diedrich et al. 1993). In addition a significant increase in HSP70 RNA expression demonstrated in mice brain that infected with scrapie forms (Kenward et al. 1994). Tamguney et al. conducted a study on 20 potential genes candidates that could regulate the replication of prions in mice infected by scrapie or cow 301V prions. They worked in a mice infected with scrapie or cow 301V prions model potential genes that may regulate replication of prions examined and overexpression of human HSP70 did not show effect on prion disease onset (Tamguney et al. 2008). To our knowledge, the mechanism of the aggregation of those proteins and interventions with chaperons is not exactly understood.
Conclusions
HSP70 has an important role on the immunopathogenesis of multiple neurological conditions. It has neuroprotective effects in various diseases. We think that, treatment of many neurological diseases with HSP70 overexpression, will be possible in the future.
Abbreviations
- Aβ:
-
Amiloid beta
- ALS:
-
Amyotrophic Lateral Sclerosis
- CJD:
-
Creutzfeldt-Jakob disease
- DA:
-
Dopamine
- EAE:
-
Experimental allegic encephalomyelitis
- FFI:
-
Fatal familial insomnia
- GSS:
-
Gerstmann-Sträussler-Scheinker syndrome
- HD:
-
Huntington disease
- HSP:
-
Heat shock protein
- LRRK2:
-
Leucine-rich repeat kinase-2
- MG:
-
Myasthenia gravis
- MS:
-
Multiple sclerosis
- MTS:
-
Mesial temporal sclerosis
- PD:
-
Parkinson’s disease
- PINK1:
-
PTEN-induced putative kinase 1
- polyQ:
-
Poly-glutamine
- PrPC:
-
Cellular prion associated proteins
- PrPSc:
-
Disease associated prion proteins
- SNCA:
-
Alpha-synuclein
- TDP-43:
-
Tar DNA binding protein 43
- UPS:
-
Ubiquitin-proteasome system
- vCJD:
-
Variant Creutzfeldt-Jakob disease
References
Ahmad, A. (2010). DnaK/DnaJ/GrpE of HSP70 system have differing effects on alpha-synuclein fibrillation involved in Parkinson’s disease. International Journal of Biological Macromolecules, 46(2), 275–279.
Ammon-Treiber, S., Grecksch, G., Angelidis, C., et al. (2007). Pentylenetetrazol kindling in mice overexpressing heat shock protein 70. Naunyn-Schmiedeberg’s Arch Pharmacol, 375, 115–112.
Auluck, P. K., Chan, H. Y., Trojanowski, J. Q., et al. (2002). Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science, 295(5556), 865–868.
Bersuker, K., Hipp, M. S., Calamini, B., et al. (2013). Heat shock response activation exacerbates inclusion body formation in a cellular model of Huntington disease. The Journal of Biological Chemistry, 288, 23633–23638.
Boiocchi, C., Monti, M. C., Osera, C., et al. (2016). Heat shock protein 70-hom gene polymorphism and protein expression in multiple sclerosis. Journal of Neuroimmunology, 298, 189–193.
Budka, H. (2003). Neuropathology of prion diseases. British Medical Bulletin, 66, 121–130.
Cassu, D., Masala, S., Frau, J., et al. (2013). Anti Mycobacterium avium subsp. Paratuberculosis heat shock protein 70 antibodies in sera of Sardinian patients with multiple sclerosis. Journal of the Neurological Sciences, 355(1–2), 131–133.
Chen, S., & Brown, I. R. (2007). Neuronal expression of constitutive heat shock proteins: Implications for neurodegenerative diseases. Cell Stress & Chaperones, 12(1), 51–58.
Chiba, S., Yokota, S., Yonekura, K., et al. (2006). Autoantibodies against HSP70 family proteins were detected in the cerebrospinal fluid from patients with multiple sclerosis. Journal of the Neurological Sciences, 241(1–2), 39–43.
Ciechanover, A., & Kwon, Y. T. (2015). Degradation of misfolded proteins in neurodegenerative diseases: Therapeutic targets and strategies. Experimental & Molecular Medicine, 47, 147.
Coban, P., Çe, P., Erkizan, O., & Gedizlioglu, M. (2011). Heat shock protein 27 in migraine patients. Journal of Neurological Sciences [Turkish], 28(1), 28–34.
Davies, S. W., Turmaine, M., Cozens, B. A., et al. (1997). Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell, 90(3), 537–548.
Davies, S. W., Beardsall, K., Turmaine, M., et al. (1998). Are neuronal intranuclear inclusions the common neuropathology of triplet-repeat disorders with polyglutamine-repeat expansions? Lancet, 351, 131.
Desler, C., Lillenes, M. S., Tønjum, T., et al. (2017). The role of mitochondrial dysfunction in the progression of Alzheimer’s disease. Current Medicinal Chemistry. https://doi.org/10.2174/0929867324666170616110111. [Epub ahead of print].
Diedrich, J. F., Carp, R. I., & Haase, A. T. (1993). Increased expression of heat shock protein, transferrin, and beta 2-microglobulin in astrocytes during scrapie. Microbial Pathogenesis, 15, 1–6.
Fiszer, U., Fredrikson, S., & Członkowska, A. (1996). Humoral response to HSP 65 and HSP 70 in cerebrospinal fluid in Parkinson’s disease. Journal of the Neurological Sciences, 139(1), 66–70.
Gómez-Chocoa, M., Doucerain, C., Urra, X., et al. (2014). Presence of heat shock protein 70 in secondary lymphoid tissue correlates with stroke prognosis. Journal of Neuroimmunology, 270(1–2), 67–74.
Halliday, G. M., Holton, J. L., Revesz, T., et al. (2011). Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathologica, 122, 187–204.
Helgeland, G., Petzold, A., Hoff, J. M., et al. (2010). Anti-heat shock protein 70 antibody levels are increased in myasthenia gravis and Guillain-Barré syndrome. Journal of Neuroimmunology, 225(1–2), 180–183.
Hernández-Pedro, N. Y., Espinosa-Ramirez, G., de la Cruz, V. P., et al. (2013). Initial immunopathogenesis of multiple sclerosis: Innate immune response. Clinical and Developmental Immunology. Article ID 413465, 15 pages.
Ho, A. K., & Hocaoglu, M. B. (2011). Impact of Huntington’s across the entire disease spectrum: The phases and stages of disease from the patient perspective. Clinical Genetics, 80(3), 235–239.
Ho, S. L., Poon, C. Y., Lin, C., et al. (2015). Inhibition of β-amyloid aggregation By albiflorin, aloeemodin and neohesperidin and their neuroprotective effect on primary hippocampal cells against β-amyloid induced toxicity. Current Alzheimer Research, 12(5), 424–433.
Huang, C., Cheng, H., Hao, S., et al. (2006). Heat shock protein 70 inhibits alpha-synuclein fibril formation via interactions with diverseintermediates. Journal of Molecular Biology, 364(3), 323–336.
Hung, S. Y., & Fu, W. M. (2017). Drug candidates in clinical trials for Alzheimer’s disease. Biomedical Science, 24(1), 47.
Huntington, G. (1872). Med Surg Report 26, 320.
Jones, G., Song, Y., Chung, S., et al. (2004). Propagation of Saccharomyces cerevisiae [PSI+] prion is impaired by factors that regulate HSP70 substrate binding. Molecular and Cellular Biology, 24(9), 3928–3937.
Kacimi, R., & Yenari, M. A. (2015). Pharmacologic heat shock protein 70 induction confers cytoprotection against inflammation in gliovascular cells. Glia, 63(7), 1200–1212.
Kalmar, B., & Greensmith, L. (2017). Cellular chaperones as therapeutic targets in ALS to restore protein homeostasis and improve cellular function. Frontiers in Molecular Neuroscience, 10, 251. https://doi.org/10.3389/fnmol.2017.00251.
Kandratavicius, L., Hallak, J. E., Carlotti, C. G., et al. (2014). Hippocampal expression of heat shock proteins in mesial temporal lobe epilepsy with psychiatric comorbidities and their relation to seizure outcome. Epilepsia, 55, 1834–1843.
Kazemi-Esfarjani, P., & Benzer, S. (2002). Suppression of polyglutamine toxicity by a Drosophila homolog of myeloid leukemia factor 1. Human Molecular Genetics, 11(21), 2657–2672.
Kenward, N., Hope, J., Landon, M., et al. (1994). Expression of polyubiquitin and heat-shock protein 70 genes increases in the later stages of disease progression in scrapie-infected mouse brain. Journal of Neurochemistry, 62, 1870–1877.
Kim, J. Y., Kim, N., Zheng, Z., et al. (2016). 70kDa heat shock protein downregulates dynamin in experimental stroke: A new therapeutic target? Stroke, 47(8), 2003–2011.
King, C. Y., Tittmann, P., Gross, H., et al. (1997). Prion-inducing domain 2-114 of yeast Sup35 protein transforms in vitro into amyloid-like filaments. Proceedings of the National Academy of Sciences of the United States of America, 94(13), 6618–6622.
Klucken, J., Shin, Y., Masliah, E., et al. (2004). HSP70 reduces alpha-synuclein aggregation and toxicity. The Journal of Biological Chemistry, 279(24), 25497–25502.
Krüger, R., Kuhn, W., Müller, T., et al. (1998). Ala30Pro mutation in the gene encoding alpha synuclein in Parkinson’s disease. Nature Genetics, 18(2), 106–108.
Lu, R. C., Tan, M. S., Wang, H., et al. (2014). Heat shock protein 70 in Alzheimer’s disease. BioMed Research International, 2014, 435203. https://doi.org/10.1155/2014.
Lucchinetti, C., Brück, W., Parisi, J., et al. (2000). Heterogenity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Annals of Neurology, 47(6), 707–717.
Mansilla, M. J., Costa, C., Eixarch, H., et al. (2014). HSP70 regulates immune response in experimental autoimmune encephalomyelitis. PLoS One, 9(8). https://doi.org/10.1371/journal.pone.0105737.
Monsellier, E., Redeker, V., Ruiz-Arlandis, G., et al. (2015). Molecular interaction between the chaperone Hsc70 and the N-terminal flank of huntingtin exon 1 modulates aggregation. The Journal of Biological Chemistry, 290(5), 2560–2576.
Muchowski, P. J., & Wacker, J. L. (2005). Modulation of neurodegeneration by molecular chaperones. Nature Reviews. Neuroscience, 6(1), 11–22.
Munakata, S., Chen, M., Aosai, F., et al. (2008). The clinical significance of anti-heat shock cognate protein 71 antibody in myasthenia gravis. Journal of Clinical Neuroscience, 15(2), 158–165.
Namba, Y., Tomonaga, M., Ohtsuka, K., et al. (1991). HSP 70 is associated with abnormal cytoplasmic inclusions characteristic of neurodegenerative diseases. Nō to Shinkei, 43(1), 57–60.
Patterson, K. R., Ward, S. M., Combs, B., et al. (2011). Heat shock protein 70 prevents both tau aggregation and the inhibitory effects of preexisting tau aggregates on fast axonal transport. Biochemistry, 50(47), 10300–10310.
Pratt, W. B., Gestwicki, J. E., Osawa, Y., et al. (2015). Targeting proteostasis through the protein quality control function of the HSP90/HSP70-based chaperone machinery for treatment of adult onset neurodegenerative diseases. Annual Review of Pharmacology and Toxicology, 55, 353–371.
Prusiner, S. B. (2001). Shattucklecture – neurodegenerative diseases and prions. The New England Journal of Medicine, 344(20), 1516–1526.
Roodveldt, C., Bertoncini, C. W., Andersson, A., et al. (2009). Chaperone proteostasis in Parkinson’s disease: Stabilization of the HSP70/alpha-synuclein complex by Hip. The EMBO Journal, 28(23), 3758–3770.
Sabirzhanov, B., Stoica, B. A., Hanscom, M., et al. (2012). Over-expression of HSP70 attenuates caspase-dependent and caspase-independent pathways and inhibits neuronal apoptosis. Journal of Neurochemistry, 123(4), 542–554.
Selmaj, K., Brosnan, C. F., & Raine, C. S. (1991). Immunology. Proceedings of the National Academy of Sciences of the United States of America, 88, 6452–6456.
Shevtsov, M. A., Nikolaev, B. P., Yakovleva, L. Y., et al. (2014). Neurotherapeutic activity of the recombinant heat shock protein HSP70 in a model of focal cerebral ischemia in rats. Drug Design Development and Therapy, 8, 639–650.
Suhr, S. T., Senut, M. C., Whitelegge, J. P., et al. (2001). Identities of sequestered proteins in aggregates from cells with induced polyglutamine expression. The Journal of Cell Biology, 153(2), 283–294.
Tagawa, K., Marubuchi, S., Qi, M. L., et al. (2007). The induction levels of heat shock protein 70 differentiate the vulnerabilities to mutant huntingtin among neuronal subtypes. The Journal of Neuroscience, 27(4), 868–880.
Talla, V., Porciatti, V., Chiodo, V., et al. (2014). Gene therapy with mitochondrial heat shock protein 70 suppresses visual loss and optic atrophy in experimental autoimmune encephalomyelitis. Investigative Ophthalmology & Visual Science, 55(8), 5214–5226.
Tamguney, G., Giles, K., Glidden, D. V., et al. (2008). Genes contributing to prion pathogenesis. The Journal of General Virology, 89, 1777–1788.
Vonsattel, J. P., & DiFiglia, M. (1998). Huntington disease. Journal of Neuropathology and Experimental Neurology, 57(5), 369–384.
Whyte, L. S., Lau, A. A., Hemsley, K. M., et al. (2017). Endo-lysosomal and autophagic dysfunction: A driving factor in Alzheimer’s disease? Neurochemistry, 140(5), 703–717.
Yon, M. I., Titiz, A. P., Bilen, S., et al. (2016). Elevated interictal serum HSP-70 levels as an indicator of neurodegeneration for chronic migraine. The Journal of the Pakistan Medical Association, 66(6), 677–681.
Zhou, Y., Gu, G., Goodlett, D. R., et al. (2004). Analysis of alpha-synuclein-associated proteins by quantitative proteomics. The Journal of Biological Chemistry, 279(37), 39155–39164.
Zoghbi, H. Y., & Orr, H. T. (2000). Glutamine repeats and neurodegeneration. Annual Review of Neuroscience, 23, 217–247.
Acknowledgements
We would like to thank the editorial staff for the opportunity of being able to be among the authors of the book.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Ortan, P., Akan, O.Y., Hosgorler, F. (2018). Heat Shock Protein70 in Neurological Disease. In: Asea, A., Kaur, P. (eds) HSP70 in Human Diseases and Disorders. Heat Shock Proteins, vol 14. Springer, Cham. https://doi.org/10.1007/978-3-319-89551-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-89551-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-89550-5
Online ISBN: 978-3-319-89551-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)